Abstract
Undifferentiated carcinomas with osteoclastic giant cells of the pancreas (OGC) are rare tumors. The current impression in the literature is that they are highly aggressive tumors similar in prognosis to ductal adenocarcinomas. In this study, the clinicopathologic characteristics of 38 resected OGCs were investigated and contrasted with 725 resected pancreatic ductal adenocarcinomas without osteoclastic cells (PDCs). The frequency among systematically reviewed pancreatic cancers was 1.4%. OGCs showed a slight female predominance (62.9%, vs 51.4% in PDCs). The mean age was 57.9 years (vs 65.0). The mean size of invasive cancer was 5.3 cm (vs 3.2). They were characterized by nodular, pushing-border growth, and 8 arose in tumoral intraepithelial neoplasms [4 in mucinous cystic neoplasms (MCN), 4 in intraductal papillary mucinous neoplasms (IPMN) type lesions] and 23 (61%) also showed prominent intraductal/intracystic growth. Twenty nine (76%) had an invasive ductal/tubular adenocarcinoma component. Osteoid was seen in 12. Despite of their larger size, perineural invasion and nodal metastasis were uncommon (31.6 and 22.6%, vs 85.5 and 64.0% respectively). Immunohistochemistry performed on 24 cases revealed that osteoclastic cells expressed the histiocytic marker CD68, and background spindle cells and pleomorphic/giant carcinoma cells often showed p53 and often lacked cytokeratin. Survival of OGCs was significantly better than that of PDCs (5-year, 59.1 vs 15.7%, respectively, p=0.0009). In conclusion, pancreatic OGCs present with larger tumor size and in slightly younger patients than PDC, 21% arise in MCN/IPMN, and 61% show intraductal/intracystic polypoid growth. OGCs have a significantly better prognosis than is currently believed in the literature.
Keywords: undifferentiated, osteoclast, pancreas, intraductal, sarcomatoid carcinoma
Introduction
Undifferentiated carcinoma with osteoclastic giant cells of the pancreas (OGC) is a distinctive tumor type in the pancreas. It is regarded as a variant of sarcomatoid carcinoma with striking chemotaxis of osteoclastic giant cells. The biologic nature of these tumors has yet to be unraveled. These tumors have been noted to coexist with garden-variety ductal adenocarcinomas or mucinous cystic neoplasms (MCNs) (1–3).
The data on the prognosis of OGCs is fairly limited. The overall impression in the literature is that it is a highly aggressive tumor, with an even worse prognosis than ordinary pancreatic ductal adenocarcinoma (4–7) although some observers noted a more protracted course. This is partly attributable to the fact that pleomorphic undifferentiated carcinomas of NOS type (poorly differentiated version of ductal adenocarcinomas) have often been classified and analyzed along with OGCs. In the current reference texts that are most widely used by practicing pathologists including the AFIP fascicle and WHO blue book, the mean survival of OGCs is reported as 12 months (8–11). On the other hand, when the publications are carefully dissected, it is revealed that several cases have been noted with long-term survival, including some beyond 10-years (12–15).
In this study, we investigated the clinicopathologic characteristics and prognosis of pancreatic undifferentiated (sarcomatoid) carcinomas defined by the presence of osteoclastic giant cells.
Material and methods
The study was conducted in accordance with the requirements of the Institutional Review Board.
Case selection
The surgical pathology databases of Emory University, Winship Cancer Center (Atlanta, GA), Samsung Medical Center (Seoul, Korea), Wayne State University, Karmanos Cancer Center (Detroit, MI) and Memorial Sloan Kettering Cancer Center (New York, NY) as well as the consultation files of the authors were searched for resected OGCs. During this search period, 2346 consecutive pancreatectomies were performed at Emory University Hospital between 2000 to 2015. These were systematically reviewed by the authors, and it was this systematically reviewed group that was utilized to determine the relative frequency of this tumor type among other pancreatic neoplasia. Additionally, 725 resected pancreatic ductal adenocarcinomas without OGC (PDCs) were identified as a control group for comparison of the clinicopathologic characteristics. Undifferentiated carcinoma without osteoclastic giant cells were excluded from this study. The macroscopic and clinicopathologic characteristics of the tumors were analyzed. Follow-up data were obtained by contacting the primary physicians or reviewing patients’ charts.
Pathological examinations
The cases included in this study were defined as primary pancreatic tumors which, in addition to having at least some osteoclastic giant cells, also showed distinctive nodular growth with pushing-border infiltration. The presence of any OGC elements was acknowledged and such cases were included in the study. There were 5 cases with sarcomatoid undifferentiated carcinoma that displayed all the other characteristics of OGCs, but no osteoclastic giant cells could be identified despite thorough analysis, and so these cases were excluded from the study. Of note, these cases displayed abundance of neutrophils or eosinophils instead, in addition to more discohesive cells and rhabdoid morphology (Fig. 1). All available pathology material on the 38 qualified cases (with osteoclastic cells) was reviewed. A mean of overall 21 slides per case (range, 1–72) and 11 lesional slides per case (range, 1–42) were available for histopathologic examination. Size of the tumor was recorded.
Figure 1.
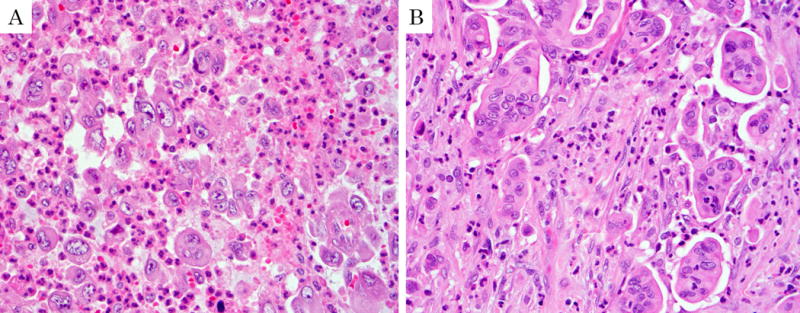
Examples of undifferentiated carcinoma excluded from the study due to absence of conventional osteoclastic giant cells. A: This case illustrates an undifferentiated carcinoma with large multi-nucleated giant cells that mimic osteoclasts but are not osteoclasts. Of note, the 5 cases that were excluded due to lack of OGC typically had neutrophilic/eosinophilic infiltrates instead of osteoclasts as seen in this example. B: Also carefully excluded from the study were cases like this in which micropapillary carcinoma clusters mimicked osteoclastic cells.
The presence or absence of an intraepithelial neoplasm was investigated. Pancreatic intraepithelial neoplasms (Pan-INs), intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs) were defined and graded per the current literature (16–18). In the cases with associated tumoral intraepithelial neoplasms, i.e., IPMN or MCN, the size of invasive cancer was recorded separately. The growth patterns of the tumors were noted. In cases with ordinary tubular (ductal adenocarcinoma) component, the proportion (%) of this component was recorded.
A histologic grading of OGCs was performed using the criteria employed in bone and soft tissues. For this, the grading system proposed by the Netherlands Committee on Bone Tumours appeared to be the most applicable (19). The details of this grading scheme are provided in Fig. 2. Additionally, the conventional pathologic prognostic parameters including vascular and perineural invasion, spread of the tumor, T-stage, and lymph nodes status were analyzed.
Figure 2.
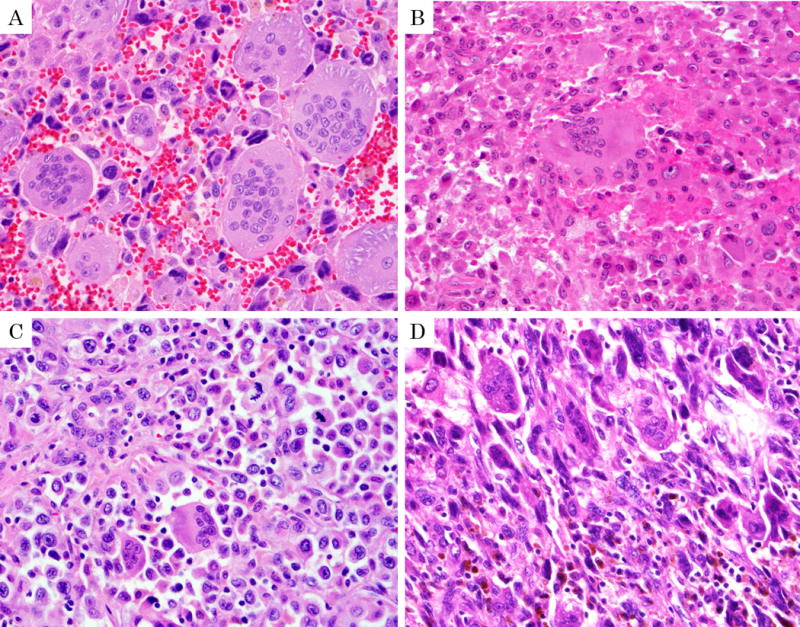
The grading of undifferentiated carcinoma with osteoclastic giant cells. (Modified based on histopathologic grading system for giant cell tumors of bone by Netherlands Committee on Bone Tumors.) A: Grade I; Numerous, evenly distributed and large osteoclastic giant cells are noted. Background cells (histocyte-like sarcomatoid carcinoma cells and pleomorphic giant carcinoma cells) have only rare mitotic figure. B: Grade II; Osteoclastic cells are less abundant. Back ground cells are prominent with identifiable mitotic figure, but less than 1 mitosis/HPF. C: Grade III; Osteoclastic cells are less numerous. Background cells are more abundant and have substantial pleomorphism and atypia and at least 1 mitosis /HPF. D: Grade IV; Background cells are overtly pleomorphic and atypical and accompanied by only scanty osteoclastic cells.
Immunohistochemistry
Tissue was available for immunohistochemistry in 24 OGCs. Immunohistochemical stains were performed using the avidin–biotin complex technique with no biotin blocking with antibodies for cytokeratin AE1/AE3 (Dako Corporation, Carpinteria, CA, USA: clones AE1/3, Dilution 1:100), CD68 (Dako Corporation, Carpinteria, CA, USA: clones KP-1, Dilution 1:3000), p53 (Dako Corporation, Carpinteria, CA, USA: clones DO-7, no dilution), and Ki-67 (Dako Corporation, Carpinteria, CA, USA: Mib-1 clone, Dilution 1:160). Negative and positive controls were included with each batch of slides tested.
Statistical analysis
Various clinico-pathological factors association between OGCs and PDCs was analyzed by Fisher’s exact test. Differences between groups in continuous variables were analyzed by Mann-Whitney U-test. Overall survival and median survival were estimated by the Kaplan Meier method, and survival curves were compared by Log-rank test. A value of p<0.05 was considered significant. All statistical analysis was performed with a statistical software package (JMP12, SAS institute Japan, Tokyo, Japan).
Results
Frequency
Of the 38 cases included in this study, 10 were identified among the 2346 pancreatectomies systematically reviewed by the authors in order to determine the relative frequency of this tumor type, thus placing the frequency of OGCs in this systematically analyzed cohort at 0.4% in all pancreatectomies, and 1.4% in 735 invasive pancreatic carcinomas (ductal adenocarcinoma and its variants), 0.8% in 1260 pancreata with neoplastic lesions.
Clinical findings
Table 1 shows the clinicopathological characteristics of the 38 resected patients with OGCs in the study. The mean age at resection was a decade younger than PDCs (mean, 57.9 years-old, range, 29 to 86 years-old, vs mean, 65.0, range, 29 to 89, respectively, p=0.0059). Female to male ratio was 2 to 1 (62.9% in female vs. 51.4% in PDC).
Table 1.
Clinicopathological characteristics of 38 cases of resected undifferentiated carcinoma with osteoclastic giant cells of the pancreas (OGCs) and 725 cases of resected pancreatic ductal adenocarcinoma without osteoclastic giant cells (PDCs).
| OGCs n=38 |
PDCs n=725 |
p-value | |
|---|---|---|---|
| Age (year) | |||
| mean±SEM, year (range) | 57.9±2.6 (29–86) | 65.0±0.4 (29–89) | 0.0059 |
| Gender | |||
| Male:Female | 13:22 | 352:373 | 0.1870 |
| NA | 3 | 0 | |
| Distribution of neoplasm | |||
| Head | 22 (61.1%) | 607 (83.7%) | 0.0004 |
| Distal (body/tail) | 13 (36.1%)* | 116 (16.0%) | |
| Head to Distal | 1 (2.8%) | 2 (0.3%) | |
| NA | 2 | 0 | |
| Size of invasive carcinoma | |||
| mean±SEM, cm (range) | 5.3±5.9 (0.6–12.0) | 3.2±0.6 (0.1–10.6) | 0.0004 |
| T stage | |||
| T1, 2 | 5 (15.2%) | 134 (18.6%) | 0.6213 |
| T3, 4 | 28 (84.9%) | 588 (81.4%) | |
| TX, NA | 5 | 3 | |
| Lymph node metastasis | |||
| Positive | 7 (22.6%) | 461 (64.0%) | <0.0001 |
| Negative | 24 (77.4%) | 259 (36.0%) | |
| NA, no dissected | 7 | 5 | |
| Surgical margin | |||
| Positive | 4 (12.9%) | 177 (24.5%) | 0.1385 |
| Negative | 27 (87.1%) | 545 (75.5%) | |
| NA, cannot be assessed | 7 | 3 | |
| Lymphovascular invasion | |||
| Positive | 22 (62.9%) | 409 (62.2%) | 0.9338 |
| Negative | 13 (37.1%) | 249 (37.8%) | |
| NA | 3 | 67 | |
| Perineural invasion | |||
| Positive | 12 (31.6%) | 611 (85.5%) | <0.0001 |
| Negative | 23 (67.7%) | 104 (14.6%) | |
| NA | 4 | 10 | |
| Associated tumoral intraepithelial lesions | |||
| MCN | 4 (10.5%) | 6 (0.8%) | <0.0001 |
| IPMN | 4 (10.5%) | 30 (4.1%) | 0.0539 |
| Neoadjuvant therapy | |||
| Received | 1 (2.6%) | 105 (14.5%) | 0.0395 |
NA: not available data. MCN: mucinous cystic neoplasm, IPMN: intraductal papillary mucinous neoplasm. Statistical analyzed by Mann-Whitney U test in continuous variables and Fisher’s exact test in no continuous variables.
In one case, tumor was located mainly in spleen. This lesion may originated from ectopic pancreas tissue in spleen or pancreatic tail extend to spleen.
The preoperative clinical data was available for 29 patients. Presenting symptoms included abdominal or back pain (n=18, 62%), weight loss (n=11, 38%), nausea (n=8, 28%), tarry stool and jaundice, each in 4 patients (14%) and diabetes mellitus (n=3, 10%). Of note, 1 case with intraductal growth pattern (see below) had a history of 3 episodes of acute pancreatitis for the 3 years prior to surgery. Serum CA19-9 levels were elevated in 73% (8/11) of patients with available data. Of 15 who underwent pre-operative biopsy of the lesion (fine needle aspiration in 11, endoscopic forceps’ biopsy from duodenum in 3, core biopsy in 1), only 4 received an “OGC” diagnosis, while 6 were diagnosed as “adenocarcinoma” (the DA component in 5 and MCN with high-grade dysplasia in 1, in the resection.) and 4 were diagnosed as “malignant neoplasm”. One was negative for malignant cells (0.6cm of the invasive component in the resection).
Radiologic findings
The pre-operative radiologic studies were available in 11 patients (5 computed tomography, 5 magnetic resonance images, and 1 with both). All images showed mass lesions with delayed enhancement. Of the 8 patients who proved later to have intraductal/intracystic growth pathologically, 4 showed the findings compatible with intraductal/intracystic growth on the retrospective analysis of radiologic images as well (Fig. 3A, 4B), although this was not noted in the original radiology report in any of these cases. One case showed prominent invasion into duodenum also forming a large polypoid protrusion into the duodenal lumen identifiable in radiologic images (Fig. 5A). In 1 case, the tumor appeared predominantly cystic (Fig. 4A).
Figure 3.
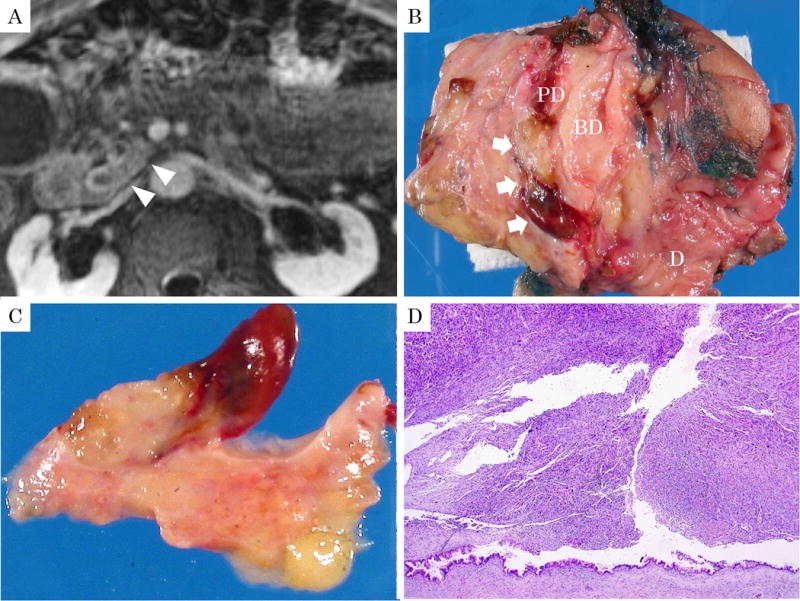
86-year-old, male with undifferentiated carcinoma with osteoclastic giant cells. A: Magnetic resonance image revealed a tumor within the dilated main pancreatic duct (arrowheads) along with thickening of pancreatic duct wall on delay phase of T1-weighted image with fat suppression. B, C: Macroscopically, the polypoid tumor projected into the dilated pancreatic duct (arrow). The polyp was soft and yellow with hemorrhagic foci and a thin stalk. (BD: common bile duct, PD: main pancreatic duct, D: duodenum) D: Macroscopic appearance of the same polypoid lesion showing sheet-like growth of sarcomatoid tumor with characteristic OGC pattern. Preserved duct epithelium is seen at the base and shows low-grade PanIN. (H&E stain, ×40)
Figure 4.
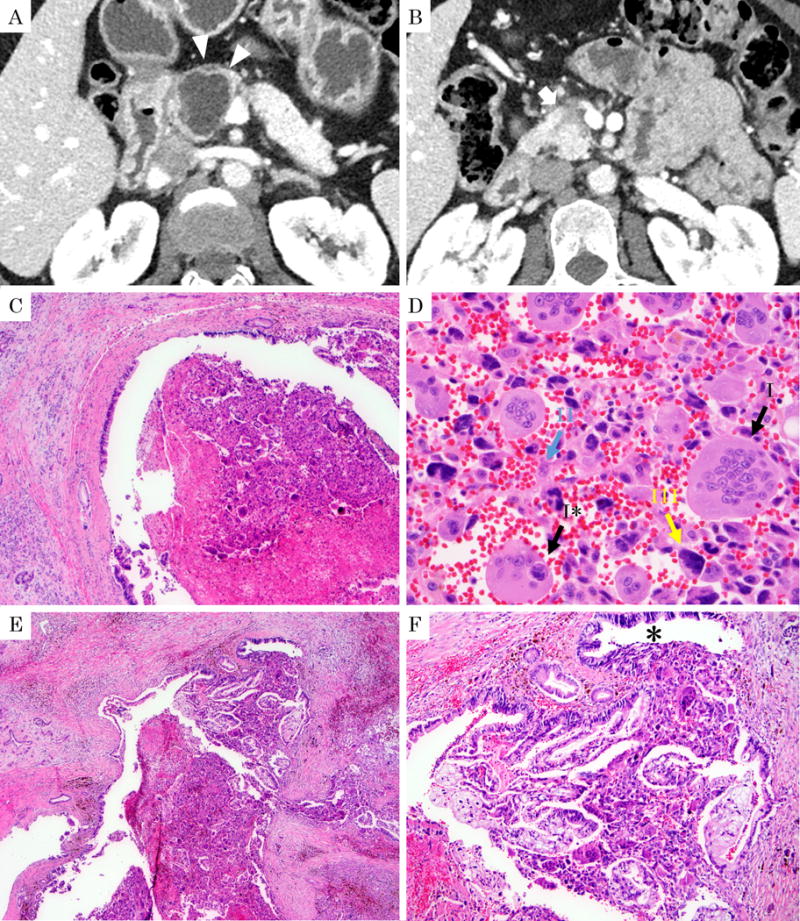
51-year-old, female with undifferentiated carcinoma with osteoclastic giant cells. A: Computed tomography (arterial phase) revealed cystic lesion in the pancreatic head (arrowheads). B: A hypo-vascular solid component is noted along the Santorini duct (arrow). C: Histologic section of the same tumor shows that the pancreatic duct is filled with solid tumor. (H&E stain, ×40) D: On high magnification, three different cell types are noted: I. Osteoclastic cells characterized by multinucleated giant cells with the nuclei clustered mostly at the center and showing the typical nuclear morphology including pale chromatin and small nucleolus (black arrow I). Tumor cell “cannibalism” (engulfment of the sarcomatoid carcinoma cells by the osteoclastic cells) is depicted (black arrow I*). II. Histiocyte-like sarcomatoid carcinoma cells characterized by relatively small, spindle-to plumb shaped cells (blue arrow II) intermixed with erythrocytes. III. Pleomorphic/undifferentiated malignant cells (yellow arrow III). (H&E stain, ×400) E: This tumor grew within the pancreatic duct. (H&E stain, ×40) F: On higher magnification in this polypoid lesion the OGC pattern is intermixed with ductal (tubular) adenocarcinoma component and the duct lining shows high-grade PanIN (PanIN-3; *). (H&E stain, ×100).
Figure 5.
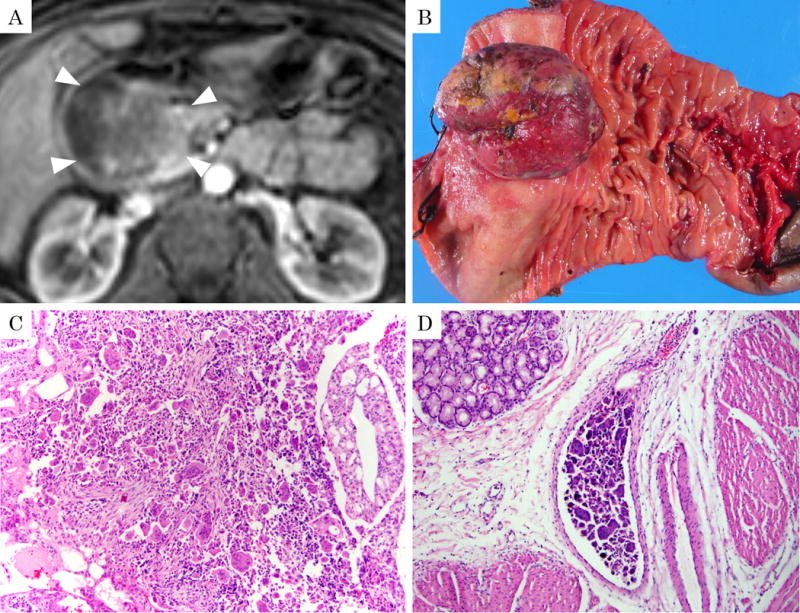
65-year-old, female with undifferentiated carcinoma with osteoclastic giant cells (OGC). A: Magnetic resonance image revealed that the tumor originating in the pancreas was also massively protruding into the duodenum in a polypoid fashion (arrowheads) on venous phase of T1-weighted image with fat suppression. B: Macroscopic examination of this polypoid component in the duodenum leading to atrophy of the adjacent duodenal mucosa. C: Histologic examination reveals the classical OGC pattern intermixed with ductal (tubular) adenocarcinoma elements. (H&E stain, ×100) D: Vascular invasion of OGC was seen. (H&E stain, ×100) Of note, despite the massive nature of the tumor, as well as ductal adenocarcinoma component and vascular invasion, this patient was in fact still alive 65 months after surgery.
Macroscopic Features
About a third (36.1%) of resected OGCs occurred in the body/tail (36.1% vs 16.0% for PDCs, respectively, p=0.0004). The tumor size was also significantly larger than PDCs (mean, 5.3 vs 3.2 cm, respectively, p=0.0004). Adequate gross information was documented in 33 cases. Twenty (61%) exhibited cyst formation. In 11 (33%) OGCs, prominent intraductal/intracystic growth was noted grossly, forming smooth-surfaced, focally hemorrhagic polypoid lesions within the ducts/cysts (Fig. 3B, C). Four (12%) tumors also protruded from the pancreatic duct into the duodenal lumen in a polypoid fashion (Fig. 5B).
Histopathologic Features
All cases showed characteristic morphology of OGC, including compact cellular and hemorrhagic nodules essentially composed of three distinct cell populations (Fig. 3–6).: I. Osteoclastic giant cells: These showed all the characteristic features of this cell type seen in the bone, including large cells with densely acidophilic cytoplasm, and multiple relatively uniform and bland nuclei, often clustering in the central aspect of the cell. II. Histiocyte-like sarcomatoid carcinoma cells (HSCs): These cells were relatively small, round-to-ovoid, often resembling synovial-like histiocytes, non-cohesive, variable in number, and were at times overshadowed by the surrounding osteoclastic giant cells. III. Pleomorphic giant carcinoma cells (PCs): These highly atypical cells had large irregular hyperchromatic nuclei, some with lobulations (Fig. 4D).
Figure 6.
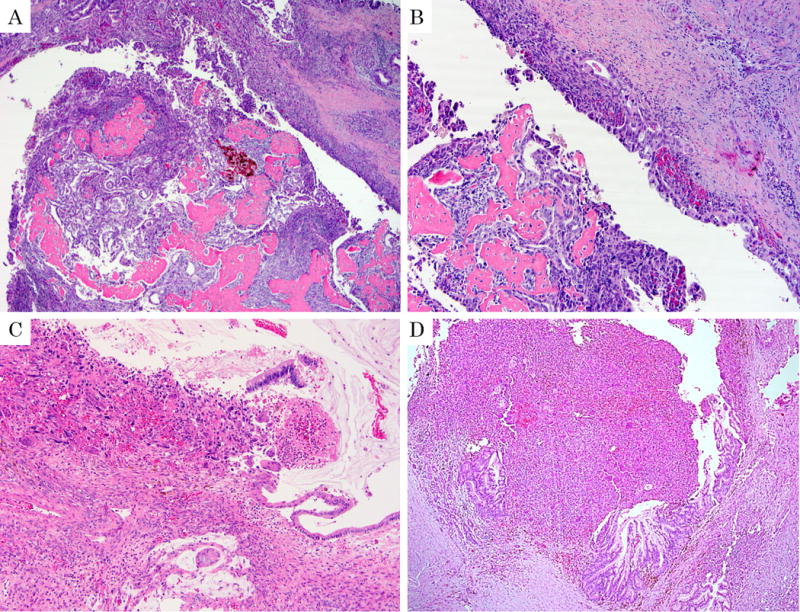
Undifferentiated carcinoma with osteoclastic giant cells (OGC). A: The polypoid tumor within the duct shows extensive osteoid formation. (H&E stain, ×40) B: High-grade glandular units admixed with sarcomatoid elements are forming a band on the duct wall (arrow head). C: OGC growing towards the lumen of a mucinous cystic neoplasm (with ovarian type stroma evident). (H&E stain, ×100) D: OGC associated with intraductal papillary mucinous neoplasm in a male patient, without ovarian stroma. (H&E stain, ×100)
The OGC component (with osteoclastic cells) ranged from minimal (less than 5% of the tumor) to 100% (all) of the invasive tumor with a mean of 57.1%. The osteoclastic giant cell-rich areas of the tumor were typically demarcated, nodular and frequently hemorrhagic with abundant extravasated red blood cells. Some of these nodules proved to be ducts filled with, and replaced almost entirely by OGCs, which on deeper step sections and leveling of the tumor revealed their peripheral duct lining.
Lymph-vascular invasion was present in 22 (62.9%) tumors (Fig. 5D) including a tumor thrombus in superior mesenteric vein (n=1) and splenic vein (n=1). Their cells consisted of OGCs in 13, HSCs and/or PCs without osteoclastic cells in 5 and DA in 4.
Perineural invasion was seen in 12 (31.6%), which was significantly less frequent than PDCs (85.5%, respectively, p<0.0001). Their cells consisted of OGCs in 4, HSCs and/or PCs without osteoclastic cells in 3 and DA in 8, including both of DA and OGCs in 1, DA and HSCs and/or PCs without osteoclastic cells in 2.
Invasion of adjacent organs was seen in 15 cases (40%), including the duodenum (n=10), stomach (n=3), spleen (n=2), colon (n=1) and liver (n=1), including 1 in both stomach and liver and 1 in both stomach and spleen.
Despite their larger size, the frequency of lymph node metastasis was significantly less than PDCs (22.6% vs 64.0%, respectively, p<0.0001). The type of cells with lymph node metastasis showed HSCs and/or PCs without osteoclastic cells in 3 and DA in 4.
Twenty nine (76%) OGCs were associated with an invasive ductal (tubular, pancreatobiliary type) adenocarcinoma (DA) component. The DA component ranged from minimal (less than 5% of the tumor) to 80% of the tumor (mean, 21%). These components were intermixed with each other in variable patterns (Fig. 5C). In addition to DA, 4 (11%) OGCs arose within an MCN (Fig. 6C) and 4 (11%) also had prominent intraductal papillary lesions that would histologically qualify as IPMN (Fig. 6D), although only 1 of these were recognized clinically/grossly as IPMN; in some, the IPMN component was presumably obscured by the OGC invasion. All MCNs and IPMN-like components had high-grade dysplasia (“carcinoma in situ”). In all 8, the OGC component was admixed with the papillary in-situ component of the tumor.
Additionally, 23 (61%) OGCs displayed histologic evidence of intraductal/intracystic growth showing polypoid, focally hemorrhagic lesions within the ducts or cysts (Fig. 3D, 4C–F, 6A, B, D). The cases with intraductal/intracystic growth had lymph-vascular invasion less often than those without (50% vs 85%, respectively, p=0.0406).
The high-grade PanIN-3/CIS was identified in 18 (47%) cases. OGCs and PanIN lesions were often intermixed (Fig. 4E, F, 6B).
Twelve (32%) tumors contained osteoid (Fig. 6A, B). This osteoid appeared to be metaplastic, mature (well-formed) bone tissue (heterologous elements) as is often seen in sarcomatoid carcinomas of other organs such as mixed mullerian tumors. In none of the cases was there an overgrowth of malignant osteoid (osteosarcoma pattern).
While many of the cases showed changes of chronic injury in the vicinity of the tumor, it was impossible to distinguish whether this was peri-tumoral pancreatitis (induced by the tumor) or an underlying pancreatic disorder.
Follow up biopsy of 8 cases with distant metastatic tumors was available in 8 cases (6 from liver, 1 from peritoneum and colonic mesentery each). These biopsies showed DA (tubular adenocarcinoma) pattern in 4, and sarcomatoid OGC pattern (with osteoclastic cells also present) in 4.
Immunohistochemical Features
In all 24 cases analyzed (Table.2), all of the osteoclastic cells and most of the histiocyte-like carcinoma cells (HSCs) (19 of 20) expressed CD68 while the pleomorphic/bizarre cells (PC) were mostly negative for this marker (Fig. 7A). In contrast, osteoclastic giant cells were negative for the epithelial marker AE1/AE3, while HSCs showed focal (mostly weak) labeling in 9, and in PCs in 3 (Fig. 7B). Both of the HSCs and PCs had a high Ki-67 proliferation index (mean, 20.7% and 26.2%, range 1.4–44.2 and 0.0–60.8%, respectively), while osteoclastic giant cells had a very low Ki-67 proliferation index (Fig. 7C). Along the same lines, p53 was expressed frequently in PCs and HSCs, while osteoclastic giant cells were uniformly negative (Fig. 7D).
Table 2.
Immunohistochemical staining characteristics of 24 cases of undifferentiated carcinoma with osteoclastic giant cells of the pancreas.
| CD68 | AE1/AE3 | Ki-67 labeling index* | p53 (>50%) |
|
|---|---|---|---|---|
| Osteoclastic Giant Cells |
20/20 | 0/23 | 0 (0%) |
0/18 |
| Histiocyte-like Carcinoma Cells |
19/20 | 9/23 (focal) |
20.7% (1.4–44.2%) |
9/18 |
| Pleomorphic Carcinoma Cells |
1/20 (focal) |
3/23 (focal) |
26.2% (0.0–60.8%) |
7/18 |
n=19, mean, (range)
Figure 7.
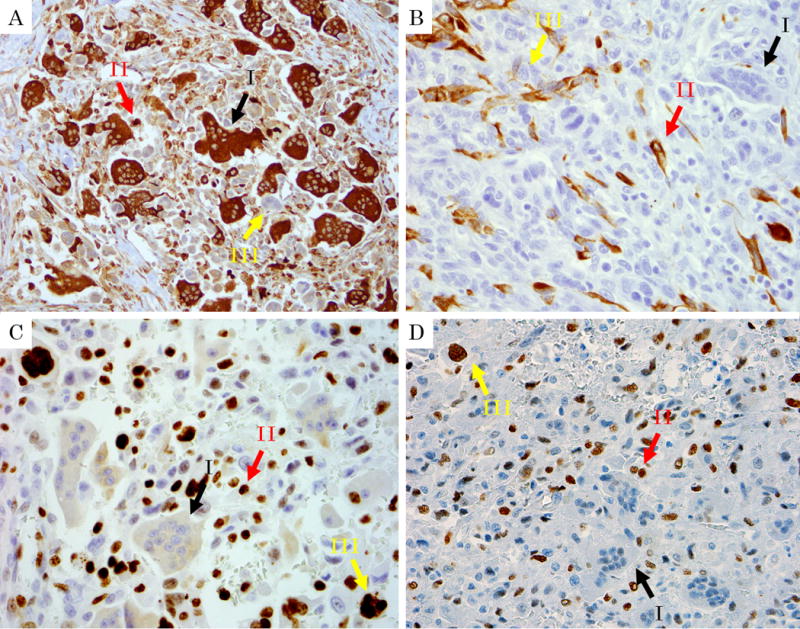
Immunohistochemical findings of undifferentiated carcinoma with osteoclastic giant cells. Black arrow I: Osteoclastic cells. Red Arrow II: Histiocyte-like sarcomatoid carcinoma cells (HSCs). Yellow arrow III: Pleomorphic/undifferentiated malignant cells (PCs). A: Immunolabelling for CD68 (lysosome, macrophage marker) labels osteoclastic cells strongly, and also marks HSCs albeit more weakly. Note that pleomorphic/undifferentiated malignant cells are being engulfed by the osteoclastic cells are not stained. (CD68, ×400) B: In contrast, AE1/AE3 is negative in osteoclastic cells and HSCs, but show focally positivity in HSCs and PCs (AE1/AE3, ×400) C, D: HSCs and PCs had a high expression of p53 and a high proliferation rate with Ki-67 stain, while osteoclastic cells were negative for both. (C: Ki-67, ×400, D: p53, ×400)
Clinical Course
One patient had neo-adjuvant chemotherapy before surgery, while 15 patients had adjuvant chemotherapy and/or radiotherapy after surgery. In the remainder, post-operative treatment data was not adequately accessible.
Five-year overall survival of the all OGCs was 59.1% and median survival period was 7.67 years which was significantly better than that of PDCs (15.7% and 1.59 years, p=0.0009) (Fig. 8A). By applying a modified version of the grading system employed for osseous osteoclastic giant cell tumors by Netherlands Committee on Bone Tumours, the pancreatic OGCs that qualified as Grades I (n=12) and II (n=14) appeared to have a better survival (5-year, 73.1%) than those that qualified as Grades III (n=10) and IV (n=2) (5-year, 28.6%, Log-rank test p=0.0288) (Fig. 8B). Similarly, Grades I/II cases seemed more prone to fall into the long-term survivor category than Grades III/IV cases (p=0.1469) (Table 3).
Figure 8.
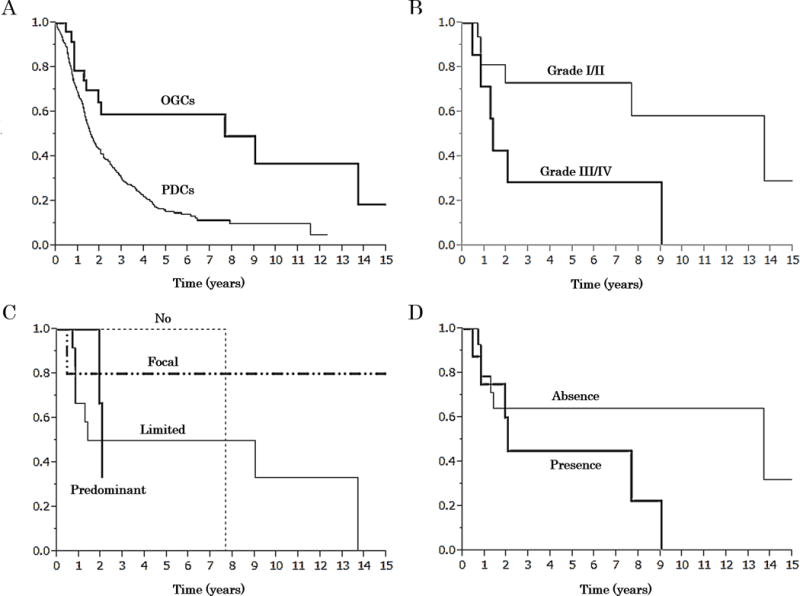
Kaplan-Meier overall survival curves. A: A comparison between of patients with undifferentiated carcinoma with osteoclastic giant cells of the pancreas (OGCs) and that of pancreatic ductal adenocarcinoma without osteoclastic giant cells (PDCs). Survival of OGCs was significantly better than that of PDCs (5-year survival rate; 59.1 vs 15.7%, median; 7.67 vs 1.59 years, respectively, Log-rank test p=0.0009). B: A comparison of grades I, II versus III, IV per modified Netherlands Committee of Bone Tumours. Survival of grade I (n=12) and II (n=14) was significantly better than that of Grade III (n=10) and IV (n=2) (5-year survival rate; 73.1 vs 28.6%, median; 13.7 vs 1.4 years, respectively, Log-rank test p=0.0288). C: Survival of cases with different amount of ductal component. [No (n=9): 0%, Limited (n=16): 0%>, ≦10%, Focal (n=9): 10%<, <50%, Predominant (n=4): ≧50%, 5-year survival rate; 100 vs 50.0 vs 80.0% (the predominant group did not reach follow up periods), median; 7.7 years vs 5.2 years vs not reach vs 2.1 years, respectively, Log-rank test p=0.5385]. D: There was no significant difference in survival between cases with versus those without osteoid (5-year survival rate; 45.0 vs 64.3%, median; 2.0 vs 13.7 years, respectively, Log-rank test p=0.2521).
Table 3.
A comparison of clinicopathological characteristics of resected undifferentiated carcinoma with osteoclastic giant cells of the pancreas between short and long-term survivor.
| Short-term survivor n=9 |
Long-term survivor n=9 |
p-value | ||
|---|---|---|---|---|
| Age (year) | ||||
| mean±SEM, year (range) | 61.1±5.3 (29–86) | 53.7±5.4 (35–78) | 0.4522 | |
| Gender | ||||
| Female | 5 (56%) | 6 (67%) | 0.6287 | |
| Distribution of neoplasm | ||||
| Head | 5 (56%) | 8 (89%) | 0.1144 | |
| Size of neoplasm | ||||
| mean±SEM, cm (range) | 4.1±0.9 (0.6–10.0) | 6.8±1.2 (2.5–12.0) | 0.1110 | |
| Lymphovascular invasion | ||||
| Positive | 5/8 (63%) | 3/7 (43%) | 0.4468 | |
| Perineural invasion | ||||
| Positive | 4/8 (50%) | 1/8 (13%) | 0.1056 | |
| Lymph node metastasis | ||||
| Positive | 1/6 (17%) | 0/8 (0%) | 0.2308 | |
| Surgical margin | ||||
| Positive | 0/8 (0%) | 2/8 (25%) | 0.1306 | |
| Intraductal/cystic growth appearance | ||||
| Presence | 7 (78%) | 6 (67%) | 0.5987 | |
| Osteoid | ||||
| Presence | 4 (44%) | 3 (33%) | 0.6287 | |
| Ki-67 index | ||||
| HSCs, mean±SEM, % (range) | 19.0±4.6* (4.7–34.5) | 23.3±7.9** (5.6–44.1) | 1.0000 | |
| PCs, mean±SEM, % (range) | 26.3±4.5* (15.0–45.2) | 31.6±4.3** (25.5–44.4) | 0.5083 | |
| p53 positivity | ||||
| HSCs, >50% | 3/7 | 3/4 | 0.3031 | |
| PCs, >50% | 3/7 | 1/4 | 0.5537 | |
| Histologic grading | ||||
| Grade I+II | 4 | 7 | ||
| Grade III+IV | 5 | 2 | 0.1469 | |
| Component of DA | ||||
| No (0%) | 0 | 2 | ||
| Limited (0<, ≦10%) | 6 | 5 | ||
| Focal (10<, <50%) | 1 | 2 | ||
| Predominantly (≧50%) | 2 | 0 | 0.2191 | |
| Associated tumoral intraepithelial lesions | ||||
| HG-PanIN | 5 (56%) | 6 (75%) | 0.4024 | |
| MCN | 1 (11%) | 0 (0%) | 0.3035 | |
| IPMN | 1 (11%) | 0 (0%) | 0.3035 | |
Short-term survivor is defined as who died within 3 years after surgery. Long-term survivor is defined as who was alive after 5 years after surgery. Cases with less than 3 years follow up period were excluded. HSCs: Histocyte-like sarcomatoid carcinoma cells, PCs: Pleomorphic giant carcinoma cells. DA: ductal adenocarcinoma, HG-PanIN: high-grade pancreatic intraepithelial neoplasia, MCN: mucinous cystic neoplasm, IPMN: intraductal papillary mucinous neoplasm. Statistical analyzed by
Mann-Whitney U test in continuous variables and Fisher’s exact test in no continuous variables.
n=7,
n=4.
We have investigated the potential impact of the amount of the ductal adenocarcinoma component by using various thresholds and we have failed to identify any prognostic correlation with any of the cut-offs tested. Five-year survival of the 9 cases with no ductal component was 100%, for those with limited ductal component (≤ 10 % of the tumor; 16 cases), it was 50%, and for those with focal ductal component (10–50%), it was 80%. Those with a predominantly ductal component (≥ 50%) did not reach calculable survival levels. The median survival for these groups were 7.7 years, 5.2 years, did not reach, and, 2.1 years, respectively (Log-rank test p=0.5385] (Fig. 8C). Additionally, there is no significant difference between the tumors with no (0%) ductal component versus all others (p value=0.6394). No significant difference could be documented between the cases with and without intraductal/intracystic growth, MCN/IPMN, or presence of an osteoid component (Fig. 8D).
In this study, 7 out of 38 cases (18%) patients were alive over 5 years after surgery with no evidence of recurrence including 3 patients who were alive after more than 10 years. Among these 7 cases, the DA component was less than 10% of DA in 6, 4 were Grade I and there was no perineural invasion or lymph node metastasis in any of the cases.
Discussion
OGC was initially described in 1968 by Juan Rosai (20) as a distinct tumor type and a variant of undifferentiated carcinoma defined by conspicuous presence of giant cells resembling “osteoclastic cells”. This tumor is now well recognized as a distinct entity of the pancreas, although it is very rare (in this study the frequency among 2346 pancreatic resections was 0.4 %, and among cancers of ductal origin/type, it was 1.4%).
Based on previous studies as well as the results in our cohort, there is overwhelming evidence that the osteoclastic cells in this tumor are indeed osteoclastic cells, both by morphology, as well as immunoprofile (positive for CD68, negative for cytokeratins AE1/AE3 and p53, and had a low Ki-67 index) and thus are believed to be a benign population of histocytic cell lineage, that is massively recruited to this distinctive sarcomatoid neoplasm (21). The occurrence of osteoclastic cells in sarcomatoid malignancies of visceral organs is an under-appreciated phenomenon but is something that may occasionally be observed in sarcomatoid tumors of various origins, including sarcomatoid melanomas, sarcomatoid mesotheliomas, in addition to sarcomatoid carcinomas in any organs. However, it is in the pancreas (and very rarely also in the biliary tract) that these osteoclastic cells are so prominent that they can even predominate the picture thus warranting their designation as “osteoclastic giant cell carcinoma”. Based on their immunoprofile and low proliferation rate, it is safe to conclude that these osteoclastic cells are innocent recruits to this peculiar neoplasm. This has been also the conclusion in other previous studies, some with molecular analysis (21, 22). In fact, in cases with relatively uniform distribution of osteoclastic cells and without a DA component, the possibility of a “benign osteoclastoma” has even been raised (23); however, this concept was later rejected by other observers (24).
The reasons for the prolific osteoclastic giant cell chemotaxis in this region compared to its dearth in other sites warrant further investigation. Chemokine ligands have been shown to be overexpressed in some cancers including oral squamous cell carcinoma and inflammatory breast cancers which display an ability to recruit inflammatory reaction (25, 26). Such chemoattractants may perhaps be upregulated by the non-osteoclastic tumor cells in OGC, a phenomenon which warrants investigation especially when one considers that tumor cell immunology is now increasingly recognized as a factor in tumor initiation and progression in pancreatic and other cancers (27, 28), as well as potential targets in cancer treatment (25, 26). In fact, the recent literature on the programmed death-1 (PD-1) may very well be one of the most promising developments in cancer treatment, and similar approaches may have significant implications in the treatment of OGCs in the future (29, 30). Investigation of the almost exclusive occurrence of OGCs in the pancreatobiliary region which otherwise generates some of the most aggressive organ cancers in the body, may yield interesting perspectives about the nature of pancreatobiliary cancers in general as well. Along those lines, the fact that the undifferentiated carcinomas with osteoclastic cells appear to behave significantly better than those without (see further discussion below) may also be related to this peculiar biologic phenomenon.
This study also confirms that the fundamental malignant cell type in these OGCs is in fact the often-innocuous-appearing histiocyte-like (or synovial-like) sarcomatoid carcinoma cells (HSC) (22, 31, 32) between which the osteoclastic cells are suspended. These HSCs show very high proliferation index and frequent p53-positivity. Based on morphology and frequent association with hemorrhage, these cells are quite distinctive even among sarcomatoid carcinomas encountered in general pathology. They also typically lack cytokeratin expression, which can be positive in sarcomatoid carcinomas of other organs.
Perhaps the most peculiar aspect of OGCs elucidated in this study but not properly recognized in the literature is its tendency toward intraductal/intracystic growth. In addition to its nodular/pushing-border growth pattern (also under-emphasized in the literature), this tumor often shows prominent intraductal polypoid growth by the OGC elements (seen in 40%), in addition to intracystic polypoid arrangement when it arises in MCN or IPMN-type papillary intraductal lesions (21%). Sarcomatoid carcinomas in other organs such as upper aero-digestive tract, uterus and urothelium are also notorious for exhibiting polypoid growth with relatively demarcated bases (33, 34). We have also seen metaplastic (sarcomatoid) carcinomas of the breast with prominent intraductal growth (35). However, this striking propensity of pancreatic OGCs for intraductal growth is noteworthy and may be a manifestation related to the biology and relatively indolent behavior of this entity. It should also be noted here that, many of the cases with documented intraductal growth were grossed by the authors, and it is possible that this intraductal/polypoid growth may prove to be even more common if proper attention is given to it during the grossing of these cases.
That almost 20% of OGCs arise from MCN or IPMN-type lesions is also noteworthy. Recent studies have shown that 90% of invasive carcinomas arising from MCNs are tubular/ductal type but about 10% are sarcomatoid mostly of OGC type (3), and none are mucinous/colloid type. Thus OGCs appear to have some, as yet undetermined, relationship to MCN pathway. This, combined with female predominance (63%), raises the question of hormones playing a role in the pathogenesis of these tumors.
While OGC is a well-recognized entity, some of its clinicopathologic characteristics have remained somewhat elusive, partly due to its rarity, but also partly, but importantly due to the fact that it is often analyzed along with other poorly differentiated pancreatic ductal adenocarcinomas (lacking characteristic osteoclastic cells and/or HSCs) under the rubric of “undifferentiated carcinomas”. This is probably the main culprit behind OGCs’ erroneous reputation as highly aggressive neoplasms, mistakenly felt by some to be even more aggressive than ordinary ductal adenocarcinomas (8, 9). In the current AFIP fascicle and WHO Classification blue book, the mean survival of these tumors has been reported as 12 months (8–11). In this study, we found 5-year survival of 59.1%, and median survival of 7.67 years, which was incomparably better than that of PDCs (15.6% and 1.59 years), and even more surprisingly, this was also true for OGCs with a substantial DA component: overall, 7 patients were still alive with no evidence of disease, 5 years after surgery including 3 with > 10 years survival, and 5 of these had a DA component (in less than 10% of the tumor in 4 and 40% in 1).
At the same time, cases with more “osteoclastoma” pattern, i.e., the presence of more abundant and uniformly distributed large osteoclastic cells with a minimum of other components and lack of significant mitotic activity appear to signify perhaps even more protracted clinical course (19).
Along those lines, it is also noted here that the survival curve for OGC tumors is biphasic with an initial rapidly decreasing component, analogous to more typical high-grade pancreatic carcinomas, and a second component with a more indolent pattern. No specific parameters could be elucidated in this study to identify the cases that were more prone to be located in the second phase (Table.3); however, grades I/II tumors were often in the latter group (7 of 9, 78%). Per Netherlands Bone Tumour Committee scheme tested here, mitotic activity in the HSC/PC component was part of the grading system and showed some correlation with outcome when grades I and II were grouped together versus grade III/IV. On the other hand, the Ki-67 index was not found to be prognostically valuable but this may be due to the fact that a smaller number of cases (n=19) were available for Ki-67 analysis. Further studies are warranted on a large number of cases to examine this further.
The protracted clinical course of OGC demonstrated in our study appears to contrast markedly with the general impression in many current hallmark texts on this topic, for in fact, when true OGCs are carefully dissected out from the other PDCs in the literature, it becomes clear that OGCs do indeed have more indolent behavior even in the published case series (36–38).
Other interesting clinicopathologic associations of OGCs were also elucidated in this study. In addition to female predominance, OGCs also occur on average in patients almost a decade younger than PDCs and about a third occur in the body-tail. Despite being larger tumors (even when the non-invasive components are discounted), they appear to be less invasive than PDCs, with less likelihood of perineural invasion and nodal metastasis. This may be related to their relatively pushing-border infiltration patterns and intraductal/intracystic growth, allowing them to achieve larger sizes before diagnosis. In the literature, acinar cell carcinomas with prominent intraductal growth have been shown to have more protracted clinical course, some surviving beyond 10 years (39, 40). This phenomenon has been noted in the literature in isolated OGC case reports as well (41–45).
Based on the common association with a DA component (76%), ductal precursor (MCN/IPMN) lesions (21%), and PanIN-3 (47%), as well as positivity for epithelial markers in the pleomorphic cells, and striking propensity for intraductal/intracystic growth, this study further supports OGCs’ origin from ductal epithelium (8, 13, 46–49). This is in accordance with other studies that have shown KRAS mutation in the HSCs in these tumors (21, 50). As such, OGCs can be regarded as a striking and fairly distinctive model of epithelial-mesenchymal transition. It should also be noted here that sarcomatoid carcinomas in other organs are now being found to have a very distinct molecular carcinogenesis. For example, in the breast, molecular-genetic studies have confirmed sarcomatoid/metaplastic carcinomas to be a model of epithelial-to-mesenchymal transition with stem cell characteristics at molecular level as well (51). It is safe to assume that a similar principle will apply also to OGCs but ought to be tested with further molecular analysis.
In conclusion, pancreatic “undifferentiated carcinomas with osteoclastic giant cells (OGC)” are highly distinct from other “undifferentiated” pancreatic carcinomas and should be classified separately. OGCs present as relatively large tumors and in slightly younger patients than ordinary pancreatic ductal adenocarcinomas without osteoclastic cells (PDCs). Additionally 21% arise in IPMN/MCN, and > 50% show intraductal/intracystic polypoid growth or pushing-border infiltration as in sarcomatoid carcinomas in other organs. OGCs have a better prognosis than the current impression in the literature, with estimated 5-year survival rate of 59.1%. Further studies are warranted to disclose the molecular pathogenesis of these peculiar neoplasms.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Lane RB, Jr, Sangueza OP. Anaplastic carcinoma occurring in association with a mucinous cystic neoplasm of the pancreas. Arch Pathol Lab Med. 1997;121:533–535. [PubMed] [Google Scholar]
- 2.Posen JA. Giant cell tumor of the pancreas of the osteoclastic type associated with a mucous secreting cystadenocarcinoma. Hum Pathol. 1981;12:944–947. doi: 10.1016/s0046-8177(81)80203-1. [DOI] [PubMed] [Google Scholar]
- 3.Jang KT, Park SM, Basturk O, et al. Clinicopathologic characteristics of 29 invasive carcinomas arising in 178 pancreatic mucinous cystic neoplasms with ovarian-type stroma: implications for management and prognosis. Am J Surg Pathol. 2015;39:179–187. doi: 10.1097/PAS.0000000000000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luka’s Z, Dvora’k K, Kroupova’ I, et al. Immunohistochemical and genetic analysis of the osteoclastic giant cell tumor of the pancreas. Pancreas. 2006;32:325–329. doi: 10.1097/01.mpa.0000202951.10612.fa. [DOI] [PubMed] [Google Scholar]
- 5.Lewanrowski KB, Weston L, Dickersin GR, et al. Giant cell tumor of the pancreas of mixed osteoclastic and pleomorphic cell type. Hum Pathol. 1990;21:1184–1187. doi: 10.1016/0046-8177(90)90157-z. [DOI] [PubMed] [Google Scholar]
- 6.Nai GA, Amico E, Gimenez VR, et al. Osteoclast-like giant cell tumor of the pancreas associated with mucus-secreting adenocarcinoma. Pancreatology. 2005;5:279–284. doi: 10.1159/000085283. [DOI] [PubMed] [Google Scholar]
- 7.Osaka H, Yashiro M, Nishino H, et al. A case of osteoclast-type giant cell tumor of the pancreas with high-frequency microsatellite instability. Pancreas. 2004;29:239–241. doi: 10.1097/00006676-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Hruban RH, Pitman MB, Klimstra DS. AFIP atlas of tumor pathology series 4 Tumors of the pancreas. Washington, USA: ARP Press; 2007. [Google Scholar]
- 9.Fukushima N, Hruban RH, Kato Y, et al. Ductal adenocarcinoma variants and mixed neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, et al., editors. WHO Classification of Tumours of the Digestive System. 4th. Lyon, France: IARC Press; 2010. pp. 292–295. [Google Scholar]
- 10.Dworak O, Wittekind C, Koerfgen HP, et al. Osteoclastic giant cell tumor of the pancreas. An immunohistological study and review of the literature. Pathol Res Pract. 1993;189:228–231. doi: 10.1016/S0344-0338(11)80101-9. discussion 232–234. [DOI] [PubMed] [Google Scholar]
- 11.Manci EA, Gardner LL, Pollock WJ, et al. Osteoclastic giant cell tumor of the pancreas. Aspiration cytology, light microscopy, and ultrastructure with review of the literature. Diagn Cytopathol. 1985;1:105–110. doi: 10.1002/dc.2840010205. [DOI] [PubMed] [Google Scholar]
- 12.Paal E, Thompson LD, Frommelt RA, et al. A clinicopathologic and immunohistochemical study of 35 anaplastic carcinomas of the pancreas with a review of the literature. Ann Diagn Pathol. 2001;5:129–140. doi: 10.1053/adpa.2001.25404. [DOI] [PubMed] [Google Scholar]
- 13.Molberg KH, Heffess C, Delgado R, et al. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer. 1998;82:1279–1287. doi: 10.1002/(sici)1097-0142(19980401)82:7<1279::aid-cncr10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Gao HQ, Yang YM, Zhuang Y, et al. Locally advanced undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. World J Gastroenterol. 2015;21:694–698. doi: 10.3748/wjg.v21.i2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shamblin WR, Priestley JT, Sprague RG, et al. Total pancreatectomy for pleomorphic carcinoma. A five-year cure. Arch Surg. 1966;92:315–317. doi: 10.1001/archsurg.1966.01320200155027. [DOI] [PubMed] [Google Scholar]
- 16.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic Intraepithelial Neoplasia: A New Nomenclature and Classification System for Pancreatic Duct Lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Adsay V, Mino-Kenudson M, Furukawa T, et al. Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting. Ann Surg. 2016;263:162–177. doi: 10.1097/SLA.0000000000001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basturk O, Hong SM, Wood LD, et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol. 2015;39:1730–1741. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forsyth RG, Hogendoorn PCW. Bone: Giant cell tumour. Atlas Genet Cytogenet Oncol Haematol. 2003;7:193–196. [Google Scholar]
- 20.Rosai J. Carcinoma of pancreas simulating giant cell tumor of bone. Electron-microscopic evidence of its acinar cell origin. Cancer. 1968;22:333–344. doi: 10.1002/1097-0142(196808)22:2<333::aid-cncr2820220210>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Westra WH, Sturm P, Drillenburg P, et al. K-ras oncogene mutations in osteoclast-like giant cell tumors of the pancreas and liver: genetic evidence to support origin from the duct epithelium. Am J Surg Pathol. 1998;22:1247–1254. doi: 10.1097/00000478-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Dhall D, Klimstra DS. The cellular composition of osteoclastlike giant cell-containing tumors of the pancreatobiliary tree. Am J Surg Pathol. 2008;32:335–337. doi: 10.1097/PAS.0b013e3180342793. [DOI] [PubMed] [Google Scholar]
- 23.Albores-Saavedra J, Grider DJ, Wu J, Henson DE, et al. Giant cell tumor of the extrahepatic biliary tree: a clinicopathologic study of 4 cases and comparison with anaplastic spindle and giant cell carcinoma with osteoclast-like giant cells. Am J Surg Pathol. 2006;30:495–500. doi: 10.1097/00000478-200604000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Dhall D, Klimstra DS. The cellular composition of osteoclastlike giant cell-containing tumors of the pancreatobiliary tree. Am J Surg Pathol. 2008;32:335–337. doi: 10.1097/PAS.0b013e3180342793. [DOI] [PubMed] [Google Scholar]
- 25.Pandruvada SN, Yuvaraj S, Liu X, et al. Role of CXC chemokine ligand 13 in oral squamous cell carcinoma associated osteolysis in athymic mice. Int J Cancer. 2010;126:2319–2329. doi: 10.1002/ijc.24920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohamed MM, El-Ghonaimy EA, Nouh MA, et al. Cytokines secreted by macrophages isolated from tumor microenvironment of inflammatory breast cancer patients possess chemotactic properties. Int J Biochem Cell Biol. 2014;46:138–147. doi: 10.1016/j.biocel.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid MD, Basturk O, Thirabanjasak D, et al. Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod Pathol. 2011;24:1612–1619. doi: 10.1038/modpathol.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khayyata S, Basturk O, Adsay NV. Invasive micropapillary carcinomas of the ampullo-pancreatobiliary region and their association with tumor-infiltrating neutrophils. Mod Pathol. 2005;18:1504–1511. doi: 10.1038/modpathol.3800460. [DOI] [PubMed] [Google Scholar]
- 29.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson LDR, Basturk O, Adsay NV. Pancreas. In: Mills S, Greenson JK, Hornick JL, et al., editors. Stemberg’s diagnostic surgical pathology. 6th. Philadelphia, Baltimore, New York, London, Hong Kong, Sydney, Tokyo: Wolters Kluwer Press; 2015. pp. 1577–1662. [Google Scholar]
- 32.Klimstra DS, Adsay NV. Tumors of the pancreas. In: Odze RD, Goldblum JR, editors. Surgical pathology of the GI tract, liver, biliary tract, and pancreas. 3th. Philadelphia, Baltimore, New York, London, Hong Kong, Sydney, Tokyo: Elesevier Saunders press; 2015. pp. 1081–1119. [Google Scholar]
- 33.Miettinen M, Fletcher CDM, Kindblom LG, et al. Mesenchymal tumours of the esophagus. In: Bosman FT, Carneiro F, Hruban RH, et al., editors. WHO Classification of Tumours of the Digestive System. 4th. Lyon, France: IARC Press; 2010. pp. 35–36. [Google Scholar]
- 34.Bansal A, Kumar N, Sharma SC. Sarcomatoid variant of urothelial carcinoma of the urinary bladder. J Cancer Res Ther. 2013;9:571–573. doi: 10.4103/0973-1482.126449. [DOI] [PubMed] [Google Scholar]
- 35.Lee JS, Kim YB, Min KW. Metaplastic mammary carcinoma with osteoclast-like giant cells: identical point mutation of p53 gene only identified in both the intraductal and sarcomatous components. Virchows Arch. 2004;444:194–197. doi: 10.1007/s00428-003-0953-5. [DOI] [PubMed] [Google Scholar]
- 36.Deckard-Janatpour K, Kragel S, Teplitz RL, et al. Tumors of the pancreas with osteoclast-like and pleomorphic giant cells: an immunohistochemical and ploidy study. Arch Pathol Lab Med. 1998;122:266–272. [PubMed] [Google Scholar]
- 37.Mannan R, Khanna M, Bhasin TS, et al. Undifferentiated carcinoma with osteoclast-like giant cell tumor of the pancreas: a discussion of rare entity in comparison with pleomorphic giant cell tumor of the pancreas. Indian J Pathol Microbiol. 2010;53:867–868. doi: 10.4103/0377-4929.72016. [DOI] [PubMed] [Google Scholar]
- 38.Maksymov V, Khalifa MA, Bussey A, et al. Undifferentiated (anaplastic) carcinoma of the pancreas with osteoclast-like giant cells showing various degree of pancreas duct involvement. A case report and literature review. JOP. 2011;12:170–176. [PubMed] [Google Scholar]
- 39.Basturk O, Zamboni G, Klimstra DS, et al. Intraductal and papillary variants of acinar cell carcinomas: a new addition to the challenging differential diagnosis of intraductal neoplasms. Am J Surg Pathol. 2007;31:363–370. doi: 10.1097/01.pas.0000213376.09795.9f. [DOI] [PubMed] [Google Scholar]
- 40.Ban D, Shimada K, Sekine S, et al. Pancreatic ducts as an important route of tumor extension for acinar cell carcinoma of the pancreas. Am J Surg Pathol. 2010;34:1025–1036. doi: 10.1097/PAS.0b013e3181e2bc11. [DOI] [PubMed] [Google Scholar]
- 41.Silverman JF, Finley JL, MacDonald KG., Jr Fine-needle aspiration cytology of osteoclastic giant-cell tumor of the pancreas. Diagn Cytopathol. 1990;6:336–340. doi: 10.1002/dc.2840060509. [DOI] [PubMed] [Google Scholar]
- 42.Shindoh N, Ozaki Y, Kyogoku S, et al. Osteoclast-type giant cell tumor of the pancreas: helical CT scans. Am J Roentgenol. 1998;170:653–654. doi: 10.2214/ajr.170.3.9490947. [DOI] [PubMed] [Google Scholar]
- 43.Tezuka K, Yamakawa M, Jingu A, et al. An Unusual case of undifferentiated carcinoma in situ with osteoclastlike giant cell of the pancreas. Pancreas. 2006;33:304–310. doi: 10.1097/01.mpa.0000235303.11734.2a. [DOI] [PubMed] [Google Scholar]
- 44.Weigt J, Bellutti M, Malfertheiner P. Osteoclast-like giant-cell tumour of the pancreas causing painful ampullary obstruction. Digestive and Liver Disease. 2007;39:952. doi: 10.1016/j.dld.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Naito Y, Kinoshita H, Okabe Y, et al. Pathomorphologic study of undifferentiated carcinoma in seven cases: relationship between tumor and pancreatic duct epithelium. J Hepatobiliary Pancreat Surg. 2009;16:478–484. doi: 10.1007/s00534-009-0078-6. [DOI] [PubMed] [Google Scholar]
- 46.Hansen T, Burg J, Kirkpatrick CJ, et al. Osteoclast-like giant cell tumor of the pancreas with ductal adenocarcinoma: case report with novel data on histogenesis. Pancreas. 2002;25:317–320. doi: 10.1097/00006676-200210000-00017. [DOI] [PubMed] [Google Scholar]
- 47.Hoorens A, Prenzel K, Lemoine NR, et al. Undifferentiated carcinoma of the pancreas: analysis of intermediate filament profile and K-ras mutations provides evidence of a ductal origin. J Pathol. 1998;185:53–60. doi: 10.1002/(SICI)1096-9896(199805)185:1<53::AID-PATH45>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 48.Bergmann F, Aulmann S, Wente MN, et al. Molecular characterisation of pancreatic ductal adenocarcinoma in patients under 40. J Clin Pathol. 2006;59:580–584. doi: 10.1136/jcp.2005.027292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gocke CD, Dabbs DJ, Benko FA, et al. KRAS oncogene mutations suggest a common histogenetic origin for pleomorphic giant cell tumor of the pancreas, osteoclastoma of the pancreas, and pancreatic duct adenocarcinoma. Hum Pathol. 1997;28:80–83. doi: 10.1016/s0046-8177(97)90283-5. [DOI] [PubMed] [Google Scholar]
- 50.Krasinskas AM, Moser AJ, Saka B, et al. KRAS mutant allele-specific imbalance is associated with worse prognosis in pancreatic cancer and progression to undifferentiated carcinoma of the pancreas. Mod Pathol. 2013;26:1346–1354. doi: 10.1038/modpathol.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]


