Abstract
Mental work may promote caloric intake, while exercise may offset positive energy balance by decreasing energy intake and increasing energy expenditure.
Purpose
This study aimed to replicate previous findings that mental work increases caloric intake compared to a rest condition and assess whether exercise following mental work can offset this effect.
Methods
Thirty-eight male and female university students were randomly assigned to Mental Work + Rest (MW+R) or Mental Work + Exercise (MW+E). Participants also completed a Baseline Rest (BR) visit consisting of no mental work or exercise. Visit order was counterbalanced. During the MW+R or MW+E visit, participants completed a 20 minute mental task and either 15 minutes rest (MW+R) or 15 minutes interval exercise (MW+E). Each visit ended with an ad libitum pizza lunch. A two-way repeated measures ANOVA was used to compare eating behavior between groups.
Results
Participants in the MW+R condition consumed an average of 100 more kcal compared to BR (633.3 ± 72.9 and 533.9 ± 67.7, respectively, p = 0.02), and participants in MW+E consumed an average of 25 kcal less compared to BR (432.3 ± 69.2 and 456.5 ± 64.2, respectively, p > 0.05). When including the estimated energy expenditure of exercise in the MW+E conditions, participants were in negative energy balance by an average of 98.5 ± 41.5 kcal, resulting in a significant difference in energy balance between the two groups (p = 0.001).
Conclusion
An acute bout of interval exercise following mental work resulted in significantly decreased food consumption compared to a non-exercise condition. These results suggest an acute bout of exercise may be employed to offset positive energy balance induced by mental tasks.
Keywords: interval exercise, eating, appetite, mental fatigue, energy balance
Introduction
Sustained mental work may lead to mental fatigue and overeating (5, 6). Thus, a sedentary lifestyle accompanied by increased mental work may contribute to positive energy balance and to recent rises in obesity rates (2, 5, 6, 8, 10, 36). Exercise contributes to negative energy balance and may impact appetite, thereby altering the effect of mental work on energy balance.
Chaput and Tremblay (7) observed a 200 kilocalorie increase in food consumption following a 45 minute reading task compared to a rest condition, despite actual energy expenditure differing by only 3 kilocalories in the two conditions. Similar increases in food intake were observed following reading tasks (approximately 200 kcal increase) and computerized tests (approximately 250 kcal increase) in comparison to a rest condition (5). Also, a high workload prior to NIH grant deadlines has been associated with increased energy intake compared to low workload conditions (25). Another study has suggested an increase in carbohydrate intake following a mental stress task, though protein and total energy intake did not significantly differ between stress and non-stress conditions (14). Mentally demanding tasks may impact the brain's energy demands (14) and thus localized brain glucose/glycogen availability. This may contribute to feelings of fatigue and hunger, thereby increasing the chance of consuming more calories than one expends, resulting in a positive energy balance and weight gain (6).
Exercise may serve as a way to provide additional energy for brain function in the form of glucose and lactate (3, 32) and decrease calorie intake following mental work. Exercise sessions lasting an hour or less are associated with decreased desire for food and decreased calorie intake (4, 9, 12, 19, 27, 35, 37). A moderate bout of exercise (in the first 15-20 minutes) increases blood glucose by 10 to 15% and is also associated with higher glucose tolerance (20, 39, 40). The additional blood lactate provided by exercise may cross the blood brain barrier (3, 32) and offset the brain's need or desire for food consumption following a mental task.
By receiving additional fuel in the form of glucose and lactate via exercise, the brain may be less likely to signal for increased energy via food consumption. This outlined chain of events supports the glucostatic theory of appetite, where glucose influences hunger and satiety signals to the hypothalamus, thereby influencing short-term appetite control (24). Previous studies have investigated exercise or mental work's effect on energy intake, but few studies have combined mental work with exercise. One study measured caloric intake following rest, mental work, and exercise, and observed increased relative intake following mental work for women and decreased relative intake following exercise for men and women (28). Oh and Taylor (26) observed a 50% decrease in ad libitum chocolate consumption when exercise preceded mental work. In a sample of children, Horsch and colleagues (16) reported decreased intake of food when physical activity preceded a cognitive stressor. Lemay and colleagues reported a difference in energy balance following mental work without exercise compared to mental work plus exercise, but did not observe a difference in energy consumption (21). The goal of this study is to replicate increased food consumption following a mental work task, as well as assess exercise's effect on eating behavior following mental work. This study is novel due to a more controlled and higher intensity exercise session that immediately precedes an ad libitum meal. Timing of exercise may also affect energy consumption, as exercise immediately preceding a meal has been exhibited to have a stronger anorexigenic effect than when following a meal (1). Timing effects may be of importance, as exercise following a mentally stressful task may be a more natural behavior.
Overall, previous findings support the construct of increased mental workload leading to increased calorie intake and exercise potentially counteracting this effect. This paper compares calorie consumption between a mental work scenario, which has been demonstrated to increase calorie intake, and a mental work plus exercise scenario, which may result in reduced calorie intake. It is hypothesized that participants in the mental work + exercise (MW+E) group will consume less calories than participants in the mental work + rest (MW+R) group. Exploratory analyses for blood glucose and lactate's association with eating behavior were also conducted.
Methods
Participants were recruited from the University's undergraduate population. Exclusion criteria were poor health or not able to participate in high effort exercise; pre-existing cardiovascular or orthopedic issues; diabetes mellitus; women who were pregnant; taking medications known to affect eating behavior or energy expenditure or insulin sensitivity; food allergies; and BMI greater than 35. Participants were blinded to the main hypothesis until after they had completed all phases of the study. The study was approved by the University's Institutional Review Board and informed consent was obtained from all participants.
Procedure
All study visits occurred at the University's Nutrition Sciences Department facilities within a private room or exercise testing laboratory. All participants completed a screening visit, baseline rest (BR) visit, and either a mental work + rest (MW+R) or mental work + exercise (MW+E) visit. After the screening visit, participants were randomly assigned to the MW+R or MW+E protocols, and the order of the BR visit with MW+R or MW+E was counterbalanced. Randomization and counterbalancing was done by block randomization with matched pairs and computer generated random numbers. Participants were asked to fast from everything but water for at least 2 hours prior to arrival, to maintain consistent behaviors (sleep, exercise, meals, etc.) before each visit, and to wear exercise clothing to each visit.
At the screening visit, participants’ height, weight, waist circumference, body fat percentage via skinfolds, and VO2 submax were measured. Participants were also asked preferred pizza toppings from the options available (cheese only, pepperoni, mushroom, black olives, or a combination of toppings). Female participants were asked about timing of their menstrual cycle, and future scheduling avoided the luteal phase.
The BR protocol consisted of a 35 minute rest period then self-report satiety and fatigue scales, followed by an ad libitum pizza lunch. During the rest period, the participant was asked to not engage in any mentally or physically stimulating activities (e.g., stretching, reading, conversation, etc.). The MW+R protocol began with a 20 minute mental task (described below), then a 15 minute rest period, self-report satiety and fatigue scales, and concluded with the ad libitum pizza lunch. The MW+E protocol was similar to the MW+R protocol except a 15 minute interval exercise bout took the place of the 15 minute rest period. Also, 3 micro blood draws from the finger were obtained throughout the visit. Samples were collected at three timepoints: shortly after arrival to the lab (time 1), after the mental work task (time 2), and approximately 4 minutes after completion of exercise (time 3). The BR and MW+R or MW+E visit occurred approximately one week apart.
Measures
Anthropometrics
All anthropometric measures (height, weight, waist circumference, and skinfold calipers) were conducted by the same research assistant according to guidelines detailed in “Anthropometric Standardization Reference Manual” (22). Height and weight were measured with a scale and stadiometer. Participants emptied their pockets and removed shoes before measurement. BMI was calculated as BMI = weight / height2 with measured height and weight. For waist circumference and skinfold calipers, participants removed interfering clothing. All measurements were taken on the right side of the body (when applicable). Percent body fat estimation via skinfolds was measured at 3 sites: triceps, suprailiac, and thigh for females; chest, abdomen, and thigh for males.
Submaximal VO2 assessment
VO2 submax was measured by indirect calorimetry on a treadmill using a modified Bruce Protocol (13). Heart rate monitoring was performed with a POLAR Vantage XL heart rate monitor (Gay Mills, WI, USA). Each assessment was terminated when a participant achieved 80% of age predicted maximum heart rate, calculated as participant's age subtracted from 220 then multiplied by 0.8. Participants walked for 4 minutes at a speed of 3 miles per hour (mph). After the 4 minutes, grade was increased to 2.5% for another 4 minutes. Next, grade was increased by 2.5% each minute up to 10% grade, then to 12% for the final grade increase. If a participant had not reached the target heart rate, speed was increased by 0.5 mph per minute until termination criteria was achieved. Volume of O2 and CO2 were measured continuously by open-circuit spirometry and analyzed using a AEI Technologies MAX-II metabolic cart (AEI Technologies, Inc., Pittsburgh, PA). Measurements recorded are averages from each 20 second interval.
Mental work task
The mental work task for the MW+R and MW+E visit was a sample of graduate entrance exam level reading comprehension problems and one college entrance level math problem. Participants were instructed to try their best and then given 20 minutes to complete as much of the problem set as possible. Participants answered multiple choice and open ended questions.
Exercise protocol
The exercise protocol consisted of four 2 minute and 24 second work intervals interspersed with three 1 minute and 43 second complete rest periods, totaling a 15 minute high intensity interval exercise period. Participants exercised at approximately 80-85% of estimated VO2 max during the work intervals. Determination of work level was derived from submaximal testing done at screening and measured by heart rate. This high intensity interval protocol was chosen to allow for higher workload in a shorter time period and to promote circulating blood glucose and lactate (34). The workload duration and exercise duration was based upon previous research and pilot testing with participants enrolled in the study described here.
Blood glucose and lactate
Blood samples were collected by finger prick on the non-dominant hand using sterile procedures. The first drop of blood after puncture was wiped away and sensors were applied to succeeding droplets. Glucose sampling always occurred before lactate, and a new droplet was allowed to form between samples. Glucose sampling was conducted with a One Touch Ultra 2 Blood Glucose Monitoring System (LifeScan, Inc. Milspitas, CA) and lactate sampling was conducted with a Lactate Scout + (SensLab, EKF Diagnostics Company, Barleben, Germany). The measurement after exercise occurred 4 minutes after completion of the exercise task.
Eating behavior
Participants were fed pre-made pizzas purchased from a national retail establishment. Participants were served identically topped pizzas at each feeding visit. A research assistant pre-sliced the pizza in sixteenths and weighed pizza before and after presentation to the participant. Any remaining food was added to the post-presentation weight. Participants were not informed that food consumption was being monitored. When a pizza was presented, the participant was told to have as much or as little as he/she likes, and given up to 30 minutes to eat. Twelve ounces of water was also presented to each participant, and amount of water consumed was recorded as well. Participants were not allowed to eat or drink any other food or beverages.
Self-report Scales of Satiety and Fatigue
After completing tasks for the respective visit and prior to presentation of food, participants were asked to self-report hunger and satiety levels using visual analog scales. Scales were 100mm in length and anchored by opposite meaning phrases at each end. Participants were asked to report on “How hungry are you?” “How full are you?” “How much can you eat?” and “What is your desire to eat?” Participants were instructed to make a mark on the line to indicate their response. Distance from the left most point on the line (corresponding to a response of 0) and the participant's mark was measured. This distance in millimeters was recorded as the response.
Participants were also asked to respond on a 5 option Likert-type scale to indicate whether they were “worn out,” “listless,” “fatigued,” “exhausted,” “sluggish,” “weary,” and “bushed.” Responses on the 5 option Likert-type scale ranged from “not at all” to “extremely.” A number between 1 and 5 was coded according to the participant's selection.
Estimation of Energy Expenditure During MW+R or MW+E
Estimates of energy expenditure of exercise during the MW+E condition was based on VO2 submax and heart rate values obtained during screening and the assumption of 5 kilocalories per liter of oxygen (17). Aerobic energy expenditure calculations were only applied to time spent in work intervals. Post-exercise measured blood lactate was used to estimate anaerobic energy expenditure. The formula for estimate of lactate energy expenditure was lactate post exercise – lactate pre exercise * 0.027 * measured bodyweight (11). The estimates for aerobic energy expenditure and anaerobic energy expenditure were summed to create an estimate of overall energy expenditure. This is likely a conservative measure of the energy expenditure during the high intensity interval exercise bout and is only intended as a rough estimation for comparing differences in energy balance between groups. Energy expenditures of the rest period during the MW+R condition were calculated with the revised Harris-Benedict equation (31). The estimation of energy expenditure during a 15 minute rest period was subtracted from the estimated energy expenditure of exercise to obtain an overall estimate of energy expenditure in the MW+E group above and beyond energy expenditure of the MW+R group.
Statistical Analyses
Power calculations were based on caloric intake reported in previously published literature (5, 7). A one-way analysis of variance (ANOVA) or chi-square test, dependent on variable type, was used to assess potential group differences. A one way ANOVA with repeated measures was used to assess changes in blood glucose and lactate in the MW+E protocol. A two (time, within-person variable) by two (group, between-person variable) ANOVA with repeated measures was run to assess differences in caloric intake between BR and MW+R or MW+E and differences between groups. Post hoc t-tests with Bonferroni correction were conducted on contrasts of interest. A t-test or paired t-test was run to analyze differences in overall energy balance, and self-report measures of satiety and fatigue. Descriptive statistics are presented as mean ± standard deviation, and results for variables of interest are presented as mean ± standard error. SPSS version 22 was used for all analyses and α was set at 0.05.
Results
Forty participants total (19 for MW+R and 21 for MW+E) were randomized to an experimental protocol; one was excluded due to error during testing and the other due to being a statistical outlier (greater than 3 standard deviations). All analyses were conducted with the remaining 38 participants (MW+R = 18, MW+E = 20). Descriptive statistics and comparisons of groups are presented in Table 1. Groups were not significantly different for age, sex, BMI, estimated percent body fat, measured VO2 submax, self-report of exerciser or non-exerciser. Percent body fat and other physiological factors (i.e., BMI, waist circumference, fitness level, etc.) were assessed as a covariate and did not impact overall outcomes.
Table 1.
Descriptive statistics of the sample stratified and compared by group.
| Variable | Mental Work + Rest (n = 18; 61% female) | Mental Work + Exercise (n = 20; 75% female) | p |
|---|---|---|---|
| Age | 20.5 ± 3.5 | 20.7 ± 3.2 | 0.63 |
| BMI | 24.6 ± 4.6 | 25.4 ± 5.4 | .12 |
| Estimated % Body Fat | 25.8 ± 10.3 | 32.7 ± 15.3 | .06 |
| VO2 submax (mL/kg/min) | 27.3 ± 5.5 | 25.0 ± 7.2 | .22 |
| Self-report Exerciser | 15/18 Yes, 83.3% | 13/20 Yes, 65% | .20 |
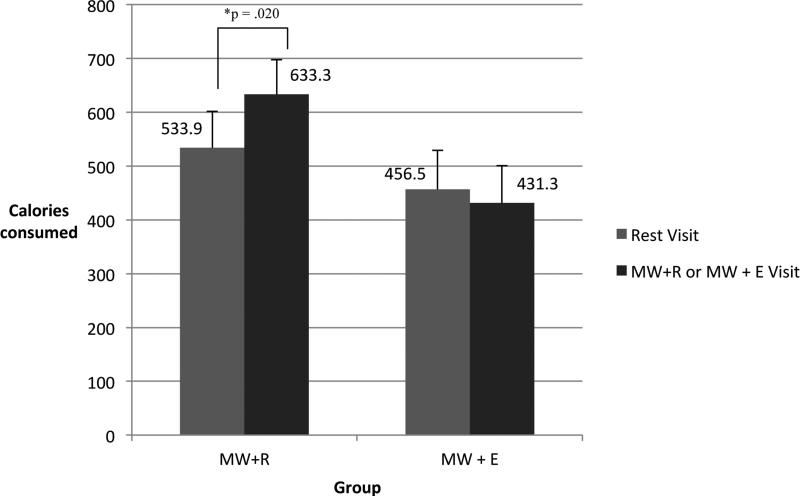
Results from the repeated measures ANOVA indicated a significant time by group interaction for caloric intake, F(1,36) = 4.84, p = .034. Participants in the MW+R group consumed approximately 100 additional kilocalories following mental work (633.3 ± 72.9) compared to their BR visit (533.9 ± 67.7), paired t(17) = −2.58, p = .020. Participants in the MW+E group decreased consumption by an estimated 25 kilocalories (BR visit = 456.5 ± 64.2, MW+E visit = 432.3 ± 69.2); this was not a statistically significant difference. Estimated caloric intake values are presented in Figure 1. There were no significant main effects of time or group.
Figure 1.
Estimated caloric intake between the Baseline Rest visit (Rest Visit) and the Mental Work + Rest (MW+R) or Mental Work + Exercise (MW+E) visit. Repeated measures ANOVA indicated a significant time by group interaction for caloric intake, F(1,36) = 4.84, p = .034. Participants in the MW+R group significantly increased intake between Baseline Rest and MW+R. Participants in the MW+E group slightly decreased intake following the MW+E visit.
Self-report satiety and fatigue did not significantly differ between groups. Participants in the MW+R group responded with lower values to “How much can you eat?” in the MW+R visit (57.89 ± 14.36) than in the BR visit (64.72 ± 13.62), paired t(17) = 2.55, p = .021. All other self-report satiety and fatigue ratings were not statistically different between the BR and MW+R visits. Participants in the MW+E group reported higher levels of “Fatigued” after the MW+E visit (2.40 ± .94) than the BR visit (1.70 ± .98), paired t(19) = −3.20, p = .005. Participants in the MW+E group also responded with higher levels of “Worn Out” after the MW+E visit (2.45 ± .94) compared to the BR visit (1.65 ± .88), paired t(19) = −3.238, p = .004. All other self-report satiety and fatigue ratings were not statistically different between the BR and MW+E visits.
Estimated energy expenditure of the interval exercise bout was calculated for participants in the MW+E condition. Energy expenditure calculations were based on VO2 submax, heart rate, and blood lactate. The average estimated energy expenditure of the exercise protocol was 90.2 ± 8.2 kcal. Energy expenditure of the 15 minute rest period was also calculated using a revised Harris-Benedict formula. Average estimated energy expenditure of the rest portion of the visit was 16.8 ± 0.7 kcal.
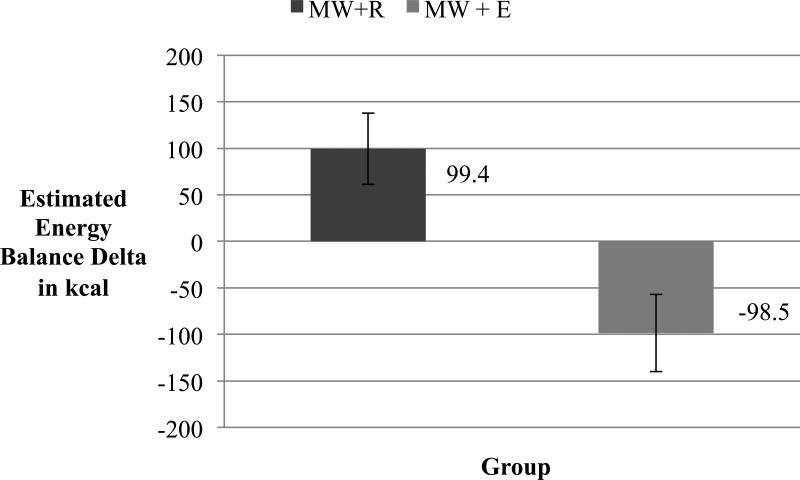
Compared to the BR visit, participants in the MW+R group were in positive energy balance by an estimated 99.4 ± 38.5 kilocalories. Participants in the MW+E group consumed less calories than during their BR visit, and factoring in the estimated energy expenditure of exercise while also subtracting energy that would have been expended at rest, participants in MW+E were in negative energy balance by an estimated −98.5 ± 41.6 kilocalories. Overall, the MW+R and MW+E group differed in estimated energy balance by 198.0 ± 57.0 kilocalories, t(36) = 3.47, p = .001. These values may vary with more precise calculations of energy expended at rest and exercise. This difference in estimated energy balance is illustrated in Figure 2.
Figure 2.
Estimated energy balance differences from Baseline Rest condition for participants in the Mental Work + Rest (MW+R) group and the Mental Work + Exercise group (MW+E), p = .001. The MW+E group includes estimation of energy expended during the exercise bout based only on work intervals and not the entire 15 minutes of exercise. The MW+E group also includes an estimation of energy that would have been expended during the 15 minute rest period; this calculation was based on a revised Harris-Benedict equation. This figure serves as an illustration of estimated mean energy balance differences between groups.
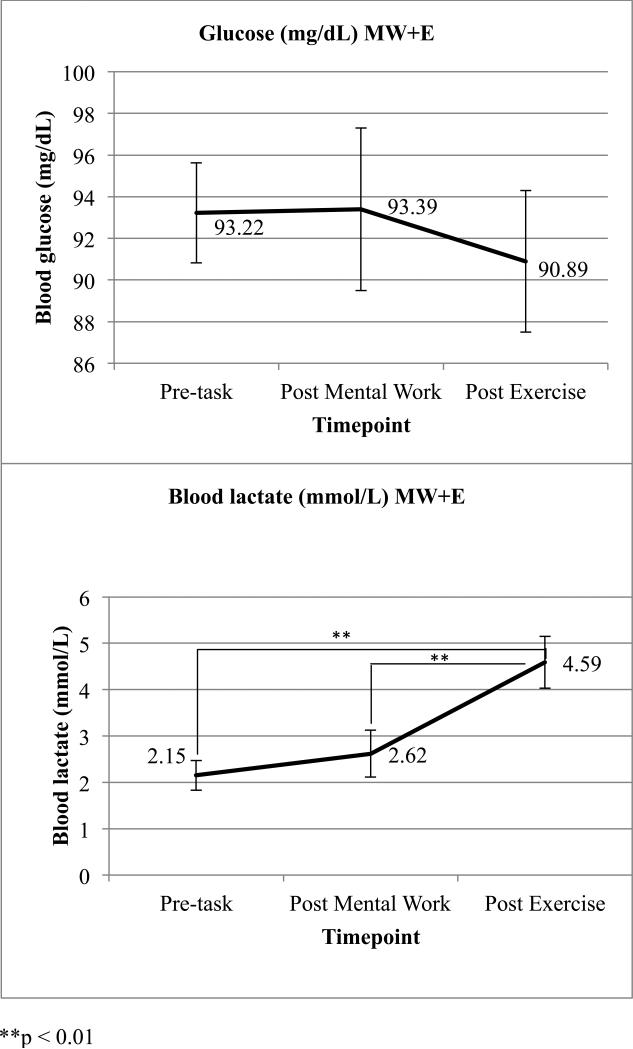
Blood glucose and lactate values are presented in Figure 3. Results for blood samples are presented for 18 of the 20 participants in the MW+E group due to a change in study protocol that occurred after 2 participants had completed the MW+E visit. Blood glucose remained stable across all 3 timepoints. Lactate significantly differed across the 3 measurement points F(1, 17) = 24.03, p = .000. Post-hoc testing indicated lactate did not significantly change between the first and second measurement but did significantly increase following exercise, paired t(17) = −4.90, p = .000 for time 1 to time 3, and paired t(17) = −3.77, p = .002 for time 2 to time 3. Changes in lactate and glucose were not significantly associated with changes in eating behavior between visits.
Figure 3.
Mean values for blood glucose and lactate measured in the MW+E condition. Measurements were taken upon arrival to the lab (time 1), after the mental work task (time 2), and 4 minutes after exercise (time 3). n = 18 due to a change in study protocol. Bars are standard error bars.
Discussion
Overall, findings suggest exercise may prevent a surplus of energy intake following mental activity. There was a significant differential effect in energy intake between participants that did or did not participate in exercise following mental work. Following mental work, participants consumed more calories than after a rest condition, resulting in a positive energy balance. Participants that exercised after mental work did not consume additional energy and were believed to be in negative energy balance. These findings suggest exercise's anorexigenic effects may decrease energy intake after activities with orexigenic effects. These findings corroborate findings by other research groups (5, 7, 16, 21, 26), and build upon them by being the first to report a statistically significant difference in energy intake between participants completing mental work and a meal, or mental work and exercise then a meal.
Reasons for the observed changes in energy intake in the MW+R and MW+E protocols are not entirely understood. One possible explanation of exercise's effects on energy intake following mental work may be due to the brain's utilization of substrates. The brain is dependent on limited glucose, glycogen, and lactate for energy, and these energy levels are tightly regulated (29, 30). Mentally demanding tasks increase brain activity, which utilizes energy stores (6, 14, 23, 33, 38). It is possible that hyperphagia following mental tasks is due to the brain attempting to replace energy supplies. Chaput and colleagues also reported fluctuations in glucose and insulin concentrations during a mental work condition, but not during a rest condition (5). Future investigations could assess glucose and insulin as a potential mechanism for the observed effects. Fluctuations of glucose and lactate have been demonstrated to impact acute appetite. These observations are best documented through Mayer's glucostatic theory (24), where a rise in plasma glucose concentrations could serve as a satiety signal. Changes in glucose were not observed in this study possibly due to collection methodology, and insulin was not measured in this protocol, though we did observe significant increases in lactate as a result of exercise.
Exercise's impact on eating behavior has also been well studied. We chose exercise as a possible means to offset hypherphagia following mental work due to previous research demonstrating exercise sessions of varying intensity and lasting an hour or less decreased desire for food and calorie intake (4, 9, 12, 19, 27, 35, 37). Additional studies have reported decreased energy intake in addition to the energy expense of exercise, which augments the negative energy balance results of exercise (16, 18, 26). The mechanism(s) of exercise's appetite suppressing effects are unclear, though much emphasis has been placed on exercise's effect on hunger and satiety hormones. However, there are numerous documented influences on eating behavior, and any number of those influences (e.g., hedonic value of food, reduced inhibition, palpability, and other behavior or social reasons), could have impacted our results as well.
An acute, intense bout of exercise can increase blood glucose and lactate concentrations (20, 39, 40). Blood glucose levels did not increase after exercise as hypothesized, but blood lactate did. Since insulin action plays a role in transport of glucose across the blood brain barrier and exercise is known to improve glucose uptake, it is possible that brain glucose may have increased despite no change in the blood glucose. Timing and location of blood sampling may have impacted blood glucose results. Given the study methods, it is not possible to do more than associate blood lactate increases with decreases in food consumption, but measurement of brain lactate, glucose, and glycogen in future studies warrants consideration. In addition, other influences on appetite should be considered and measured in future studies.
Exercise and mental work's impacts on energy intake are possibly due to the brain's need to replenish fuel sources, or due to decreases in self-control, attention, etc. Not only would a person be drawn to food for increasing glucose levels, but reduced inhibition may lead to consumption of more food. That is, food consumption may occur for energy demand purposes or may also be due to reduced willpower to resist a food option that is typically viewed as appealing but unhealthy (15). This may especially be the case in our current abundant food environment, and exercise may aid against positive energy balance not only through increased energy expenditure, but also by affecting caloric intake.
The current findings are limited to an acute setting and comparisons between two ad libitum meal sessions with only one food type. Participants were also assigned to different intervention procedures, so the lack of a full cross-over design and variability between participants may have impacted the outcomes, but the significant findings indicate a possible relationship between mental work, exercise, and energy intake. Figure 2 includes an estimation of these energy balance differences, but we did not assess energy expenditure in all conditions. Future studies should include a more accurate measurement of energy expenditure in all settings. Future studies should also include more precise measurements of glucose, lactate, and insulin levels in all conditions. Other factors that have previously been demonstrated to relate to eating behavior such as sex, restrained eating, BMI and other biomarkers may be highly valuable in understanding the mechanisms for the observed outcomes. In addition, though attempts were made to standardize participant behavior before each visit, caloric intake at breakfast or energy expenditure prior to arriving in the lab was not completely controlled. Follow-up measurement of food intake would also be beneficial to determine if compensation occurs.
In conclusion, a high intensity exercise bout after mental work may offset increased energy intake. Exercise after mental work, instead of food consumption, may serve as a way to regulate energy intake. The modern work environment is highly sedentary and cognitively demanding. The cognitive demand may influence the individual's appetite and lend itself towards positive energy balance. If an individual seeking to take a break from mental work chooses to participate in exercise before consuming food, this may prevent the positive energy balance. This relationship between mental work, exercise, and energy intake may be impactful in reducing the positive energy balance responsible for modern obesity trends.
Acknowledgments
The authors would like to thank David B. Allison, David Bryan, Brandon Kane, and Gabrielle Pybus. Also this work was supported by Grant Number T32HL105349 from the National Heart, Lung, and Blood Institute, and pilot funding from the Department of Human Studies at the University of Alabama at Birmingham. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the University of Alabama at Birmingham.
Footnotes
Conflict of Interest
The authors have no conflicts to disclose.
The results of the present study do not constitute endorsement by ACSM.
References
- 1.Albert MH, Drapeau V, Mathieu ME. Timing of moderate-to-vigorous exercise and its impact on subsequent energy intake in young males. Physiol Behav. 2015;151:557–562. doi: 10.1016/j.physbeh.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 2.Archer E, Shook RP, Thomas DM, et al. 45-year trends in women's use of time and household management energy expenditure. PloS One. 2013;8(2):e56620. doi: 10.1371/journal.pone.0056620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boumezbeur F, Petersen K, Cline G, et al. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13c nuclear magnetic resonance spectroscopy. J Neurosci. 2010;30(42):13983–91. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozinovski NC, Bellissimo N, Thomas SG, Pencharz PB, Goode RC, Anderson GH. The effect of duration of exercise at the ventilation threshold on subjective appetite and short-term food intake in 9 to 14 year old boys and girls. Int J Behav Nutr Phys Act. 2009;6:66. doi: 10.1186/1479-5868-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaput JP, Drapeau V, Poirier P, Teasdale N, Tremblay A. Glycemic instability and spontaneous energy intake: Association with knowledge-based work. Psychosom Med. 2008;70(7):797–804. doi: 10.1097/PSY.0b013e31818426fa. [DOI] [PubMed] [Google Scholar]
- 6.Chaput JP, Klingenberg L, Astrup A, Sjodin AM. Modern sedentary activities promote overconsumption of food in our current obesogenic environment. Obes Rev. 2011;12(5):e12–20. doi: 10.1111/j.1467-789X.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 7.Chaput JP, Tremblay A. Acute effects of knowledge-based work on feeding behavior and energy intake. Physiol Behav. 2007;90(1):66–72. doi: 10.1016/j.physbeh.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Church TS, Thomas DM, Tudor-Locke C, et al. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PloS One. 2011;6(5):e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evero N, Hackett LC, Clark RD, Phelan S, Hagobian TA. Aerobic exercise reduces neuronal responses in food reward brain regions. J Appl Physiol (1985) 2012;112(9):1612–9. doi: 10.1152/japplphysiol.01365.2011. [DOI] [PubMed] [Google Scholar]
- 10.Flegal K, Carroll M, Ogden C, Curtin L. Prevalence and trends in obesity among us adults, 1999-2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 11.Gladden LB, Welch HG. Efficiency of anaerobic work. J Appl Physiol. 1978;44(4):564–70. doi: 10.1152/jappl.1978.44.4.564. [DOI] [PubMed] [Google Scholar]
- 12.Hanlon B, Larson MJ, Bailey BW, LeCheminant JD. Neural response to pictures of food after exercise in normal-weight and obese women. Med Sci Sports Exerc. 2012;44(10):1864–70. doi: 10.1249/MSS.0b013e31825cade5. [DOI] [PubMed] [Google Scholar]
- 13.Hellerstein HK, Franklin BA. Exercise Testing and Prescription. In: Wenger NK, Hellerstein HK, editors. Rehabilitation of the Coronary Patient. John Wiley & Sons, Inc; New York, NY: 1984. pp. 197–284. [Google Scholar]
- 14.Hitze B, Hubold C, van Dyken R, et al. How the selfish brain organizes its supply and demand. Front Neuroenergetics. 2010;2:7. doi: 10.3389/fnene.2010.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollmann M, Hellrung L, Pleger B, et al. Neural correlates of the volitional regulation of the desire for food. Int J Obes (Lond) 2012;36(5):648–55. doi: 10.1038/ijo.2011.125. [DOI] [PubMed] [Google Scholar]
- 16.Horsch A, Wobmann M, Kriemler S, et al. Impact of physical activity on energy balance, food intake and choice in normal weight and obese children in the setting of acute social stress: A randomized controlled trial. BMC Pediatrics. 2015;15(1):326. doi: 10.1186/s12887-015-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter GR, Seelhorst D, Snyder S. Comparison of metabolic and heart rate responses to super slow vs. traditional resistance training. J Strength Cond Res. 2003;17(1):76–81. doi: 10.1519/1533-4287(2003)017<0076:comahr>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Imbeault P, Saint-Pierre S, Almeras N, Tremblay A. Acute effects of exercise on energy intake and feeding behaviour. Br J Nutr. 1997;77(4):511–21. doi: 10.1079/bjn19970053. [DOI] [PubMed] [Google Scholar]
- 19.King J, Wasse LK, Broom DR, Stensel DJ. Influence of brisk walking on appetite, energy intake, and plasma acylated ghrelin. Med Sci Sports Exerc. 2010;42(3):485–92. doi: 10.1249/MSS.0b013e3181ba10c4. [DOI] [PubMed] [Google Scholar]
- 20.LeBlanc J, Nadeau A, Richard D, Tremblay A. Studies on the sparing effect of exercise on insulin requirements in human subjects. Metabolism. 1981;30(11):1119–1124. doi: 10.1016/0026-0495(81)90057-3. [DOI] [PubMed] [Google Scholar]
- 21.Lemay V, Drapeau V, Tremblay A, Mathieu ME. Exercise and negative energy balance in males who perform mental work. Pediatr Obes. 2014;9(4):300–9. doi: 10.1111/j.2047-6310.2013.00158.x. [DOI] [PubMed] [Google Scholar]
- 22.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Human Kinetics Books; Champaign, IL: 1988. p. 177. [Google Scholar]
- 23.Madsen PL, Cruz NF, Sokoloff L, Dienel GA. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normal during recovery: excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. J Cereb Blood Flow Metab. 1999;19(4):393–400. doi: 10.1097/00004647-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Mayer J. Regulation of energy intake and the body weight: The glucostatic theory and the lipostatic hypothesis. Annals N Y Acad Sci. 1955;63(1):15–43. doi: 10.1111/j.1749-6632.1955.tb36543.x. [DOI] [PubMed] [Google Scholar]
- 25.McCann B, Warnick G, Knopp R. Changes in plasma lipids and dietary intake accompanying shifts in perceived workload and stress. Psychosom Med. 1990;52(1):97–108. doi: 10.1097/00006842-199001000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Oh H, Taylor AH. Brisk walking reduces ad libitum snacking in regular chocolate eaters during a workplace simulation. Appetite. 2012;58(1):387–92. doi: 10.1016/j.appet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Panek LM, Jones KR, Temple JL. Short term aerobic exercise alters the reinforcing value of food in inactive adults. Appetite. 2014;81:320–29. doi: 10.1016/j.appet.2014.06.102. [DOI] [PubMed] [Google Scholar]
- 28.Perusse-Lachance E, Brassard P, Chaput JP, et al. Sex differences in the effects of mental work and moderate-intensity physical activity on energy intake in young adults. ISRN Nutr. 2013;2013:723250. doi: 10.5402/2013/723250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters A. The selfish brain: competition for energy resources. Am J Hum Biol. 2011;23(1):29–34. doi: 10.1002/ajhb.21106. [DOI] [PubMed] [Google Scholar]
- 30.Peters A, Schweiger U, Pellerin L, et al. The selfish brain: competition for energy resources. Neurosci Biobehav Rev. 2004;28(2):143–80. doi: 10.1016/j.neubiorev.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr. 1984;40(1):168–82. doi: 10.1093/ajcn/40.1.168. [DOI] [PubMed] [Google Scholar]
- 32.Salmina AB, Kuvacheva NV, Morgun AV, et al. Glycolysis -mediated control of blood-brain barrier development and function. Int J Biochem Cell Biol. 2015;64:174–84. doi: 10.1016/j.biocel.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Swanson R, Morton M, Sagar S, Sharp F. Sensory stimulation induces local cerebral glycogenolysis: Demonstration by autoradiography. Neuroscience. 1992;51(2):451–461. doi: 10.1016/0306-4522(92)90329-z. [DOI] [PubMed] [Google Scholar]
- 34.Swart J, Lamberts RP, Lambert MI, et al. Exercising with reserve: evidence that the central nervous system regulates prolonged exercise performance. Br J Sports Med. 2009;43(10):782–8. doi: 10.1136/bjsm.2008.055889. [DOI] [PubMed] [Google Scholar]
- 35.Taylor AH, Oliver AJ. Acute effects of brisk walking on urges to eat chocolate, affect, and responses to a stressor and chocolate cue. An experimental study. Appetite. 2009;52(1):155–60. doi: 10.1016/j.appet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Thorp AA, Healy GN, Winkler E, et al. Prolonged sedentary time and physical activity in workplace and non-work contexts: a cross-sectional study of office, customer service and call centre employees. Int J Behav Nutr Phys Act. 2012;9:128. doi: 10.1186/1479-5868-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unick JL, Otto AD, Goodpaster BH, Helsel DL, Pellegrini CA, Jakicic JM. Acute effect of walking on energy intake in overweight/obese women. Appetite. 2010;55(3):413–9. doi: 10.1016/j.appet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlassenko AG, Rundle MM, Mintun MA. Human brain glucose metabolism may evolve during activation: Findings from a modified fdg pet paradigm. Neuroimage. 2006;33(4):1036–41. doi: 10.1016/j.neuroimage.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 39.Zinker B, Britz K, Brooks G. Effects of a 36-hour fast on human endurance and substrate utilization. J Appl Physiol (1985) 1990;69(5):1849–1855. doi: 10.1152/jappl.1990.69.5.1849. [DOI] [PubMed] [Google Scholar]
- 40.Zinker B, Dallman P, Brooks G. Augmented glucoregulatory hormone concentrations during exhausting exercise in mildly iron-deficient rats. Am J Physiol. 1993;265(4 Pt 2):R863–71. doi: 10.1152/ajpregu.1993.265.4.R863. [DOI] [PubMed] [Google Scholar]