Abstract
Background
Evidence demonstrates that the T allele of the single-nucleotide polymorphism rs405509 in the apolipoprotein E (APOE) promoter is a risk factor for Alzheimer’s disease (AD). However, it is unknown whether rs405509 T allele synergizes to the APOE ε4 allele in influencing cognition and brain structure.
Methods
We analyzed the interaction of the rs405509 T allele and the APOE ε4 allele on cognitive ability and brain gray matter volume (GMV) among the elderly people. The subjects were grouped into four groups according to APOE and rs405509 genotypes.
Results
Significant interactions were found between rs405509 and APOE on general mental status, memory and attention. Analysis of the whole brain gray matter showed a significantly positive interaction between rs405509 and APOE on the right inferior temporal gyrus and the right fusiform gyrus (alphasim correction p<0.001).Additionally, there was a significant relationship between cognitive ability and GMV.
Conclusions
The data indicates that the APOE rs405509 T homozygote modulates effect of APOE ε4 on both cognitive performance and brain gray matter structure.
Keywords: APOE, rs405509, cognitive impairment, interaction, voxel-based morphometry, Alzheimer’s Disease, risk factor, MRI
Introduction
Alzheimer’s disease (AD) is a complex neurodegenerative disease, commonly associated with episodic memory and other cognitive impairment, as well as brain structure alterations such as gray matter atrophy (1). Many genetic risk factors have been identified in late-onset AD, with apolipoprotein E (APOE) ε4 being the most established one (2). Research efforts are on-going to explore additional gene risk factors and to confirm those that have been recently explored (3, 4).
Rs405509 single-nucleotide polymorphism (SNP), located at the APOE promoter, is known as −219 G/T or as Th1/E47cs owing to its location within a potential Th1/E47 transcription factor-binding site (5, 6). The T to G substitution at rs405509 provoked an increase of 169% in promoter activity (7), and the median onset age of AD in patients homozygous for rs405509 minor allele G was 5 years later than that of those homozygous for the major allele T (8). Additionally, the rs405509 T homozygote increased risk for developing AD compared with the rs405509 T/G+G/G group in a case-control study (9), and it was associated with a significant risk of sporadic AD (10). Lambert et al. (11) divided 74 AD patients into two groups (≤70 years and > 70 years), and the results suggested that rs405509 T/T patients had higher amounts of Aβ42 and total Aβ than those with G/T or G/G genotypes. As reported in the literature, rs405509 and APOE may share pathways involved in the processing of β amyloid. Regarding this process, many studies agreed that rs405509 was associated with the serum concentration of apoE, with the T allele causing the most reduced apoE concentration in vivo (12, 13). The amount of senile plaque increased significantly for individuals who were also homozygous for the T allele of rs405509 in hippocampus CA1 and subiculum, and there is a larger amount of plaque in the superior temporal cortex (14). This finding provided reliable pathological evidence that rs405509 contributed to β amyloid pathology in specific brain region by modulating APOE gene. Since many AD risk genes may contribute to AD pathology, exploring gene-gene interaction on potential AD biomarkers including neuroimaging measures gives us a better understanding part of the causes underlying disease heterogeneity. For instance, the interaction effect of APOE and TOMM40 impacts on hippocampus volume and white matter integrity in old adults (15), and two risk variants of AGTR1 gene interacted with the APOE4 allele to accelerate right hippocampal volume loss(16). Recently, a study reported interactive effects of PICALM and APOE on brain atrophy and cognitive impairment in Alzheimer’s disease (17). On the other hand, genes which are associated with increased AD onset risk does not always interact with each other (18). Interestingly, previous studies also reported rs405509’s interaction with APOE in terms of AD risk. Its TT genotype was associated with a significantly increased risk of dementia in people with APOE ε3/ε3 genotype (19). And a more recent study reported both TT and GG genotype of rs405509 with higher odds ratio for AD compared with GT group in the APOE ε4 carriers, however, in ε3/ε3 carriers, both TT and GG showed lower frequency than GT in AD patient(20). In another study, the rs405509 T-APOEε4 haplotype could increase the risk of AD (21).
Based on previous researches, there are two questions to be answered. First, does rs405509 TT interact with APOE ε4 on cognitive performance in old but cognitive normal subjects? Second, how is the rs405509 × APOE interaction associated with the brain gray matter structure? So in current study a large sample of cognitively normal individuals were recruited and voxel-based morphometry (VBM) technique(22) was applied to investigate the gray matter volumein a subset of these study participants. To the best of our knowledge, this is the first study investigating the interaction between APOE and rs405509 using neuropsychological tests and VBM approach.
Materials and methods
Participants
Participants in the present study were from the Beijing Ageing Brain Rejuvenation Initiative (BABRI), which is an ongoing longitudinal study that plans to collect cognitive behavioral, sociodemographics, potential influencing factor, and clinical data from Beijing’s elderly adults in five time-windows over 10 years and there were more than 1600 eligible urban participants. The study were undertaken with the understanding and written consent of each subject, and the study conformed to the guidelines set out in the 2013 World Medical Association Declaration of Helsinki. The use of human subjects for this study has been approved by institutional review board (IRB) at the Imaging Center for Brain Research at Beijing Normal University.
Currently, 1211 urban elderly living members in Beijing, China, were enrolled in the BABRI dataset. In this report, 836 elderly participants (mean age 65.2 ± 7.5)were included (Figure 1).Individuals were included if they met the following criteria: (a) age of 55–85 years, (b) at least six years of education, and (c) a score of 20 or higher on the Chinese version of the Mini-Mental Status Examination (MMSE). The exclusion criteria were as follow: (a) a previous diagnosis of a brain structural abnormality other than cerebrovascular lesions, such as tumors, psychotic disorders including schizophrenia or bipolar disorder, multiple sclerosis, motor neuron disease or developmental disability, (b) genotyping failure, (c) history of addictions neurologic or psychiatric diseases, or treatments that would affect cognitive function and (d) repeated data or other doubtful data.
Figure 1. Participant flow chart.
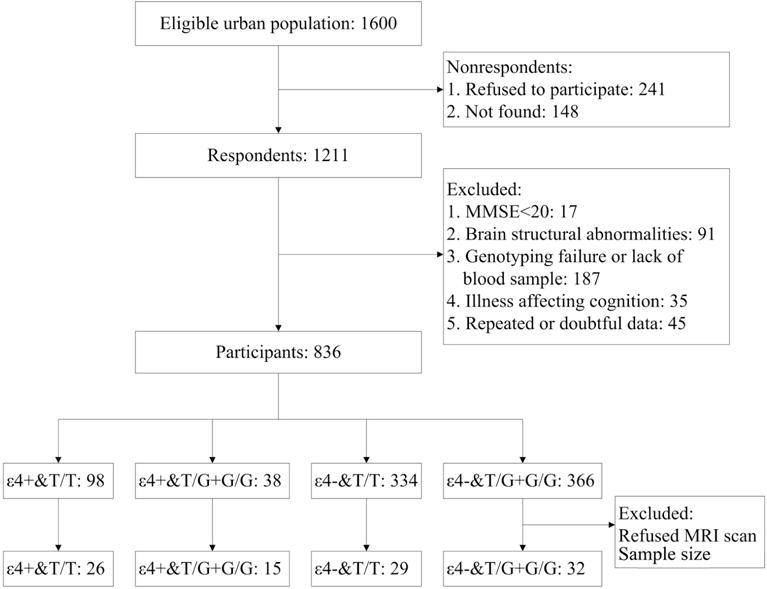
Abbreviations: MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; ε4+, APOE ε4 carriers; ε4−, APOE ε4 non-carriers.
There were only 38 participants who were both APOE ε4 and G carriers and the rs405509 TT genotype is associated with a significantly increased risk of AD whereas TG and GG genotypes are not (15).So we merged the rs405509 TG and GG groups and APOE into ε4 carriers and non-carriers. Overall, there were 136 APOE ε4 carriers, 98 with the rs405509 TT genotype and 38 with the rs405509 TG+GG group; and there were 693 APOEε4 non-carriers, 332 with the rs405509 TT genotype and 361 with the rs405509 TG+GG genotype. Age and education were matched among the four groups, whereas gender was not matched (χ2=8.912, p=0.03) (Table 1).
Table 1.
Demographics and neuropsychological test results for all participants who completed neuropsychological testsa
| APOEε4 non-carriers | APOE main effect | Rs405509 main effect | ANOVA/interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rs405509,TG+GG | Rs405509,TT | Rs405509,TG+GG | F | p | F | p | F/χ2 | P value | |
| 38 | 332 | 361 | |||||||
| 12:26 | 142:190 | 116:245 | 8.912 | 0.030 | |||||
| 64.68±7.05(55–80) | 65.68±7.37(55–85) | 64.85±7.44(55–82) | 0.827 | 0.479 | |||||
| 11.22±3.53(6–18) | 10.79±3.60(6–19) | 10.88±3.61(6–20) | 0.336 | 0.799 | |||||
|
| |||||||||
| 27.68±2.41(23–30) | 27.41±2.51(20–30) | 27.52±2.64(20–30) | 7.82 | 0.005 | 10.741 | 0.001 | 10.852 | 0.001* | |
| 5.85±2.34(1–10) | 5.57±2.47(0–11) | 5.71±2.53(0–11) | 4.062 | 0.044 | 4.778 | 0.029 | 4.629 | 0.032* | |
| 29.35±8.76(15–50) | 29.49±9.25(5–54) | 30.24±9.20(7–50) | 5.773 | 0.016 | 2.391 | 0.122 | 1.796 | 0.181 | |
| 13.57±6.63(2–32) | 13.23±6.22(0–30) | 13.51±6.15(0–30) | 0.299 | 0.584 | 0.731 | 0.393 | 0.538 | 0.463 | |
| 32.92±3.09(25–36) | 32.96±3.50(15–36) | 33.12±3.93(10–36) | 1.829 | 0.177 | 1.243 | 0.265 | 0.502 | 0.479 | |
| 25.05±3.01(18–30) | 24.69±3.39(14–30) | 24.44±4.046(0–30) | 0.172 | 0.678 | 1.468 | 0.226 | 3.396 | 0.066 | |
| 81.60±37.17(44–240) | 78.42±24.54(40–219) | 78.70±22.91(29–179) | 1.078 | 0.299 | 0.600 | 0.439 | 0.009 | 0.925 | |
| 45.51±3.02(40–50) | 44.31±4.82(12–50) | 44.37±4.49(23–50) | 1.462 | 0.227 | 1.262 | 0.262 | 1.301 | 0.254 | |
| 181.70±60.67(91–318) | 189.96±82.96(70–585) | 179.72±71.45(64–481) | 1.121 | 0.290 | 3.029 | 0.082 | 0.543 | 0.461 | |
| 23.14±4.08(14–29) | 22.88±3.72(10–30) | 23.04±3.72(11–30) | 0.151 | 0.698 | 1.437 | 0.231 | 0.178 | 0.673 | |
| 44.78±8.21(29–64) | 44.33±9.11(12–70) | 44.55±9.00(23–71) | 1.063 | 0.303 | 1.106 | 0.293 | 1.212 | 0.271 | |
| 32.76±11.81(11–54) | 33.35±11.41(9–64) | 35.07±11.63(8–68) | 8.019 | 0.005 | 1.904 | 0.168 | 0.239 | 0.625 | |
| 58.70±15.03(25–94) | 61.05±24.37(24–177) | 60.95±23.85(22–159) | 1.773 | 0.183 | 3.336 | 0.068 | 4.698 | 0.030* | |
Abbreviations: MMSE, Mini-Mental State Examination; AVLT, Auditory Verbal Learning Test; AVLT-T, AVLT-total; ROCF, Rey-Osterrieth Complex Figure; CDT, Clock-Drawing Test; CVFT, Category Verbal Fluency Test; BNT, Boston Naming Test; SDMT, Symbol Digit Modalities Test; TMT, Trail Making Test.
Values are mean ± standard deviation. The main effects of rs405509 and APOE and their interaction on cognitive ability were performed by MANCOVA, age, gender and education as covariates.
To further investigate the association of rs405509 and APOE on gray matter, we used a 2×2 design pattern. A total of 102 of the 836 participants received MRI scans (high-resolution three-dimensional T1-weighted anatomical images).There were 15 participants in the APOE ε4 carriers&rs405509 TT group, 26 participants in the APOE ε4 carriers&rs405509 TG+GG group and 61 in the APOE ε4 non-carriers, with 29 with the rs405509 TT genotype and 32 with the rs405509 TG+GG genotype, and age, gender and education level were matched among the 4 groups (Table S2).
Neuropsychological testing
To evaluate the general mental status and other cognitive functions, participants underwent a series of neuropsychological tests involving 5 cognition domains, including episodic memory, attention and processing speed, visual-spatial ability, language ability, and executive function. General mental status was assessed with the Chinese version of the Mini-Mental State Examination (MMSE) (23). Episodic memory tests were comprised of the Auditory Verbal Learning Test (AVLT) (24) and Recall component of Rey-Osterrieth Complex Figure Test (ROCF) (25). Attention and processing speed tests were comprised of the Trail Making Test A (TMT-A) (26) and Symbol Digit Modalities Test (SDMT) (27). Visual-spatial tests were comprised of the Copy component of ROCF (25) and Clock-Drawing Test (CDT) (28). Language ability tests were comprised of the Boston Naming Test (BNT) (29). Executive function tests were comprised of the Trail Making Test B (TMT-B) (26) and Stroop Color-word Test (Stroop) (30).
Genotype and quality control
The Custom Taqman SNP Genotyping Assays (Applied Biosystems, Foster City, USA) were used to prescreen for the rs405509 genotype in the participants. The extra two SNPs, rs429358 and rs7412, which jointly defined the APOE ε2 (with haplotype of rs429358-rs7414: T-T), ε3 (T-C), and ε4 alleles (C-C), were also genotyped. The sample success rates for all of the SNPs were 100%, and the reproducibility of all of the genotyping was 100% according to a duplication analysis of at least 10% of the genotypes. According to the Hapmap database, the rs405509 is in high LD (D′>0.8) with rs7412 but not with rs429358. For both rs405509 and APOE, we did not encounter significant deviations from Hardy-Weinberg equilibrium at an alpha level of 0.05. Rs405509-APOE combined genotypes were defined by Plink (31). Totally, there were 12 combined genotypes (Table S1).
Structural image acquisition
Magnetic resonance imaging data acquisition was performed using a Siemens Trio 3.0 Tesla scanner (Trio; Siemens, Erlangen, Germany) in the Imaging Center for Brain Research at Beijing Normal University. Foam padding and headphones were used to reduce head motion and scanner noise. The T1-weighted structural images were acquired using 3-dimensional (3D) magnetization prepared rapid gradient echo sequences: 176 sagittal slices, TR=1900 ms, TE=3.44 ms, slice thickness=1 mm, flip angle=9°, FOV=256 mm×256 mm and gap=9.
Structural image preprocessing
All subjects’ individual structural images were preprocessed using the VBM8 software package (http://dbm.neuro.uni-jena.de/vbm/). The preprocessing of structural images used default parameters except estimation using “ICBM space template-East Asian brains” and extended options using “thorough clean up”. Images were bias-corrected, tissue classified, and normalized to MNI-space using affine and nonlinear transformations to compare the absolute amount of tissue (32).
Homogeneity of gray matter images was checked using the covariance structure of each image with all other images, as implemented in the check data quality function. Two subjects with severe white matter lesion and three with excessive head motion (translation > 3mm or rotation >3°) were identified and excluded. The images of remaining 97 subjects were free of such problems. The modulated gray matter images were smoothed with a Gaussian kernel of 8 mm FWHW. Group statistics were calculated using SPM8 (Statistical Parametric Mapping, www.fil.ion.ucl.ac.uk/spm). In the study, we utilized the mean GM map (threshold = 0.2) of all of the patients to obtain a group based brain mask and used it for subsequent analysis.
Statistical Analysis
The influence of the rs405509-APOE combined genotype on cognitive ability was tested using one-way ANCOVA. The subjects’ age, sex and education were entered as covariates to control their effects in the analysis. Post-hoc comparisons were performed using Fisher’s LSD test. The main effects of the rs405509 and APOE genotypes, as well as the effects of the genotypes on cognitive performance, were examined with a two factor (rs405509 TT vs GT+GG) via two (APOE ε4 carriers vs ε4 non-carriers) analyses of variance, with age, gender, and education years as covariates using SPSS 20.0.0.0 (IBM Corporation; Armonk, NY).
A two-way ANCOVA was performed to investigate the main effects of the rs405509 and APOE genotype as well as interactions on GM volume, with age, gender, education level and total intracranial volume as covariates. These evaluations were performed using SPM8, with a full factorial design on a voxel-by-voxel basis. The resulting areas for the interaction of rs405509 and APOE in VBM analysis were extracted as ROI masks using REST v1.8 (http://www.restfmri.net). To extract the GMV (gray matter value) of ROIs, we used the SurfStat toolbox (http://www.math.mcgill.ca/keith/surfstat/) for MATLAB. The statistical results of brain GMV were viewed using Brainnet Viewer (33). Partial correlation between cognitive ability and GMV of ROIs was examined using SurfStat toolbox, with age, gender and education level as covariates.
After examining rs405509 × APOE interaction on cognitive performance and GM, we performed a series of mediation analyses (34) to identify if the gray matter volume could mediate the role of risk allele in cognitive decline. A mediator model typically consists of one (or more) independent (or exogenous) variable(s), a mediator variable, and a dependent variable. Both the independent and the mediator variables are assumed to influence the dependent variable. Accordingly, in the present study, the rs405509 × APOE carrying status constituted the independent variable, disrupted cognitive performance was the dependent variable and GMV were the mediator variables. Therefore, we would assume that the association between genotype and cognitive performance is mediated by the altered brain structure. The mediation analyses were performed with SPSS software.
Results
Haplotype analysis
The number of carriers of each APOE genotype is ε2ε2: ε2ε3: ε2ε4: ε3ε3: ε3ε4: ε4ε4 = 1: 113: 9: 586: 119: 8(0.12%, 13.52%, 1.07%, 70.09%, 14.24%, 0.95%). The frequencies of the ε2, ε3, and ε4 alleles were 7.1%, 84.3%, and 8.6%, respectively. As for rs405509, TT: GT: GG = 432: 347:57 (51.6%, 41.5%, 6.8%), and frequency of T allel is 72.4%.An ANCOVA analysis was performed (N=836, TT/ε3ε2, TG/ε2ε2 and GG/ε2ε2 groups were excluded because the number of people were less than 5) to find the difference in cognitive ability between all the rs405509-APOE combined genotypes. Although there were no differences among groups in sex, age or education, the analyses still included sex, age and education as covariates. The differences of MMSE (F=5.738, p<0.001), AVLT-delay recall (F=2.782, p=0.005), AVLT-total (F=2.460, p=0.012) and SDMT (F=2.179, p=0.027) scores were significant among the groups (Table S1), and the results of MMSE, AVLT-delay recall and AVLT-total survived a multiple comparison correction FDR (false discovery rate) p<0.05. The post hoc analysis indicated that the double homozygous genotype (−219TT/ε4ε4) results in a significantly reduced cognitive ability, including general mental status, memory, attention and processing speed (see Figure S1).
Rs405509 × APOE effects on cognitive ability
After merging GG and GT carriers together, we further analyzed the interaction between rs405509 and APOE using a 2×2 design of 836 participants, with gender and education as covariates. The results showed that the rs405509 genotype and APOE status demonstrated a significant interaction effect on MMSE (F=10.852, p=0.001), which achieved a FDR p<0.05. AVLT delay recall (F=4.629, p=0.032), and TMT-A performance (F=4.698, p=0.030) were both with uncorrected p<0.05. Other cognitive tests did not show significant interaction results. For detailed F and p values, see (Table 1, Figure 2).
Figure 2. Diagrams of group-specific MMSE, AVLT delay recall, CDT and TMT-A time in a sample of 836 participants.
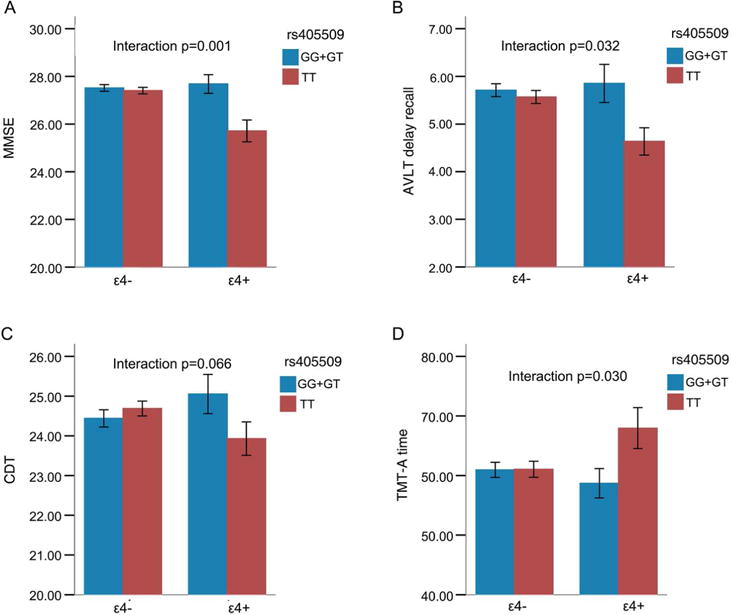
X axis: left, APOE Ɛ4 carriers, right APOE Ɛ4 non-carriers. Blue, rs405509 G/G+G/T group; Red, rs405509 T/T group. Error bars represent the SE.
In the sample of 102 participants with MRI data, the two-way ANCOVA analysis showed that the rs405509 genotype and APOE status showed significant interaction effects on MMSE (F=14.106, p<0.001) and AVLT delay recall (F=6.054, p=0.016), which was similar to the overall larger group results. The rs405509 genotype and APOE status showed significant interaction effects on SDMT performance (F=5.395, p=0.022), which reflects attention and processing speed ability; this was also similar to the overall larger group. The rs405509 genotype and APOE status showed significant interaction effects on CDT (F=4.470, p=0.037) (Table S2, Figure S2). Other psychological tests did not show significant interaction effect.
APOE main effect and rs405509 main effect on gray matter volume
Compared with APOE ε4 non-carriers, APOE ε4 carriers showed smaller gray matter at the fusiform gyrus (R), inferior temporal gyrus (R), superior and middle frontal gyrus (L), angular gyrus(R), cingulate gyrus and precuneus (L) (alphasim correction p<0.001) (Table S3, Figure 3). The rs405509 did not show significant main effect on gray matter volume analysis.
Figure 3. T-statistical difference maps in gray matter volume between APOE ε4 carriers and APOE ε4 non-carriers.
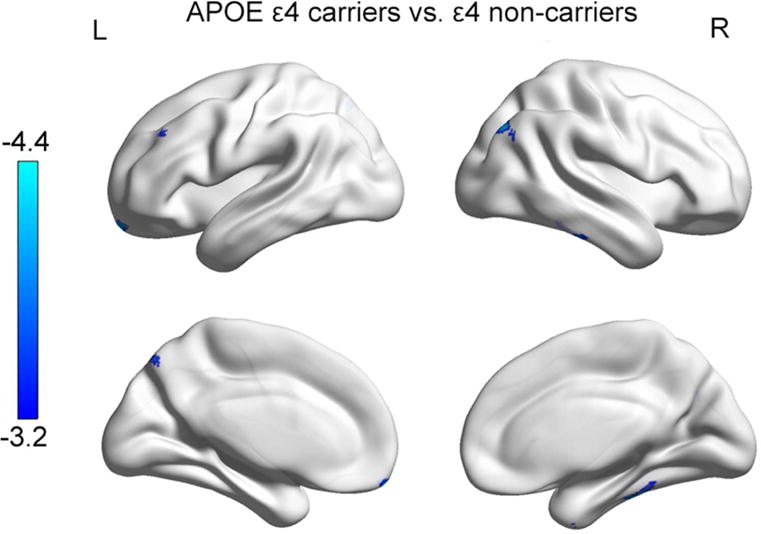
a. Maps are displayed at a threshold of p<0.001, alphasim corrected.
b. Blue: T value, showing area influenced by APOE.
Rs405509 × APOE effects on gray matter volume
The rs405509 × APOE were significant associated with the GMV of the right inferior temporal gyrus and right fusiform gyrus (alphasim correction p<0.001) (Table S4, Figure 4).Post hoc analysis showed that mean GMV of the right inferior temporal gyrus in the TT &ε4− carriers group was significantly lower than the other three groups; APOE ε4 carriers showed lower GMV of the right inferior temporal gyrus (F=4.500, p=0.037) than the non-carriers, whereas the main effect of rs405509 was not significant. The mean GMV of the fusiform gyrus in the TT/ε4− carriers group was significantly lower than the other three groups, and the main effects of rs405509 and APOE were not significant.
Figure 4. Interaction between rs405509 and APOE on gray matter volume.
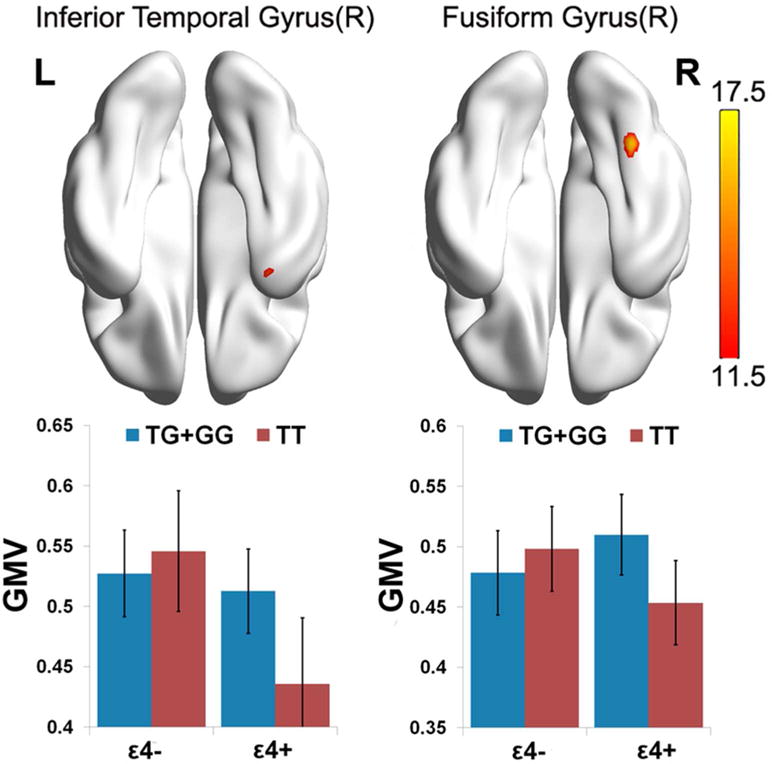
a. Maps are displayed at a threshold of p<0.001, alphasim corrected.
b. Red: F value, showing rs405509 and APOE interaction area.
c. Bottom bar chart: Diagrams of group-specific right inferior temporal gyrus GMV and Right fusiform gyrus GMV. X axis: left, APOE ε4 carriers, right APOE ε4 non-carriers; Blue, rs405509 G/G+G/T group; Red, rs405509 T/T group.
Correlation between GMV and neuropsychological tests and Mediation Analyses
To identify the correlation between GMV and cognitive performance, we calculated the partial correlation between GMV and all cognitive measures, controlling the effects of age, gender, education and group. GMV of the right inferior temporal gyrus was significantly correlated with MMSE (r=0.345, p<0.001) and SDMT (r=0.229, p=0.024). GMV of the right fusiform gyrus was also correlated with MMSE (r=0.243, p=0.016) and SDMT (r=0.274, p=0.003) (Figure 5). In the mediation analysis, the independent factor was genotype group and dependent variables were cognitive measures that showed significant genotype differences, such as MMSE, SDMT and TMT-a. The proposed mediators were the GMV of fusiform and inferior temporal gyrus, which showed significant interaction effects in the above analyses. Mediation analysis indicated that the GMV of the right inferior temporal gyrus mediated the effect of rs405509 × APOE variants on SDMT score (Z=2.09, P =0.037), and MMSE (Z=2.07, p=0.039). No other significant mediation effects were found.
Figure 5. Partial correlation between GMV and cognitive performance.
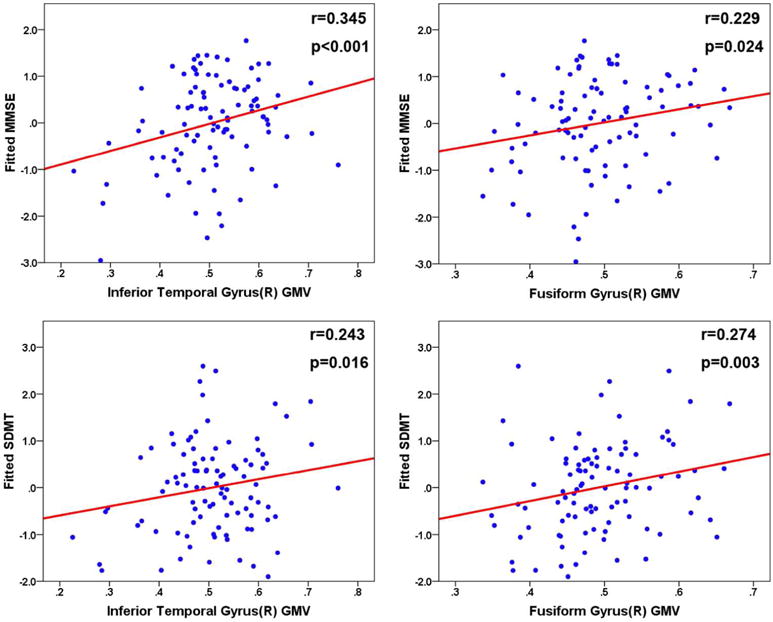
a. Partial correlation between GMV and cognitive performance, controlling the effects of age, gender, education and group: A. partial correlation between GMV of Inferior Temporal Gyrus(R) and MMSE; B. partial correlation between GMV of Fusiform Gyrus(R) and MMSE; C. partial correlation between GMV of Inferior Temporal Gyrus(R) and SDMT; D. partial correlation between GMV of Fusiform Gyrus(R) and SDMT.
Discussion
To the best of our knowledge, our study is among the first to discover the interaction between the APOE genotype and its promoter rs405509 polymorphisms on both cognitive performance and regional brain gray matter volume. Compared with other genotype carriers, the participants carrying both rs405509 T/T and APOE ε4 showed more severe cognitive impairment in general cognition, episodic memory, processing speed and executive function, as well as reduced GMV in the inferior temporal gyrus and fusiform gyrus. More importantly, we also discovered a significant correlation between cognition phenotype and brain alterations. These results suggest that the APOE × rs405509 interaction has a strong association with the AD risk of healthy elderly carriers.
We found that participants carrying both rs405509 TT and APOE ε4 exhibited more severe cognitive impairment in several cognitive domains. Significant cognitive impairments in the elderly were reported to be associated with a higher risk of AD(10), and APOE ε4 was considered to be the most influential gene for elderly cognitive decline (35). Consistent with previous studies in AD population, these results show similar interactive effects in the elderly cognitively intact population. Various genotype combinations were associated with different degrees of cognitive impairment. Considering the relationship between cognitive decline and AD (36), the interaction between the two genes on the elderly’s cognition might also affect the disease’s onset and development. Although we did not found significant interaction besides MMSE, as an exploratory study, these results could serve implications for the gene-gene interaction on cognition impairment.
Following the concern that cognitive performance is strongly associated with brain impairment and disease stage (36), we then calculated GMV and found several regions where APOE ε4 carriers showed more severe atrophy. Similar to those of a previous study that reported wide spread atrophy primarily located in the temporal and parietal regions in healthy elderly people (37). Moreover, we found regions that showed significant interaction between APOE and rs405509 in the inferior temporal gyrus and fusiform gyrus, where the ε4+&T/T group showed smallest GMV.
The inferior temporal region was associated with significant pathological changes and is among the first affected regions with gray matter structural changes in AD onset (38, 39). The fusiform gyrus was a region with both structural and functional changes in MCI and early AD (40). Interestingly, a study reported that both the inferior temporal and fusiform gyrus show the earliest changes before the diagnosis of AD (41).Hippocampus, whose atrophy was often considered a hallmark of progression in AD pathology(42), was not a significant interaction region in the present study. As study reported, in the preclinical stage of AD hippocampus volume is not significantly affected (43). The medial temporal region first revealed structural abnormality, then pathology (tau and/or Aβ) infecting the connected regions including the hippocampus (44), causing observable structural deficits. Taken above neuroimaging evidence together, present structural damage could reflect significant pre-clinical AD characteristics and suggests that ε4+ & T/T carriers have greater risk than either ε4 allele carriers or T/T haplotype carriers alone.
ApoE protein functions as a traffic molecule for lipids throughout the brain and can bind Aβ depending on lipidation status (45). The latter largely determines whether the interaction of APOE and Aβ leads to an efflux of Aβ from the brain or, alternatively, to Aβ aggregation and plaque formation. Although APOE has been associated with the modulation of brain Aβ peptide levels, its ε4 isoform increases the propensity of Aβ to be deposited and reduces Aβ efflux from the brain. The accumulation of Aβ, specifically of soluble Aβ forms, has been shown to impair synaptic function, such as via binding to insulin receptors and disruption to insulin signaling. A post-mortem study found that in ε4 allele carriers, the amounts of senile plaque were 4 to 7 times higher than non-carrier, in the hippocampal sub-region cornu ammonis 1 (CA1), subiculum, isocortex and entorhinal cortex. The rs405509 has been shown to have a major effect on gene expression levels (7, 46, 47)., and with the increase of APOE ε4 allele dose, apoE level was reduced in the hippocampus and the frontal cortex (45). Importantly, the single nucleotide changes in the promoter region may impact transcriptional activity through the direct binding of transcription factors (48) or indirectly through changing the secondary structure of DNA and influencing the combination of transcription factors (49). Artiga et al (7) also confirmed the effects of the differences in transcription factors’ access on APOE transcriptional activity.
Therefore, both APOE ε4 and rs405509 are involved in Aβ processing and are negatively affected at variable level. Largely consistent with the findings mentioned above, a combination of APOE ε4 and rs405509 T/T in the elderly population exhibited severe cognitive impairment and smaller regional brain GMV. Therefore, this polymorphism may play a role in APOE-involved diseases, such as AD. Previous studies described that rs405509 was associated with an increased risk of AD (5, 50). As a result, the combination of APOE ε4 allele and rs405509 T/T haplotype could lead to a significantly increased risk for AD.
Correlation analyses between neuroimaging and cognitive measures further enhanced our understanding of the neural integration that underlie multiple cognitive abilities. Notably, GMV of two brain regions with smaller GMV (the right inferior temporal gyrus and the right fusiform gyrus) were significantly correlated with both MMSE and SDMT. This finding suggested that these gray matter alterations in different brain regions might account for specific cognitive impairment.
The rs405509 and APOE ε4 interaction results in GMV may be implicative a direct result of harboring clinically silent but higher amyloid burden in TT & ε4 carriers who are supposed more likely to develop AD in the future. However, such hypothesis should be examined together with direct measure of beta-amyloid by, e.g., florbetapir PET technique in future studies in rs405509 and APOE ε4 carriers and non-carriers throughout longitudinal research to confirm the relationship between APOE-rs405509 interaction with AD onset. And the limited sample size made the results requiring significant replication in large independent cohorts, because many genetic associations in small samples disappear in larger groups.
Supplementary Material
Acknowledgments
This work was supported by the State Key Program of National Natural Science of China(Grant No.81430100), the National Science Foundation of China (Grant No.81173460, and No. 81274001), Beijing New Medical Discipline Based Group (Grant No.100270569), Project of Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences (Grant No.Z0175 and No.Z0 288), Program for New Century Excellent Talents in University (Grant No.NCET-10-0249), and by National Institute on Aging (R01AG031581).
Footnotes
Statement of interest: All authors declare that they have no conflicts of interest.
References
- 1.Forero DA, Pinzon J, Arboleda GH, Yunis JJ, Alvarez C, Catano N, et al. Analysis of common polymorphisms in angiotensin-converting enzyme and apolipoprotein e genes and human longevity in Colombia. Arch Med Res. 2006 Oct;37(7):890–4. doi: 10.1016/j.arcmed.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.van der Flier WM, Pijnenburg YA, Fox NC, Scheltens P. Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE varepsilon4 allele. Lancet neurology. 2011 Mar;10(3):280–8. doi: 10.1016/S1474-4422(10)70306-9. [DOI] [PubMed] [Google Scholar]
- 3.Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nature genetics. 2011 May;43(5):436–41. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA: the journal of the American Medical Association. 2010 May 12;303(18):1832–40. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert JC, Pasquier F, Cottel D, Frigard B, Amouyel P, Chartier-Harlin MC. A new polymorphism in the APOE promoter associated with risk of developing Alzheimer’s disease. Hum Mol Genet. 1998 Mar;7(3):533–40. doi: 10.1093/hmg/7.3.533. [DOI] [PubMed] [Google Scholar]
- 6.Lambert JC, Berr C, Pasquier F, Delacourte A, Frigard B, Cottel D, et al. Pronounced impact of Th1/E47cs mutation compared with −491 AT mutation on neural APOE gene expression and risk of developing Alzheimer’s disease. Hum Mol Genet. 1998 Sep;7(9):1511–6. doi: 10.1093/hmg/7.9.1511. [DOI] [PubMed] [Google Scholar]
- 7.Artiga MJ, Bullido MJ, Sastre I, Recuero M, Garcia MA, Aldudo J, et al. Allelic polymorphisms in the transcriptional regulatory region of apolipoprotein E gene. FEBS letters. 1998 Jan 9;421(2):105–8. doi: 10.1016/s0014-5793(97)01543-3. [DOI] [PubMed] [Google Scholar]
- 8.Jones EL, Mok K, Hanney M, Harold D, Sims R, Williams J, et al. Evidence that PICALM affects age at onset of Alzheimer’s dementia in Down syndrome. Neurobiology of aging. 2013 Oct;34(10):2441, e1–5. doi: 10.1016/j.neurobiolaging.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert JC, Araria-Goumidi L, Myllykangas L, Ellis C, Wang JC, Bullido MJ, et al. Contribution of APOE promoter polymorphisms to Alzheimer’s disease risk. Neurology. 2002 Jul 9;59(1):59–66. doi: 10.1212/wnl.59.1.59. [DOI] [PubMed] [Google Scholar]
- 10.Maloney B, Ge YW, Petersen RC, Hardy J, Rogers JT, Perez-Tur J, et al. Functional characterization of three single-nucleotide polymorphisms present in the human APOE promoter sequence: Differential effects in neuronal cells and on DNA-protein interactions. Am J Med Genet B Neuropsychiatr Genet. 2010 Jan 5;153B(1):185–201. doi: 10.1002/ajmg.b.30973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert JC, Mann D, Goumidi L, Harris J, Amouyel P, Iwatsubo T, et al. Effect of the APOE promoter polymorphisms on cerebral amyloid peptide deposition in Alzheimer’s disease. Lancet. 2001 Feb 24;357(9256):608–9. doi: 10.1016/S0140-6736(00)04063-0. [DOI] [PubMed] [Google Scholar]
- 12.Lambert JC, Brousseau T, Defosse V, Evans A, Arveiler D, Ruidavets JB, et al. Independent association of an APOE gene promoter polymorphism with increased risk of myocardial infarction and decreased APOE plasma concentrations-the ECTIM study. Hum Mol Genet. 2000 Jan 1;9(1):57–61. doi: 10.1093/hmg/9.1.57. [DOI] [PubMed] [Google Scholar]
- 13.Moreno JA, Lopez-Miranda J, Marin C, Gomez P, Perez-Martinez P, Fuentes F, et al. The influence of the apolipoprotein E gene promoter (−219G/T) polymorphism on postprandial lipoprotein metabolism in young normolipemic males. J Lipid Res. 2003 Nov;44(11):2059–64. doi: 10.1194/jlr.M300124-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Berr C, Lambert JC, Sazdovitch V, Amouyel P, Chartier-Harlin MC, Mohr M, et al. Neuropathological epidemiology of cerebral aging: a study of two genetic polymorphisms. Neurobiology of aging. 2001 Mar-Apr;22(2):227–35. doi: 10.1016/s0197-4580(00)00227-x. [DOI] [PubMed] [Google Scholar]
- 15.Lyall DM, Harris SE, Bastin ME, Munoz Maniega S, Murray C, Lutz MW, et al. Alzheimer’s disease susceptibility genes APOE and TOMM40, and brain white matter integrity in the Lothian Birth Cohort 1936. Neurobiology of aging. 2014 Jun;35(6):1513, e25–33. doi: 10.1016/j.neurobiolaging.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zannas AS, McQuoid DR, Payne ME, MacFall JR, Ashley-Koch A, Steffens DC, et al. Association of gene variants of the renin-angiotensin system with accelerated hippocampal volume loss and cognitive decline in old age. Am J Psychiatry. 2014 Nov 1;171(11):1214–21. doi: 10.1176/appi.ajp.2014.13111543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgen K, Ramirez A, Frolich L, Tost H, Plichta MM, Kolsch H, et al. Genetic interaction of PICALM and APOE is associated with brain atrophy and cognitive impairment in Alzheimer’s disease. Alzheimers Dement. 2014 Mar 6; doi: 10.1016/j.jalz.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Carrasquillo MM, Belbin O, Hunter TA, Ma L, Bisceglio GD, Zou F, et al. Replication of BIN1 association with Alzheimer’s disease and evaluation of genetic interactions. J Alzheimers Dis. 2011;24(4):751–8. doi: 10.3233/JAD-2011-101932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heijmans BT, Slagboom PE, Gussekloo J, Droog S, Lagaay AM, Kluft C, et al. Association of APOE epsilon2/epsilon3/epsilon4 and promoter gene variants with dementia but not cardiovascular mortality in old age. Am J Med Genet. 2002;107(3):201–8. doi: 10.1002/ajmg.10142. [Journal Article; Research Support, Non-U.S. Gov’t; Research Support, U.S. Gov’t, P.H.S. %/Copyright 2001 Wiley-Liss, Inc.] 2002-01-22. [DOI] [PubMed] [Google Scholar]
- 20.Bizzarro A, Seripa D, Acciarri A, Matera MG, Pilotto A, Tiziano FD, et al. The complex interaction between APOE promoter and AD: an Italian case–control study. Eur J Hum Genet. 2009;17(7):938–45. doi: 10.1038/ejhg.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lescai F, Chiamenti AM, Codemo A, Pirazzini C, D’Agostino G, Ruaro C, et al. An APOE haplotype associated with decreased epsilon4 expression increases the risk of late onset Alzheimer’s disease. J Alzheimers Dis. 2011;24(2):235–45. doi: 10.3233/JAD-2011-101764. [Journal Article; Research Support, Non-U.S. Gov’t] 2011-01-20. [DOI] [PubMed] [Google Scholar]
- 22.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000 Jun;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975 Nov;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg SJ, Ryan JJ, Prifitera A. Rey Auditory-Verbal Learning Test performance of patients with and without memory impairment. Journal of clinical psychology. 1984 May;40(3):785–7. doi: 10.1002/1097-4679(198405)40:3<785::aid-jclp2270400325>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Tupler LA, Welsh KA, Asare-Aboagye Y, Dawson DV. Reliability of the Rey-Osterrieth Complex Figure in use with memory-impaired patients. Journal of clinical and experimental neuropsychology. 1995 Aug;17(4):566–79. doi: 10.1080/01688639508405146. [DOI] [PubMed] [Google Scholar]
- 26.Gordon NG. The Trail Making Test in neuropsychological diagnosis. Journal of clinical psychology. 1972 Apr;28(2):167–9. doi: 10.1002/1097-4679(197204)28:2<167::aid-jclp2270280212>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Sheridan LK, Fitzgerald HE, Adams KM, Nigg JT, Martel MM, Puttler LI, et al. Normative Symbol Digit Modalities Test performance in a community-based sample. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 2006 Jan;21(1):23–8. doi: 10.1016/j.acn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Ishiai S, Sugishita M, Ichikawa T, Gono S, Watabiki S. Clock-drawing test and unilateral spatial neglect. Neurology. 1993 Jan;43(1):106–10. doi: 10.1212/wnl.43.1_part_1.106. [DOI] [PubMed] [Google Scholar]
- 29.Knesevich JW, LaBarge E, Edwards D. Predictive value of the Boston Naming Test in mild senile dementia of the Alzheimer type. Psychiatry research. 1986 Oct;19(2):155–61. doi: 10.1016/0165-1781(86)90008-9. [DOI] [PubMed] [Google Scholar]
- 30.Koss E, Ober BA, Delis DC, Friedland RP. The Stroop color-word test: indicator of dementia severity. The International journal of neuroscience. 1984 Aug;24(1):53–61. doi: 10.3109/00207458409079534. [DOI] [PubMed] [Google Scholar]
- 31.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007 Sep;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001 Jul;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 33.Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PloS one. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol. 2014 Nov;67(3):451–70. doi: 10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- 35.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature reviews Neurology. 2013 Feb;9(2):106–18. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology. 2005 Jul;19(4):520–31. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 37.Espeseth T, Westlye LT, Fjell AM, Walhovd KB, Rootwelt H, Reinvang I. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiology of aging. 2008 Mar;29(3):329–40. doi: 10.1016/j.neurobiolaging.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 38.Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex. 1994 Mar-Apr;4(2):138–50. doi: 10.1093/cercor/4.2.138. [DOI] [PubMed] [Google Scholar]
- 39.Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. NeuroImage. 2005 Oct 1;27(4):934–46. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Convit A, De Leon MJ, Tarshish C, De Santi S, Tsui W, Rusinek H, et al. Specific hippocampal volume reductions in individuals at risk for Alzheimer’s disease. Neurobiology of aging. 1997 Mar-Apr;18(2):131–8. doi: 10.1016/s0197-4580(97)00001-8. [DOI] [PubMed] [Google Scholar]
- 41.Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007 Jul;130(Pt 7):1777–86. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014 Jun;13(6):614–29. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 43.Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005 Aug 9;65(3):404–11. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009 Jul;11(7):909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertrand P, Poirier J, Oda T, Finch CE, Pasinetti GM. Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Brain research Molecular brain research. 1995 Oct;33(1):174–8. doi: 10.1016/0169-328x(95)00097-c. [DOI] [PubMed] [Google Scholar]
- 46.Campillos M, Lamas JR, Garcia MA, Bullido MJ, Valdivieso F, Vazquez J. Specific interaction of heterogeneous nuclear ribonucleoprotein A1 with the −219T allelic form modulates APOE promoter activity. Nucleic Acids Res. 2003;31(12):3063–70. doi: 10.1093/nar/gkg435. [Journal Article; Research Support, Non-U.S. Gov’t] 2003-06-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramos MC, Matias S, Artiga MJ, Pozueta J, Sastre I, Valdivieso F, et al. Neuronal specific regulatory elements in apolipoprotein E gene proximal promoter. Neuroreport. 2005 Jun 21;16(9):1027–30. doi: 10.1097/00001756-200506210-00029. [DOI] [PubMed] [Google Scholar]
- 48.Angotti E, Mele E, Costanzo F, Avvedimento EV. A polymorphism (G–>A transition) in the −78 position of the apolipoprotein A–I promoter increases transcription efficiency. J Biol Chem. 1994 Jul 1;269(26):17371–4. [PubMed] [Google Scholar]
- 49.Smith JD, Brinton EA, Breslow JL. Polymorphism in the human apolipoprotein A–I gene promoter region. Association of the minor allele with decreased production rate in vivo and promoter activity in vitro. J Clin Invest. 1992 Jun;89(6):1796–800. doi: 10.1172/JCI115783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bullido MJ, Artiga MJ, Recuero M, Sastre I, Garcia MA, Aldudo J, et al. A polymorphism in the regulatory region of APOE associated with risk for Alzheimer’s dementia. Nature genetics. 1998 Jan;18(1):69–71. doi: 10.1038/ng0198-69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


