Abstract
Dendritic cell (DC)-based vaccine strategies aimed at targeting cancer stem-like cells (CSC) may be most efficacious if deployed in the adjuvant setting. In this study, we offer preclinical evidence this is the case for a CSC-DC vaccine as tested in murine models of SCC7 squamous cell cancer and D5 melanoma. Vaccination of mice with an ALDHhigh SCC7 CSC-DC vaccine after surgical excision of established SCC7 tumors reduced local tumor relapse and prolonged host survival. This effect was augmented significantly by simultaneous administration of anti-PD-L1, an immune checkpoint inhibitor. In the minimal disease setting of D5 melanoma, treatment of mice with ALDHhigh CSC-DC vaccination inhibited primary tumor growth, reduced spontaneous lung metastases and increased host survival. In this setting, CCR10 and its ligands were downregulated on ALDHhigh D5 CSCs and in lung tissues respectively after vaccination with ALDHhigh D5 CSC-DC. RNAi-mediated attenuation of CCR10 blocked tumor cell migration in vitro and metastasis in vivo. T cells harvested from mice vaccinated with ALDHhigh D5 CSC-DC selectively killed ALDHhigh D5 CSCs, with additional evidence of humoral immunological engagement and a reduction in ALDHhigh cells in residual tumors. Overall, our results offered a preclinical proof of concept for the use of ALDHhigh CSC-DC vaccines in the adjuvant setting to more effectively limit local tumor recurrence and spontaneous pulmonary metastasis, as compared with traditional DC vaccines, with increased host survival further accentuated by simultaneous PD-L1 blockade.
Keywords: cancer stem cell, dendritic cell, vaccine, immunotherapy, adjuvant
Introduction
Although surgical resection has been a standard treatment for solid malignancies, therapeutic efficacy is limited by both local and distant recurrence (1–3). There are many factors associated with tumor recurrence (4, 5). Several reports have described strategies to eliminate residual tumor cells after surgery (3, 6). However, effectively preventing local tumor recurrence remains a significant challenge. The existence of micro metastasis at the time of tumor resection represents an even greater therapeutic challenge, since 90% of tumor deaths are due to tumor metastasis. There is increasing evidence that many cancers are driven and maintained by a subpopulation of cells that display stem cell properties. Cancer stem cells (CSCs) can self-renew, mediate tumor growth and contribute to tumor recurrence and metastasis (7–9). Targeting CSCs may thus increase the therapeutic efficacy of current cancer treatment.
ALDEFLUOR/ALDH (aldehyde dehydrogenase) activity has been successfully used as a marker to enrich CSC populations in a variety of cancers (10–17). We previously demonstrated that ALDHhigh murine squamous carcinoma SCC7 and D5 melanoma cells were highly enriched for tumor initiating capacity (15). Their protective immunogenicity was evaluated by administering CSC-based dendritic cell (DC) vaccines in syngeneic immunocompetent hosts (15). In a recent study (17), we demonstrated significant therapeutic efficacy conferred by an ALDHhigh CSC-DC vaccine in the treatment of established tumors following localized radiation therapy (RT).
Eliminating microscopic residual disease in the tumor bed is important in preventing local disease recurrence. Administration of CSC-based vaccines after surgical excision of tumor, where local recurrence is high, may reduce local tumor relapse and distant metastasis and possibly improve survival. Furthermore, since CSCs mediate tumor metastasis, targeting this cell population in the adjuvant setting may eliminate micro metastasis prolonging survival. In this study, we evaluated the potential therapeutic efficacy of this approach in the adjuvant setting using CSC-DC vaccination following surgical resection of the tumor, or by treatment of minimal disease.
We developed a vaccination strategy utilizing cell lysates from ALDHhigh SCC7 or D5 CSCs to pulse dendritic cells (CSC-DC). DCs pulsed with ALDHlow SCC7 or D5 non-CSC lysate (ALDHlow-DC), or with heterogeneous, unsorted cell lysate (H-DC) served as controls. Vaccination with ALDHhigh CSC-DC in immunocompetent mice significantly inhibited SCC7 local tumor recurrence after surgery, and inhibited minimal D5 tumor growth with prolonged survival significantly more than either ALDHlow-DC or H-DC vaccination. Furthermore, this effect was accentuated by simultaneous PD-L1 immune checkpoint blockade.
Materials and Methods
Mice
Female C3H/HeNCr MTV (C3H) mice and C57BL/6 (B6) mice were purchased from Jackson lab and Charles River Laboratories (15). The University of Michigan Laboratory of Animal Medicine approved all animal protocols.
Culture of Tumor cells
The squamous carcinoma cell line, SCC7, produces a poorly immunogenic tumor and is syngeneic to C3H mice. D5 is a clone of the melanoma cell line B16, which is syngeneic to B6 mice, and was originally established by our laboratory. The cell lines were grown in complete medium consisting of RMPI 1640 and supplements (15).
ALDEFLUOR assay
The ALDEFLUOR™ Kit (StemCell Technologies, British Columbia, Canada) was used to isolate ALDEFLUOR+/ALDHhigh CSCs from the SCC7 and D5 cells (15).
Preparation of Dendritic Cell vaccine
Tumor cell lysates of unsorted, ALDEFLUOR+/ALDHhigh or ALDEFLUOR−/ALDHlow SCC7 and D5 cells were prepared as previously described (15). Bone marrow-derived murine cells were cultured in 10 ml complete medium (CM) supplemented with 20 ng/mL GM-CSF at a concentration of 0.2 – 0.4 × 106 cells/ml in non-tissue culture petri dishes (Corning, Tewksbury , MA) on day 0. Fresh medium supplemented with 20 ng/mL GM-CSF was added On days 3, 10 ml. On day 6 and day 8, 10 ml of cultured cell suspension was taken from each dish respectively, centrifuged, and the pellet re-suspended in 10 ml of fresh CM with 20 ng/ml of GM-CSF, and added back to each dish. On day 10, DCs were harvested by dispenser and enriched by Opti-Prep density gradient medium. Lysate of unsorted, ALDHlow or ALDHhigh cells was added to DCs at a 1:3 cell equivalent ratio. The DCs were then incubated at 37°C for 24 h with 5% CO2. After incubation, the unsorted tumor cell lysate-pulsed DCs (H-DC), ALDHlow lysate-pulsed DCs (ALDHlow-DC) or ALDHhigh lysate-pulsed DCs (ALDHhigh-DC, e.g. CSC-DC) will be used as vaccine as specified in he subsequent experiments.
Tumor model and treatment protocols
C3H mice were inoculated subcutaneously (s.c.) with 0.5 million SCC7 cells on day 0. On day 21, the mice were subjected to surgical tumor resection except for one group serving as control. The animals with the s.c. tumor removed were then divided into 4 groups (n=5), and administrated with PBS, H-DC, ALDHlow SCC7-DC and ALDHhigh SCC7 CSC-DC vaccine respectively 24 hours after tumor resection. The vaccination was repeated on day 29 and day 36 respectively. Each mouse was inoculated s.c with 2 million DCs per vaccine. In additional experiments when SCC7 CSC-DC is used in combination with an anti-PD-L1 antibody (MedImmune Inc., Gaithersburg, MD), the vaccination was only repeated once on day 29 with anti-PD-L1 administration. In the minimal tumor model, B6 mice were inoculated s.c. with 5,000 D5 cells. The 1st vaccine was administered s.c. 24 hours after tumor inoculation, followed by a 2nd vaccine on day 8. Each vaccine comprised 2 million DCs. The long and short diameters of tumor masses were measured, as well as the tumor volumes measured three times per week. The volumes were calculated as: tumor volume = (width2 * length)/2. Survival was monitored and recorded as the percentage of survivors after tumor inoculation.
Hematoxylin and eosin (H&E) staining for histologic analysis
At the conclusion of the experiments, the lungs were harvested and stained with H&E to discern the histopathological response.
Measurement of chemokine receptor and PD-L1 expression on tumor cells
Freshly harvested s.c. D5 tumors were disaggregated into single cell suspensions (18). Unsorted, ALDHhigh and ALDHlow D5 cells were then respectively incubated with PE-anti-CCR10 for flow cytometry analysis with a BD LSR-cytometer. To evaluate the PD-L1 levels in the CSC and non-CSC populations post-treatment, D5 tumors were harvested at the end of therapy to prepare tumor cell suspensions. These tumor cells were then incubated with PE-anti-PD-L1 (BioLegend, San Diego, CA), followed by staining with ALDEFLUOR (FITC) for ALDHhigh and ALDHlow population isolation as described in ALDEFLUOR assay. The ALDHhigh (CSC) and ALDHlow (non-CSC) D5 tumor cells were then examined by flow cytometry for PD-L1 expression.
Detection of chemokine expression in lung tissues
The mRNA expression levels of chemokines CCL27 and CCL28 in lung tissues were analyzed using real time quantitative PCR (qRT-PCR) (17). The preparations of the total RNA and cDNA were previously described (19). The data was expressed as the relative fold changed.
CCR10 gene silencing
Equal doses of CCR10 siRNA and negative siRNA (QIAGEN Sciences, Germantown, MD) were used according to the manufacture’s instructions to transfer unsorted, ALDHhigh, and ALDHlow D5 cells for 48 hours to inhibit CCR10 expression. 106 cells were then resuspended and RNA extracted using RNeasy Mini Kit (QIAGEN Sciences). Five mg of total RNA was reverse transcribed (M-MLV, Invitrogen, Carlsbad, CA) to generate cDNA for subsequent RT-PCR. Platinum Sybr Supermix (Invitrogen) was used to amplify sequences for CCR10 (forward: CAGTCTTCGTGTGGCTGTTGTC and reverse TCACAGTCTGCGTGAGGCTTTC) and GAPDH (forward TGAAGCAGGCATCTGAGGG and reverse CGAAGGTGGAAGAGTGGGAG ) using a standard 3-step protocol (35 cycles of: 30 seconds each of 95°C , 58°C , 72°C). Melting point analysis verified the presence of single products.
Chemotaxis assay
500 μl RMPI 1640 containing 1×106 D5 cells or CCR10 siRNA transferred D5 cells were added to the upper chamber of a transwell (insert pore size, 8μm; Corning, New York, NY). Chemokines CCL27 and CCL28 (R&D Systems, Minneapolis, MN) were added to the lower chamber in a volume of 750μl RMPI 1640 which contained 20% FBS. After 37°C incubation for 27 hours, the cells that migrated to the lower surface of membrane were stained with Diff-QuikTM set (Siemens Healthcare Diagnostics Inc, Newark, DE). Cells were photographed under the microscope at 200×magnifications, and counted in 5 fields of triplicate membranes.
Purification and culture of host B cells and T cells
Spleens were harvested from animals subjected to various treatments at the end of the experiments. Splenic B cells were purified and activated in CM supplemented with lipopolysaccharide (LPS, SIGMA, St. Louis, MO), anti-CD40 (AdipoGen, San Diego, CA) and IL-2 (Prometheus Laboratories Inc., San Diego, CA) (15). The culture supernatants were collected and stored at −20°C for future experiments. Splenic T cells were purified and activated to generate CTLs that were analyzed in LDH cytotoxicity assays (15).
CSC binding by immune supernatant and antibody/complement mediated cytotoxicity
Sorted ALDHihgh or ALDHlow D5 cells were incubated with the immune supernatants collected from the cultured B cells with equal quantities of IgG followed by incubation with the second FITC-conjugated anti-mouse IgG. The binding of supernatant antibody to ALDHhigh vs. ALDHlow D5 cells was assessed using flow cytometry (15). Antibody and complement-mediated cytotoxicity against CSCs was measured as previously described (15).
Statistics
Survival analysis was determined by the log-rank test. Analysis for the presence of lung metastasis was performed using the Fisher exact test. Other data were evaluated by unpaired Student’s t-test (2 cohorts) or one-way analysis of variance (ANOVA) (> 2 cohorts).
Results
1. An ALDHhigh CSC-DC vaccine significantly inhibited tumor recurrence and prolonged animal survival after surgical resection of head and neck SCC7 tumors
We previously demonstrated that administration of ALDHhigh SCC7 CSC-DC vaccines in immunocompetent mice induces protection against subsequent SCC7 challenge (15). In this study, we examined the therapeutic potential of CSC-DC vaccination to prevent local tumor recurrence, reduce metastasis, and prolong survival when deployed in the adjuvant /early disease settings. The first model employed surgical excision of SCC7 s.c head and neck squamous carcinomas, a tumor in which local recurrence contributes to patient mortality and morbidity (20, 21). C3H mice were inoculated s.c. with 0.5 × 106 SCC7 tumor cells. Resulting tumors were surgically excised 21 days after inoculation, followed by vaccination with DCs pulsed with lysates of heterogeneous unsorted SCC7 cells (H-DC), ALDHlow SCC7 cells (ALDHlow-DC) or ALDHhigh SCC7 cells (ALDHhigh –DC). Vaccines were administrated once per week for 3 weeks starting on the second day post-surgery. Mice were subsequently monitored for local tumor recurrence and survival.
As shown in Figure 1, there was 100% mortality in tumor bearing mice without tumor resection by day 40 due to progressive tumor growth. In PBS control mice, tumor recurrence was noted beginning on day 30 and all mice ultimately died by day 55 due to tumor growth. The H-DC and ALDHlow-DC vaccination delayed tumor recurrence, resulting in prolonged animal survival compared with control mice. More importantly, the ALDHhigh-DC (CSC-DC) vaccine significantly reduced tumor recurrence compared with the PBS control (p<0.0001), H-DC (p=0.0221) and ALDHlow-DC (p=0.0495) vaccination, respectively (Figure 1A). As a result, the ALDHhigh-DC treatment significantly increased animal survival compared to the other treatments or control mice (Figure 1B). While only a 50% of the mice in H-DC and ALDHlow-DC treated groups survived to day 65 all of the mice treated with the ALDHhigh-DC vaccine survived to that time point. These results demonstrate the ability of the ALDHhigh-DC vaccine to reduce local recurrence and prolong survival in this model of SCC.
Fig. 1.
(A) DCs pulsed with ALDHhigh SCC7 CSCs significantly inhibited tumor recurrence. Twenty-one days after inoculation of SCC7 cells, s.c, tumors were surgically removed and animals were treated with different vaccines as indicated on day 22, day 29 and day 36 except for the “no tumor resection” group as a control. Tumor volume (mean±SEM) is shown. (B) DCs pulsed with ALDHhigh SCC7 CSCs significantly prolonged the animal survival after surgical resection of the s.c. SCC7 tumors. Data are representative of three experiments independently performed. (C) Administration of anti-PD-L1 significantly inhibited tumor recurrence in animals treated with suboptima1 doses (2 vs. 3 in A) ALDHhigh SCC7 CSC-DC vaccinations after surgical resection of the s.c. SCC7 tumors. SCC7 s.c tumors were surgically excised 21 days after inoculation as in (A). Animals were then treated with different vaccines as indicated on day 22 and day 29 except for the “no tumor resection” group as a control. In addition, anti-PD-L1 (0.05mg/mouse) was intraperitoneally injected on days 22 and 25, days 29 and 32, either alone or with the ALDHhigh-DC vaccine. (D) Administration of anti-PD-L1 significantly prolonged the survival of animals treated in (C). Two experiments were independently performed.
One of the major recent advances in tumor immunotherapy has been the development of strategies to block the immunosuppressive components of the tumor microenvironment (22, 23). We next performed experiments where SCC7 s.c tumors were surgically excised as in Figure 1A, and animals were treated as indicated in Figure 1C with or without anti-PD-L1 administration. SCC7 ALDHhigh-DC (CSC-DC) vaccination plus anti-PD-L1 administration significantly inhibited tumor relapse (Figure 1C) and prolonged animal survival (Figure 1D) compared to either treatment alone. These experiments clearly demonstrate that immunologically targeting CSCs, while simultaneously blocking PD-1/PD-L1-mediated immune suppression, has the potential to significantly enhance the efficacy of cancer immunotherapies.
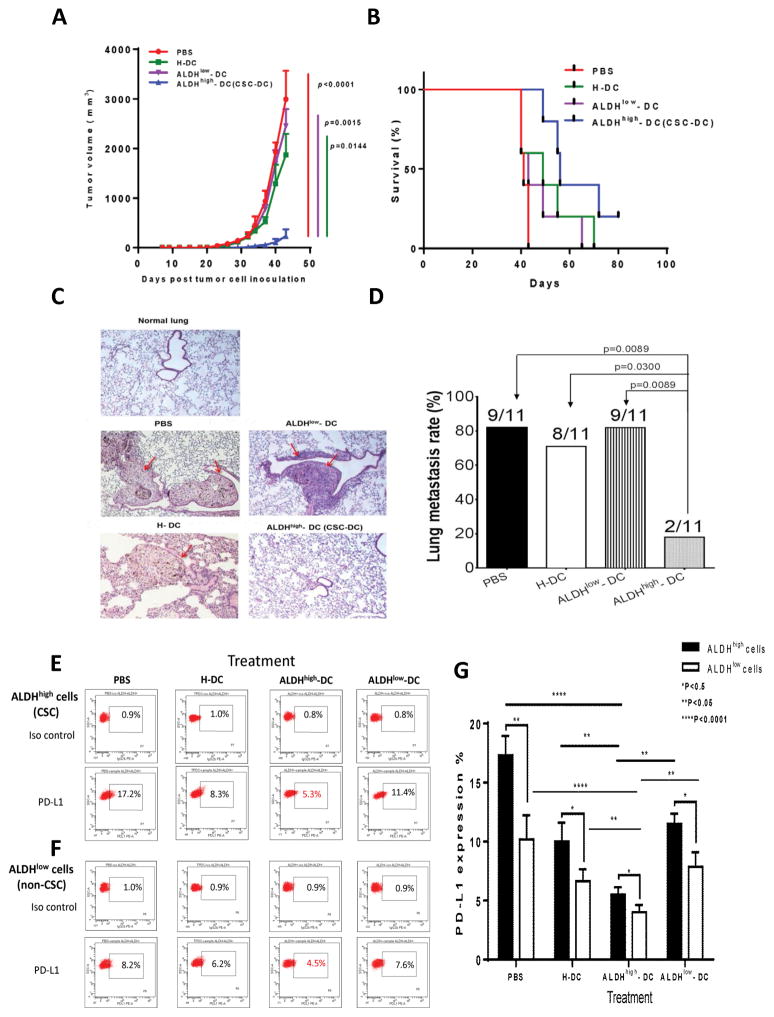
2. CSC-DC vaccination inhibited tumor growth and prevented spontaneous lung metastasis in D5 melanoma
To evaluate the therapeutic efficacy of the CSC-DC vaccine in the setting of micrometastatic disease, we utilized the highly metastatic D5 mouse melanoma model. In order to test the efficacy of the CSC-DC vaccine in treating micro-metastatic disease, it was administered 24 hours following inoculation of tumor cells. Syngeneic B6 mice were inoculated with 5,000 D5 melanoma cells s.c. followed by vaccination 24 hours later (day 1) with DCs pulsed with the lysate of ALDHhigh D5 CSCs (CSC-DC), ALDHlow D5 cell lysate (ALDHlow-DC), heterogeneous unsorted D5 cell lysate (H-DC), or with PBS, respectively. The treatment was repeated on day 8. As shown in Figure 2A, no significant difference in primary tumor growth was observed among PBS, H-DC or ALDHlow-DC treated mice. However, administration of the CSC-DC vaccine treatment resulted in significant inhibition of tumor growth compared to controls (p<0.02 vs. all other groups). The CSC-DC treated mice also survived longer than controls (Figure 2B). These data indicate that treatment of s.c tumor-bearing mice in the setting of minimal tumor with CSC-DC vaccination generated significant antitumor immunity, resulting in inhibited s.c. tumor growth and prolonged survival of the tumor-bearing hosts.
Fig. 2.
In the minimal D5 tumor model, the ALDHhigh CSC–DC vaccine significantly inhibited tumor growth and prolonged the survival which was associated with prevented lung metastasis. (A) The CSC-DC vaccination significantly inhibited subcutaneous tumor growth. 24 hours after s.c inoculation of D5 cells, animals were treated with different vaccines as indicated, and the treatment was repeated one week later. Tumor volumes (mean ± SEM) are shown. (B) CSC-DC vaccine significantly prolonged the survival of s.c D5-bearing mice. (C, D) CSC-DC vaccination significantly prevented the lung metastasis of the s.c injected tumor. (C) Lung metastasis was verified by hematoxylin and eosin (H&E) staining. Representative graphs show the histologic alternation of the lung tissues. Lung tissue harvested from a normal B6 mouse served as control. The red arrows point to the tumor lesions in the lung tissues. (D) p values comparing lung metastasis (n=11) among groups treated as indicated. Data are representative of three independent experiments performed. (E, F) Representative flow cytometry data of one of the three experiments performed to show decreased PD-L1 expression on ALDHhigh cells (CSC, E) and ALDHlow cells (non-CSC, F) after ALDHhigh-DC vaccination. (G) Statistically, ALDHhigh-DC vaccination significantly (p<0.05) reduced the PD-L1 expression on both ALDHhigh cells (CSC) and ALDHlow (non-CSC) cells.
To investigate the effect of these treatments on the development of lung metastases, we harvested the lungs at the end of the experiments and accessed lung metastases. Representative histology of the lungs is shown in Figure 2C. Mice subjected to PBS treatment, H-DC or ALDHlow-DC vaccine all displayed numerous large lung metastases. In contrast, there were significantly reduced lung metastases detected in the lungs harvested from ALDHhigh CSC-DC vaccinated hosts (Figure 2C). The ALDHhigh-DC vaccine significantly inhibited tumor metastasis to the lung compared with PBS, H-DC and ALDHlow-DC vaccine treatments (p<0.05, Figure 2D). Only 2 of 11 total mice developed lung metastasis after ALDHhigh-DC vaccination, while 9 of 11 mice treated with PBS or ALDHlow-DC; and 8 of 11 mice treated with H-DC developed lung metastases (Figure 2D). Together, these results indicated that CSC-DC vaccine significantly inhibited tumor growth and lung metastases resulting in increased animal survival. In addition, as shown in Figure 2E, after ALDHhigh-DC vaccination, PD-L1 expression on ALDHhigh cells (CSC) was decreased to 5.3% compared to PBS (17.2%), H-DC (8.3%) or ALDHlow-DC (11.4%) treatment. Similarly, PD-L1 expression on ALDHlow cells (non-CSC) was decreased after ALDHhigh-DC vaccination to 4.5% (Figure 2F) compared to PBS (8.2%), H-DC (6.2%) or ALDHlow-DC (7.6%) treatment. Statistically, when the mean+/−SE of the three experiments’ data were compared (Figure 2G), ALDHhigh-DC vaccination significantly (p<0.05) reduced the PD-L1 expression on both ALDHhigh cells (CSC) and ALDHlow (non-CSC) cells.
3. CSC-DC vaccination significantly down-regulated CCR10 expression on ALDHhigh CSCs
Chemokines play a significant role in tumor metastasis (24–26). We examined the expression of CCR10 on tumor cells from mice treated in the minimal disease setting, and compared its expression in ALDHhigh CSCs vs. ALDHlow non-CSCs. CCR10 expression was accessed by flow cytometry in D5 tumors harvested from animals subjected to various vaccines (Figure 3A and 3B). CSC-DC vaccination significantly decreased expression of CCR10 in unsorted bulk tumor cells (p< 0.01 vs. all other groups). With CSC-DC vaccination, the expression of CCR10 on D5 tumor cells was significantly decreased to approx. 3% compared with PBS treatment (>20%), or with H-DC and ALDHlow-DC vaccination (both around 15%) (Figure3A, 3B). We then sorted ALDHhigh and ALDHlow cells from freshly harvested D5 tumors subjected to vaccine therapy, and assessed their CCR10 expression. We found that the expression of CCR10 was significantly (p<0.0001) higher on D5 ALDHhigh CSCs (>60%) than on ALDHlow non-CSCs (<20%) (Figure 3C, 3D, PBS groups). ALDHhigh CSC-DC vaccination significantly decreased the expression of CCR10 on D5 ALDHhigh as well as on ALDHlow cells (Figure 3C, 3D). Finally, using qRT-PCR, we found that mRNA for the corresponding chemokine ligands for CCR10 in the lung tissues, CCL27 and CCL28, were both significantly decreased after ALDHhigh CSC-DC vaccine treatment (p<0.01 vs. all other groups) (Figure 3E). Collectively, these data suggest that CSC-DC vaccination may inhibit pulmonary metastasis of the local tumor by significantly down-regulating the expression of CCR10 on primary tumor cells, particularly on the ALDHhigh CSCs in the primary tumor, as well as reducing the production of CCR10 ligands, CCL27 and CCL28 in the lung tissues.
Fig. 3.
The expression of CCR10 was significantly down-regulated on ALDHhigh D5 cancer stem cells in animals subjected to ALDHhigh D5 CSC-DC vaccine treatment. (A) Flow cytometry graphs of CCR10, which were generated using mixed D5 cells harvested from multiple animals in each treatment group as indicated. (B) Bar graph shows the p value with SE using the D5 cells harvested from each experiment group. Data are representative of two independently performed experiments. (C) Flow cytometry graphs of CCR10 expression on D5 ALDHhigh-CSCs vs. ALDHlow-non CSCs post treatment of the minimal tumor with PBS, ALDHlow-DC, H-DC, and ALDHhigh-DC respectively. (D) p values comparing CCR10 expression on D5 ALDHhigh-CSCs vs. D5 ALDHlow-non CSCs from animals treated as indicated. (E) PCR analyses showed that the ALDHhigh-DC (CSC-DC) vaccine significantly reduced the mRNA levels of CCR10 ligands, e.g. CCL27 and CCL28, in lung tissues harvested from D5-bearing host subjected to treatments in minimal disease.
4. The role played by CCR10 in the metastasis of tumor cells was significantly blocked by CCR10 siRNA inhibition
To substantiate the role for CCR10 and its ligands in tumor metastasis, we used CCR10 siRNA to inhibit CCR10 gene expression as described in Materials and Methods. To test the effect of CCR10 siRNA on the inhibition of D5 cell migration in vitro, we carried out a chemotaxis assay. As shown in Figure 4A, CCR10 siRNA treated D5 cells demonstrated significantly (p<0.001) less migration ability than non-treated D5 cells towards the CCL27 and CCL28 added to the bottom of the transwell at the concentrations as indicated. To test the effect of CCR10 siRNA on the inhibition of ALDHlow and ALDHhigh D5 cell migration in vivo, we compared the metastasis of non-treated ALDHlow and ALDHhigh D5 cells with that of CCR10 siRNA transferred ALDHlow and ALDHhigh D5 cells when 1×106 cells of each group were i.v. injected into the normal B6 mice. As expected, ALDHhigh D5 cells generated significantly (p=0.03) more metastasis than ALDHlow D5 cells (Figure 4B). Importantly, CCR10 siRNA-treated ALDHlow and ALDHhigh D5 cells generated significantly less metastasis than non-treated ALDHlow (p=0.0002) or ALDHhigh (p=0.0003) D5 cells respectively. These experiments strongly suggest that CCR10 plays an important role in the migration and therefore the metastasis of D5 tumor cells.
Fig. 4.
CCR10 siRNA significantly blocked the role played by CCR10 in tumor metastasis. (A) The effect of CCR10 siRNA on the inhibition of D5 cell migration in vitro in a Chemotaxis assay. Data are representative of three chemotaxis assays independently performed. (B) The effect of CCR10 siRNA on the inhibition of metastasis of ALDHlow and ALDHhigh D5 cells. (C) Equal doses of CCR10 siRNA and control siRNA were used to transfer unsorted D5 cells for various periods of time as indicated to inhibit CCR10 expression. The data was expressed as the relative fold changed. Relative fold changes of CCR10 gene expression as shown in C represent the averages of 3 replicates for each group analyzed via the 2-ΔΔCT method. (D). ALDHlow and ALDHhigh D5 cells showed significantly down-regulated CCR10 gene expression by CCR10 siRNA treatment. Data as shown represent the averages of 3 replicates for each group analyzed via the 2-ΔΔCT method.
To confirm the efficacy of CCR10 siRNA in CCR10 gene silencing, equal doses of CCR10 siRNA and control siRNA were used to transfer unsorted D5 cells for various time periods, e.g. 24, 48, and 72 hours. Figure 4C shows that transfer of unsorted D5 cells for 48 hours begin to demonstrate significantly (p=0.0005) silenced CCR10 gene. We therefore transferred unsorted D5 cells for 48 hours in Figure 4A as well as for ALDHhigh and ALDHlow D5 cells in Figure 4B. In addition, Figure 4D revealed that CCR10 gene expression in ALDHhigh D5 cells is higher (p<0.0001) than that in ALDHlow D5 cells. Importantly, CCR10 siRNA treated ALDHlow and ALDHhigh D5 cells showed significantly down-regulated CCR10 gene expression compared with non-treated ALDHlow (p<0.05) and ALDHhigh (p<0.05) D5 cells, respectively.
5. CSC-DC vaccination conferred host CSC-specific antibody responses
To provide experimental evidence that CSC-DC vaccination induces specific anti-CSC immunity, we collected the spleens following the full treatment course in the minimal D5 tumor model, purified splenic B cells, and activated them in vitro with LPS and anti-CD40. We then accessed the specificity of CSC-DC vaccine-primed antibody by binding assays of the B cell culture supernatants to ALDHhigh D5 CSCs vs. ALDHlow D5 non-CSCs respectively. Immune supernatants produced by B cells from mice that received ALDHhigh-DC treatment bound to ALDHhigh D5 CSCs (60.8%) (Figure 5A) much more effectively than the binding of immune supernatants collected from PBS-treated (12.3%), H-DC vaccinated (29.8%), or ALDHlow-DC treated (15.7%) mice. In contrast, the immune supernatants produced by B cells harvested from H-DC or ALDHlow-DC vaccinated mice bound to the ALDHlow non-CSCs (45.8% and 50.2% respectively) significantly more than the binding of immune supernatants produced by B cells harvested from the CSC-DC vaccinated mice (6.8%) or from PBS-treated controls (18.8%). Figure 5B shows the results of multiple binding assays, indicating that the immune supernatants produced by CSC-DC vaccine-primed B cells bound to the ALDHhigh D5 CSCs much more effectively (p<0.01 vs. all other groups). In contrast, the ALDHlow-DC vaccine-primed immune supernatants bound to the ALDHlow non-CSCs similar to the binding by H-DC vaccine-primed immune supernatants, but significantly more than the binding of PBS or CSC-DC vaccine-primed immune supernatants (Figure 5C); demonstrating CSC-DC vaccine induced CSC-specific humoral immunity.
Fig. 5.
Antibody produced by D5 CSC-DC vaccine-primed B cells bound and killed D5 CSCs specifically. (A) Flow cytometry histograms using culture supernatant of mixed B cells from each treatment group. (B) Statistic analysis of the binding to ALDHhigh CSCs by immune supernatants primed by PBS, H-DC, ALDHlow-DC or ALDHhigh-DC respectively. (C) Statistical analysis of the binding to ALDHlow D5 cells by immune supernatants primed as indicated. Binding experiments were repeated three times. (D) CSC-DC vaccine-primed antibody selectively targeted CSCs via CDC. 105 viable ALDHhigh or ALDHlow D5 cells were incubated with the culture immune supernatants of purified and activated spleen B cells collected from the animals subjected to treatments as indicated. The cells were then incubated with rabbit complement for 1 hour. The trypan blue staining was used to assess the cell lysis, which was expressed as: % viable cells = the number of viable cells after immune supernatant and complement incubation/105. Each experiment was repeated once.
To examine the functional consequence of CSC-specific antibody induced by CSC-DC vaccination, we performed antibody and complement-dependent cytotoxicity (CDC) assays (Figure 5D). ALDHhigh CSC-DC vaccine-primed immune supernatant killed ALDHhigh D5 CSCs significantly more than the immune supernatants collected from other groups (p<0.001 vs. all other groups). In contrast, the immune supernatant harvested from H-DC or ALDHlow non-CSC vaccinate-treated host resulted in significant ALDHlow D5 cell lysis, while the immune supernatant from the ALDHhigh CSC-DC vaccinated hosts produced minimal lysis of the ALDHlow targets. Together these data support the conclusion that ALDHhigh D5 CSC-DC vaccine confers significant host anti-CSC humoral immunity by producing D5 CSC-specific antibodies that specifically bind and kill D5 CSCs.
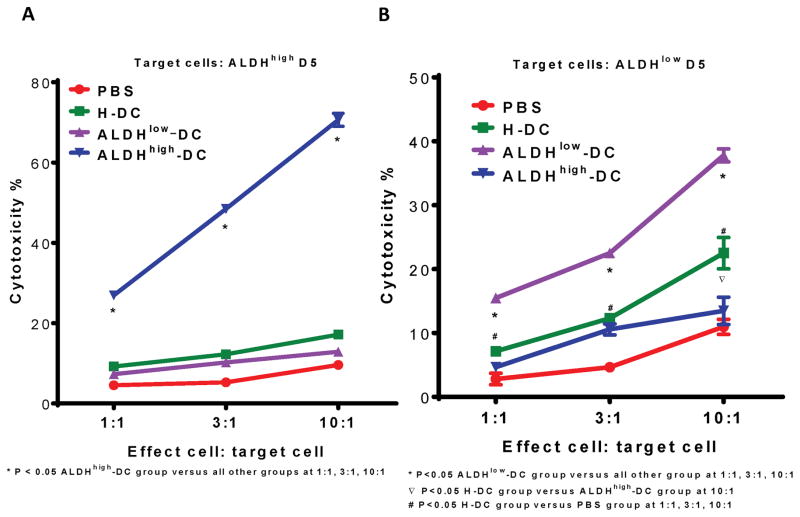
6. CSC-DC vaccination conferred host CSC-specific CTL function
We next examined the ability of CSC-DC vaccination to generate host CSC-specific CTL activity. As in Fig. 5, we collected the spleens at the end of the treatment in the minimal D5 tumor model; purified splenic T cells, and activated them in vitro with anti-CD3/anti-CD28 followed by expansion in IL-2. This activation procedure generates cytotoxic T cells (>95 CD3 cells) (15). We then measured the CTL activity of these T cells on ALDHhigh D5 CSCs vs. ALDHlow D5 non-CSCs respectively. D5 ALDHhigh-DC-primed CTLs mediated significantly greater cytotoxicity in D5 ALDHhigh CSCs at all E:T ratios compared with the CTLs generated from PBS, H-DC or ALDHlow DC-primed CTLs (p<0.05 Figure 6A). In contrast, CTLs generated from splenocytes of mice subjected to ALDH low DC and H-DC vaccination selectively killed ALDHlow D5 cells (Figure 6B). These experiments indicate that CSC-DC vaccination conferred host CTL reactivity as well as humoral responses against CSCs in the treatment of minimal tumor disease.
Fig. 6.
T cells harvested from D5 ALDHhigh DC vaccinated animals selectively and significantly killed the ALDHhigh D5 cells. CTLs were generated as described in the Materials and Methods from the spleens harvested from the animals subjected to PBS, H-DC, ALDHlow–DC or ALDHhigh–DC vaccination respectively. Cytotoxicity mediated by CTLs was measured by LDH release assay. (A) ALDHhigh–DC (CSC-DC)-primed CTLs selectively and significantly killed the ALDHhigh D5 CSCs (p<0.05 compared with all other groups). (B) CTLs generated from the splenocytes of mice vaccinated with ALDHlow–DC or H-DC killed ALDHlow D5 cells specifically.
7. CSC-DC vaccination significantly reduced the population of ALDHhigh CSCs in vivo
As described above, ALDHhigh CSC-DC vaccination induced significant host cellular and humoral immune responses against CSCs. To confirm that CSCs are effectively targeted by CSC-induced immunity, we determined the effect of CSC-DC vaccination on the proportion of ALDHhigh CSC in vivo. Assessment of the ALDHhigh population was performed by flow cytometry using the Aldefluor assays as previously described (15). We mixed the tumor cells from mice of each experimental group, and generated representative flow cytometric graphs to demonstrate the ALDHhigh populations in each group (Figure 7A). Subcutaneous tumors harvested from CSC-DC treated mice contained only 1.7% ALDHhigh cells, which was significantly less than that present in the s.c. tumors subjected to PBS (13.4%), H-DC (7.5%) or ALDHlow-DC (8.3%) treatments. As shown in Figure 7B, CSC-DC vaccination significantly reduced the percentage of ALDHhigh populations compared with PBS, H-DC, or ALDHlow-DC treatments (p=0.0002, 0.0002 and 0.0029, respectively) in this tumor model. Together, these studies demonstrate that in the D5 minimal disease model, CSC-DC vaccine elicits both humoral and cellular immune responses reducing the proportion of CSC, resulting in decreased tumor growth, lung metastases and prolonged survival.
Fig. 7.
The ALDHhigh CSC-DC vaccine treatment significantly decreased the percentage of ALDHhigh cells in the s.c minimal residual tumors. (A) Representative flow cytometry showing the percentage of ALDHhigh cells in the residual tumor after different treatment as indicated. (B) The bar graph shows the mean+/−SE and p values using multiple animals from each group.
Discussion
Utilizing two tumor models, we demonstrate the efficacy of a CSC-DC vaccine when used to treat minimal disease in the adjuvant setting. Several reports have described the generation of CSC-specific CD8 T effector cells in vitro (27–30); the killing of CSCs via non-specific immune effector cells (31–34) as well as by oncolytic viruses (35) and antibodies (36). We previously reported therapeutic efficacy of CSC-DC vaccination in the treatment of established tumors following localized radiation therapy (RT) (17). However, since CSC may be responsible for local tumor recurrence following resection (37) as well as mediating tumor metastasis (38–41), CSC targeted therapeutics may have their greatest utility when they are utilized in the adjuvant-minimal disease setting. We examined this utilizing two different mouse tumor models.
The SCC7 squamous carcinoma model was designed to determine the efficacy of CSC-DC vaccination following surgical removal of the primary tumor. This model is clinically relevant since in squamous carcinoma of the head and neck, resection of bulky SCC primary tumors has been associated with a high rate of local tumor recurrence associated with significant morbidity and mortality (20, 21). Using the murine SCC7 tumor model, we found that the SCC7 ALDHhigh CSC-DC vaccine significantly inhibited tumor recurrence and prolonged animal survival following surgical resection compared with SCC7 H-DC or SCC7 ALDHlow-DC vaccinations. Simultaneous administration of an anti-PD-L1 mAb significantly enhanced the therapeutic efficacy of SCC7 CSC-DC vaccine in the adjuvant setting.
The second model involved treatment of D5 murine melanoma in an early disease setting 24 hours after tumor inoculation. While the H-DC vaccination and the ALDHlow-DC had minimal effects on the local tumor growth and only modestly prolonged survival, the ALDHhigh CSC-DC vaccine was significantly more effective in inhibiting tumor growth, resulting in prolonged survival.
To date, the mechanisms that are involved in CSC-DC vaccine-mediated therapeutic efficacy have not been fully defined, and limited experimental evidence was provided for direct targeting of CSCs by CSC-DC vaccine-induced anti-CSC immunity. CSCs are responsible for tumor metastasis and progression (38–41). In this study we found that the therapeutic efficacy of CSC-DC vaccine was associated with significantly inhibited metastasis of the s.c. tumor to the lung. A number of studies have suggested that tumor cell metastasis is determined by the expression level of chemokine receptors on the malignant tumor cells and the expression of corresponding chemokine ligands in the target organs (24–26, 42–46).
We demonstrated high levels of CCR10 (>20%) in tumor cells isolated from control mice. CSC-DC vaccination significantly reduced the expression of CCR10 to 3%. More importantly, we found that the expression of CCR10 was significantly higher on D5 ALDHhigh CSCs (>60%) than on ALDHlow non-CSCs (<20%), and ALDHhigh CSC-DC vaccine significantly decreased the expression of CCR10 on D5 ALDHhigh cells to <15%. In a group of experiments, we found that CCR10 siRNA treatment to inhibit CCR10 gene expression significantly blocked tumor cell migration in vitro and metastasis in vivo. In addition, ligands for CCR10, including CCL27 and CCL28 were significantly decreased in the lung tissues harvested from the animals treated with CSC-DC vaccination. These data suggest that decreased CCR10, CCL27 and CCL28 may play an important role in CSC-DC vaccination-induced inhibition of tumor metastasis. Chemokine receptors can activate downstream effectors, such as mitogen-activated protein kinases, by complex mechanisms (47). The molecular and biochemical signaling pathways by which CSC-DC vaccination induces down-regulation of CCR10, CCL27 and CCL28 remain to be identified.
We examined the ability of CSC-DC vaccines to elicit CSC specific humoral and cellular immune responses. Using splenocytes collected from the treated mice, we generated CTLs. D5 ALDHhigh-DC-primed CTLs significantly killed the D5 ALDHhigh CSCs compared with the CTLs generated from PBS, H-DC or ALDHlow DC-primed CTLs. In contrast, CTLs generated from splenocytes of mice subjected to ALDHlow DC and H-DC vaccination selectively killed ALDHlow D5 cells. These experiments indicate that CSC-DC vaccination confers host CTL activity that specifically target CSCs. In addition, the ALDHhigh CSC-DC vaccine-primed host B cells produced antibody specifically bound to ALDHhigh CSCs (>60%), which was significantly higher than the binding by antibodies produced PBS, H-DC or ALDHlow-DC-primed B cells (10–30%). In contrast, H-DC or ALDHlow-DC vaccine-primed B cells produced antibody preferentially bound to ALDHlow D5 cells (45–50%), which was significantly higher than the binding by antibodies produced by PBS or ALDHhigh CSC-DC vaccine-primed B cells (18% and 6% respectively). The immunological consequence of antibody binding of the CSCs was the lysis of the CSCs in the presence of complement, demonstrating that CSC-DC vaccines elicit significant humoral immune responses against CSCs as well as CSC specific cellular immune responses. While we demonstrated that antibodies and CTLs were induced against cancer stem cells, the identity of any recognized antigens has yet to be elucidated. Identification of CSC antigen(s) represents an active research focus in our lab and warrants further investigation.
The induction of cytotoxic T cells to CSCs has been observed in 2 different animal histologies using the CSC lysate vaccine both in our previous protection study (15) and in this therapeutic study. To date, we have not observed immune tolerance in our model system of CSC-DC vaccination. However, an immune adjuvant may enhance the induction of a tumor lysate-DC vaccine. We previously reported that the use of a second signal agent such as anti-4-1BB mAb augmented the anti-tumor efficacy of DC-based vaccines (48). We did not use this approach in this report in order to focus on the use of CSC-DC vaccines by themselves. Nevertheless, the use of adjuvant agents may enhance T, B cell activation as a method to improve CSC-DC vaccine-induced anti-CSC immunity.
Our experiments provide direct evidence that CSC-DC vaccine can induce anti-CSC immunity by targeting CSCs. As a result, ALDHhigh CSC populations in the residual tumor of the mice subjected to CSC-DC vaccine were significantly decreased to <2% compared with the PBS-treated control (~15%), and was significantly lower than those of the animals subjected to H-DC or ALDHlow-DC treatment (7–9%). In our previous publications we have demonstrated that a reduction in ALDH expression is strongly associated with reduction in tumor initiating capacity as accessed by extreme limiting dilution analysis (49, 50). The values of reduction of ALDH associated with treatments shown in this study are highly statistically significant. Future studies accessing the ability of CSC-DC vaccines to reduce tumor initiating capacity of treated tumor cells transplanted into secondary animals are warranted. We propose that the significant reduction of the residual CSCs after CSC-DC immunotherapy is due to CSC-DC vaccine-induced cellular and humoral targeting of CSCs. Together these studies suggest the potential clinical efficacy of utilizing CSC-DC vaccines in the adjuvant/early tumor setting, a strategy that may be augmented by PD-l/PD-L1 immune checkpoint blockade.
Acknowledgments
Financial support: This work was supported by the Elsa U. Pardee Foundation, partially supported by the Gillson Longenbaugh Foundation and the University of Michigan MICHR Grant UL1TR000433, as well as the National Science Fund of China (81072170 and 81202093) and NCI research grant 1R-35CA 197585 (MW).
We thank Jill Granger for valuable assistance in editing the manuscript.
Footnotes
Conflicts of interest: All authors have declared there are no conflicts of interest in regards to this work.
References
- 1.Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19(11):1821–8. doi: 10.1093/annonc/mdn386. [DOI] [PubMed] [Google Scholar]
- 2.Mueller JL, Fu HL, Mito JK, Whitley MJ, Chitalia R, Erkanli A, et al. A quantitative microscopic approach to predict local recurrence based on in vivo intraoperative imaging of sarcoma tumor margins. Int J Cancer. 2015;137(10):2403–12. doi: 10.1002/ijc.29611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acasigua GA, Warner KA, Nor F, Helman J, Pearson AT, Fossati AC, et al. BH3-mimetic small molecule inhibits the growth and recurrence of adenoid cystic carcinoma. Oral Oncol. 2015;51(9):839–47. doi: 10.1016/j.oraloncology.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tham M, Khoo K, Yeo KP, Kato M, Prevost-Blondel A, Angeli V, et al. Macrophage depletion reduces postsurgical tumor recurrence and metastatic growth in a spontaneous murine model of melanoma. Oncotarget. 2015;6(26):22857–68. doi: 10.18632/oncotarget.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofer SO, Molema G, Hermens RA, Wanebo HJ, Reichner JS, Hoekstra HJ. The effect of surgical wounding on tumour development. Eur J Surg Oncol. 1999;25(3):231–43. doi: 10.1053/ejso.1998.0634. [DOI] [PubMed] [Google Scholar]
- 6.Miwa S, De Magalhaes N, Toneri M, Zhang Y, Cao W, Bouvet M, et al. Fluorescence-guided surgery of human prostate cancer experimental bone metastasis in nude mice using anti-CEA DyLight 650 for tumor illumination. J Orthop Res. 2016;34(4):559–65. doi: 10.1002/jor.22973. [DOI] [PubMed] [Google Scholar]
- 7.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–61. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien CA, Kreso A, Jamieson CH. Cancer stem cells and self-renewal. Clin Cancer Res. 2010;16(12):3113–20. doi: 10.1158/1078-0432.CCR-09-2824. [DOI] [PubMed] [Google Scholar]
- 9.Yue L, Huang ZM, Fong S, Leong S, Jakowatz JG, Charruyer-Reinwald A, et al. Targeting ALDH1 to decrease tumorigenicity, growth and metastasis of human melanoma. Melanoma Res. 2015;25(2):138–48. doi: 10.1097/CMR.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 10.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, et al. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32(9):1195–201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y, Dallaglio K, Chen Y, Robinson WA, Robinson SE, McCarter MD, et al. ALDH1A isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells. 2012;30(10):2100–13. doi: 10.1002/stem.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boonyaratanakornkit JB, Yue L, Strachan LR, Scalapino KJ, LeBoit PE, Lu Y, et al. Selection of tumorigenic melanoma cells using ALDH. J Invest Dermatol. 2010;130(12):2799–808. doi: 10.1038/jid.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Magnen C, Bubendorf L, Rentsch CA, Mengus C, Gsponer J, Zellweger T, et al. Characterization and clinical relevance of ALDHbright populations in prostate cancer. Clin Cancer Res. 2013;19(19):5361–71. doi: 10.1158/1078-0432.CCR-12-2857. [DOI] [PubMed] [Google Scholar]
- 15.Ning N, Pan Q, Zheng F, Teitz-Tennenbaum S, Egenti M, Yet J, et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 2012;72(7):1853–64. doi: 10.1158/0008-5472.CAN-11-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuda K, Torigoe T, Morita R, Kuroda T, Takahashi A, Matsuzaki J, et al. Ovarian cancer stem cells are enriched in side population and aldehyde dehydrogenase bright overlapping population. PLoS One. 2013;8(8):e68187. doi: 10.1371/journal.pone.0068187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu L, Tao H, Chang AE, Hu Y, Shu G, Chen Q, et al. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. Oncoimmunology. 2015;4(3):e990767. doi: 10.4161/2162402X.2014.990767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, Ito F, Davis MA, McGinn CJ, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63(23):8466–75. [PubMed] [Google Scholar]
- 19.Wang W, Lv L, Pan K, Zhang Y, Zhao JJ, Chen JG, et al. Reduced expression of transcription factor AP-2alpha is associated with gastric adenocarcinoma prognosis. PLoS One. 2011;6(9):e24897. doi: 10.1371/journal.pone.0024897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tupchong L, Scott CB, Blitzer PH, Marcial VA, Lowry LD, Jacobs JR, et al. Randomized study of preoperative versus postoperative radiation therapy in advanced head and neck carcinoma: long-term follow-up of RTOG study 73-03. Int J Radiat Oncol Biol Phys. 1991;20(1):21–8. doi: 10.1016/0360-3016(91)90133-o. [DOI] [PubMed] [Google Scholar]
- 21.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328(3):184–94. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 22.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73(6):1733–41. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25(4):345–56. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 25.Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118(6):915–22. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- 26.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 27.Brown CE, Starr R, Martinez C, Aguilar B, D'Apuzzo M, Todorov I, et al. Recognition and killing of brain tumor stem-like initiating cells by CD8+ cytolytic T cells. Cancer Res. 2009;69(23):8886–93. doi: 10.1158/0008-5472.CAN-09-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jachetti E, Mazzoleni S, Grioni M, Ricupito A, Brambillasca C, Generoso L, et al. Prostate cancer stem cells are targets of both innate and adaptive immunity and elicit tumor-specific immune responses. Oncoimmunology. 2013;2(5):e24520. doi: 10.4161/onci.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao T, Kaufmann AM, Qian X, Sangvatanakul V, Chen C, Kube T, et al. Susceptibility to cytotoxic T cell lysis of cancer stem cells derived from cervical and head and neck tumor cell lines. J Cancer Res Clin Oncol. 2013;139(1):159–70. doi: 10.1007/s00432-012-1311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visus C, Wang Y, Lozano-Leon A, Ferris RL, Silver S, Szczepanski MJ, et al. Targeting ALDH(bright) human carcinoma-initiating cells with ALDH1A1-specific CD8(+) T cells. Clin Cancer Res. 2011;17(19):6174–84. doi: 10.1158/1078-0432.CCR-11-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castriconi R, Daga A, Dondero A, Zona G, Poliani PL, Melotti A, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182(6):3530–9. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 32.Contag CH, Sikorski R, Negrin RS, Schmidt T, Fan AC, Bachireddy P, et al. Definition of an enhanced immune cell therapy in mice that can target stem-like lymphoma cells. Cancer Res. 2010;70(23):9837–45. doi: 10.1158/0008-5472.CAN-10-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R, et al. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190(5):2381–90. doi: 10.4049/jimmunol.1201542. [DOI] [PubMed] [Google Scholar]
- 34.Todaro M, D'Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182(11):7287–96. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 35.Bach P, Abel T, Hoffmann C, Gal Z, Braun G, Voelker I, et al. Specific elimination of CD133+ tumor cells with targeted oncolytic measles virus. Cancer Res. 2013;73(2):865–74. doi: 10.1158/0008-5472.CAN-12-2221. [DOI] [PubMed] [Google Scholar]
- 36.Skubitz AP, Taras EP, Boylan KL, Waldron NN, Oh S, Panoskaltsis-Mortari A, et al. Targeting CD133 in an in vivo ovarian cancer model reduces ovarian cancer progression. Gynecol Oncol. 2013;130(3):579–87. doi: 10.1016/j.ygyno.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi GH, Kim GI, Yoo JE, Na DC, Han DH, Roh YH, et al. Increased Expression of Circulating Cancer Stem Cell Markers During the Perioperative Period Predicts Early Recurrence After Curative Resection of Hepatocellular Carcinoma. Ann Surg Oncol. 2015;22(Suppl 3):1444–52. doi: 10.1245/s10434-015-4480-9. [DOI] [PubMed] [Google Scholar]
- 38.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796(2):293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin. 2013;34(6):732–40. doi: 10.1038/aps.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enderling H, Hlatky L, Hahnfeldt P. Cancer Stem Cells: A Minor Cancer Subpopulation that Redefines Global Cancer Features. Front Oncol. 2013;3:76. doi: 10.3389/fonc.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schatton T, Frank MH. Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res. 2008;21(1):39–55. doi: 10.1111/j.1755-148X.2007.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben-Baruch A. Site-specific metastasis formation: chemokines as regulators of tumor cell adhesion, motility and invasion. Cell Adh Migr. 2009;3(4):328–33. doi: 10.4161/cam.3.4.9211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dobner BC, Riechardt AI, Joussen AM, Englert S, Bechrakis NE. Expression of haematogenous and lymphogenous chemokine receptors and their ligands on uveal melanoma in association with liver metastasis. Acta Ophthalmol. 2012;90(8):e638–44. doi: 10.1111/j.1755-3768.2012.02515.x. [DOI] [PubMed] [Google Scholar]
- 44.Murakami T, Cardones AR, Hwang ST. Chemokine receptors and melanoma metastasis. J Dermatol Sci. 2004;36(2):71–8. doi: 10.1016/j.jdermsci.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Kai H, Kadono T, Kakinuma T, Tomita M, Ohmatsu H, Asano Y, et al. CCR10 and CCL27 are overexpressed in cutaneous squamous cell carcinoma. Pathol Res Pract. 2011;207(1):43–8. doi: 10.1016/j.prp.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Simonetti O, Goteri G, Lucarini G, Filosa A, Pieramici T, Rubini C, et al. Potential role of CCL27 and CCR10 expression in melanoma progression and immune escape. Eur J Cancer. 2006;42(8):1181–7. doi: 10.1016/j.ejca.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 47.Salazar N, Castellan M, Shirodkar SS, Lokeshwar BL. Chemokines and chemokine receptors as promoters of prostate cancer growth and progression. Crit Rev Eukaryot Gene Expr. 2013;23(1):77–91. doi: 10.1615/critreveukaryotgeneexpr.2013006905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ito F, Li Q, Shreiner AB, Okuyama R, Jure-Kunkel MN, Teitz-Tennenbaum S, et al. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004;64(22):8411–9. doi: 10.1158/0008-5472.CAN-04-0590. [DOI] [PubMed] [Google Scholar]
- 49.Ke J, Zhao Z, Hong SH, Bai S, He Z, Malik F, et al. Role of microRNA221 in regulating normal mammary epithelial hierarchy and breast cancer stem-like cells. Oncotarget. 2015;6(6):3709–21. doi: 10.18632/oncotarget.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'Angelo RC, Ouzounova M, Davis A, Choi D, Tchuenkam SM, Kim G, et al. Notch reporter activity in breast cancer cell lines identifies a subset of cells with stem cell activity. Mol Cancer Ther. 2015;14(3):779–87. doi: 10.1158/1535-7163.MCT-14-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]