Abstract
During development oligodendrocyte precursor cells (OPCs) rapidly proliferate and migrate throughout the central nervous system. The mobilization of OPCs is followed by terminal differentiation into mature oligodendrocytes and the subsequent myelination of axons. Differentiation of OPCs is CNS-wide and robust, and yet spatially and temporally restricted. What factors control this precise and coordinated differentiation effort? We discuss evidence for both intrinsic and extrinsic cues in regulating OPC differentiation and gather that extrinsic cues play the leading role in regulating the differentiation of OPCs into mature oligodendrocytes.
INTRODUCTION
One of the most exquisite neuronal-glial interactions is the myelination of axons by oligodendrocytes. Oligodendrocytes concentrically wrap their plasma membranes around the axons of neurons in order to produce myelin, a multi-layered, compact sheath that insulates the axon and allows for rapid conduction of electrical impulses. Myelination regulates the activity and timing of neural circuits [1–3], while also providing metabolic support [4]. Oligodendrocytes arise from oligodendrocyte precursor cells (OPCs) that multiply and populate the CNS during development until they mature into myelinating oligodendrocytes. Differentiation of OPCs is CNS-wide and robust, and yet spatially and temporally restricted. What factors control this precise and coordinated differentiation effort? It is clear that several transcriptional, chromatin remodeling, and epigenetic factors are involved in the process of oligodendrocyte differentiation [5–7], but whether differentiation is initiated by intrinsic or extrinsic factors—or a combination of both is still a matter of debate. In this review, we will discuss evidence that points to either intrinsic or extrinsic cues in governing the differentiation of OPCs into mature oligodendrocytes and contend that extrinsic signals are ultimately the main regulators of differentiation. Importantly, while oligodendrocyte differentiation and myelination have yet to be mechanistically uncoupled as separate processes, for the purposes of this review we will assume that differentiation and myelination are independently controlled and focus solely on differentiation.
Intrinsic control of differentiation
Does oligodendrocyte differentiation occur intrinsically via a pre-program under the cells own control? If a pre-program exists one main criteria must be met – namely that some distinguishable intrinsic differences among the OPC population will allow some OPCs to differentiate during development, while others will not and perhaps never will. Differences within the OPC population have been identified and it is now well established that OPCs are generated from three regions in the brain and two regions in the spinal cord at different times during development [8]. The neural stem cells that give rise to each wave of OPCs can be identified using different molecular markers indicating that the cells start out with substantial differences in gene expression. These differences could potentially result in different populations of OPCs – at least five main populations corresponding to their place and time of birth. However, it has also been demonstrated that any one region can take over the production of OPCs if any of the other regions fail to generate them resulting in a functionally normal oligodendroglial population [9]. This suggests that the differences associated from their place and time of birth may not have a significant impact on OPC function and oligodendrocyte development and implies a relatively homogeneous population.
While birth origin may not lead to OPC heterogeneity, other studies have suggested that the final destination of OPCs does, namely gray matter versus white matter [10]. One study finds that oligodendroglia from white matter differentiate similarly when transplanted into either gray or white matter, but that oligodendroglia from gray matter will only differentiate when transplanted into white matter [11]. The authors conclude that there are intrinsic differences in white matter versus gray matter oligodendroglia, although they note that the white matter environment seems to better support differentiation. It is important to note that this study was conducted using oligodendroglia from adult mice and therefore these cells may have already been largely influenced by their microenvironments to produce the differences observed by the authors. A different transplant study using explants from young postnatal pups also analyzed the differences between white and gray matter OPCs and found that white matter OPCs are more responsive, this time to the growth factor platelet-derived growth factor (PDGF), than gray matter-derived OPCs [12]. However, since this study used explants rather than dissociated cells to transplant onto slice cultures, it is possible that the respective white and gray matter microenvironments were transplanted along with the OPCs and therefore continued environmental influence cannot be ruled out as the source for the differences.
A recent study using single cell gene expression analysis identified six different subclasses of oligodendroglial cells in gray matter including somatosensory cortex and hippocampus of the mouse brain [13]. One caveat to this study is that it is not absolutely clear whether these six types of oligodendroglial cells represent different cell types or different developmental stages, although the authors suggest that based on their gene expression patterns the cells likely represent different developmental stages. All six subclasses were found in both areas of gray matter analyzed except for one type that seemed to be enriched in the somatosensory cortex. This enrichment suggests a heavy environmental influence and it would be illuminating to determine whether these six subclasses are also found in white matter and whether the same subclasses are found throughout development. If these subclasses do indeed represent different developmental stages and not oligodendroglial cell types it would be difficult to conclude that a heterogenous population of OPCs exists.
It is important to note that even if a heterogeneous population of cells is identified based on transcriptional profiling, morphology and function, it does not implicitly indicate intrinsic “preprogramming”. While it is difficult to say for certain that a pre-program of OPC differentiation exists, heterogeneity of OPCs can just as easily be accomplished by specific environmental cues. Astrocytes, another glial type in the CNS, offer valuable insight into this issue, as they are thought to be highly heterogeneous throughout the nervous system based on specific transcriptional profiles, morphology and function [14]. A recent study revealed just how heterogeneity can be influenced by environmental signals–illustrating that velate astrocytes (VA) located in a regionally distinct area from the bergman glial (BG) astrocytes can obtain BG-like properties when subjected to increased levels of Sonic hedgehog (Shh) signaling [15]. The well-known morphogen is highly secreted by Purkinje neurons that are located alongside BG. The study also revealed that BG adopted VA-like properties if Shh signaling was attenuated. While OPCs do not posses the large amount of heterogeneity seen in astrocytes, there is a great deal of evidence that demonstrates the necessity of extrinsic stimuli to OPCs and how it can influence their differentiation. Furthermore, it has long been proposed that OPCs contain an internal timer that governs cell cycle progression and therefore determines when OPCs divide and when they will differentiate [16]. However, as mentioned earlier, even the concept of an intrinsic timer can be coupled with extrinsic signals to finely tune not only the “when” but also the “where” these processes occur. Therefore, an intrinsic mechanism makes more sense when combined with environmental signals that will ultimately control the spatiotemporal differentiation of oligodendrocytes [16,17].
Extrinsic control of differentiation
There are various known molecular inhibitors and to a lesser extent, promoters of oligodendrocyte differentiation. Inhibitors can be molecules that promote proliferation such as the mitogen PDGF. Since proliferation and differentiation are mutually exclusive processes, a factor that promotes proliferation cannot at the same time directly promote differentiation. However, numerous studies show that factors that promote proliferation subsequently also promote differentiation. For instance, over-expression of PDGF in neurons promotes a significant increase in OPC numbers as well as early and widespread differentiation of OPCs [18]. Moreover, Shh signaling, known to be important for OPC specification [19,20], has also been shown to promote OPC proliferation [21,22,20]. However studies in models of demyelination have shown that increased Shh signaling can also promote OPC differentiation [23]. One particular study using the cuprizone model of demyelination showed that while loss of Shh signaling did not have an effect on differentiation of OPCs within the lesion, removal of one of its downstream effectors, Gli1, enhanced differentiation, and that this differentiation was further enhanced by over-activation of Shh signaling [24]. The transcription factor Gli1 is well known to be activated by Shh signaling [25], so these results are surprising, but they do point to Shh-independent regulation of Gli1. Ultimately, the mechanism by which these factors, PDGF and Shh, induce both OPC proliferation and differentiation is not well understood and perhaps Gli1 may play a role in determining which process occurs, but it may be that the enhanced differentiation occurs as a consequence of the increased population of OPCs, possibly via enhanced depletion of available mitogens or increased spatial constraints as discussed later in the review, and not from the mitogenic signals directly.
Another known inhibitor of oligodendrocyte differentiation is Wnt signaling. Early studies show that over-activation of the Wnt pathway via expression of a constitutively active beta-catenin delays differentiation while inhibiting the pathway via receptor antagonists promotes differentiation [26,27]. More recently, activation of Wnt signaling was also shown to increase OPC generation and proliferation [28]. However, this has become more complicated as later studies have conversely shown that activation of Wnt signaling decreases OPC specification and that disruption causes a decrease in OPC differentiation [29]. This discrepancy may be due to the existence of multiple downstream pathways [30]. It has now been shown that one of the extrinsic factors that can activate Wnt signaling is hypoxia via upregulation of hypoxia inducible factor (HIF) [31]. OPCs require substantial metabolic support to differentiate [32], which means that access to the vasculature and proper oxygenation is crucial. In fact, a recent study showed that OPCs spend a great deal of time associating with and migrating on vasculature [33]. Taken together, without proper vascularization, OPCs become hypoxic and upregulate HIF expression, which then leads to Wnt activation to inhibit differentiation until proper support is available [31].
Certain astroglial extracellular matrix (ECM) components can block oligodendrocyte differentiation. Chondroitin sulfate proteoglycans (CSPGs) and the glycoprotein Tenascin C, largely found on astrocyte ECM have been found to inhibit OPC differentiation [34]. Additionally, astrocytes secrete a high molecular weight form of the glycosoaminoglycan, hyaluronan (HA), into the ECM which can inhibit the differentiation of OPCs when cleaved by OPC-secreted hyaluronidases [35–37]. Interestingly, HA also accumulates in white matter lesions in the EAE model and various demyelinating diseases in humans and is considered to be one of the factors that inhibits remyelination [35,38]. The demyelinated lesion environment contains several more factors that inhibit OPC differentiation including semaphorins [39,40], bone morphogenic proteins (BMPs) secreted by reactive astrocytes [41], fibronectin [42], and myelin debris [43].
Known promoters of differentiation are less numerous. Several studies are now examining the role of neuronal activity in OPC differentiation and myelination. It has been established that neurons make functional synapses onto OPCs [17], and it has been hypothesized that the purpose of this intimate interaction is for neurons to communicate to OPCs about where and when to differentiate and myelinate. If this is true, perhaps increasing neural activity promotes differentiation. OPCs express neurotransmitter receptors including the glutamate receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) [17]. In vitro studies have shown that blocking activity or AMPAR increased OPC proliferation and differentiation [44]. Other studies have shown that increasing glutamate release or activating NMDAR promoted OPC differentiation, although effects on proliferation were not analyzed [45,46]. However, other research shows that blocking or knocking out NMDAR has no effect on OPC proliferation or differentiation [44,47]. In an in vivo gain of function experiment, optogenetics was used to increase neuronal activity in mouse premotor cortical neurons [48]. Interestingly, increased activity led to enhanced OPC proliferation throughout the stimulated circuit followed by increased differentiation along with hyper-myelination. Conversely, recent research in zebrafish in which neuronal activity was pharmacologically increased or inhibited showed that modulating activity had little to no effect on OPC proliferation and differentiation, but it did modulate the extent of myelination [49,50]. While these studies are not all in perfect agreement, it is interesting to note that when an increase in OPC differentiation is observed it is preceded by OPC proliferation.
There is evidence that increased spatial constraints, which can be brought about by increasing cellular density, for instance, can promote the differentiation of OPCs. In vitro studies have shown that OPCs seeded onto neurons at higher densities differentiate earlier than OPCs seeded at lower densities [51]. These researchers also found that OPCs seeded at low densities can be induced to differentiate earlier when the OPCs were seeded along with another cell type (Schwann Cells) or even polystyrene beads [51] thus pointing to a mechanical induction of differentiation as the most probable mechanism. Similarly, other studies have shown that substrate rigidity can play a strong role in regulating OPC differentiation – stiffer substrates promote differentiation better than softer ones [52,53]. How might this mechanical induction of differentiation be accomplished? Recent work has shown that certain protein structures that connect the cell membrane to nuclear components may be at play. Researchers knocked down the expression of Syne1 (synaptic nuclear envelope protein 1), a component of the Linker of the Nucleoskeleton and the Cytoskeleton (LINC) complex, and found that increased spatial constraints, via addition of polystyrene beads or membrane compression, no longer promoted differentiation [54]. They also saw that when wild type OPCs were spatially constrained a complete reorganization of nuclear chromatin structure was observed. They propose that mechanical forces act on rigid cytoskeletal structures that are linked to nuclear components, which interact with epigenetic machinery to induce transcriptional changes that lead to differentiation. It is possible then that perhaps this mechanism, which promotes differentiation of OPCs subjected to increased spatial constraints, is the same mechanism by which increasing proliferation of OPCs, as with addition of mitogen or increasing neuronal activity, also increases differentiation, since increased cellular density will increase the physical forces acting on each OPC.
Environment rules
The nature versus nurture debate is one that has been around for centuries and will likely continue to be debated for centuries to come as new discoveries place more weight on one over the other. As it stands now, most scholars will agree that both genes and the environment are important in governing many aspects of biological and cellular behaviors. However, when it comes to oligodendrocyte differentiation the evidence seems to lie heavily on the side of extrinsic environmental cues determining where and when OPC differentiation occurs. Previous observations that may at first seem to imply intrinsic control of oligodendrocyte development, such as OPC heterogeneity, can much better be explained by extrinsic influences. This is even more clearly seen now that epigenetic studies have shown several ways in which external stimuli can affect epigenetic and transcriptional machinery [6,55,56]. However, while environmental signals may play the chief role in regulating OPC differentiation, they do not exclude the possibility that intrinsic mechanisms exist. Importantly, epigenetic modifications can be inherited and passed on to generations of daughter cells, thus potentially causing population wide effects which may appear to be intrinsic to the cell and which can also be reversed [56].
We’ve discussed several extrinsic signals that seem to play a role in regulating OPC differentiation (Figure 1), several other extrinsic signals were not discussed, but common to all known factors is that their individual effects are not very convincing on their own. At most their actions either delay or promote early differentiation, yet differentiation always occurs. Is there an as yet undiscovered extrinsic trigger or combination of triggers for OPC differentiation? What is their source and are they the same in development and in adulthood? Are the triggers promoters or inhibitors of differentiation, or both (Figure 2)? There is a great need for remyelination therapies for diseases that generate demyelinating lesions in the CNS. As briefly discussed, it is well known that these lesions are made up of largely inhibitory factors for OPC differentiation. It is therefore imperative to discover strategies that either remove the inhibitory signals or overcome them with factors that promote OPC differentiation. Numerous puzzles and questions remain and we look forward to many new discoveries in the field that will illuminate the answers.
Figure 1. Extrinsic regulation of OPC differentiation.
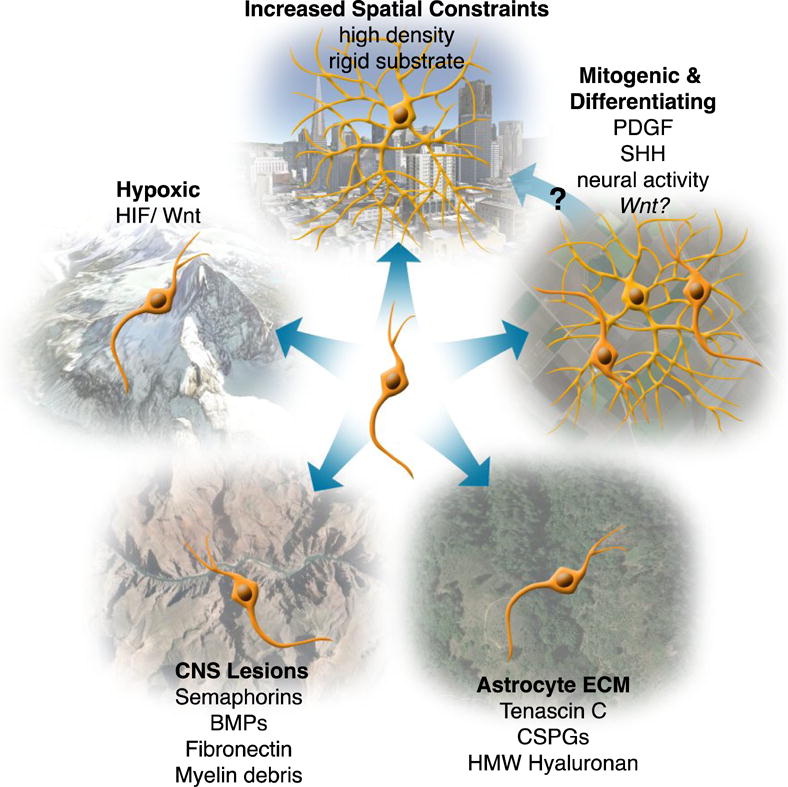
OPCs encounter different environments throughout their life-time. These environments can express cues that determine whether an OPC will differentiate or not. Most extrinsic cues are inhibitory to differentiation as seen when OPCs are found in hypoxic, lesioned, or astrocyte-rich environments, thereby maintaining OPCs as OPCs. Some environments contain cues that promote both proliferation and differentiation such as certain mitogenic environments. Environments that have increased spatial constraints, due to high cellular density or substrate rigidity, promote differentiation. In this way mitogenic environments, which promote proliferation, may subsequently promote differentiation by increasing cellular density. HMW = high molecular weight. Environmental images captured on Google Earth and are attributed to Google; Sanborn; Cybercity; DigitalGlobe.
Figure 2. Signals that promote or inhibit oligodendrocyte differentiation.
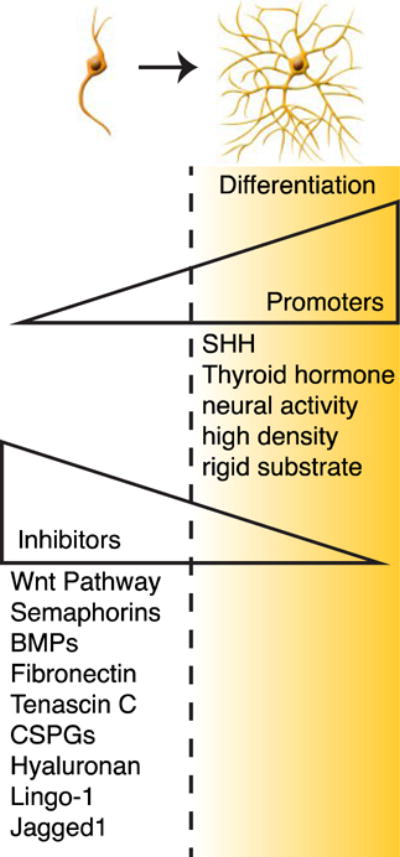
OPCs experience the presence of both promoters and inhibitors of differentiation simultaneously therefore differentiation may only be triggered when the actions of the promoters outweigh the inhibitors.
HIGHLIGHTS.
OPC differentiation is largely initiated by extrinsic signals.
The majority of extrinsic signals that regulate OPC differentiation are inhibitory.
Extrinsic differentiation cues include mechanical deformation of the cell membrane.
Overcoming extrinsic inhibitory cues is crucial for the repair of myelin lesions.
Acknowledgments
We thank R. R. Mayoral for his assistance in creating the illustrations in Figure 1 and Figure 2. We also thank the members of the Chan laboratory for their constructive and critical feedback on the manuscript. This review was supported by the NMSS Research Grants (RG4541A3 and RG5203A4), NIH/NINDS (R01NS062796), the Rachleff Family Endowment to JRC and NIH/NIGMS (K12GM081266) IRACDA Postdoctoral Fellowship to SRM. We respectfully apologize to colleagues whose relevant work was not discussed due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there is no conflict of interest in the submitted review.
References
- 1.Tomassy GS, Dershowitz LB, Arlotta P. Diversity Matters: A Revised Guide to Myelination [Internet] Trends Cell Biol. 2015 doi: 10.1016/j.tcb.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fields RD. A new mechanism of nervous system plasticity: activity-dependent myelination. [Internet] . Nat Rev Neurosci. 2015;16:756–67. doi: 10.1038/nrn4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michalski J-P, Kothary R. Oligodendrocytes in a Nutshell. [Internet] Front Cell Neurosci. 2015;9:340. doi: 10.3389/fncel.2015.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang P-W, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. [Internet] Nature. 2012;487:443–8. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuchero JB, Barres BA. Intrinsic and extrinsic control of oligodendrocyte development. [Internet] Curr Opin Neurobiol. 2013;23:914–20. doi: 10.1016/j.conb.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyon S, Liang J, Casaccia P. Epigenetics in NG2 glia cells. [Internet] Brain Res. 2015 doi: 10.1016/j.brainres.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emery B, Lu QR. Transcriptional and Epigenetic Regulation of Oligodendrocyte Development and Myelination in the Central Nervous System. [Internet] Cold Spring Harb Perspect Biol. 2015;7:a020461–. doi: 10.1101/cshperspect.a020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. [Internet] Nat Rev Neurosci. 2006;7:11–8. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. [Internet] Nat Neurosci. 2006;9:173–9. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viganò F, Dimou L. The heterogeneous nature of NG2-glia. [Internet] Brain Res. 2015 doi: 10.1016/j.brainres.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Viganò F, Möbius W, Götz M, Dimou L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. [Internet] Nat Neurosci. 2013;16:1370–2. [Google Scholar]
- 12.Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A. NG2 Cells in White Matter But Not Gray Matter Proliferate in Response to PDGF [Internet] J Neurosci. 2013;33:14558–14566. doi: 10.1523/JNEUROSCI.2001-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Zeisel A, Manchado ABM, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq [Internet] Science (80-.) 2015;347:1138–1142. doi: 10.1126/science.aaa1934. Using single cell gene expression analysis this study identifies six different subclasses of oligodendroglial cells likely representing sequential steps in the process of maturation, from OPC to terminally differentiated oligodendrocyte. [DOI] [PubMed] [Google Scholar]
- 14.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte development and heterogeneity. [Internet] Cold Spring Harb Perspect Biol. 2015;7:a020362. doi: 10.1101/cshperspect.a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Farmer WT, Abrahamsson T, Chierzi S, Lui C, Zaelzer C, Jones EV, Bally BP, Chen GG, Theroux J-F, Peng J, et al. Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling [Internet] Science (80-.) 2016;351:849–854. doi: 10.1126/science.aab3103. In this paper researchers show how the secretion of Shh from Purkinjie neurons is crucial to the production of Bergman glia (BG), a particular type of astocyte that resides adjacent to the neurons. Inhibition of Shh signaling leads to the misdevelopment of BG into a more Velate astrocyte (VA)-like cell. VAs reside in a regionally distinct area from BG. Researchers also found that activating Shh signaling in VAs induces them to develop into BG-like cells. [DOI] [PubMed] [Google Scholar]
- 16.Durand B, Raff M. A cell-intrinsic timer that operates during oligodendrocyte development. [Internet] Bioessays. 2000;22:64–71. doi: 10.1002/(SICI)1521-1878(200001)22:1<64::AID-BIES11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Bergles DE, Richardson WD. Oligodendrocyte Development and Plasticity. [Internet] Cold Spring Harb Perspect Biol. 2015;8:a020453–. doi: 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calver aR, Hall aC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. [Internet] Neuron. 1998;20:869–82. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- 19.Yu K, McGlynn S, Matise MP. Floor plate-derived sonic hedgehog regulates glial and ependymal cell fates in the developing spinal cord. [Internet] Development. 2013;140:1594–604. doi: 10.1242/dev.090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortega JA, Radonjić NV, Zecevic N. Sonic hedgehog promotes generation and maintenance of human forebrain Olig2 progenitors. [Internet] Front Cell Neurosci. 2013;7:254. doi: 10.3389/fncel.2013.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loulier K, Ruat M, Traiffort E. Increase of proliferating oligodendroglial progenitors in the adult mouse brain upon Sonic hedgehog delivery in the lateral ventricle. [Internet] J Neurochem. 2006;98:530–42. doi: 10.1111/j.1471-4159.2006.03896.x. [DOI] [PubMed] [Google Scholar]
- 22.Merchán P, Bribián A, Sánchez-Camacho C, Lezameta M, Bovolenta P, de Castro F. Sonic hedgehog promotes the migration and proliferation of optic nerve oligodendrocyte precursors. [Internet] Mol Cell Neurosci. 2007;36:355–68. doi: 10.1016/j.mcn.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Ferent J, Zimmer C, Durbec P, Ruat M, Traiffort E. Sonic Hedgehog signaling is a positive oligodendrocyte regulator during demyelination. [Internet] J Neurosci. 2013;33:1759–72. doi: 10.1523/JNEUROSCI.3334-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Samanta J, Grund EM, Silva HM, Lafaille JJ, Fishell G, Salzer JL. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. [Internet] Nature. 2015;526:448–52. doi: 10.1038/nature14957. Researchers show that inhibition of the Shh-signaling mediator, Gli1, promotes oligodendrodendrocyte differentiation in vivo. They also show that inhibition of Shh signaling does not have the same effect which implies Shh-independent regulation of Gli1 and demonstrates that Shh signaling is more complex than previously thought. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz i Altaba A, Palma V, Dahmane N. Hedgehog-Gli signalling and the growth of the brain. [Internet] Nat Rev Neurosci. 2002;3:24–33. doi: 10.1038/nrn704. [DOI] [PubMed] [Google Scholar]
- 26.Fancy SPJ, Baranzini SE, Zhao C, Yuk D-I, Irvine K-A, Kaing S, Sanai N, Franklin RJM, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. [Internet] Genes Dev. 2009;23:1571–85. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu T, Kagawa T, Wada T, Muroyama Y, Takada S, Ikenaka K. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. [Internet] Dev Biol. 2005;282:397–410. doi: 10.1016/j.ydbio.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Ortega F, Gascón S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. [Internet] Nat Cell Biol. 2013;15:602–13. doi: 10.1038/ncb2736. [DOI] [PubMed] [Google Scholar]
- 29.Dai Z-M, Sun S, Wang C, Huang H, Hu X, Zhang Z, Lu QR, Qiu M. Stage-specific regulation of oligodendrocyte development by Wnt/β-catenin signaling. [Internet] J Neurosci. 2014;34:8467–73. doi: 10.1523/JNEUROSCI.0311-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo F, Lang J, Sohn J, Hammond E, Chang M, Pleasure D. Canonical Wnt signaling in the oligodendroglial lineage–puzzles remain. [Internet] Glia. 2015;63:1671–93. doi: 10.1002/glia.22813. [DOI] [PubMed] [Google Scholar]
- 31*.Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SPJ, Zahed H, Maltepe E, Rowitch DH. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. [Internet] Cell. 2014;158:383–96. doi: 10.1016/j.cell.2014.04.052. This report shows for the first time a link between oligodendroglial cells and the vasculature. OPCs produce angiogenic factors to encourage vascularization during a critical window of early postnatal development that is essential for myelination and the integrity of axons in the white matter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris JJ, Attwell D. The energetics of CNS white matter. [Internet] J Neurosci. 2012;32:356–71. doi: 10.1523/JNEUROSCI.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai H-H, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien A-C, Kuo CJ, Chan JR, Daneman R, et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system [Internet] Science (80-.) 2016;351:379–384. doi: 10.1126/science.aad3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harlow DE, Macklin WB. Inhibitors of myelination: ECM changes, CSPGs and PTPs. [Internet] Exp Neurol. 2014;251:39–46. doi: 10.1016/j.expneurol.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Back SA, Tuohy TMF, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. [Internet] Nat Med. 2005;11:966–72. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 36.Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2 [Internet] Proc Natl Acad Sci. 2010;107:11555–11560. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preston M, Gong X, Su W, Matsumoto SG, Banine F, Winkler C, Foster S, Xing R, Struve J, Dean J, et al. Digestion products of the PH20 hyaluronidase inhibit remyelination. [Internet] Ann Neurol. 2013;73:266–80. doi: 10.1002/ana.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bugiani M, Postma N, Polder E, Dieleman N, Scheffer PG, Sim FJ, van der Knaap MS, Boor I. Hyaluronan accumulation and arrested oligodendrocyte progenitor maturation in vanishing white matter disease. [Internet] Brain. 2013;136:209–22. doi: 10.1093/brain/aws320. [DOI] [PubMed] [Google Scholar]
- 39.Syed YA, Hand E, Möbius W, Zhao C, Hofer M, Nave KA, Kotter MR. Inhibition of CNS remyelination by the presence of semaphorin 3A. [Internet] J Neurosci. 2011;31:3719–28. doi: 10.1523/JNEUROSCI.4930-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaguchi W, Tamai R, Kageura M, Furuyama T, Inagaki S. Sema4D as an inhibitory regulator in oligodendrocyte development. [Internet] Mol Cell Neurosci. 2012;49:290–9. doi: 10.1016/j.mcn.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR, Cao QL. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. [Internet] J Neurosci. 2011;31:6053–8. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoffels JMJ, de Jonge JC, Stancic M, Nomden A, van Strien ME, Ma D, Sisková Z, Maier O, Ffrench-Constant C, Franklin RJM, et al. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. [Internet] Brain. 2013;136:116–31. doi: 10.1093/brain/aws313. [DOI] [PubMed] [Google Scholar]
- 43.Plemel JR, Manesh SB, Sparling JS, Tetzlaff W. Myelin inhibits oligodendroglial maturation and regulates oligodendrocytic transcription factor expression. [Internet] Glia. 2013;61:1471–87. doi: 10.1002/glia.22535. [DOI] [PubMed] [Google Scholar]
- 44.Fannon J, Tarmier W, Fulton D. Neuronal activity and AMPA-type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. [Internet] Glia. 2015;63:1021–35. doi: 10.1002/glia.22799. [DOI] [PubMed] [Google Scholar]
- 45.Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. [Internet] Science. 2011;333:1647–51. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C, Xiao L, Liu X, Yang W, Shen W, Hu C, Yang G, He C. A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. [Internet] Glia. 2013;61:732–49. doi: 10.1002/glia.22469. [DOI] [PubMed] [Google Scholar]
- 47.De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, Bergles DE. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. [Internet] J Neurosci. 2011;31:12650–62. doi: 10.1523/JNEUROSCI.2455-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. [Internet] Science. 2014;344:1252304. doi: 10.1126/science.1252304. Researchers use optogenetics to increase neural activity in mouse premotor cortex and find that the increased activity leads to increased OPC proliferation, differentiation, and hypermyelination within the actived motor circuit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. [Internet] Nat Neurosci. 2015;18:628–30. doi: 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo [Internet] Nat Neurosci. 2015;18:683–689. doi: 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenberg SS, Kelland EE, Tokar E, De La Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation [Internet] Proc Natl Acad Sci. 2008;105:14662–14667. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. [Internet] Biomaterials. 2009;30:6867–78. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Jagielska A, Norman AL, Whyte G. Mechanical Environment Modulates Biological Properties of Oligodendrocyte Progenitor Cells [Internet] Stem Cells Dev. 2012;21:2905–2914. doi: 10.1089/scd.2012.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Hernandez M, Patzig J, Mayoral SR, Costa KD, Chan JR, Casaccia P. Mechanostimulation Promotes Nuclear and Epigenetic Changes in Oligodendrocytes [Internet] J Neurosci. 2016;36:806–813. doi: 10.1523/JNEUROSCI.2873-15.2016. OPC differentiation can be induced by packing constrains resulting from intercellular interactions that are biophysical in nature. This paper reports that the spatial constrains are mechanotransduced by the actin cytoskeleton and LINC complex, that mediate nuclear changes in oligodendrocyte progenitors that favor a default pathway of differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. [Internet] Nat Genet. 2003;33 (Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 56.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. [Internet] Cell. 2014;157:95–109. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


