Abstract
Flavonoids are a large class of polyphenolic compounds ubiquitously distributed in dietary plants with an array of biological activities. Flavonols are a major sub-class of flavonoids featuring a hydroxyl group at C-3. Certain natural flavonols, such as quercetin and fisetin, have been shown by in vitro cell-based and in vivo animal experiments to be potential anti-prostate cancer agents. However, the Achilles' heel of flavonols as drug candidates is their moderate potency and poor pharmacokinetic profiles. This study aims to explore the substitution effect of 3-OH in flavonols on the in vitro anti-proliferative potency against both androgen-sensitive and androgen-insensitive human prostate cancer cell lines. Our first lead flavonol (3′,4′-dimethoxyflavonol), eight 3-O-alkyl-3′,4′-dimethoxyflavonols, and six 3-O-aminoalkyl-3′,4′-dimethoxyflavonols have been synthesized through aldol condensation and the Algar-Flynn-Oyamada (AFO) reaction. The WST-1 cell proliferation assay indicates i) that all synthesized 3-O-alkyl-3′,4′-dimethoxyflavonols and 3-O-aminoalkyl-3′,4′-dimethoxyflavonols are more potent than the parent 3′,4′-dimethoxyflavonol and the natural flavonol quercetin in suppressing prostate cancer cell proliferation; and ii) that incorporation of a dibutylamino group to the 3-OH group through a three- to five-carbon linker leads to the optimal derivatives with up to 292-fold enhanced potency as compared with the parent flavonol. Flow cytometry analysis showed that the most potent derivative 22 can activate PC-3 cell cycle arrest at the G2/M phase and induce PC-3 cell apoptosis. No inhibitory ability of 22 up to 50 μM concentration was observed against PWR-1E normal human epithelial prostate cells, suggesting its in vitro safety profile. The results indicate that chemical modulation at 3-OH is a vital strategy to optimize flavonols as anti-prostate cancer agents.
Keywords: Flavonol; Prostate cancer; Cell proliferation; 3′,4′-Dimethoxyflavonol; Quercetin
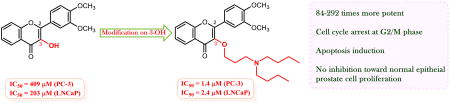
Graphical abstract
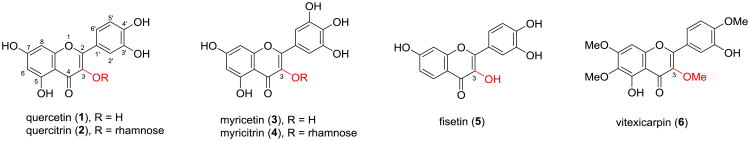
Flavonoids, with two aromatic rings and a central heterocyclic ring, belong to a large class of naturally occurring polyphenolic compounds. Recently, the potential of flavonoids in preventing and treating prostate cancer has received considerable attention due to an inverse correlation between the intake of flavonoid-enriched diet and the incidence of prostate cancer.1-8 Flavonols, featuring a hydroxyl group at C-3 in ring C of flavone skeleton, are one of the major subclasses of flavonoids ubiquitously distributed in dietary foods.9,10 The moderate in vitro antiproliferative and cytotoxic potency of certain flavonols, such as quercetin (1), myricetin (3), fisetin (5), vitexicarpin (6), etc. (Figure 1), against various prostate cancer cell lines has been identified.1,11,12 Among them, the anti-prostate tumor efficacy of quercetin and fisetin has been confirmed by in vivo studies on animal-based models.13-15 However, the moderate potency and poor pharmacokinetic profiles of these flavonols have hindered their further advancement as chemotherapeutic agents.
Figure 1. Structures of some naturally occurring flavonols.
Flavonols have thus been regarded as promising lead structures for the development of anti-prostate cancer agents. It is well-known that poor bioavailability of naturally occurring flavonols is largely attributed to their polyphenolic structures that are highly susceptible to first-pass metabolism of glucuronidation and sulfation.16 One strategy to simultaneously improve activity and bioavailability of flavonols is to modify the phenolic groups. We envision that modification on the 3-OH, the unique group in the flavonol core structure, is thus a starting point for the development of flavonol-based anti-prostate cancer agents. Incorporation of a sugar unit (e.g. rhamnoside in quercitrin (2) and myricitrin (4)) on the 3-OH was found to be unfavorable to the in vitro cytotoxic and antiproliferative potency on prostate cancer cell models.17,18 However, vitexicarpin (6) that possesses a methoxyl group instead of a hydroxyl group at C-3 showed promising capability in suppressing prostate cancer cell proliferation with IC50 values ranging from 1.3-29 μM.19-21 These data suggest that 3-OH substitution on a lead flavonol could significantly alter its anticancer activities.
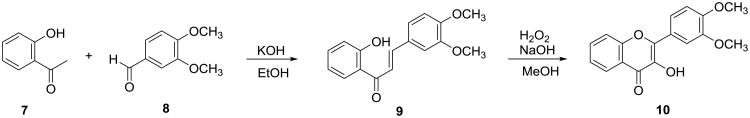
As part of our ongoing project to develop anti-prostate cancer agents through optimization of dietary natural products, this study aims to explore the substitution effect of 3-OH in flavonols on the in vitro anti-proliferative activity against both androgen-sensitive and androgen-insensitive human prostate cancer cell lines. Given that substituents (either OH or OMe) on ring B have proven beneficial to the in vitro antiproliferative activity toward prostate cancer cells,1 3′,4′-dimethoxyflavonol (10) was chosen as our first lead flavonol because it contains two substituents on ring B but lacks a catechol moiety. This lead flavonol was expected to overcome, to some degree, pharmacokinetic limitations caused by the catechol moiety and to provide ample flexibility to incorporate a basic nitrogen-containing group to 3-OH without the possible complications due to the acidity of the catechol unit. No in vitro or in vivo studies have so far been reported on the anti-prostate cancer potential of 3′,4′-dimethoxyflavonol and its 3-O-substituted derivatives.
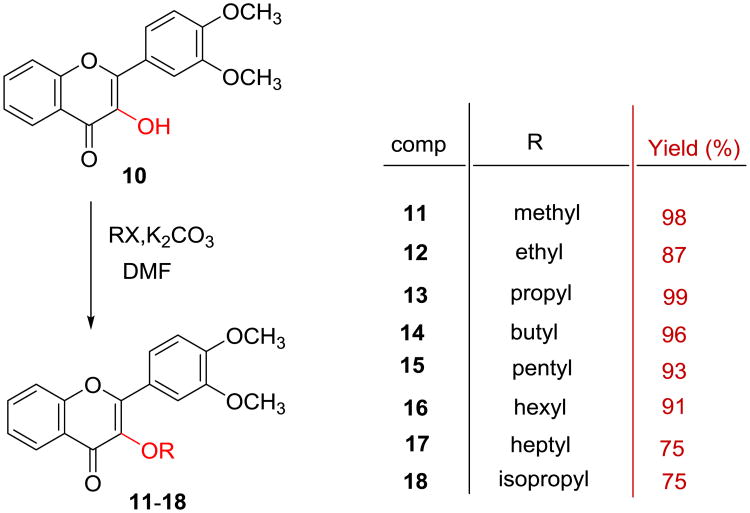
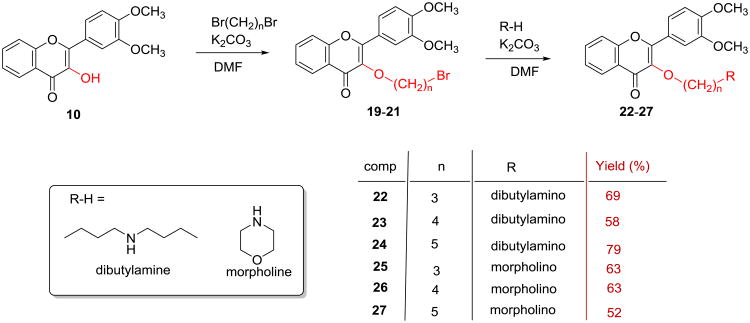
3′,4′-Dimethoxyflavonol (10) was synthesized by a two-step transformation as illustrated in Scheme 1 according to the reported procedure.22-24 Specifically, the intermediate 3′,4′-dimethoxychalcone (9) was prepared by aldol condensation of 2′-hydroxyacetophenone (7) with 3,4-dimethoxybenzaldehyde (8), which was directly subjected to the Algar-Flynne-Oyamada (AFO) reaction without further purification to furnish 3′,4′-dimethoxyflavonol (10) in 34% overall yield for two steps. Eight 3-O-alkyl-3′,4′-dimethoxyflavonols (11-18) were prepared in 75-99% yields via O-alkylating 3′,4′-dimethoxyflavonol (10) with an appropriate alkyl iodide or alkyl bromide mediated by potassium carbonate in DMF (Scheme 2). Six 3-O-aminoalkyl-3′,4′-dimethoxyflavonols (19-24) were prepared in 52-79% overall yields from 3′,4′-dimethoxyflavonol via two sequential alkylation reactions as shown in Scheme 3. Both O-alkylation and N-alkylation reactions used potassium carbonate as base and DMF as a polar aprotic solvent. The crude product obtained from the first step O-alkylation reaction was directly used for the second step N-alkylation reaction without further purification.
Scheme 1. Synthesis of 3′,4′-dimethoxyflavonol.
Scheme 2. Synthesis of 3-O-alkyl-3′,4′-dimethoxyflavonols.
Scheme 3. Synthesis of 3-O-aminoalkyl-3′,4′-O-dimethoxyflavonols.
To determine the in vitro antiproliferative activity of 3′,4′-O-dimethoxyflavonol (10) and its 3-O-substituted derivatives (11-18, 22-27) toward both androgen-insensitive PC-3 and androgen-sensitive LNCaP human prostate cancer cell lines, we used the WST-1 cell proliferation assay to monitor the viability of PC-3 and LNCaP cells. The prostate cancer cells were treated with 3′,4′-dimethoxyflavonol (10), eight 3-O-alkyl-3′,4′-dimethoxyflavonols (11-18), or six 3-O-aminoalkyl-3′,4′-dimethoxyflavonols (22-27) separately at five different doses for three days to determine the IC50 values. Equal volumes of DMSO were used as vehicle control and quercetin was used as a positive control. The IC50 values were calculated from the dose-response curves and summarized in Table 1, from which we can conclude that 3′,4′-dimethoxyflavonol as the parent flavonol is only weakly active, with IC50 values of 409 μM and 203 μM against PC-3 and LNCaP cells, respectively. All synthetic 3-O-substituted-3′,4′-dimethoxyflavonols (11-18, 22-27) are more potent than the parent flavonol 10 and the natural flavonol quercetin (1) in inhibiting prostate cancer cell proliferation as measured by the IC50 values. Alkylation of 3-OH in the parent flavonol 10 results in a 5-fold to 41-fold increase in the inhibitory activity against prostate cancer cell proliferation. Introduction of a basic dibutylamino moiety to 3-OH through a 3- to 5-carbon linker bestows 102-292 and 41-84 fold enhancement in antiproliferative effect toward androgen-insensitive PC-3 and androgen-sensitive LNCaP prostate cancer cells, respectively. These data suggest that androgen-insensitive PC-3 prostate cancer cells are more susceptible than androgen-sensitive LNCaP cells to these three derivatives (22-24), a surprising yet highly desirable outcome. The difference in IC50 values implies that the potency of derivatives 22-24 is slightly decreased with increasing length of the linker. The in vitro aniproliferative potency of 3′,4′-dimethoxyflavonol (10) can also be improved by 14-91 times through incorporating a basic morpholino unit to 3-OH via a 3- to 5-carbon bridge.
Table 1. Antiproliferative activities (IC50a) of the flavonols (10-18, 22-27) against prostate cancer cell lines.
| Comp. | IC50 (μM) | IC50(10)/IC50(derivative) | ||
|---|---|---|---|---|
|
|
||||
| PC-3b | LNCaPc | PC-3 | LNCaP | |
| 10 | 409.0 ± 1.8 | 202.5 ± 32.2 | 1 | 1 |
| 11 | 30.0 ± 15.0 | 16.2 ± 9.4 | 14 | 12.5 |
| 12 | 13.7 ± 9.3 | 9.8 ± 0.6 | 30 | 21 |
| 13 | 10.1 ± 3.6 | 6.4 ± 2.2 | 41 | 32 |
| 14 | 16.8 ± 6.7 | 8.1 ± 3.1 | 24 | 25 |
| 15 | 16.8 ± 4.6 | 14.2 ± 7.6 | 24 | 14 |
| 16 | 14.0 ± 6.5 | 12.6 ± 5.8 | 29 | 16 |
| 17 | 86.3 ± 21.2 | 43.9 ± 3.3 | 5 | 5 |
| 18 | 33.0 ± 7.7 | 10.1 ± 4.3 | 12 | 20 |
| 22 | 1.4 ± 0.2 | 2.4 ± 1.5 | 292 | 84 |
| 23 | 2.7 ± 0.8 | 4.3 ± 1.3 | 152 | 47 |
| 24 | 4.0 ± 1.0 | 5.0 ± 1.3 | 102 | 41 |
| 25 | 10.9 ± 4.7 | 14.9 ± 12.1 | 38 | 14 |
| 26 | 9.1 ± 1.8 | 8.5 ± 6.2 | 45 | 24 |
| 27 | 4.5 ± 1.0 | 7.7 ± 3.5 | 91 | 26 |
| 1 | >100 | 45.5 ± 1.3 | - | - |
IC50 is the drug concentration effective in inhibiting 50% of the cell viability measured by WST-1 cell proliferation assay after 3 days of exposure. The data were presented as the mean ± standard error of the mean. See the supplementary data for experimental details associated with this assessment.
Human androgen-insensitive prostate cancer cell line.
Human androgen-sensitive prostate cancer cell line.
The anti-proliferative effect of naturally occurring flavonols quercetin (1), fisetin (5), and vitexicarpin (6) in androgen-insensitive PC-3 human prostate cancer cells has been demonstrated to be associated with cell apoptosis activation.21,25,26 The violet excitable dye F2N12S can detect membrane asymmetry changes during apoptosis, and SYTOX AADVanced dead cell stain can be used for the detection of late apoptotic and necrotic cells. The F2N12S and SYTOX AADVanced double staining assay in a flow cytometer was employed for the discrimination between early apoptotic PC-3 cells and late apoptotic/necrotic PC-3 cells when treated with derivative 22 at the concentrations spe cified in Figure 3 for 16 h. Staurosporine, a known apoptotic inducer, was used as positive apoptotic control in all experiments (data not shown). The data illustrated in Figure 3 indicate that derivative 22 can significantly activate apoptotic cell death in the androgen-insensitive PC-3 prostate cancer cell line in a dose-dependent manner after a 16-hour treatment. Specifically, treatment of 22 at 5 μM can lead to 45% of PC-3 cells in early phase of apoptosis as compared with control cells; exposure of PC-3 cells to derivative 22 at 10 μM induces 80% early apoptotic cells together with 5% late apoptotic/necrotic cells; 20 μM of 22 activates notable apoptosis as well, with 93% early apoptotic cells and 4% late apoptotic/necrotic cells. Both apoptotic and necrotic cell populations increased in response to increasing concentration of 22 (0-100 μM final concentration range). The maximal population for early apoptotic cells was reached when PC-3 prostate cancer cells were treated with 22 at 20-30 μM concentrations.
Figure 3.
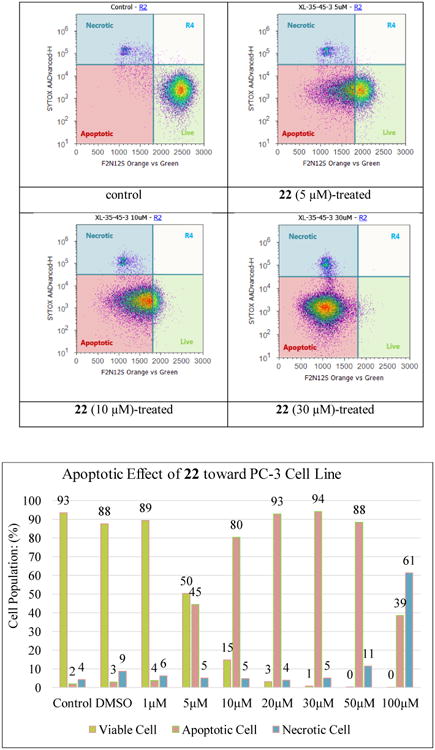
Evolution of viable, apoptotic, and necrotic PC-3 cells populations in response to derivative 22 at 1-100 μM concentrations.
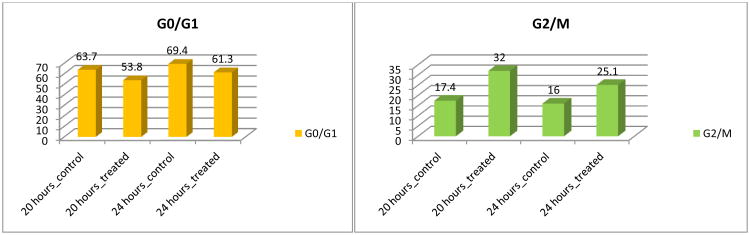
Two natural flavonols, quercetin (1) and fisetin (5), have been revealed to have cell cycle perturbation properties in the androgen-insensitive PC-3 human prostate cancer cell line by arresting cell cycle at G2/M phase.11,27 The effect of derivative 22 on the PC-3 cell cycle was evaluated using flow cytometric analysis with propidium iodide NDA staining. As illustrated in Figure 4, this flavonol derivative induces cell cycle arrest at the G2/M phase by increasing PC-3 cell population in the G2/M phase, while fewer cells were abserved in the G0/G1 phase. Specifically, the G2/M PC-3 cells were increased from 17% and 16% in control cells at 20 hours and 24 hours, respectively, t o 32% and 25% in derivative-treated cells at both time points (Figure 4). The cell population in G0/G1 phase slightly decreased from 64% in control cells to 54% at 20 hours, and from 69% in control cells to 61% in derivative-treated cells at 24 hours.
Figure 4.
Cell cycle analysis of PC-3 cells. PC-3 cancer cells were untreated or treated with 22 at 10 μM. Cells were harvested after 20 and 24 hours, fixed, stained, and analyzed for DNA content.
Flavonol 22, the most promising derivative, was selected for further evaluation against PWR-1E normal epithelial prostate cells. No anti-proliferative activity was observed on treating the cells with both 10 and 22 at 10-50 μM concentrations, implying the in vitro safety profile of 22 in the cell model. In summary, 3′,4′-dimethoxyflavonol, eight 3-O-alkyl-3′,4′-dimethoxyflavonols, and six 3-O-aminoalkyl-3′,4′-dimethoxyflavonols have been synthesized for the evaluation of their ability in inhibiting cell proliferation against both androgen-insensitive PC-3 and androgen-sensitive LNCaP prostate cancer cells. All 3-O-alkyl-3′,4′-dimethoxyflavonols and 3-O-aminoalkyl-3′,4′-dimethoxyflavonols exhibit superior potency of cell proliferation supression relative to that of parent flavonol 3′,4′-dimethoxyflavonol (10) and the naturally occurring flavonol quercetin (1). Incorporation of a dibutylamino group to 3-OH through a three- to five-carbon linker leads to the optimal derivatives with up to 292-fold enhanced potency when compared with the parent compound. The preliminary structure-activity relationship data indicate (i) that 3-O-substituted-3′,4′-dimethoxyflavonols represent a potential scaffold for the development of flavonol-based agents with substantially improved antiproliferative potency in prostate cancer cell models; (ii) that alkyl and basic aminoalkyl groups serve as the beneficial substituents for the scaffold; and (iii) that dibutylaminoalkyl groups in derivatives 22-24 act as the most favorable substituents for the potency. Flow cytometry analysis shows that the antiproliferative ability of derivative 22 toward PC-3 prostate cancer cells is associated with its activation of cell cycle arrest at G2/M phase and cell apoptosis. Both 3′,4′-dimethoxyflavonol (10) and 22 at 10-50 μM concentrations did not exhibit any obvious cell proliferation inhibition toward normal PWR-1E prostate epitherial cells, suggesting their safty profiles in normal cells. These findings warrant in-depth optimization of lead flavonols through chemical manipulations on 3-OH to further exploit their therapeutic merits for the treatment of prostate cancer.
Supplementary Material
Acknowledgments
We are grateful to California State University (CSU)-Fresno for financial support of this research. The HRMS data were supported by the NIH RCMI program at Xavier University of Louisiana through Grant 2G12MD007595 (G.W.) and NIH-NIGMS through Grant 1U54GM104940 (G.W.). We are also grateful to the Graduate Net Initiative at CSU-Fresno for a Graduate Research Grant (to X.Z.).
Footnotes
Supplementary data: Supplementary data (synthetic procedures and structural characterization) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2016
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Vue B, Zhang S, Chen QH. Anti-Cancer Agents Med Chem. 2016;16 doi: 10.2174/1871520615666151008122622. [DOI] [PubMed] [Google Scholar]
- 2.Lee MM, Gomez SL, Chang JS, Wey M, Wang RT, Hsing AW. Cancer Epidemiol, Biomarkers Prev. 2003;12:665. [PubMed] [Google Scholar]
- 3.McCann SE, Ambrosome CB, Moysich KB, Brasure J, Marshall JR, Freudenheim JL, Wilkinson GS, Graham S. Nutr Cancer. 2005;53:33. doi: 10.1207/s15327914nc5301_4. [DOI] [PubMed] [Google Scholar]
- 4.Bosetti C, Bravi F, Talamini R, Parpinel M, Gnagnarella P, Negri E, Montella M, Lagiou P, Franceschi S, La Vecchia C. Nutr Cancer. 2006;56:123. doi: 10.1207/s15327914nc5602_1. [DOI] [PubMed] [Google Scholar]
- 5.Geybels MS, Verhage BAJ, Arts ICW, van Schooten FJ, Goldbohm RA, van den Brandt PA. Am J Epidemiol. 2013;177:1388. doi: 10.1093/aje/kws419. [DOI] [PubMed] [Google Scholar]
- 6.Liu HL, Jiang WB, Xie MX. Recent Pat Anticancer Drug Discov. 2010;5:152. doi: 10.2174/157489210790936261. [DOI] [PubMed] [Google Scholar]
- 7.Ravishankar D, Rajora AK, Greco F, Osborn HML. Int J Biochem Cell Biol. 2013;45:2821. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Kelly GE, Husband AJ. Prostate Cancer Methods and Protocols. Springer; 2003. p. 377. [DOI] [PubMed] [Google Scholar]
- 9.Mullie P, Clarys P, Deriemaeker P, Hebbelinck M. Plant Foods Hum Nutr. 2007;62:93. doi: 10.1007/s11130-007-0047-7. [DOI] [PubMed] [Google Scholar]
- 10.Mullie P, Clarys P, Deriemaeker P, Hebbelinck M. Int J Food Sci Nutr. 2008;59:291. doi: 10.1080/09687630701539293. [DOI] [PubMed] [Google Scholar]
- 11.Haddad AQ, Venkateswaran V, Viswanathan L, Teahan SJ, Fleshner NE, Klotz LH. Prostate Cancer Prostatic Dis. 2006;9:68. doi: 10.1038/sj.pcan.4500845. [DOI] [PubMed] [Google Scholar]
- 12.Britton RG, Horner-Glister E, Pomenya OA, Smith EE, Denton R, Jenkins PR, Steward WP, Brown K, Gescher A, Sale S. Eur J Med Chem. 2012;54:952. doi: 10.1016/j.ejmech.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 13.Poyil P, Budhraja A, Son YO, Wang X, Zhang Z, Ding S, Wang L, Hitron A, Lee JC, Xu M, Chen G, Luo J, Shi X. PLoS One. 2012;7:e47516. doi: 10.1371/journal.pone.0047516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Firdous A, Sharmila G, Balakrishnan S, Raja Singh P, Suganya S, Srinivasan N, Arunakaran J. Food Function. 2014;5:2632. doi: 10.1039/c4fo00255e. [DOI] [PubMed] [Google Scholar]
- 15.Khan N, Asim M, Afaq F, Abu ZM, Mukhtar H. Cancer Res. 2008;68:8555. doi: 10.1158/0008-5472.CAN-08-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu B, Kulkarni K, Basu S, Zhang S, Hu M. J Pharm Sci. 2011;100:3655. doi: 10.1002/jps.22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stolarczyk M, Piwowarski JP, Granica S, Stefanska J, Naruszewica M, Kiss AK. Phytother Res. 2013;27:1842. doi: 10.1002/ptr.4941. [DOI] [PubMed] [Google Scholar]
- 18.Xu R, Zhang Y, Ye X, Xue S, Shi J, Pan J, Chen Q. Food Chem. 2013;138:48. doi: 10.1016/j.foodchem.2012.09.102. [DOI] [PubMed] [Google Scholar]
- 19.Diaz F, Yang JB, Yang CH, Jiang Y, Zhou YF, Yu B, Yang H. J Nat Prod. 2003;66:865. [Google Scholar]
- 20.Huo CH, Zhang ML, Wang YF, Zhang Q, Qin F, Shi QW, Kjyota H. Chem Nat Comp. 2013;48:958. [Google Scholar]
- 21.Meng FM, Yang JB, Yang CH, Jiang Y, Zhou YF, Yu B, Yang H. Asian Pac J Cancer Prev. 2012;13:6369. doi: 10.7314/apjcp.2012.13.12.6369. [DOI] [PubMed] [Google Scholar]
- 22.Dias TA, Duarte CL, Lima CF, Proenca MF, Pereira-Wilson C. Eur J Med Chem. 2013;65:500. doi: 10.1016/j.ejmech.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 23.Bennett M, Burke AJ, O'Sullivan WI. Tetrahedron. 1996;52:7163. [Google Scholar]
- 24.Hasan A, Sadiq A, Abbas A, Mughal E, Khan KM, Ali M. Nat Prod Res. 2010;24:995. doi: 10.1080/14786410902847302. [DOI] [PubMed] [Google Scholar]
- 25.Vijayababu MR, Kanagaraj P, Arunkumar A, Ilangovan R, Aruldhas MM, Arunakaran J. J Cancer Res Clin Oncol. 2005;131:765. doi: 10.1007/s00432-005-0005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haddad AQ, Fleshner N, Nelson C, Saour B, Musquera M, Venkateswaran V, Klotz L. Nutr Cancer. 2010;62:668. doi: 10.1080/01635581003605524. [DOI] [PubMed] [Google Scholar]
- 27.Khan N, Afaq F, Syed DN, Mukhtar H. Carcinogenesis. 2008;29:1049. doi: 10.1093/carcin/bgn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.