Abstract
Neurons are organized and connected into functional circuits by axons that conduct action potentials. Many vertebrate axons are myelinated and further subdivided into excitable domains that include the axon initial segment (AIS) and nodes of Ranvier. Nodes of Ranvier regenerate and propagate action potentials, while AIS regulate action potential initiation and neuronal polarity. Two distinct cytoskeletons control axon structure and function: 1) a submembranous ankyrin/spectrin cytoskeleton that clusters ion channels and provides mechanical support, and 2) a microtubule-based cytoskeleton that controls selective trafficking of dendritic and axonal cargoes. Here, we review recent studies that provide significant additional insight into the cytoskeleton-dependent mechanisms controlling the functional organization of axons.
INTRODUCTION
Neurons have two main anatomical and functional domains: an input domain including the soma and dendrites, and an output domain consisting of the axon. While dendrites are typically short, axons can be orders of magnitude longer (up to 1 m in humans) since they may connect cells that are located far from one another. These long distances present significant challenges to the neuron. For example, axons require the sorting and delivery of specific axonal proteins, synthesized in the neuronal cell body, to distant locations like the pre-synaptic terminals, while excluding those proteins that normally function in dendrites. Also, the farther apart neurons and their effector cells (e.g. muscle cells) are located from one another, the longer it takes for action potentials to carry information. Because of their length and trajectories throughout the body, axons are also exposed to mechanical forces that without structural support would cause them to break. In this review we discuss recent discoveries that have dramatically expanded our understanding of how cytoskeletal actin filaments, ankyrin/spectrin protein complexes, and microtubules help to overcome these challenges.
Cytoskeletal control of neuronal polarity
How do neurons control the sorting and delivery of specific axonal and dendritic proteins? The axon initial segment (AIS) is a specialized ~30 µm long membrane domain at the proximal part of the axon where voltage-gated ion channels are clustered (Fig. 1A). It is both the functional and anatomical bridge between the input and output domains of a neuron. It is defined molecularly by ankyrinG (ankG) and βIV spectrin, which together bind AIS Na+ channels and link them to the underlying actin cytoskeleton. Genetic ablation of ankG blocks AIS Na+ channel clustering [•• 1,2] and the recruitment of all other AIS proteins [3]. The AIS functions as the site of action potential initiation due to the high density of channels [4]. The AIS also controls neuronal polarity by functioning 1) as a membrane barrier that restricts the lateral mobility of membrane proteins and ion channels, thereby maintaining their asymmetric distribution [5,6], and 2) a cytoplasmic gate that ensures only axonal cargoes are transported into the axon [7]. These AIS functions depend on actin since depolymerizing actin disrupts polarity and permits the mixing of somatodendritic and axonal proteins and cargoes [5,7]. However, how actin does this remains unknown.
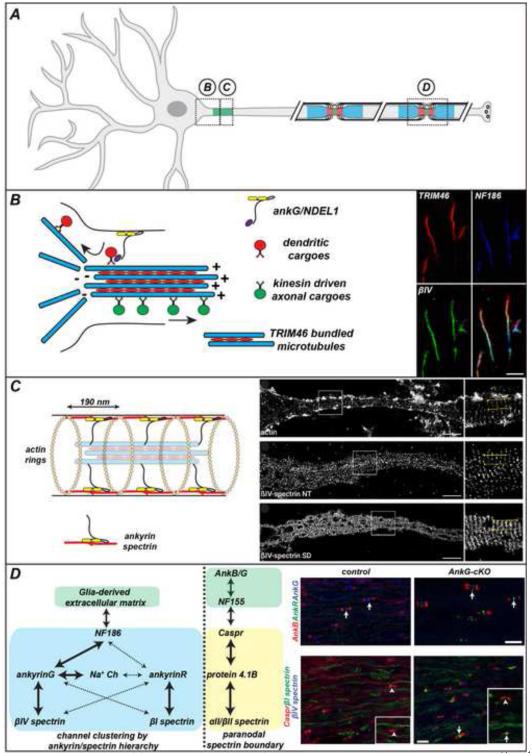
Figure 1.
A) Domain organization of the axon with axon initial segments (regions B and C) and nodes of Ranvier (region D).
B) Sorting of axonal and dendritic cargoes is a microtubule-dependent mechanism. Recent studies have shown that TRIM46 is found at and just proximal to the AIS (right) and is responsible for the bundling of microtubules. The dynein regulator NDEL1 is also important for sorting of vesicles and functions to exclude vesicles from the AIS. Scale bar = 10 µm.
C) The actin/spectrin/ankyrin submembranous cytoskeleton is organized as a periodic lattice of proteins that alternate between actin rings and spectrin/ankyrin complexes. STORM super-resolution imaging has given this unprecedented level of structural and molecular detail (right). Immunostaining pictures from [10].
D) Two cytoskeleton-dependent mechanisms are responsible for ion channel clustering at nodes of Ranvier: an active clustering mechanism mediated by a hierarchy of ankyrins and spectrins, and a paranodal exclusion barrier mediated by αII and βII spectrin. In nodes lacking ankG, ankR and βI spectrin compensate for the loss of ankG and βIV spectrin. Adapted from [1].
One major impediment to understanding actin function at the AIS has been the lack of detailed knowledge about its subcellular distribution within the AIS. To address this problem, Watanabe et al. [8] used correlative light and scanning electron microscopy to visualize discrete patches of actin filaments (several microns in diameter) in the AIS of dissociated cortical neurons. Live imaging of vesicles carrying dendritic proteins were found to stop at these actin patches rather than enter the axon, suggesting that they may constitute a vesicle filter. However, other imaging methods failed to reveal similar actin patches in locations where they would function as a vesicle filter. For example, Jones et al. [9] used platinum replica electron microscopy (PREM) to visualize the actin cytoskeleton in the AIS. They were unable to find actin patches and instead reported short, stable actin filaments and slightly longer dynamic actin filaments. The use of super-resolution microscopy techniques revealed actin patches at the AIS, but only at the periphery of the axon near membrane rather than centrally located within the axoplasm [• 10] (Fig. 1B). Furthermore, these patches colocalized with presynaptic scaffolding proteins such as bassoon, suggesting that these sites correspond to pre-synaptic boutons rather than vesicle filters [11]. Thus, it is not clear how actin patches could function as the gate for vesicle entry into axons. More will be said about the structure and organization of actin within the AIS below.
In contrast to the model where actin functions as the gate, other studies suggest that polarized axonal trafficking depends on microtubules and the selective activities of motor proteins associated with those microtubules and vesicles. For example, live imaging of vesicles containing dendritic proteins showed that they fail to enter the axon and instead reverse direction before entering the AIS [12]. Furthermore, the constitutive addition of an axon-specific motor protein forces vesicles containing dendritic cargoes into the axon [13].
The concept of a microtubule-based gate fits well with the intrinsic polarity of microtubules and the observation that microtubule stabilization promotes axon specification and neuronal polarity [14]. Historically, the earliest recognized ultrastructural feature of the AIS (prior to the discovery of ankG) was the bundling of microtubules linked by molecularly undefined cross-bridges [15]. Recently, van Beuningen et al. [•• 16] discovered that the cross-linked AIS microtubules depend on tripartite motif containing protein 46 (TRIM46), which functions to bundle microtubules into closely spaced arrays with their plus-end out (Fig. 1B). Remarkably, expression of TRIM46 in non-neuronal cells is sufficient to bundle and induce the formation of cross-bridges between microtubules. TRIM46 is enriched in the proximal axon and its distribution overlaps with ankG at the AIS, but it can also be detected just a few microns proximal to ankG in the same location where vesicles reverse direction to move away from the axon [• 12,13]. Consistent with a role in controlling the sorting of vesicular cargoes, TRIM46 is only found at the AIS and not at other ankG-enriched domains like the nodes of Ranvier where sorting need not take place. TRIM46 is an early marker of neuronal polarity since it localizes to the nascent axon before ankG clustering. Loss of TRIM46 disrupts axon formation, ankG clustering, microtubule bundling, and the efficient sorting of vesicles into their appropriate axonal or dendritic compartments. Conversely, loss of ankG disrupts the bundling of microtubules [17].
The observations described above indicate that the assembly of ankG-dependent AIS Na+ channel clusters and TRIM46-based bundling of AIS microtubules are reciprocally dependent on one another, and that together these protein complexes regulate neuronal polarity. However, ankG forms part of the submembranous cytoskeleton together with βIV spectrin and actin, while TRIM46 is associated with cytoplasmic microtubules. How are actin/βIV spectrin/ankG complexes and microtubules linked? While the answer to this question remains unknown, important clues include the fact that the proteins EB1 and EB3 are found at the AIS and bind to both microtubules and ankG [18]. Even more striking is the recent discovery that the dynein regulator NDEL1 binds directly to ankG, is enriched at the AIS, and its loss permits somatodendritic proteins to appear in the axon [•• 19]. Thus, NDEL1 functions at the AIS to promote dynein-dependent transport of somatodendritic vesicles out of the axon (Fig. 1B), but the molecular mechanisms controlling recognition of somatodendritic cargoes by NDEL1 remain unknown. Furthermore, since live imaging of dendritic cargoes show that they never enter the axon and reverse direction before they even enter the AIS [• 12,13] suggests that there are additional NDEL1-independent mechanisms controlling sorting. Since loss of ankG causes axons to acquire dendritic features [20], rather than dendrites acquiring axonal features, we propose that the major vesicle sorting mechanism in neurons is exclusion of dendritic cargoes from axons rather than preferential trafficking of vesicles to axons. However, exceptions to this idea include the observation that Nav1.6 Na+ channels are preferentially targeted to the axon and inserted into the AIS where they are immobilized through their interaction with ankG [21].
The actin/spectrin/ankyrin cytoskeleton in axons
If microtubule-based mechanisms control the sorting of axonal and somatodendritic vesicles, then what is actin's role and where is it located in the axon, and the AIS in particular? Super-resolution imaging of the actin cytoskeleton suggests a surprising and remarkable answer to this question. Using STochastic Optical Reconstruction Microscopy (STORM), a super-resolution microscopy technique, Xu et al. [22] found that axonal actin filaments form submembranous rings that are periodically and regularly spaced along the axonal shaft (Fig. 1C). These observations were later confirmed and shown to be ubiquitous throughout the nervous system and in many cell types including both neurons and oligodendrocytes using a different form of super-resolution microscopy: STimulated Emission Depletion (STED) microscopy [11,23,24]. During neuronal development these actin rings appear immediately after the breaking of symmetry and axon specification [• 25]. The periodicity of the actin rings was highly regular, with a spacing of approximately 190 nm (Fig. 1C). The rings colocalized with the actin capping protein adducin, and alternated with the actin-crosslinking protein βII spectrin, the primary spectrin found in the distal axon [26]. Spectrins consist of two α- and two β-subunits, bind to actin, and have a predicted length of ~190 nm. The coincident spacing and length of the spectrin tetramer suggest that spectrins dictate the assembly of the periodic cytoskeleton. However, additional experiments showed the organization of these rings is far more complicated than originally thought because the periodic structure of the actin/spectrin cytoskeleton was disrupted by actin depolymerizing agents, silencing of βII spectrin expression, and even the disruption of microtubules [• 25].
Spectrins bind not only actin, but are anchored to the plasma membrane through interactions with ankyrins. In the distal axon ankyrinB (ankB) also takes up a periodic organization with a similar spacing as that for actin and βII spectrin. Leterrier et al. [• 10] used STORM imaging to visualize components of the AIS and found that ankG, βIV spectrin, Na+ channels, and the AIS cell adhesion molecule neurofascin (NF) 186 are all found in a similar periodic structure (Fig. 1C). The use of different antibodies directed against distinct and widely spaced epitopes found in ankG and βIV spectrin revealed in unprecedented detail the organization of the AIS submembranous cytoskeleton. As predicted, βIV spectrin subunits associate in a head-to-head orientation immediately beneath the plasma membrane, with the N-terminus of ankG also located in the submembrane skeleton. However, the C-terminus of ankG extended on average 32 nm into the axoplasm where it presumably interacts with other proteins [• 10]. Consistent with the idea that the C-terminus of ankG plays important roles in AIS assembly, Jenkins et al. [•• 27] showed that mice lacking the so-called 'giant' exon of ankG near its C-terminus fail to form AIS. Thus, interactions between the C-terminus of ankG and other, as yet undefined AIS proteins are critical for normal AIS assembly.
What is the function of the periodic cytoskeleton? Spectrins are comprised of many (from 17 to 22) three-helix spectrin repeats in each subunit. These repeats are elastic [28], permitting the submembranous cytoskeleton to be flexible. Thus, the periodic spectrin cytoskeleton confers flexibility and elasticity on the axon. Consistent with this idea, loss of β spectrin from c. Elegans results in axons that break under mechanical strain [29]. Future studies of mice lacking spectrins will be important to confirm a similar role in vertebrate axons.
Although actin, spectrins, and ankyrins all form a periodic cytoskeleton in axons, distinct ankyrin/spectrin complexes are found at discrete sites along the axon. As emphasized already, βIV spectrin and ankG are at the AIS; no α spectrin has yet been reported there. In unmyelinated axons, the distal submembranous cytoskeleton consists of ankB, αII spectrin and βII spectrin; this cytoskeleton participates in AIS assembly through the generation of an intra-axonal boundary that restricts ankG and βIV spectrin to the proximal axon [26]. βIII spectrin is located in the dendrites of Purkinje neurons, and its loss results in Purkinje cell degeneration and spinocerebellar ataxia type 5 [30].
In myelinated axons, nodes of Ranvier have a cytoskeletal specialization much like the AIS. Nodes of Ranvier are primarily responsible for the rapid and efficient propagation of action potentials along axons (Fig. 1A). The nodal cytoskeleton consists of ankG and βIV spectrin, but there is no known specialized microtubule organization. STED imaging revealed a periodic organization for nodal ankG and βIV spectrin, just like the AIS [11,24]. AnkG is clustered at nodes of Ranvier by two glia-dependent mechanisms (Fig. 1D). First, ankG is clustered through interactions with the axonal cell adhesion molecule NF186. NF186 accumulates adjacent to elongating myelin sheaths and at nascent nodes of Ranvier through interactions with glia-derived extracellular matrix molecules [31,32]. NF186 then functions as a membrane receptor for the binding of ankG. Na+ channels then accumulate at nodes because they bind with high affinity to the N-terminal segment of ankG in a phosphorylation dependent manner [33,34]. To directly test this accepted model, we recently used a conditional allele of ankG to eliminate ankG's expression from peripheral sensory neurons and retinal ganglion cells. Surprisingly, we found that Na+ channels still clustered at nodes. This unexpected result led to additional experiments that revealed a second clustering mechanism consisting of ankR and βI spectrin that could compensate for loss of ankG or βIV spectrin [•• 1] (Fig. 1D). Although ankR/βI spectrin protein complexes are normally expressed in neurons, they do not contribute to clustering of Na+ channels due to their lower affinity for Na+ channels and NF186 as compared to ankG/βIV spectrin. Thus, a hierarchy of ankyrin/spectrin interactions functions to cluster Na+ channels at nodes of Ranvier (Fig. 1D).
The second mechanism responsible for nodal Na+ channel clustering consists of an axoglial junction between axons and myelinating Schwann cells in the PNS and oligodendrocytes in the CNS. These 'paranodal' axoglial junctions form the largest known vertebrate intercellular junction which, through as yet undetermined mechanisms, acts as lateral diffusion barrier to restrict ion channels to nodal and juxtaparanodal domains; juxtaparanodes are short regions beneath the myelin sheath adjacent to the paranodal junctions where Kv1 K+ channels are clustered. Disruption of the paranodal junctions permits juxtaparanodal Kv1 channels to enter into paranodal regions and causes reduced action potential conduction velocity [32]. On the other hand, Na+ channels remain clustered due to the NF186/ankG mechanism. The paranodal axoglial junctions consists of both glial and axonal cell adhesion molecules, each of which is anchored to cytoskeletal proteins (Fig. 1D). For example, in myelinating glia a 155 kilodalton glia-specific splice variant of Neurofascin (NF155) functions as the cell adhesion molecule at the paranodal junction. We recently showed that in the PNS NF155 binds to ankB, and in the CNS NF155 binds to ankG. Conditional knockout of ankG from myelinating oligodendrocytes delays paranodal junction formation and slows action potential propagation [35]. On the axonal side, a heterodimeric cell adhesion complex consisting of caspr and contactin are linked to an αII spectrin and βII spectrin-dependent cytoskeleton by protein 4.1B [36]. Intriguingly, this axonal organization of the spectrin cytoskeleton is reminiscent of the organization observed at the AIS: βIV spectrin flanked by αII spectrin and βII spectrin [26]. Since βIV spectrin and ankG are restricted to the AIS by an αII spectrin and βII spectrin-dependent intra-axonal boundary, we wondered if a paranodal spectrin-dependent intra-axonal boundary also exists to help organize the axonal membrane. To test this directly, we used a conditional allele for βII spectrin and found that although the paranodal junction itself remained intact in mice lacking axonal βII spectrin, Kv1 K+ channels were no longer restricted to juxtaparanodes but extended into paranodal and even nodal domains [•• 37]. These observations immediately led to the conceptually important insight that the molecular basis of the paranodal lateral diffusion barrier in myelinated axons is not the junction itself, but rather the paranodal cytoskeleton whose initial formation depends on the paranodal junction.
If the paranodal cytoskeleton functions as an intra-axonal boundary to restrict ion channels to specific domains in the axonal plasma membrane, why are Na+ channels still clustered at nodes in βII spectrin-deficient mice? We speculate that the answer to this question is that the NF-186/glial ECM mechanism is sufficient to cluster Na+ channels and ankG in the absence of the paranodal cytoskeleton. However, the only way to prove this will be to construct mice lacking both mechanisms. For example, if a dual mechanism model is correct and the paranodal cytoskeleton can function as a second clustering mechanism, then mice lacking both axonal NF186 and βII spectrin are predicted to lack Na+ channel and ankG clustering at nodes of Ranvier. Future experiments will be needed to test these possibilities.
In conclusion, the functional organization and structural integrity of axons depends on a complex set of interactions among actin, microtubules, spectrins and ankyrins. The importance of these cytoskeletal elements is only now becoming apparent as their interactions, accessory proteins, and functions are discovered. We predict that elucidating the neurobiological mechanisms whereby axonal cytoskeletons control axon structure and function will provide essential insights into future therapeutic interventions for human diseases and injuries of the nervous system.
Highlights.
Neuronal polarity and polarized trafficking of vesicles depends on microtubule specializations at the axon initial segment.
The submembranous axonal cytoskeleton is organized as a periodic structure consisting of rings of actin spaced by spectrin/ankyrin protein complexes.
Ion channels are clustered at nodes of Ranvier by a hierarchy of ankyrin/spectrin protein complexes.
The molecular basis of the membrane boundary flanking nodes of Ranvier is the βII spectrin-dependent paranodal cytoskeleton.
Acknowledgements
This work was supported by grants from the US National Institutes of Health (NS069688 and NS044916 to M.N.R.) and the Dr. Miriam and Sheldon Adelson Medical Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests
REFERENCES
- 1••.Ho TS, Zollinger DR, Chang KJ, Xu M, Cooper EC, Stankewich MC, Bennett V, Rasband MN. A hierarchy of ankyrin-spectrin complexes clusters sodium channels at nodes of Ranvier. Nat Neurosci. 2014;17:1664–1672. doi: 10.1038/nn.3859. This paper demonstrates that ankyrins are required for node of Ranvier formation. However, it also shows that ankG is dispensable due to compensation by ankR. Furthermore, it reveals the existence of a heirarchy of ankyrins and spectrins in axons that, based on their relative affinities for each other and Na+ channels, participate in node of Ranvier formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedstrom KL, Xu X, Ogawa Y, Frischknecht R, Seidenbecher CI, Shrager P, Rasband MN. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol. 2007;178:875–886. doi: 10.1083/jcb.200705119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- 5.Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- 6.Nakada C, Ritchie K, Oba Y, Nakamura M, Hotta Y, Iino R, Kasai RS, Yamaguchi K, Fujiwara T, Kusumi A. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat Cell Biol. 2003;5:626–632. doi: 10.1038/ncb1009. [DOI] [PubMed] [Google Scholar]
- 7.Song A-H, Wang D, Chen G, Li Y, Luo J, Duan S, Poo M-M. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136:1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, Al-Bassam S, Miyazaki Y, Wandless TJ, Webster P, Arnold DB. Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell Rep. 2012;2:1546–1553. doi: 10.1016/j.celrep.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones SL, Korobova F, Svitkina T. Axon initial segment cytoskeleton comprises a multiprotein submembranous coat containing sparse actin filaments. J Cell Biol. 2014;205:67–81. doi: 10.1083/jcb.201401045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Leterrier C, Potier J, Caillol G, Debarnot C, Rueda Boroni F, Dargent B. Nanoscale Architecture of the Axon Initial Segment Reveals an Organized and Robust Scaffold. Cell Rep. 2015;13:2781–2793. doi: 10.1016/j.celrep.2015.11.051. This paper uses super-resolution microscope to reveal in unprecedented detail the position and orientations of the key AIS cytsokeletal proteins ankG and βIV spectrin. [DOI] [PubMed] [Google Scholar]
- 11.D'Este E, Kamin D, Gottfert F, El-Hady A, Hell SW. STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Rep. 2015;10:1246–1251. doi: 10.1016/j.celrep.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 12•.Petersen JD, Kaech S, Banker G. Selective microtubule-based transport of dendritic membrane proteins arises in concert with axon specification. J Neurosci. 2014;34:4135–4147. doi: 10.1523/JNEUROSCI.3779-13.2014. This paper refutes the idea of an actin-based selectivity filter as the basis for sorting of dendritic and axonal proteins and shows that selective transport of vesicles arises before AIS assembly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farias GG, Guardia CM, Britt DJ, Guo X, Bonifacino JS. Sorting of Dendritic and Axonal Vesicles at the Pre-axonal Exclusion Zone. Cell Rep. 2015;13:1221–1232. doi: 10.1016/j.celrep.2015.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witte H, Neukirchen D, Bradke F. Microtubule stabilization specifies initial neuronal polarization. J Cell Biol. 2008;180:619–632. doi: 10.1083/jcb.200707042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palay SL, Sotelo C, Peters A, Orkand PM. The axon hillock and the initial segment. J Cell Biol. 1968;38:193–201. doi: 10.1083/jcb.38.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Van Beuningen SF, Will L, Harterink M, Chazeau A, van Battum EY, Frias CP, Franker MA, Katrukha EA, Stucchi R, Vocking K, et al. TRIM46 Controls Neuronal Polarity and Axon Specification by Driving the Formation of Parallel Microtubule Arrays. Neuron. 2015;88:1208–1226. doi: 10.1016/j.neuron.2015.11.012. The authors show that TRIM46 is the molecular basis of microtubule bundling at the AIS. They also show that the bundling of microtubules is necessary for axon specification and to establish neuronal polarity. [DOI] [PubMed] [Google Scholar]
- 17.Sobotzik JM, Sie JM, Politi C, Del Turco D, Bennett V, Deller T, Schultz C. AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc Natl Acad Sci U S A. 2009;106:17564–17569. doi: 10.1073/pnas.0909267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leterrier C, Vacher H, Fache MP, d'Ortoli SA, Castets F, Autillo-Touati A, Dargent B. End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proc Natl Acad Sci U S A. 2011;108:8826–8831. doi: 10.1073/pnas.1018671108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Kuijpers M, van de Willige D, Freal A, Chazeau A, Franker MA, Hofenk J, Rodrigues RJ, Kapitein LC, Akhmanova A, Jaarsma D, et al. Dynein Regulator NDEL1 Controls Polarized Cargo Transport at the Axon Initial Segment. Neuron. 2016;89:461–471. doi: 10.1016/j.neuron.2016.01.022. The authors show that NDEL1 binds to ankG, is restricted to the AIS, and functions there as a mechanism to cause dynein-dependent trafficking of vesicles containing somatodendritic cargoes out of the axon. [DOI] [PubMed] [Google Scholar]
- 20.Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183:635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akin EJ, Sole L, Dib-Hajj SD, Waxman SG, Tamkun MM. Preferential targeting of Nav1.6 voltage-gated Na+ Channels to the axon initial segment during development. PLoS One. 2015;10:e0124397. doi: 10.1371/journal.pone.0124397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu K, Zhong G, Zhuang X. Actin, Spectrin, and Associated Proteins Form a Periodic Cytoskeletal Structure in Axons. Science. 2013;339:30495–30501. doi: 10.1126/science.1232251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukinavicius G, Reymond L, D'Este E, Masharina A, Gottfert F, Ta H, Guther A, Fournier M, Rizzo S, Waldmann H, et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat Methods. 2014;11:731–733. doi: 10.1038/nmeth.2972. [DOI] [PubMed] [Google Scholar]
- 24.D'Este E, Kamin D, Velte C, Gottfert F, Simons M, Hell SW. Subcortical cytoskeleton periodicity throughout the nervous system. Sci Rep. 2016;6:22741. doi: 10.1038/srep22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Zhong G, He J, Zhou R, Lorenzo D, Babcock HP, Bennett V, Zhuang X. Developmental mechanism of the periodic membrane skeleton in axons. Elife. 2014:3. doi: 10.7554/eLife.04581. This paper shows the actin/spectrin-periodic cytoskeleton arises very early after axon specification and its integrity depends on both βII spectrin and intact microtubules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galiano MR, Jha S, Ho TS, Zhang C, Ogawa Y, Chang KJ, Stankewich MC, Mohler PJ, Rasband MN. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell. 2012;149:1125–1139. doi: 10.1016/j.cell.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Jenkins PM, Kim N, Jones SL, Tseng WC, Svitkina TM, Yin HH, Bennett V. Giant ankyrin-G: a critical innovation in vertebrate evolution of fast and integrated neuronal signaling. Proc Natl Acad Sci U S A. 2015;112:957–964. doi: 10.1073/pnas.1416544112. This paper demonstrates the essential role the giant exon in ankG has for AIS assembly. It further reveals an unexpected and phosphorylation-dependent binding-site for βIV spectrin in the giant exon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paramore S, Ayton GS, Mirijanian DT, Voth GA. Extending a spectrin repeat unit. I: linear force-extension response. Biophys J. 2006;90:92–100. doi: 10.1529/biophysj.105.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammarlund M, Jorgensen EM, Bastiani MJ. Axons break in animals lacking beta-spectrin. J Cell Biol. 2007;176:269–275. doi: 10.1083/jcb.200611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarkson YL, Perkins EM, Cairncross CJ, Lyndon AR, Skehel PA, Jackson M. beta-III spectrin underpins ankyrin R function in Purkinje cell dendritic trees: protein complex critical for sodium channel activity is impaired by SCA5-associated mutations. Hum Mol Genet. 2014;23:3875–3882. doi: 10.1093/hmg/ddu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinberg K, Eshed-Eisenbach Y, Frechter S, Amor V, Salomon D, Sabanay H, Dupree JL, Grumet M, Brophy PJ, Shrager P, et al. A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier. Neuron. 2010;65:490–502. doi: 10.1016/j.neuron.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Susuki K, Chang KJ, Zollinger DR, Liu Y, Ogawa Y, Eshed-Eisenbach Y, Dours-Zimmermann MT, Oses-Prieto JA, Burlingame AL, Seidenbecher CI, et al. Three mechanisms assemble central nervous system nodes of Ranvier. Neuron. 2013;78:469–482. doi: 10.1016/j.neuron.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, Wei Z, Chen K, Ye F, Yu C, Bennett V, Zhang M. Structural basis of diverse membrane target recognitions by ankyrins. Elife. 2014;3 doi: 10.7554/eLife.04353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu M, Cooper EC. An Ankyrin-G N-terminal Gate and Protein Kinase CK2 Dually Regulate Binding of Voltage-gated Sodium and KCNQ2/3 Potassium Channels. J Biol Chem. 2015;290:16619–16632. doi: 10.1074/jbc.M115.638932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang KJ, Zollinger DR, Susuki K, Sherman DL, Makara MA, Brophy PJ, Cooper EC, Bennett V, Mohler PJ, Rasband MN. Glial ankyrins facilitate paranodal axoglial junction assembly. Nat Neurosci. 2014;17:1673–1681. doi: 10.1038/nn.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, Susuki K, Peles E, Stankewich MC, Rasband MN. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J Neurosci. 2006;26:5230–5239. doi: 10.1523/JNEUROSCI.0425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Zhang C, Susuki K, Zollinger DR, Dupree JL, Rasband MN. Membrane domain organization of myelinated axons requires betaII spectrin. J Cell Biol. 2013;203:437–443. doi: 10.1083/jcb.201308116. The authors show that the paranodal spectrin cytoskeleton is the molecular basis for the paranodal lateral diffusion barrier that restricts Kv1 K+ channels to juxtaparanodes. [DOI] [PMC free article] [PubMed] [Google Scholar]