Abstract
Purpose
Natural killer T (NKT) cells are important mediators of anti-tumor immune responses. We have previously shown that ovarian cancers shed the ganglioside GD3, which inhibits NKT cell activation. Ovarian cancers also secrete high levels of vascular endothelial growth factor (VEGF). In this study, we sought to test the hypothesis that VEGF production by ovarian cancers suppresses NKT cell-mediated anti-tumor responses.
Experimental Design
To investigate the effects of VEGF on CD1d-mediated NKT cell activation, a conditioned media model was established wherein the supernatants from ovarian cancer cell lines (OV-CAR-3 and SK-OV-3) were used to treat CD1d-expressing antigen presenting cells (APC) and co-cultured with NKT hybridomas. Ovarian cancer associated-VEGF was inhibited by treatment with Bevacizumab and Genistein, conditioned medium was collected and CD1d-mediated NKT cell responses were assayed by ELISA.
Results
Ovarian cancer tissue and ascites contain lymphocytic infiltrates, suggesting that immune cells traffic to tumors, but are then inhibited by immunosuppressive molecules within the tumor microenvironment. OV-CAR-3 and SK-OV-3 cell lines produce high levels of VEGF and GD3. Pretreatment of antigen presenting cells with ascites or conditioned medium from OV-CAR-3 and SK-OV-3 blocked CD1d-mediated NKT cell activation. Inhibition of VEGF resulted in a concomitant reduction in GD3 levels and restoration of NKT cell responses.
Conclusions
We found that VEGF inhibition restores NKT cell function in an in-vitro ovarian cancer model. These studies suggest that the combination of immune modulation with anti-angiogenic treatment has therapeutic potential in ovarian cancer.
Introduction
In the United States, ovarian cancer is the fifth most common cause of cancer death among women (1). In fact, >120,000 women worldwide die each year from this disease that has the highest fatality-to-incidence of all gynecologic malignancies (2). The major clinical challenge for this disease is that the majority of patients present with late stage disease − 70% of patients have stage III or IV disease at the time of diagnosis. Despite improvements in treatment, even with aggressive cytoreduction combined with chemotherapy, five- year survival rates of patients with advanced ovarian cancer remain less than 50% (3, 4). The lack of effective treatment options for relapse requires the development of alternative interventions against this recalcitrant disease.
In ovarian cancer, immune function is central to response to treatment and prognosis (5-11). Several groups have reported that long-term survivors (>10 years) have higher levels of T cell infiltrates in their tumors. However, the immune response is more nuanced. The presence or absence of specific T cells subsets has been correlated to survival (7). Tumor infiltration by regulatory T cells (CD4+CD25+ T cells) is indicative of reduced survival, whereas the presence of intraepithelial CD8+ T cells is associated with favorable prognosis in ovarian cancer (8).
Escape from the host's immune system is crucial for cancer growth and development of metastasis. Identification of immunosuppressive factors produced within the tumor microenvironment, and the ability to target these factors could enhance anti-tumor immune responses. Several studies have focused on tumor-associated immune suppression mediated by T regulatory (Treg) cells, myeloid derived suppressor cells (MDSC), immunosuppressive dendritic cells, immune-inhibitory receptors, and inhibitory factors, including TGF-β, prostaglandins, and adenosine (12-17). In addition, components of ovarian ascites fluid have also been shown to inhibit immune function (18). Recently, it was reported that phosphatidylserine present in extracellular vesicles (EV) harvested from ovarian tumor ascites fluids and from solid ovarian tumors induces TCR signaling arrest (19). In addition, we have shown that ganglioside (GD3) produced by ovarian cancer cells is present in ascites fluid and can inhibit antitumor natural killer T (NKT) cell responses (20). Similarly, it has been reported that higher levels of gangliosides, specifically GD3, are present in sera of ovarian cancer patients compared to healthy donors due to ganglioside shedding from the surface of tumor cells (21).
VEGF levels in the ascites of ovarian cancer patients are much higher (up to tenfold higher) than levels in ascites associated with other solid tumors (22). These high ascites VEGF levels in patients with ovarian cancer have also been shown to be inversely correlated with survival (23, 24), correlate directly with invasion and metastasis of ovarian cancer cells and further play a role in the formation of ovarian cancer related ascites (25, 26). Huang and colleagues demonstrated that low-dose anti-VEGF antibody therapy helps to facilitate the penetration of immune effector elements into the tumor parenchyma (27, 28). VEGF also reduces T cell cytotoxic activity increasing VEGF production by tumor cells, creating a negative feedback loop that my be harnessed to improve treatment or help overcome chemotherapy resistance (29, 30). In the present study, we sought to characterize the interaction between angiogenesis and immune function in ovarian cancer cells.
Materials and Methods
Patient samples
Ovarian cancer associated ascites were collected from patients undergoing primary cytoreductive surgery by the Kelly Gynecologic Oncology Service at Johns Hopkins Medical Institutions and at the Marlene and Stewart Greenebaum Cancer Center at the University of Maryland School of Medicine. All donors gave written informed consent before enrolling in the study. The Institutional Review Boards of Johns Hopkins Medical Institutions and the University of Maryland Medical Center approved this investigation. Ovarian cancer samples were obtained from the tissue bank and 4μm-sections of paraffin-embedded tissues were deparaffinized and rehydrated, and an antigen retrieval procedure performed according the manufacturer's instructions using Target-Retrieval Solution (DAKO).
Cell Lines
Human ovarian cancer cells, SK-OV-3, and endometrial cancer cell line, HEC-1A, were cultured in McCoy's 5a Modified Medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. OV-CAR-3, purchased from the ATCC (Manassas, VA), was grown in in RPMI-1640 medium supplemented with 20% fetal bovine serum, 0.01 mg/mL bovine insulin, and penicillin/streptomycin. The cell lines were used within six months of purchase.
Murine L cells transfected with vector alone (Lvector) or the WT cd1d1 cDNA in pcDNA3.1-neo (Invitrogen) (LCD1dwt) were kindly provided by R.R. Brutkiewicz (Indiana University School of Medicine, Indianapolis, IN) (31), and were cultured in DMEM media, supplemented with 2 mM L-glutamine, 10% FBS, 500 μg/mL G418 and penicillin/streptomycin. The cell lines used have been tested and authenticated routinely by staining for stable cell surface expression of CD1d, compared to isotype control staining, and also compared to cells stably transfected with the empty control vector.
The Vα14+ NKT cell hybridoma cell lines DN32.D3, (32, 33), N38-2C12, N38-2H4, N38-3C3, and the CD1d-specific NKT cell hybridoma N37-1A12 (Vα5+), have all been described (34) and were cultured in IMDM medium supplemented with 5% FBS and 2 mM L-glutamine. The NKT cells are tested for specificity to CD1d in each experiment via functional T cell assay.
VEGF inhibition
VEGF was purchased from Sigma (#V7259) and reconstituted as per manufacturer's instructions. Genistein was purchased from Sigma (#G6649) and reconstituted in DMSO. Bevacizumab/ Avastin (Genentech) was supplied reconstituted by the manufacturer in water supplemented with salts. OV-CAR-3 cells were seeded in a flask, allowed to grow to 70% confluence and subsequently treated with increasing concentrations of Genistein and vehicle (DMSO) for 3 days, with daily medium and drug change, followed by a 24 hour fresh medium recovery phase. Similarly, OV-CAR-3 cells were treated with Bevacizumab and vehicle (PBS) for 3 days with daily medium and drug exchange. Following a 24-hour recovery period, supernatants were harvested and used in conditional medium experiments.
Inhibition of GD3
OV-CAR-3 cells were infected with adenovirus encoding for human NEU3 (AdNEU3) or GFP plasmid (AdGFP), as a control at a multiplicity of infection (MOI) of 100 for 20-24h at 37°C. Plasmid construction and packaging are previously described (35). OV-CAR-3 cells were grown to confluence and allowed to recover for one day with fresh medium not containing the virus. Medium was collected and used in conditioned medium experiments as described.
Generation of artificial APC
The preparation of CD1d-Ig–based aAPC was carried out according to the previously described method (36). The hCD1d-aAPCs were loaded with lipid antigen and α-GalCer (5 μg/mL in 1 mL PBS containing 5 × 107 beads).
Treatment of cells with tumor-cell supernatants
The APCs were treated with the clarified supernatants for 4 hours at 37°C, unless otherwise indicated. The APCs were subsequently washed extensively with PBS, and cocultured with NKT hybridomas (0.5-1 X 105) for 20 to 24 hours at 37°C. Cytokine release was measured as an indication of NKT cell activation and was measured by standard sandwich ELISA (Biolegend). For the ovarian cancer cocultures, ovarian cancer cell lines were fixed in 0.05% paraformaldehyde for 20 min at room temperature, washed, pulsed with α-GC and co-cultured with NKT cells. Supernatants were harvested after 16 hours. The VEGF ELISA was performed according the manufacturer's instructions (R&D Systems).
Immunoblotting
L-CD1d cells were pretreated with the indicated ascites for 4 hours. The cells were washed in PBS and lysed. Equal amounts of protein were then resolved on a 10% SDS-PAGE gel and transferred to a polyvinylidene difluoride membrane. The blot was processed using anti-p38, anti-JNK, or anti-ERK1/2 Abs specific for the phosphorylated forms and developed using chemiluminescence before exposure on film. The blot was then stripped and reprobed with Abs for the detection of total p38 and ERK1/2 (Cell Signaling Technologies).
Intracellular Staining
Intracellular staining was performed according to the BD Transcription Buffer Set (BD Pharmingen) protocol. GD3 mAb (Abcam) was used at a concentration of 1 μg/mL. ERK and pERK antibodies were purchased from Cell Signaling and were used at 1:50 dilution. Secondary antibody for GD3 (PE-anti-mouse IgG; Biolegend); secondary antibody for ERK/pERK (APC-anti-rabbit IgG; Life Technologies) were used at a 1:50 dilution. Isotype control Abs (mouse IgG3 and rabbit IgG purchased from Biolegend) were used as indicated.
Statistical analysis
Data analysis was conducted by Prism software (version 5.02 for Windows; GraphPad). Parametric statistics were used to analyze differences between experimental groups, when needed. Where multiple groups existed within a single experiment, multiple between-group comparisons were made by ANOVA with the Bonferroni post-test, with the following designations for p values: *p<0.05, **p<0.01, and ***p<0.001. A p value less than 0.05 was considered significant. The error bars in the bar graphs indicate S.E.M.
Results
Conditioned medium from ovarian cancer cell lines inhibit NKT cell activation
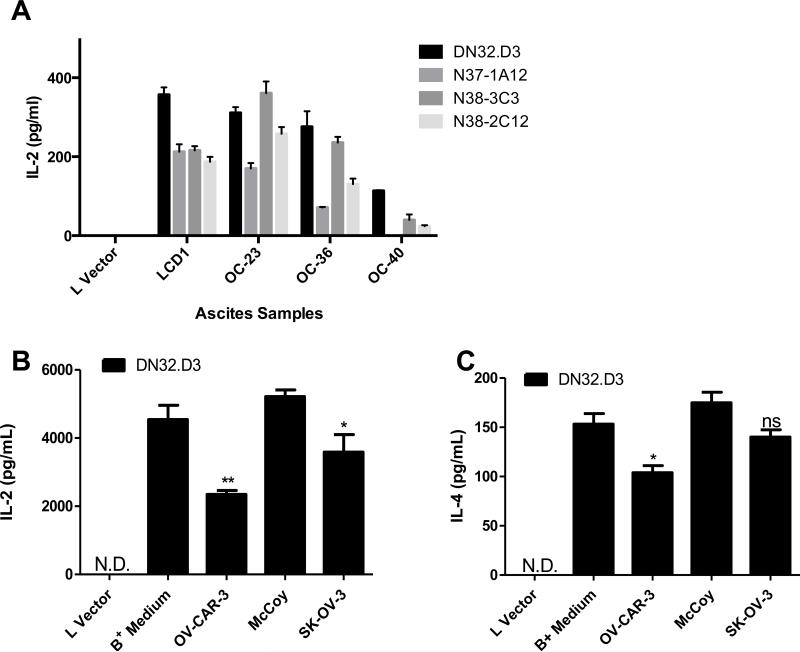
We previously reported that ovarian cancer tumor cells in ascites fluid shed soluble factors that inhibit CD1d-mediated activation of NKT cells (18, 20). In the current study, we confirmed those findings (Fig 1A) and examined whether treatment with conditioned medium from ovarian cancer cell lines would inhibit the ability of CD1d-expressing cells to stimulate NKT hybridomas. Mouse fibroblasts expressing high levels of CD1d (LCD1dwt) were incubated with cell-free supernatants from ovarian cancer cell lines OV-CAR-3 and SK-OV-3 cultured to confluence. Treatment with conditioned medium treatment inhibited NKT cell activation, as evidenced by decreased IL-2 (Fig 1B) and IL-4 (Fig 1C) production. These data demonstrate established ovarian cancer cell lines secrete soluble factors that block CD1d-mediated antigen presentation to NKT cells.
Figure 1. Tumor Ascites and Conditioned medium from ovarian cancer cell lines inhibits NKT cell activation.
(A) LCD1d cells were treated with control medium or with ovarian cancer ascites fluid from patients for 4 h, then washed extensively and cocultured with a panel of NKT cell hybridomas (DN32.D3, N37-1A12, N38-3C3 and N38-2C12). After 20-24 h, IL-2 was measured as an indication of NKT cell activation using standard cytokine ELISA. (B, C) LCD1d cells were treated with supernatants from confluent ovarian cancer cell lines OVCAR-3 and SK-OV-3 for 4 hours at 37°C and washed extensively following treatment. Control LCD1d cells were concurrently treated with RPMI (B+ Medium) and McCoy media. Lvector cells serve as a negative control. N.D.=not detectable. Following treatment, LCD1d cells were co-cultured with NKT cell hybridomas, DN32.D3, and incubated for 24 hours at 37° C. Standard ELISA was performed to measure cytokine production (B) IL-2 (C) IL-4. Data are shown as mean ±S.E.M. of one experiment set up in triplicate. The experiments were performed at least twice with each ascites sample and three times with conditioned medium. T-tests were performed to compare medium vs. conditioned medium groups, yielding p-value of p=0.0061 for IL-2 and p=0.0187 for IL-4.
Lymphocytes are present within the tumor microenvironment
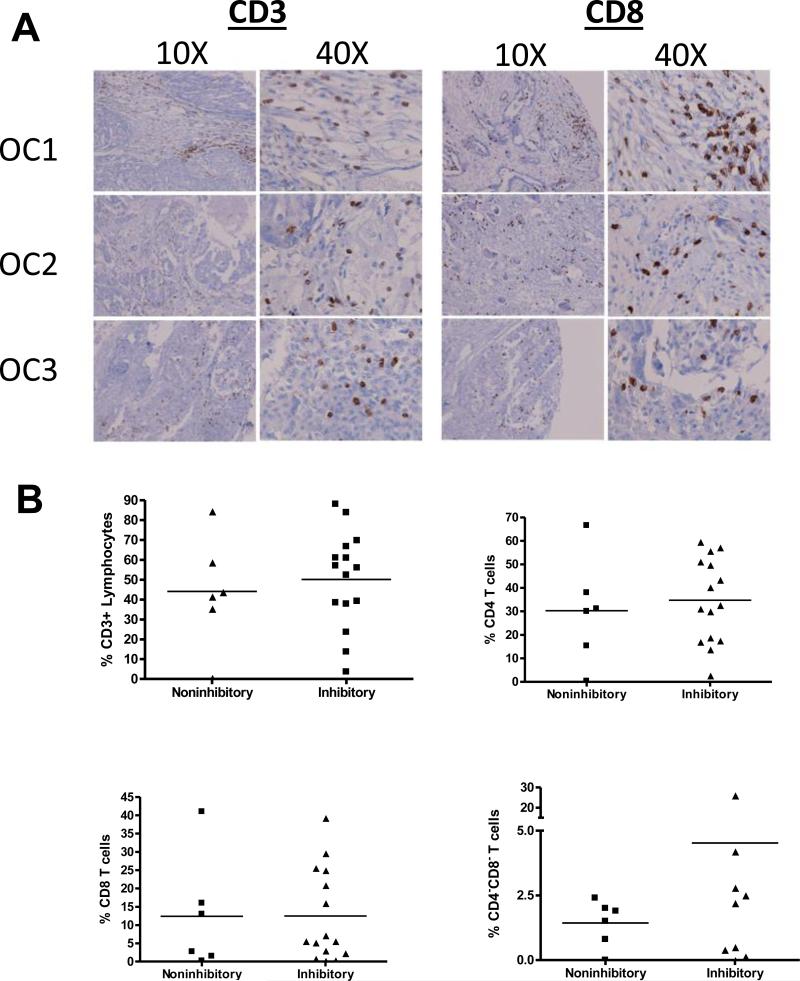
We next assessed whether there were T cells within the tumor microenvironment that could be influenced by the immunosuppressive factors produced by ovarian cancers. Ovarian cancer samples obtained from the tissue bank were examined for CD3 and CD8 expression. As shown in Figure 2A, large numbers of lymphocytes infiltrate the tumor microenvironment. Of note, there was a higher percentage of CD4−CD8− double negative T lymphocytes present in NKT cell inhibitory ascites, compared to non-inhibitory ascites fluid (Fig 2B). These data suggest that lymphocytes within the tumor microenvironment may be actively suppressed by factors produced by ovarian cancers. To define the factors responsible for inhibiting CD1d-mediated NKT cell activation, ovarian cancer associated-ascites was passed through size exclusion columns and treated with proteinase K (Supplemental Figures 1 A-C). Filtration of the ascites did not alter its inhibitory properties; however, treatment with proteinase K resulted in a loss of suppressive activity. These data suggest that in addition to the previously identified factor, ganglioside G3, one or more other soluble factor(s), likely a protein, produced by ovarian cancers modulates CD1d-mediated presentation to NKT cells.
Figure 2. Lymphocytes are present within the tumor microenvironment.
(A) Representative images of patient tissue sections from confirmed cases of ovarian cancer were stained for CD3 and CD8. (B) Flow cytometry was performed to assess T cell subpopulations within the ascites. CD1d-expressing cells were treated with cell-free ascites for 4 hours, then washed extensively and cocultured with a panel of NKT cell hybridomas. Samples that caused <10% inhibition of IL-2 production by NKT cells were deemed non-inhibitory, the others are referred to as inhibitory.
High VEGF levels in patient ascites and ovarian cancer cell conditioned medium
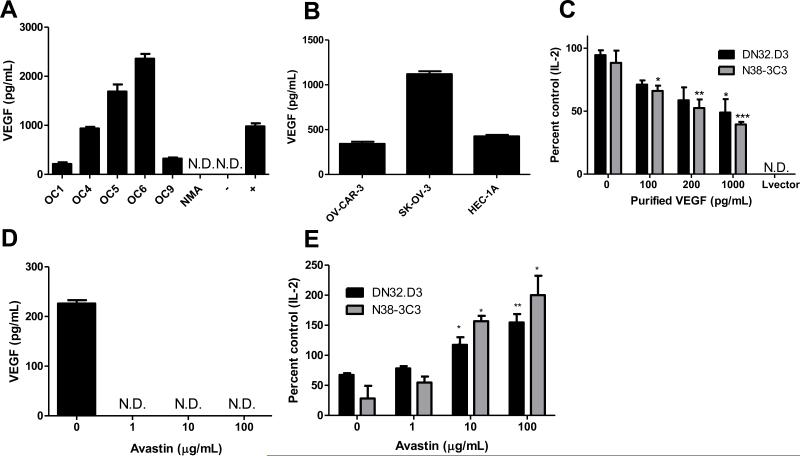
Given the critical role of growth factors in the biology of epithelial ovarian cancer, we measured VEGF levels in ascites fluid and conditioned medium. In Fig 3A, donors OC1, OC4, OC5, and OC9 had primary disease. Patient OC6 had recurrent disease and another donor was diagnosed as low malignant potential (NMA). VEGF was present in the ascites of ovarian cancer patients (Fig 3A) and conditioned medium from ovarian cancer cell lines (Figure 3B). HEC-1-A, an endometrial cancer cell line was included as it has been reported to secrete VEGF (37). Moreover, treatment of CD1d-expressing cells with comparable levels of recombinant VEGF resulted in a dose-dependent decrease in NKT cell activation (Figure 3C).
Figure 3. VEGF inhibits NKT cell function.
(A) VEGF levels in ovarian cancer patient ascites and (B) OV-CAR-3, SK-OV-3 and endometrial cancer cell lines HEC-1 were measured by ELISA. In (A), non-malignant ascites (NMA) represents VEGF levels in non-cancer-associated ascites. The negative control is the assay diluent used in the ELISA assay and the positive control was recombinant VEGF (1000 pg/mL). ND=not detectable. (C) LCD1d cells were treated with the indicated concentrations of recombinant VEGF for 4 hours, then washed extensively and cocultured with NKT cell hybridomas, DN32.D3 and N38-3C3. (D) OV-CAR-3 cells were treated with increasing concentrations of Bevacizumab/Avastin and VEGF levels in the cell culture supernatant were assessed after 24 hours by ELISA. (E) OV-CAR-3 cells were treated with increasing concentrations of Bevacizumab/Avastin for 3 days, following a one-day recovery period in fresh culture medium. Pretreatment of CD1d-expressing cells with conditioned medium were performed as described above. ANOVA with Bonferroni post-test confirmed significance. *p<0.05; **p<0.01; and ***p<0.001 for treatment groups compared to control.
VEGF inhibition in ovarian cancer cells restores NKT responses
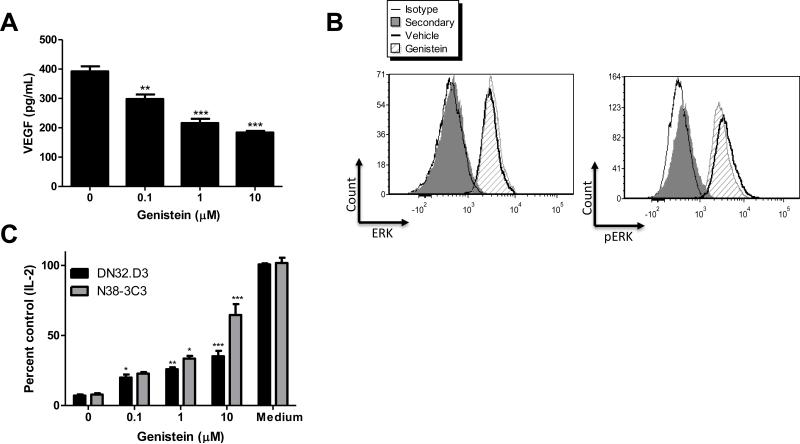
To confirm a role for VEGF in suppressing NKT cell function, we introduced a neutralizing anti-VEGF antibody, Bevacizumab/Avastin (Figure 3D). Immuno-blockade of VEGF dose-dependently restored NKT cell function, indicating that VEGF is directly responsible for NKT cell suppression (Figure 3E). To further establish a role for ovarian cancer-associated VEGF in suppressing NKT cell function, we used a flavonoid known to inhibit VEGF in ovarian cancer cells lines, Genistein (Figure 4A) (38). Genistein is a tyrosine kinase inhibitor (Figure 4B) and similar to Avastin, treatment of ovarian cancer cell lines with Genistein restored CD1d-mediated antigen presentation to NKT cells (Figure 4C).
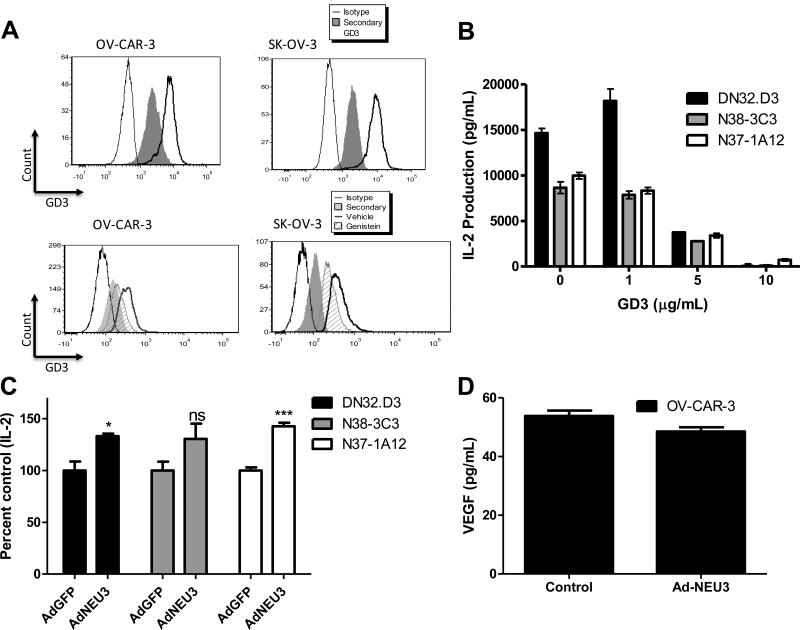
Figure 4. Inhibiting VEGF blocks GD3 in ovarian cancer cells.
(A) OV-CAR-3 cells were treated with Genistein and VEGF levels in cell culture supernatants were measured 24 hours post treatment. (B) As a control for Genistein, ERK and phosphorylated ERK (pERK) were assessed by flow cytometry in OV-CAR-3 cells treated with 10 μM Genistein for 24 hours. (C) OV-CAR-3 cells were treated with increasing concentrations of Genistein or vehicle (DMSO) for 3 days, followed by a one-day recovery period. Conditioned medium from the vehicle and Genistein-treated cells were used to treat LCD1d cells for 4 hours. Following treatment, LCD1d cells were co-cultured with NKT cell hybridomas DN32.D3 and N38-3C3. IL-2 production was measured by standard ELISA. ANOVA compared treatment groups to the vehicle-treated group and Bonferroni post-test confirmed significance.
Previous studies showed that the ganglioside GD3 in ovarian cancer ascites was responsible, at least in part, in inhibiting CD1d-mediated NKT cell activation (20). Accordingly, we examined ovarian cancer cell lines OV-CAR-3 and SK-OV-3 for GD3 expression. As shown in top panels of Fig 5A, flow cytometric analysis indicated that GD3 is present in these cells. We then asked whether VEGF and ganglioside synthesis pathways might be linked, working in tandem to suppress immune responses. To establish crosstalk between VEGF and GD3, we asked whether VEGF inhibition alters GD3 expression in ovarian cancer cell lines. We found that GD3 expression was reduced after 72 hours of Genistein treatment (Figure 5A), and treatment with GD3 inhibited CD1d-mediated NKT cell activation (Fig 5B). To establish that Genistein-mediated GD3 inhibition is responsible for restoring NKT cell responses, we overexpressed the plasma membrane-associated sialidase NEU3 in ovarian cancer cells. NEU3 has been shown to decrease GD3 (39). Following infection with adenovirus encoding for human NEU3 (AdNEU3), we harvested cell culture supernatants and utilized the conditioned medium. Supernatants from NEU3-overexpressing cells inhibited NKT cell function to a lesser extent than did controls (Figure 5C). Other groups have shown an activating role for GD3 (40, 41). We hypothesized that the source of GD3 may influence its functional impact. We treated CD1d-expressing cell lines and artificial antigen presenting cells (aAPC) with GD3 from different sources. It was found that GD3 from bovine brain (source of our original stock) was inhibitory, compared to GD3 isolated from buttermilk (Supplemental Figures 2A-B). In addition, we examined whether there may be reciprocal regulation between VEGF and GD3 by comparing VEGF levels in conditioned medium from ovarian cancer cells infected with control adenovirus and AdNEU3. However, VEGF levels were similar in the presence and absence of NEU3 ectopic expression (Fig 5D). Taken together, these data suggest that VEGF can modulate GD3 expression and confirm that ovarian cancer-associated GD3 is responsible for suppressing CD1d-mediated NKT cell activation.
Figure 5. Ovarian cancer-associated GD3 inhibits NKT cell responses.
(A) GD3 expression in ovarian cancer cell lines was assessed using flow cytometry. OV-CAR-3 and SK-OV-3 cells were treated with vehicle or 10 μM Genistein for 72 hours. Cells were fixed, permeabilized, and stained either with primary anti-GD3 antibody alone or with primary and PE-conjugated secondary antibodies. (B) CD1d-expressing cells were treated with GD3 at the indicated concentrations for 4 hours, washed and co-cultured with NKT cell hybridomas, DN32.D3, N37-1A12, and N38-3C3. Treatment with GD3 significantly inhibited CD1d-mediated NKT cell activation. (C) Adenovirus encoding for human NEU3 (AdNEU3) or GFP (AdGFP) was used to infect OV-CAR-3 cells. Following infection and a one-day recovery period, supernatants from confluent cells were used in conditioned medium experiments. ANOVA compared treatment groups to the vehicle-treated group, with Bonferroni post-test confirming significance. *p<0.05 and ***p<0.001 for AdNEU3 compared to AdGFP. (D) Following infection and a one-day recovery period, supernatants from confluent cells were assessed for VEGF levels.
Ovarian cancer cells can act as antigen presenting cells to NKT cells
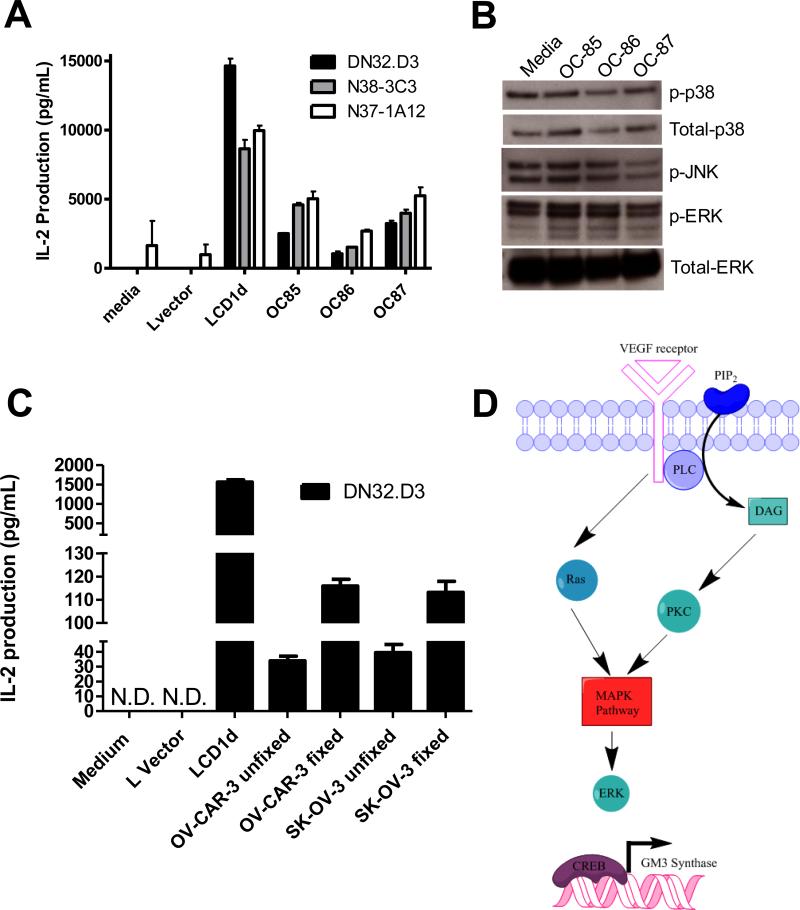
We have found that conditioned medium from ovarian cancer cell lines and ascites fluid (Fig 6A) inhibits CD1d-mediated NKT cell activation. In addition to studying active competition for glycolipid antigen bound to CD1d (20), we investigated whether treatment with ascites fluid directly modulated MAPK signaling cascades. As shown in Fig 6A, pretreatment with ascites from patients OC85-87 inhibited CD1d-mediated NKT cell activation; however, we observed ascites-specific differences in MAPK activation (Figure 6B). Treatment with OC-85 resulted in an increase in p38, OC-86 had a decrease in ERK, and OC87 had a decrease in phosphorylated JNK. Ovarian cancers express CD1d and utilize different pathways to evade immune detection. We asked whether ovarian cancers could serve as antigen presenting cells in the absence of these soluble factors. Ovarian cancer cell lines, OV-CAR-3 and SK-OV-3, were fixed with paraformaldehyde and co-cultured with NKT cells (Figure 6C). Importantly, we found that fixation of ovarian cancer cell lines resulted in a >2 fold increase in their ability to activate NKT cells.
Figure 6. Ovarian cancer cells can present antigen to NKT cells.
(A) LCD1dwt cells were treated with control medium or with ovarian cancer ascites fluid from patients for 4 h, then washed extensively and cocultured with a panel of NKT cell hybridomas (DN32.D3, N38-3C3 and N37-1A12). After 20-24 h, IL-2 was measured as an indication of NKT cell activation using standard cytokine ELISA. (B) Ascites pretreatment induces MAPK signaling. LCD1d1wt cells were incubated with ascites for 4 hours, the cells were lysed and then equal amounts of protein were loaded per well for the detection of phosphorylated and total p38, phosphorylated JNK, and ERK1/2 expression by Western blot analysis. (C) Fixation of OV-CAR-3 and SK-OV-3 cells restored their antigen presentation capabilities. The ovarian cancer cells were fixed in 0.05% paraformaldehyde, pulsed with α-GC and co-cultured with NKT cells. Supernatants were harvested after 16 hours. (D) Proposed model of crosstalk between the VEGF, MAPK, and GD3 signaling pathways. We postulate that activation of VEGF receptor signaling leads to the activation of MAPK signaling. MAPK signaling can induce GM3 synthase, which will lead to the production of GD3. Fixation of the ovarian cancer cells will prevent the induction of these signaling cascades, thereby removing these immunosuppressive factors from the microenvironment and allowing the presentation of glycolipid antigen to NKT cells.
In summary, we have identified a novel immunomodulatory link between VEGF and ganglioside biosynthesis pathways in ovarian cancer. In our proposed model (Fig 6D), we postulate that activation of VEGF receptor signaling activates of MAPK signaling, which in turn induces GM3 synthase. GM3 synthase produces the direct precursor to GD3, thereby leading to the synthesis of immunosuppressive GD3. Blockade of VEGF signaling therefore depletes the precursor pool and results in a decrease in GD3 shedding.
Discussion
Here, we report that treatment of CD1d-expressing cells with conditioned medium from human ovarian cancer cell lines abrogated their ability to activate both canonical and noncanonical NKT cells. Mechanistically, we found that inhibiting VEGF resulted in a decrease in ganglioside GD3 expression and restoration of NKT cell responses. In addition, we addressed the link between VEGF and lipid signaling and demonstrated that tumors may utilize multiple signaling pathways to achieve escape from immune surveillance. We have identified a novel mechanism by which angiogenic signaling pathways contribute to immune suppression through alteration of the lipid repertoire, with VEGF serving as one of the modulators of the lipid rheostat.
The level of VEGF in ovarian cancer serum and ascites fluid can be directly related to disease burden, and is inversely related to survival (42-44), thus targeting VEGF has become important to treatment of ovarian cancer. Preclinical studies with anti-VEGF antibodies have shown that inhibiting VEGF blocks angiogenesis and the formation of ascites (45). Bevacizumab has shown great promise in the treatment of recurrent and metastatic disease (46-49). Bevacizumab has also been used to palliate fluid accumulation in patients with ovarian cancer associated-ascites (30). In contrast, Gourley et al. identified a molecular signature within a subset of 284 high-grade serous cancers from the ICON7 trial in which antiangiogenic therapy might actually confer a worse progression-free survival (PFS) and overall survival (OS) when compared with chemotherapy alone (50). Specifically, in this study mRNA from 265 HGSOCs from Scottish patients was compared to 283 UK samples from the ICON7 study in which patients were treated first line with paclitaxel/carboplatin +/− concomitant and maintenance with Bevacizumab for 12 months. The authors reported a 63-gene signature that identified an immune subgroup that had superior PFS and OS when compared with the two-proangiogenic subgroups combined, but which showed a decrease in survival when treated with Bevacizumab. These studies are informative and require further investigation.
Ascites, a clinical hallmark of ovarian cancer, reportedly predicts treatment benefit for Bevacizumab in epithelial ovarian cancer (51). More than one third of ovarian cancer patients present with ascites. Several types of pro-inflammatory and tumor-promoting factors have been identified in ovarian cancer ascites fluid. For example, pro-inflammatory cytokines IL-6 and IL-10 are detectable in ascites. Of the multitude of cytokines present in ascites (52), several have been shown to inhibit T cell function. Alteration of T cell function by ovarian cancer cells and ascites is known to contribute to poor prognosis (20, 23). Based on the well-described relationship between advanced ovarian cancer, ascites, VEGF and T cell function, we postulated that VEGF plays a central role in mediating this immune response. Our data support the concept that inhibition of VEGF not only affects angiogenesis, but also has an unexpected effect on the shedding of GD3. Notably, ovarian cancer is heterogeneous and the genomic landscape of epithelial ovarian cancer varies depending on the tumor stage, grade, and sensitivity to chemotherapy. Due to tumor cell heterogeneity and redundancy in angiogenic pathways, it is likely that combining therapies, such as targeted therapy and immunotherapy, will be necessary to overcome resistance. Further studies are needed and biomarkers to help identify individuals most likely to benefit from such therapies are essential.
In other disease states, interactions have been reported between VEGF and immune function. A positive correlation between peripheral blood Treg concentrations and baseline VEGF has been demonstrated in stage IV melanoma patients (53). In good agreement, a recent study by Farsaci et al. (54) showed that using antiangiogenic tyrosine kinase inhibitors in combination with a therapeutic vaccine increased CD3+ tumor infiltrating lymphocytes (TILs) and tumor antigen–specific CD8+ T cells. Furthermore, Gavalas et al. demonstrated that ascites-derived VEGF directly suppressed T cell activation and reduces T cell proliferation in a dose-dependent manner (55). Conversely, blockade of VEGF receptor on the surface of T cells restored T cell proliferation. Moreover, T cell-mediated cytotoxicity was suppressed by the addition of VEGF. Taken together, these studies implicate a role for VEGF in directly modulating T cell responses in ovarian cancer.
To determine whether VEGF might have comparable effects on NKT cells, we ascertained that primary human NKT cells express the VEGF receptor (data not shown). This finding suggests that VEGF may suppress NKT cell function both directly (via binding to VEGF receptor on NKT cell surface) and indirectly (by altering tumor ganglioside shedding). Future studies will determine whether VEGF functions through inhibition of NKT cells directly, or through alteration of antigen presentation. These studies suggest that targeting of tumor VEGF production is a rational approach to restore anti-tumor immune responses.
It is well known that tumors alter between distinct different pathways can promote tumorigenesis. VEGF receptor engagement activates ERK and may thus be responsible for activation of GM3 synthase-mediated synthesis of the direct precursor to GD3. Chung et al. demonstrated that ERK is responsible for activation of GM3 synthase (56). VEGF inhibition may suppress GM3 activity and thus deplete precursor pool, leading to a decrease in GD3 levels. Notably, ovarian cancer associated GD3 may undergo modifications, such as acetylation, that cause immunosuppression. We have tested GD3 preparations from different labs and different companies and have obtained strikingly distinct results with comparable amounts of lipid.
In summary, we have found that VEGF inhibition suppresses GD3 and hypothesize that VEGF receptor-mediated activation of ERK induces ganglioside shedding by ovarian cancer cells. However, further work elucidating the link between VEGF and GD3 axis is needed. Finally, fixation of ovarian cancer cells, which abrogated their VEGF secretion, restored their ability to present antigen to NKT cells. These data demonstrate that VEGF suppresses NKT cell function and that modulation of VEGF secretion via Genistein and direct blockade of VEGF with Bevacizumab restores NKT cell responses.
Supplementary Material
Translational Relevance.
Vascular endothelial growth factor (VEGF) inhibition has become important to treatment of ovarian cancer. VEGF expression is inversely correlated with survival in ovarian cancer patients. Ovarian cancer associated ascites contains much higher levels of VEGF compared to ascites found in patients with other solid tumors. Bevacizumab, a monoclonal antibody that binds to VEGF and prevents the interaction of VEGF with its receptors, has demonstrated activity in the treatment of recurrent and metastatic ovarian cancer. Bevacizumab is also useful in palliation of ascites in patients with advanced and recurrent ovarian cancer where ascites is a hallmark of disease. Since avoiding detection by the host's immune system is crucial for the growth and metastasis of cancer, we sought to examine the effects of ovarian cancer associated VEGF on CD1d-mediated antigen presentation to natural killer T (NKT) cells. We found that inhibiting VEGF secretion by ovarian cancer cell lines restored NKT cell activation. Herein, we demonstrate a novel link between immunosuppressive ganglioside shedding and VEGF production by ovarian cancers. By establishing a mechanism through which VEGF impairs anti-tumor immune responses, our studies have the potential to enhance the clinical therapeutic possibilities for women with this disease.
Acknowledgements
The authors would like to thank the patients who allowed their samples to be studied. The authors would also like to thank Dr. Hans Spiegel for careful reading of the manuscript and helpful critiques.
Grant Support
This work was supported by grants from the HERA foundation, and NIH/NCI K01 CA131487, R21 CA162273, R21 CA16227 to T.J. Webb, 2004 Gynecologic Cancer Foundation/Ann Schreiber Ovarian Cancer Research Grant to R.L. Giuntoli II, and NIH AI 44129, CA 108835, and P01 AI072677 to J.P. Schneck.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors (TJW, MO, & JPS) have a patent on the use of GD3 as a biomarker or therapeutic target for ovarian cancer. The other authors have no competing financial interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Scarlett UK, Conejo-Garcia JR. Modulating the tumor immune microenvironment as an ovarian cancer treatment strategy. Expert Rev Obstet Gynecol. 2012;7:413–9. doi: 10.1586/eog.12.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heintz AP, Odicino F, Maisonneuve P, Beller U, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. Int J Gynaecol Obstet. 2003;83(Suppl 1):135–66. doi: 10.1016/s0020-7292(03)90118-4. [DOI] [PubMed] [Google Scholar]
- 4.Chu CS, Kim SH, June CH, Coukos G. Immunotherapy opportunities in ovarian cancer. Expert Rev Anticancer Ther. 2008;8:243–57. doi: 10.1586/14737140.8.2.243. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 6.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomsova M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108:415–20. doi: 10.1016/j.ygyno.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–59. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams SF, Levine DA, Cadungog MG, Hammond R, Facciabene A, Olvera N, et al. Intraepithelial T cells and tumor proliferation: impact on the benefit from surgical cytoreduction in advanced serous ovarian cancer. Cancer. 2009;115:2891–902. doi: 10.1002/cncr.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Buckanovich RJ, et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68:7684–91. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, Martinez DG, Engle X, Gewirtz AT, et al. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 2009;69:7329–37. doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209:495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cubillos-Ruiz JR, Rutkowski M, Conejo-Garcia JR. Blocking ovarian cancer progression by targeting tumor microenvironmental leukocytes. Cell Cycle. 2010;9:260–8. doi: 10.4161/cc.9.2.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landskron J, Helland O, Torgersen KM, Aandahl EM, Gjertsen BT, Bjorge L, et al. Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients. Cancer Immunol Immunother. 2015;64:337–47. doi: 10.1007/s00262-014-1636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyne HE, Stone PJ, Burnett AF, Cannon MJ. Ovarian tumor ascites CD14+ cells suppress dendritic cell-activated CD4+ T-cell responses through IL-10 secretion and indoleamine 2,3-dioxygenase. J Immunother. 2014;37:163–9. doi: 10.1097/CJI.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webb TJ, Giuntoli RL, 2nd, Rogers O, Schneck J, Oelke M. Ascites specific inhibition of CD1d-mediated activation of natural killer T cells. Clin Cancer Res. 2008;14:7652–8. doi: 10.1158/1078-0432.CCR-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelleher RJ, Jr., Balu-Iyer S, Loyall J, Sacca AJ, Shenoy GN, Peng P, et al. Extracellular Vesicles Present in Human Ovarian Tumor Microenvironments Induce a Phosphatidylserine-Dependent Arrest in the T-cell Signaling Cascade. Cancer Immunol Res. 2015 doi: 10.1158/2326-6066.CIR-15-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb TJ, Li X, Giuntoli RL, 2nd, Lopez PH, Heuser C, Schnaar RL, et al. Molecular Identification of GD3 as a Suppressor of the Innate Immune Response in Ovarian Cancer. Cancer Res. 2012;72:3744–52. doi: 10.1158/0008-5472.CAN-11-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyatlovitskaya EV, Andreasyan GO, Malykh Ya N, Rylova SN, Somova OG. Ganglioside shedding and changes in ceramide biosynthesis in human ovarian tumors. Biochemistry (Mosc) 1997;62:557–61. [PubMed] [Google Scholar]
- 22.Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol. 1999;6:373–8. doi: 10.1007/s10434-999-0373-0. [DOI] [PubMed] [Google Scholar]
- 23.Bamias A, Koutsoukou V, Terpos E, Tsiatas ML, Liakos C, Tsitsilonis O, et al. Correlation of NK T-like CD3+CD56+ cells and CD4+CD25+(hi) regulatory T cells with VEGF and TNFalpha in ascites from advanced ovarian cancer: Association with platinum resistance and prognosis in patients receiving first-line, platinum-based chemotherapy. Gynecol Oncol. 2008;108:421–7. doi: 10.1016/j.ygyno.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Rudlowski C, Pickart AK, Fuhljahn C, Friepoertner T, Schlehe B, Biesterfeld S, et al. Prognostic significance of vascular endothelial growth factor expression in ovarian cancer patients: a long-term follow-up. Int J Gynecol Cancer. 2006;16(Suppl 1):183–9. doi: 10.1111/j.1525-1438.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 25.Ueda M, Terai Y, Kumagai K, Ueki K, Yamaguchi H, Akise D, et al. Vascular endothelial growth factor C gene expression is closely related to invasion phenotype in gynecological tumor cells. Gynecol Oncol. 2001;82:162–6. doi: 10.1006/gyno.2001.6229. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto S, Konishi I, Mandai M, Kuroda H, Komatsu T, Nanbu K, et al. Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer. 1997;76:1221–7. doi: 10.1038/bjc.1997.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, Stylianopoulos T, Duda DG, Fukumura D, Jain RK. Benefits of vascular normalization are dose and time dependent--letter. Cancer Res. 2013;73:7144–6. doi: 10.1158/0008-5472.CAN-13-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73:2943–8. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziogas AC, Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Terpos E, et al. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer. 2012;130:857–64. doi: 10.1002/ijc.26094. [DOI] [PubMed] [Google Scholar]
- 30.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 31.Sriram V, Cho S, Li P, O'Donnell PW, Dunn C, Hayakawa K, et al. Inhibition of glycolipid shedding rescues recognition of a CD1+ T cell lymphoma by natural killer T (NKT) cells. Proc Natl Acad Sci U S A. 2002;99:8197–202. doi: 10.1073/pnas.122636199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lantz O, Bendelac A. An invariant T cell receptor a chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brutkiewicz RR, Bennink JR, Yewdell JW, Bendelac A. TAP-independent, b2-microglobulin-dependent surface expression of functional mouse CD1.1. J Exp Med. 1995;182:1913–9. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, et al. Selective ability of mouse CD1 to present glycolipids: a-galactosylceramide specifically stimulates Va14+ NK T lymphocytes. J Immunol. 1998;161:3271–81. [PubMed] [Google Scholar]
- 35.Lillehoj EP, Hyun SW, Feng C, Zhang L, Liu A, Guang W, et al. Human airway epithelia express catalytically active NEU3 sialidase. Am J Physiol Lung Cell Mol Physiol. 2014;306:L876–86. doi: 10.1152/ajplung.00322.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb TJ, Bieler JG, Schneck JP, Oelke M. Ex vivo induction and expansion of natural killer T cells by CD1d1-Ig coated artificial antigen presenting cells. J Immunol Methods. 2009;346:38–44. doi: 10.1016/j.jim.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charnock-Jones DS, Sharkey AM, Rajput-Williams J, Burch D, Schofield JP, Fountain SA, et al. Identification and localization of alternately spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biology of Reproduction. 1993;48:1120–8. doi: 10.1095/biolreprod48.5.1120. [DOI] [PubMed] [Google Scholar]
- 38.Luo H, Jiang BH, King SM, Chen YC. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr Cancer. 2008;60:800–9. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- 39.Azuma Y, Sato H, Higai K, Matsumoto K. Enhanced expression of membrane-associated sialidase Neu3 decreases GD3 and increases GM3 on the surface of Jurkat cells during etoposide-induced apoptosis. Biol Pharm Bull. 2007;30:1680–4. doi: 10.1248/bpb.30.1680. [DOI] [PubMed] [Google Scholar]
- 40.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–81. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park JE, Wu DY, Prendes M, Lu SX, Ragupathi G, Schrantz N, et al. Fine specificity of natural killer T cells against GD3 ganglioside and identification of GM3 as an inhibitory natural killer T-cell ligand. Immunology. 2008;123:145–55. doi: 10.1111/j.1365-2567.2007.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraft A, Weindel K, Ochs A, Marth C, Zmija J, Schumacher P, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85:178–87. [PubMed] [Google Scholar]
- 43.Hollingsworth HC, Kohn EC, Steinberg SM, Rothenberg ML, Merino MJ. Tumor angiogenesis in advanced stage ovarian carcinoma. Am J Pathol. 1995;147:33–41. [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez AA, Krigman HR, Whitaker RS, Dodge RK, Rodriguez GC. The prognostic significance of angiogenesis in epithelial ovarian carcinoma. Clin Cancer Res. 1999;5:587–91. [PubMed] [Google Scholar]
- 45.Luo JC, Toyoda M, Shibuya M. Differential inhibition of fluid accumulation and tumor growth in two mouse ascites tumors by an antivascular endothelial growth factor/permeability factor neutralizing antibody. Cancer Res. 1998;58:2594–600. [PubMed] [Google Scholar]
- 46.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 47.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 48.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–6. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 49.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 50.Gourley C, McCavigan A, Perren T, Paul J, Michie CO, Churchman M, et al. Molecular subgroup of high-grade serous ovarian cancer (HGSOC) as a predictor of outcome following bevacizumab. J Clin Oncol (Meeting Abstracts) 2014;32:5502. [Google Scholar]
- 51.Ferriss JS, Java JJ, Bookman MA, Fleming GF, Monk BJ, J LW, et al. Ascites predicts treatment benefit of bevacizumab in front-line therapy of advanced epithelial ovarian, fallopian tube and peritoneal cancers: An NRG Oncology/GOG study. Gynecol Oncol. 2015 doi: 10.1016/j.ygyno.2015.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giuntoli RL, 2nd, Webb TJ, Zoso A, Rogers O, Diaz-Montes TP, Bristow RE, et al. Ovarian cancer-associated ascites demonstrates altered immune environment: implications for antitumor immunity. Anticancer Res. 2009;29:2875–84. [PubMed] [Google Scholar]
- 53.Agostino NM, Saraceni C, Kincaid H, Shi W, Nevala WK, Markovic S, et al. A prospective evaluation of the role of Vascular Endothelial Growth Factor (VEGF) and the immune system in stage III/IV melanoma. Springerplus. 2015;4:186. doi: 10.1186/s40064-015-0951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farsaci B, Donahue RN, Coplin MA, Grenga I, Lepone LM, Molinolo AA, et al. Immune consequences of decreasing tumor vasculature with antiangiogenic tyrosine kinase inhibitors in combination with therapeutic vaccines. Cancer Immunol Res. 2014;2:1090–102. doi: 10.1158/2326-6066.CIR-14-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Ioannou K, Ziogas AC, et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br J Cancer. 2012;107:1869–75. doi: 10.1038/bjc.2012.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung TW, Choi HJ, Lee YC, Kim CH. Molecular mechanism for transcriptional activation of ganglioside GM3 synthase and its function in differentiation of HL-60 cells. Glycobiology. 2005;15:233–44. doi: 10.1093/glycob/cwh156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.