Abstract
Cancer cachexia is a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass, which negatively impacts quality of life and portends a poor prognosis. Numerous molecular substrates and mechanisms underlie the dysregulation of skeletal muscle synthesis and degradation observed in cancer cachexia, including pro-inflammatory cytokines (TNF-α, IL-1 and IL-6), and the NF-kB, IGF1-AKT-mTOR, and myostatin/activin-SMAD pathways. Recent preclinical and clinical studies have demonstrated that anti-cachexia drugs (such as MABp1 and soluble receptor antagonist of myostatin/activin) not only prevent muscle wasting but may also prolong overall survival. In this review, we focus on the significance of cachexia signalling in cancer patients and highlight promising drugs targeting tumor cachexia in clinical development.
Background
Cancer cachexia is a complex metabolic syndrome characterized by an irreversible loss of skeletal muscle mass, which leads to progressive functional impairment (1). Cachexia is a significant cause of morbidity and mortality, affecting 60–80% of cancer patients, and is particularly common in individuals with pancreas cancer (2, 3). In addition to functional impairment, cachexia is associated with increased fatigue and emotional distress, all of which considerably compromise quality of life. Moreover, cancer patients with cachexia are less likely to respond to chemotherapy and radiation, and are more likely to endure treatment toxicities (4). Importantly, cachexia may be a direct result of malignancy as well as the chemotherapeutics (e.g. bevacizumab or sorafenib) used to treat it. Sarcopenia, a related but distinct condition, also results in loss of muscle mass, but is attributable to aging and inactivity, rather than anorexia, a feature of cachexia marked by decreased energy expenditure and reduced fat accumulation (5). The definition of cachexia has evolved in recent years (1), and much remains to be understood regarding the interplay between cachexia, anorexia, and sarcopenia and how these entities affect cancer development, treatment resistance, and patient outcomes.
Studies evaluating the metabolic alterations inducing cancer cachexia have revealed several tumor-derived cytokines and pathways implicated in skeletal muscle degradation (6), and have led to the development of promising therapies for the prevention of muscle wasting (7). However, advances in this field have been impeded by a lack of consensus regarding the clinical assessment of cancer cachexia as well as the heterogeneity of disease presentation (8). A better understanding of the molecular mechanisms underlying tumor cachexia has the potential to identify novel therapeutic targets and inform the development of successful interventions. In this review, we critically summarize cachexia signalling in cancer patients and highlight recent pre-clinical and clinical advances in the management of this paraneoplastic syndrome.
Cachexia pathways in muscle tissue
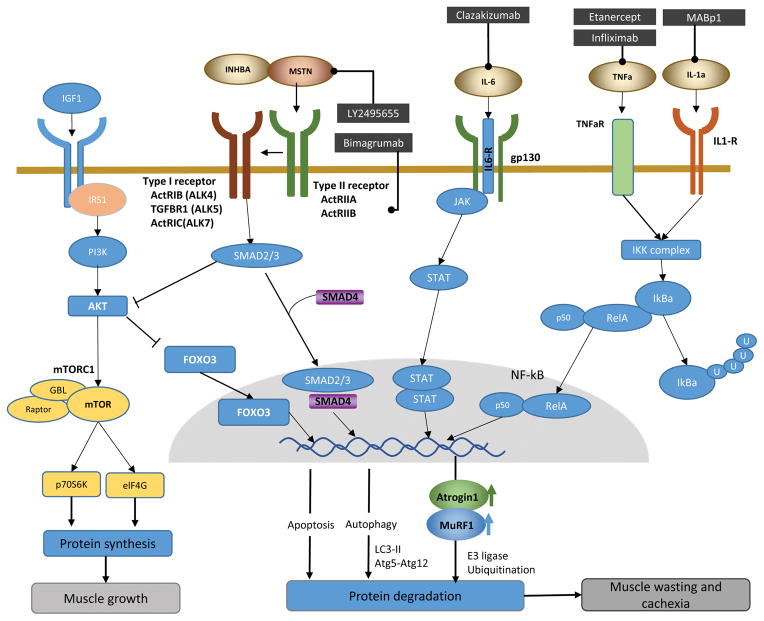
Cancer cachexia ultimately results from an imbalance in the regulation of muscle protein synthesis and degradation. Such muscle wasting is orchestrated by extracellular ligands which activate several intersecting intracellular signaling pathways (Fig. 1). In particular, pro-inflammatory cytokines derived from immune or tumor cells, including tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1) and interleukin-6 (IL-6), have been shown to trigger muscle wasting, through activation of the nuclear factor kappa B (NF-kB) and Janus Kinase–Signal Transducer and Activator of Transcription (JAK-STAT) pathways, respectively. Accumulating evidence also suggests that the insulin-like growth factor 1 (IGF1) pathway induces skeletal myogenesis, while myostatin and activin serve as negative regulators of IGF1 signaling to inhibit muscle growth and promote degradation. Other major skeletal muscle proteolytic pathways include the ubiquitin– proteasome system (UPS), as well as the autophagy-lysosomal, calpain, and the caspase pathways (9–11). The UPS has received the most attention, through which the ubiquitin E3-ligases, muscle ring-finger protein 1 (MuRF-1) and atrophy gene-1/muscle atrophy F-box (Atrogin-1/MAFbx), act as two main regulators of muscle protein breakdown.
Fig. 1. Cachexia signaling regulating protein synthesis and degradation in muscle and anti-cachexia drugs in development.
IGF-Akt-mTOR signaling: Binding of IGF-1 to IGF-1R results in phosphorylation of the insulin receptor substrate (IRS). IRS activates PI3K-Akt signaling, which then stimulates protein synthesis by activating mTOR. mTOR activates the ribosomal S6K and eukaryotic initiation factor 4E-BP-1, leading to protein synthesis. Akt also phosphorylates and inhibits FoxOs, which is a negative regulator of myogenesis.
Myostatin/activin signaling: Myostatin/activin binds to type II receptor (ActRIIB) and induces its dimerization with the activin type I receptor. Subsequent phosphorylation of Smad2/3 recruits Smad4. The Smad complex is translocated into the nucleus to induce transcriptional changes, which result in muscle wasting. Simultaneously, myostatin/activin reduces Akt activity and suppresses FoxOs phosphorylation. Dephosphorylated FoxOs are translocated into the nucleus and induce the transcription of target genes which regulate the ubiquitin–proteasome and autophagy–lysosome systems.
IL-6 signaling: The binding of IL-6 to its receptors induces homodimerization of gp130 and its complex, which activate JAK–STAT-3 signaling. Phosphorylated STAT3 forms a dimer and translocates into the nucleus, leading to increased protein degradation.
TNF-a and IL-1 signaling: Binding of TNF-a or IL-1 to its receptor activates the IKK complex which phosphorylates IκBa proteins. This signal-induced phosphorylation targets IκBa for poly-ubiquitination and subsequent degradation by the proteasome, thereby allowing the RelB/p52 complex to translocate to the nucleus to transcribe respective target genes.
Cytokines activate protein degradation in cancer cachexia
Multiple cytokines, including TNF-α, IL-1 and IL-6, have been implicated in facilitating a cachectic state (12), and their expression or upregulation is prompted by both tumor and host derived factors. High serum levels of these cytokines are present in many cancer patients with cachexia.
TNF-α has long been recognized as a mediator of cancer cachexia. Its administration leads to increased protein degradation in cultured muscle cells (13) and in rat muscle (14). In murine models, TNF-α and recombinant IL-1 act synergistically to reduce muscle protein content (15). Mechanistically, these cytokines increase NF-κB mediated transcription and subsequent ubiquitin conjugation activity (16), thereby inducing skeletal muscle protein loss. The NF-kB protein family is comprised of five transcription factors (p65 [Rel A], Rel B, c-Rel, p52, and p50), which are expressed in skeletal muscle and mediate a variety of processes depending on the cell type and upstream trigger (17). Activation of NF-kB is achieved by nuclear transport of NF-kB heterodimers, acting in concert with the ubiquitination and degradation of the NF-kB inhibitory protein, IκB. TNFα and IL-1 activate the IKK complex, which then phosphorylates IκB a proteins, marking them for poly-ubiquitination and proteasomal degradation, thereby releasing NF-κB. Activated NF-κB then activates MuRF1 and Atrogin-1 (18), both of which are ligases that promote protein degradation.
IL-6, secreted mainly from tumor and immune cells, induces activation of inflammatory and degradation pathways, resulting in suppression of protein synthesis in muscle cells. IL-6 bound to its receptor, IL-6R, recruits the associated cell-surface glycoprotein and signal transducer, gp130, to induce downstream JAK–STAT signaling. Prolonged activation of the IL-6/JAK-STAT axis is an established mechanism of tumorigenesis as well as the muscle wasting observed in cancer cachexia (12). In ApcMin, Lewis lung carcinoma (LLC) and Colon-26 tumor-implanted mice, IL-6 and STAT3 activation have been shown to be integral to loss of skeletal muscle. Furthermore, STAT3 was sufficient to induce remarkable muscle fiber atrophy, even in non-tumor bearing mice (12, 19).
Myostatin and activin signals have a distinct role in the negative regulation of myogenesis
Myostatin and activin are transforming growth factor-beta (TGF-beta) superfamily ligands involved in skeletal muscle degradation. Myostatin is predominantly secreted from muscle cells and acts as a key negative regulator of muscle growth; its genetic deficiently results in dramatic muscle hypertrophy (20, 21). Activin A is a homodimer formed from two inhibin betaA chains (22), and is involved in many physiologic functions, including embryogenesis, cell growth, differentiation, and the immune response (23). Myostatin and activin act via heteromeric complexes of two related transmembrane type I and type II serine/threonine kinase receptors to activate downstream signal transduction. Myostatin or activin A binds to its respective type II activin receptor (activin: ACVR2B or ACVR2A; myostatin: ACVR2B) on the muscle cell membrane, leading to its dimerization, and subsequent recruitment and activation of type I receptors (Activin: ALK4 or 7; myostatin: ALK5 or 7). The activated type I receptor then phosphorylates SMAD2 and SMAD3, which together with the common mediator, SMAD4, translocate to the nucleus. This SMAD complex then regulates transcriptional responses leading to muscle wasting (24).
IGF1-AKT-mTOR pathway has anabolic effects on muscle by inhibiting protein degradation and promoting myogenesis
IGF1 signaling is a major anabolic pathway involved in muscle development and regeneration (25). Several studies have shown that IGF1/Akt signaling suppresses protein breakdown and promotes muscle growth (26, 27). Binding of IGF-1 to its receptor triggers the activation of PI3K/Akt signal transduction, inducing protein synthesis by blocking the repression of mTOR. Activated mTOR phosphorylates its two major targets, S6K1 and 4E-BP1, which play a key role in myogenesis. Akt further phosphorylates and inactivates forkhead box O proteins (FoxOs: FoxO1, FoxO3 and FoxO4) by promoting their export from the nucleus to the cytoplasm (28). FoxOs are transcriptional regulators of autophagy, which promote protein ubiquitination and degradation in muscle cells. Myostatin and activin suppress Akt activity, leading to disinhibition of FoxO3 (29, 30) and subsequent upregulation of MuRF-1, Atrogin-1 (or MAFbx) and autophagy genes to induce muscle protein degradation.
Clinical-Translational Advances
Nutritional support alone, or in conjunction with anabolic drugs such as enobosarm (an oral, non-steroidal, selective androgen receptor modulator) or anamorelin (an orally active ghrelin receptor antagonist), have failed to deliver clinical benefit in cancer patients with cachexia (31). Directly targeting the cachexia pathway may indeed prove to be a more successful endeavor. To this end, recent pre-clinical and clinical studies have offered a number of drugs with promising activity against cancer-induced muscle wasting (Table 1).
Table 1.
Summary of clinical trials evaluating treatments for Cancer Cachexia
| Inhibitor | Drug | Type | Disease | Model | Outcome | Reference |
|---|---|---|---|---|---|---|
| TNF-a | Etanercept | TNF-a ligand bound to Fc-IgG11 | Cancer | Phase III RCT (placebo) | No inhibition of muscle wasting | NCT00046904 (34) |
| Etanercept | TNF-a ligand bound to Fc-IgG11 | Cancer | Phase II/III RCT (placebo) | Ongoing | NCT00127387 | |
| Infliximab | TNF-a specific mAb | NSCLC | Phase II/III RCT (placebo) +docetaxel | Trial stopped early because of decreased quality of life in infliximab-treated group | NCT00040885 (35) | |
| Infliximab | TNF-a specific mAb | PC | Phase II RCT (placebo) +Gemcitabine | No statistically significant differences in lean body mass. | NCT00060502 (36) | |
|
| ||||||
| IL-6 | Clazakizumab (ALD-518) | IL-6-specific mAb | Cancer | phase I | Well tolerated. | (40) |
| Clazakizumab (ALD-518) | IL-6-specific mAb | NSCLC | Phase II RCT (placebo) | Less loss of LBM. Increased Hb. | NCT00866970 (41, 42) | |
|
| ||||||
| IL-1a | MABp1 | IL-1a-specific mAb | Cancer | phase I | Serum IL-6 level decreased. Disease control was observed | NCT01021072 (38) |
| MABp1 | IL-1a-specific mAb | CRC | Phase III RCT (placebo) | A trend in decreased risk of death. Physical functions did not decline. | NCT01767857 (39) | |
| MABp1 | IL-1a-specific mAb | CRC | Phase III RCT (placebo) | Ongoing | NCT02138422 | |
|
| ||||||
| ActRIIB | Bimagrumab (BYM338) | ActRIIB-specific mAb | Cancer | Phase II RCT (placebo) | Ongoing | NCT01433263 |
|
| ||||||
| Myostatin | LY2495655 | myostastin-specific mAb | Cancer | Phase I | Well tolerated. Increased hand grip strength and improved in functional tests. | NCT01524224 (49) |
| LY2495655 | myostastin-specific mAb | PC | Phase II | Ongoing | NCT01505530 | |
mAb: monoclonal antibody, NSCLC: non-squamous cell lung cancer, CRC: colorectal cancer, PC: pancreatic cancer, RCT: randomized control trial, LBM: lean body mass, Hb: hemoglobin
TNF-α
Administration of TNF-α results in increased skeletal muscle proteolysis associated with higher levels of conjugated ubiquitin (32). TNF-α is also involved in anorexia associated with tumor growth, as suggested by the use of TNF inhibitors in anorectic tumor-bearing rats. Specifically, the injection of TNF inhibitor in tumor-bearing rats significantly improves food intake and body weight (33). Despite these promising preclinical data, TNF-α inhibitors have not demonstrated meaningful clinical benefit. In two phase II studies, which randomized advanced cancer patients to either etanercept (a recombinant fusion protein of TNF-α type II receptor which blocks TNF-α activity) or infliximab (a recombinant anti-TNF-α antibody) versus placebo (34, 35), there was no significant benefit with respect to reducing muscle wasting, restoring lean body mass, or improving quality of life. Likewise, the addition of infliximab to gemcitabine to treat cachexia in advanced pancreatic cancer patients did not yield any significant benefit when compared with placebo (36). In fact, a phase II/III randomized, placebo-controlled study combining infliximab with docetaxel in NSCLC patients was terminated early due to significantly worse quality of life in the experimental group. Recent data suggests that a monoclonal antibody against fibroblast growth factor-inducible 14 (Fn14), which is related to the TNF receptor superfamily and is a receptor for the TWEAK cytokine, may help prevent tumor-induced cachexia and prolong survival in C26 tumor-bearing mice (37). Interestingly, TWEAK blockade using an anti-TWEAK antibody had no effect on Fn14-induced cachexia, suggesting the presence of a second, as yet unidentified ligand for Fn14.
IL-1
An IL-1a specific humanized monoclonal antibody, MABp1, has shown promising results in patients with cancer cachexia. A phase I dose-escalation and expansion study was performed to assess the safety and tolerability of MABp1 in refractory cancer patients (38). MABp1 was well tolerated, with demonstrated efficacy on body composition and quality of life, as well as potential antitumor effects in the response analysis. The most common adverse event in this study was proteinuria (all grade, n=11; 21%). Subsequently, a phase III randomized study comparing MABp1 monotherapy to megestrol acetate was performed in advanced colorectal cancer (CRC) patients with cachexia (39). In this study, MABp1-treated patients had a trend towards improved median overall survival without worsening physical function, compared to patients receiving megestrol acetate. A placebo-controlled, double-blind phase III study in refractory CRC patients is ongoing.
IL-6
ALD518, a humanized monoclonal antibody that binds with high affinity to human IL-6, is being developed for the treatment of anemia, cachexia, and fatigue (12). A phase I study of nine patients with advanced cancer has reported statistically significant differences in hand grip strength and fatigue after ALD518 administration (40). In a phase II randomized placebo-controlled study in 124 patients with advanced NSCLC, ALD518 resulted in less lean body mass reduction, improved lung symptom scores and reversed fatigue and anemia (41, 42). ALD518 is well tolerated, with minimal adverse effects and has the potential to improve anemia and fatigue, as well as reduce cancer-related cachexia.
Myostatin/activin pathway
Several studies have suggested that serum levels of activin (43, 44) and myostatin (43) are increased in patients with cancer cachexia. In mice models, inhibition of myostatin/activin signaling has been shown to increase muscle mass and improve physical performance and muscle function (45, 46). A recombinant decoy ActRIIB antagonist which inhibits both myostatin and activin-mediated Smad2/3 signal transduction, dramatically prevented muscle wasting and prolonged survival in multiple mouse models, without affecting inflammatory cytokine levels (47). A myostatin specific antibody (PF-354) has also been shown to suppress tumor-induced muscle wasting and loss of muscle function, even in mildly cachexic mice (48). Unfortunately, clinical trials testing the ActRIIB decoy were stopped because of gum and nose bleeding events in healthy adults and boys with Duchenne muscular dystrophy. Another myostatin specific antibody (LY2495655) and its receptor ActRIIB specific antibody (bimagrumab or BYM338) showed promising results in clinical trials. A phase I study of LY2495655 in patients with advanced cancer not receiving chemotherapy showed that LY2495655 was well tolerated and provided durable improvement in hand grip strength and functional tests (49). A phase II study of LY2495655 in patients with advanced pancreatic cancer receiving standard chemotherapy is ongoing, with overall survival as the primary endpoint (NCT1505530). Similarly, BYM338 has previously shown an improvement in thigh muscle volume at eight weeks in patients with inclusion body myositis (50), and is now being tested in a randomized, double-blind, placebo-controlled trial phase II in lung and pancreatic cancer patients (NCT01433263). Interestingly, this pathway may also play an important role in prevention strategies. For example, in a study of early stage gastric cancer patients, myostatin expression was found to be upregulated in muscle tissue before the onset of significant weight loss (51), suggesting that early intervention to prevent cancer cachexia may delay tumor recurrence or progression and improve outcomes.
Little is known regarding how modulation of cachexia signaling influences tumor biology. However, studies suggest that activation of cachexia signaling may contribute to tumor progression. Gallot et al. reported on the effect of myostatin signaling on cancer biology using LLC tumor-bearing mice. In this study (52), tumor weight was significantly lower in Mstn−/− mice compared with wild type mice. In addition, gene expression analysis in tumor tissue showed this phenotype to be associated with reduced expression of genes involved in angiogenesis, tumor metabolism, activin signaling, and apoptosis. These results are consistent with studies showing that the soluble type II receptor antagonist of myostatin and activin (sActRIIB) reduced tumor weight and incidence of lung metastases (45, 53). Taken together, myostatin/activin signaling has a critical role not only in muscle cell degradation but also cancer progression, although it should be noted that these data have not been reproduced in other studies. Interestingly, myostatin/activin signaling has been associated with activation of angiogenesis. For instance, ALK5 overexpression promotes tumor angiogenesis, invasion and metastatic potential by upregulating matrix metalloproteinase-9 in tumor cells (54). Conversely, an inhibitor of the type I activin like receptor (SB431542) has been shown to decrease VEGF expression and inhibit angiogenesis. These data warrant further investigation and may lead to novel drug combinations with inhibitors of cachexia signaling.
Conclusions
The mechanisms of cancer cachexia are heterogeneous and multifactorial. Targeting the cachexia signaling pathway has shown promising results in preclinical and early clinical trials, but primarily to prevent muscle wasting rather than prolong survival. Ongoing phase III clinical trials are testing the clinical efficacy of these novel compounds. Improving the classification, objective assessment and monitoring of cancer patients with cachexia remain challenges to the clinical development of agents targeting this pathway. A refined understanding of how cancer cachexia affects oncogenic signaling in different cancer types and host status is critically needed in order to develop more successful therapeutic interventions. Identifying predictive biomarkers for these compounds, based on the precise mechanism of cachexia affected, will be essential to bringing these compounds into the clinic.
Acknowledgments
Grant Support
H.-J. Lenz was supported by the NIH under award number P30CA014089, Wunder Project, Call to Cure, and Danny Butler Memorial Fund.
Footnotes
Disclosure of Potential Conflicts of Interest
H.-J. Lenz is a consultant/advisory board member for Bayer, Boehringer Ingelheim, Celgene, Merck Serono, and Roche. No other potential conflicts of interest were disclosed.
References
- 1.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 2.Bruera E. ABC of palliative care. Anorexia, cachexia, and nutrition BMJ. 1997;315:1219–22. doi: 10.1136/bmj.315.7117.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wigmore SJ, Plester CE, Richardson RA, Fearon KC. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer. 1997;75:106–9. doi: 10.1038/bjc.1997.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–9. doi: 10.1016/s0959-8049(97)10090-9. [DOI] [PubMed] [Google Scholar]
- 5.Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr. 2014;33:737–48. doi: 10.1016/j.clnu.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Tisdale MJ. Reversing cachexia. Cell. 2010;142:511–2. doi: 10.1016/j.cell.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Han HQ, Zhou X, Mitch WE, Goldberg AL. Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol. 2013;45:2333–47. doi: 10.1016/j.biocel.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–66. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–83. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Hasselgren PO, Wray C, Mammen J. Molecular regulation of muscle cachexia: it may be more than the proteasome. Biochem Biophys Res Commun. 2002;290:1–10. doi: 10.1006/bbrc.2001.5849. [DOI] [PubMed] [Google Scholar]
- 12.Narsale AA, Carson JA. Role of interleukin-6 in cachexia: therapeutic implications. Curr Opin Support Palliat Care. 2014;8:321–7. doi: 10.1097/SPC.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li YP, Reid MB. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1165–70. doi: 10.1152/ajpregu.2000.279.4.R1165. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Martinez C, Lopez-Soriano FJ, Argiles JM. Acute treatment with tumour necrosis factor-alpha induces changes in protein metabolism in rat skeletal muscle. Mol Cell Biochem. 1993;125:11–8. doi: 10.1007/BF00926829. [DOI] [PubMed] [Google Scholar]
- 15.Fong Y, Moldawer LL, Marano M, Wei H, Barber A, Manogue K, et al. Cachectin/TNF or IL-1 alpha induces cachexia with redistribution of body proteins. Am J Physiol. 1989;256:R659–65. doi: 10.1152/ajpregu.1989.256.3.R659. [DOI] [PubMed] [Google Scholar]
- 16.Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J. 1998;12:871–80. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- 17.Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. FASEB J. 2002;16:529–38. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- 18.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–98. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012;303:E410–21. doi: 10.1152/ajpendo.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 21.Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–8. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 22.Chen YG, Lui HM, Lin SL, Lee JM, Ying SY. Regulation of cell proliferation, apoptosis, and carcinogenesis by activin. Exp Biol Med (Maywood) 2002;227:75–87. doi: 10.1177/153537020222700201. [DOI] [PubMed] [Google Scholar]
- 23.Chen YG, Wang Q, Lin SL, Chang CD, Chuang J, Ying SY. Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis. Exp Biol Med (Maywood) 2006;231:534–44. doi: 10.1177/153537020623100507. [DOI] [PubMed] [Google Scholar]
- 24.Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, et al. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol. 2009;296:C1248–57. doi: 10.1152/ajpcell.00104.2009. [DOI] [PubMed] [Google Scholar]
- 25.Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab. 2004;287:E591–601. doi: 10.1152/ajpendo.00073.2004. [DOI] [PubMed] [Google Scholar]
- 26.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–13. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 27.Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, Viereck J, et al. Fast/glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7:159–72. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–88. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 29.Sartori R, Gregorevic P, Sandri M. TGFbeta and BMP signaling in skeletal muscle: potential significance for muscle-related disease. Trends Endocrinol Metab. 2014;25:464–71. doi: 10.1016/j.tem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–70. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 31.Lok C. Cachexia: the last illness. Nature. 2015;528:182–3. doi: 10.1038/528182a. [DOI] [PubMed] [Google Scholar]
- 32.Argiles JM, Lopez-Soriano FJ. Catabolic proinflammatory cytokines. Curr Opin Clin Nutr Metab Care. 1998;1:245–51. doi: 10.1097/00075197-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Torelli GF, Meguid MM, Moldawer LL, Edwards CK, 3rd, Kim HJ, Carter JL, et al. Use of recombinant human soluble TNF receptor in anorectic tumor-bearing rats. Am J Physiol. 1999;277:R850–5. doi: 10.1152/ajpregu.1999.277.3.R850. [DOI] [PubMed] [Google Scholar]
- 34.Jatoi A, Dakhil SR, Nguyen PL, Sloan JA, Kugler JW, Rowland KM, Jr, et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: results from N00C1 from the North Central Cancer Treatment Group. Cancer. 2007;110:1396–403. doi: 10.1002/cncr.22944. [DOI] [PubMed] [Google Scholar]
- 35.Jatoi A, Ritter HL, Dueck A, Nguyen PL, Nikcevich DA, Luyun RF, et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9) Lung Cancer. 2010;68:234–9. doi: 10.1016/j.lungcan.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiedenmann B, Malfertheiner P, Friess H, Ritch P, Arseneau J, Mantovani G, et al. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J Support Oncol. 2008;6:18–25. [PubMed] [Google Scholar]
- 37.Johnston AJ, Murphy KT, Jenkinson L, Laine D, Emmrich K, Faou P, et al. Targeting of Fn14 prevents cancer-induced cachexia and prolongs survival. Cell. 2015;162:1365–78. doi: 10.1016/j.cell.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Hong DS, Hui D, Bruera E, Janku F, Naing A, Falchook GS, et al. MABp1, a first-in-class true human antibody targeting interleukin-1alpha in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15:656–66. doi: 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- 39.Fisher GA. A phase III study of xilonix in refractory colorectal cancer patients with weight loss. J Clin Oncol. 2015;33(suppl 3) abstr 685. [Google Scholar]
- 40.Clarke SJ, Smith JT, Gebbie C, Sweeney C, Olszewski N. A phase I, pharmacokinetic (PK), and preliminary efficacy assessment of ALD518, a humanized anti-IL-6 antibody, in patients with advanced cancer. J Clin Oncol. 2009;27:15s. (suppl; abstr 3025) [Google Scholar]
- 41.Rigas JR, Schuster M, Orlov SV, Milovanovic B, Prabhash K, Smith JT, et al. Efect of ALD518, a humanized anti-IL-6 antibody, on lean body mass loss and symptoms in patients with advanced non-small cell lung cancer (NSCLC): results of a phase II randomized, double-blind safety and efficacy trial. J Clin Oncol. 2010;28(suppl):15s. abstr 7622. [Google Scholar]
- 42.Schuster M, Rigas JR, Orlov SV, Milovanovic B, Prabhash K, Smith JT, et al. ALD518, a humanized anti-IL-6 antibody, treats anemia in patients with advanced non-small cell lung cancer (NSCLC): results of a phase II, randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2010;28(suppl):15s. abstr 7631. [Google Scholar]
- 43.Padrao AI, Oliveira P, Vitorino R, Colaco B, Pires MJ, Marquez M, et al. Bladder cancer-induced skeletal muscle wasting: disclosing the role of mitochondria plasticity. Int J Biochem Cell Biol. 2013;45:1399–409. doi: 10.1016/j.biocel.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Loumaye A, de Barsy M, Nachit M, Lause P, Frateur L, van Maanen A, et al. Role of Activin A and myostatin in human cancer cachexia. J Clin Endocrinol Metab. 2015;100:2030–8. doi: 10.1210/jc.2014-4318. [DOI] [PubMed] [Google Scholar]
- 45.Busquets S, Toledo M, Orpi M, Massa D, Porta M, Capdevila E, et al. Myostatin blockage using actRIIB antagonism in mice bearing the Lewis lung carcinoma results in the improvement of muscle wasting and physical performance. J Cachexia Sarcopenia Muscle. 2012;3:37–43. doi: 10.1007/s13539-011-0049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benny Klimek ME, Aydogdu T, Link MJ, Pons M, Koniaris LG, Zimmers TA. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun. 2010;391:1548–1554. doi: 10.1016/j.bbrc.2009.12.123. [DOI] [PubMed] [Google Scholar]
- 47.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–43. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Murphy KT, Chee A, Gleeson BG, Naim T, Swiderski K, Koopman R, et al. Antibody-directed myostatin inhibition enhances muscle mass and function in tumor-bearing mice. Am J Physiol Regul Integr Comp Physiol. 2011;301:R716–26. doi: 10.1152/ajpregu.00121.2011. [DOI] [PubMed] [Google Scholar]
- 49.Jameson GS, Von Hoff DD, Weiss GJ, Richards DA, Smith DA, Becerra C, et al. Safety of the antimyostatin monoclonal antibody LY2495655 in healthy subjects and patients with advanced cancer. J Clin Oncol. 2012;30(suppl) abstr 2516. [Google Scholar]
- 50.Amato AA, Sivakumar K, Goyal N, David WS, Salajegheh M, Praestgaard J, et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology. 2014;83:2239–46. doi: 10.1212/WNL.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aversa Z, Bonetto A, Penna F, Costelli P, Di Rienzo G, Lacitignola A, et al. Changes in myostatin signaling in non-weight-losing cancer patients. Ann Surg Oncol. 2012;19:1350–6. doi: 10.1245/s10434-011-1720-5. [DOI] [PubMed] [Google Scholar]
- 52.Gallot YS, Durieux AC, Castells J, Desgeorges MM, Vernus B, Plantureux L, et al. Myostatin gene inactivation prevents skeletal muscle wasting in cancer. Cancer Res. 2014;74:7344–56. doi: 10.1158/0008-5472.CAN-14-0057. [DOI] [PubMed] [Google Scholar]
- 53.Toledo M, Busquets S, Penna F, Zhou X, Marmonti E, Betancourt A, et al. Complete reversal of muscle wasting in experimental cancer cachexia: additive effects of activin type II receptor inhibition and beta-2 agonist. Int J Cancer. 2016;138:2021–9. doi: 10.1002/ijc.29930. [DOI] [PubMed] [Google Scholar]
- 54.Mori S, Matsuzaki K, Yoshida K, Furukawa F, Tahashi Y, Yamagata H, et al. TGF-beta and HGF transmit the signals through JNK-dependent Smad2/3 phosphorylation at the linker regions. Oncogene. 2004;23:7416–29. doi: 10.1038/sj.onc.1207981. [DOI] [PubMed] [Google Scholar]