Abstract
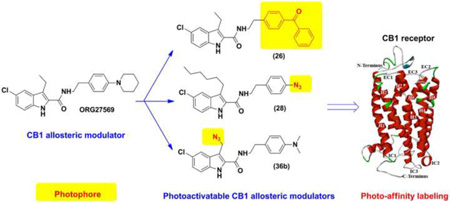
5-Chloro-3-ethyl-N-(4-(piperidin-1-yl)phenethyl)-1H–indole-2-carboxamide (ORG27569, 1) is a prototypical allosteric modulator for the cannabinoid CB1 receptor. Based on this indole-2-carboxamide scaffold, we designed and synthesized novel CB1 allosteric modulators that possess photoactivatable functionalities, which include benzophenone, phenyl azide, aliphatic azide and phenyltrifluoromethyldiazrine. To assess their allosteric effects, the dissociation constant (KB) and allosteric binding cooperativity factor (α) were determined and compared to their parent compounds. Within this series, benzophenone-containing compounds 26 and 27, phenylazide-containing compound 28, and the aliphatic azide containing compound 36b showed allosteric binding parameters (KB and α) comparable to their parent compound 1, 7, 8, and 9, respectively. We further assessed these modulators for their impact on G-protein coupling activity. Interestingly, these compounds exhibited negative allosteric modulator properties in a manner similar to their parent compounds, which antagonize agonist-induced G-protein coupling. These novel CB1 allosteric modulators, possessing photoactivatable functionalities, provide valuable tools for future photo-affinity labeling and mapping the CB1 allosteric binding site(s).
Keywords: Cannabinoid receptor, Allosteric modulator, Photoaffinity labeling, Photoactivatable ligands
Graphical Abstract
Introduction
The cannabinoid CB1 receptor is a G-protein coupled receptor (GPCR) within the endocannabinoid system and the most abundant GPCR in the brain [1]. The CB1 receptor is also widely expressed in peripheral tissues and organs [2]. In line with its distribution pattern, the CB1 receptor is involved in regulation of a variety of physiological functions that include neuronal development, food intake and energy balance, perception, immune modulation, cell apoptosis, cardiovascular and reproductive functions [3–6]. Therefore, the CB1 receptor has been proposed to be a potential therapeutic target for ameliorating a number of pathological conditions such as obesity, pain and cardiovascular diseases [5, 6].
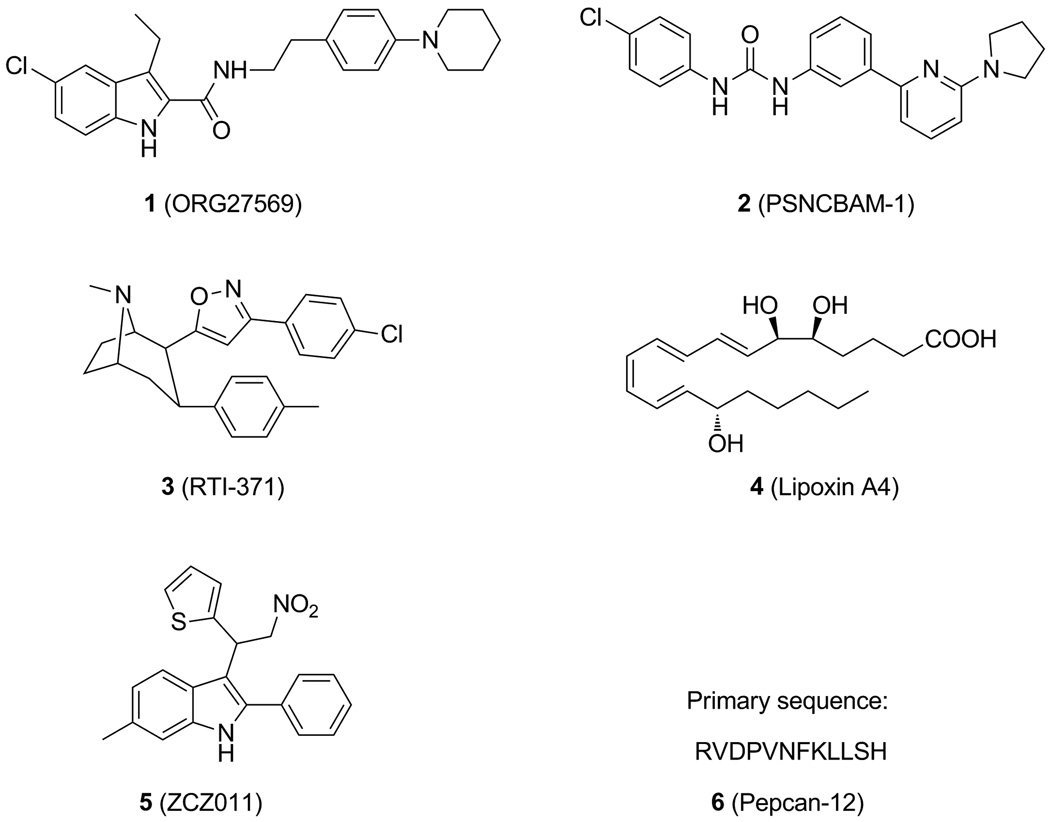
Traditionally, drug discovery, targeting the CB1 receptor, has focused on agonists and inverse agonists/antagonists that bind to the orthosteric ligand binding site where the endogenous ligands of cannabinoid receptors bind [7]. To date, two drugs developed from the class of CB1 agonists, Marinol® and Cesamet®, have been approved by FDA for the management of nausea and vomiting associated with cancer chemotherapy [8]. Additionally, an inverse agonist of the CB1 receptor, rimonabant, was marketed in Europe as an anorectic anti-obesity drug for a short period of time and later was withdrawn from clinical usage due to psychiatric side effects [9]. Recently, several small molecules have been identified as allosteric modulators of the CB1 receptor; these include ORG27569 (1) [10], PSNCBAM-1 (2) [11], RTI-371(3) [12], lipoxin A4 (4) [13], ZCZ011 (5) [14] and pepcan-12 (6) [15], whose structures are shown in Figure 1. The identification of these ligands implicates the existence of allosteric site(s) on the CB1 receptor and offers new opportunities for discovery of innovative and safer therapies by targeting the allosteric site(s), which are topographically distinct from orthosteric site(s).
Fig. 1.
Structures of representative allosteric modulators of the CB1 receptor.
Molecules targeting allosteric sites on GPCRs provide several advantages over molecules targeting orthosteric sites; these include greater receptor-subtype selectivity and a lower tendency towards target-based side effects, which are primarily due to: 1) the greater structure divergence in allosteric sites across receptor subtypes relative to the more conserved orthosteric sites; 2) the ceiling effects of receptor allosteric modulation [16, 17]. However, rational design of allosteric modulators is often hampered by a lack of structural information of the allosteric site. In the absence of a crystal structure of the receptor, an alternative approach is to identify the ligand binding domain by using a methodology that involves labeling the receptor with photoaffinity ligands followed by mass spectrometry-based proteomic studies [18]. This methodology has been successfully applied to the identification of the allosteric binding sites of nicotinic acetylcholine receptors (nAChRs) [19, 20], M1 muscarinic acetylcholine receptor [21], dopamine D2 receptors [22], GABAA receptor [23], and allosteric binding sites of other proteins [24–26]. Notably, the orthosteric binding domains of cannabinoid CB1 and CB2 receptors have been investigated using similar methodology [27, 28].
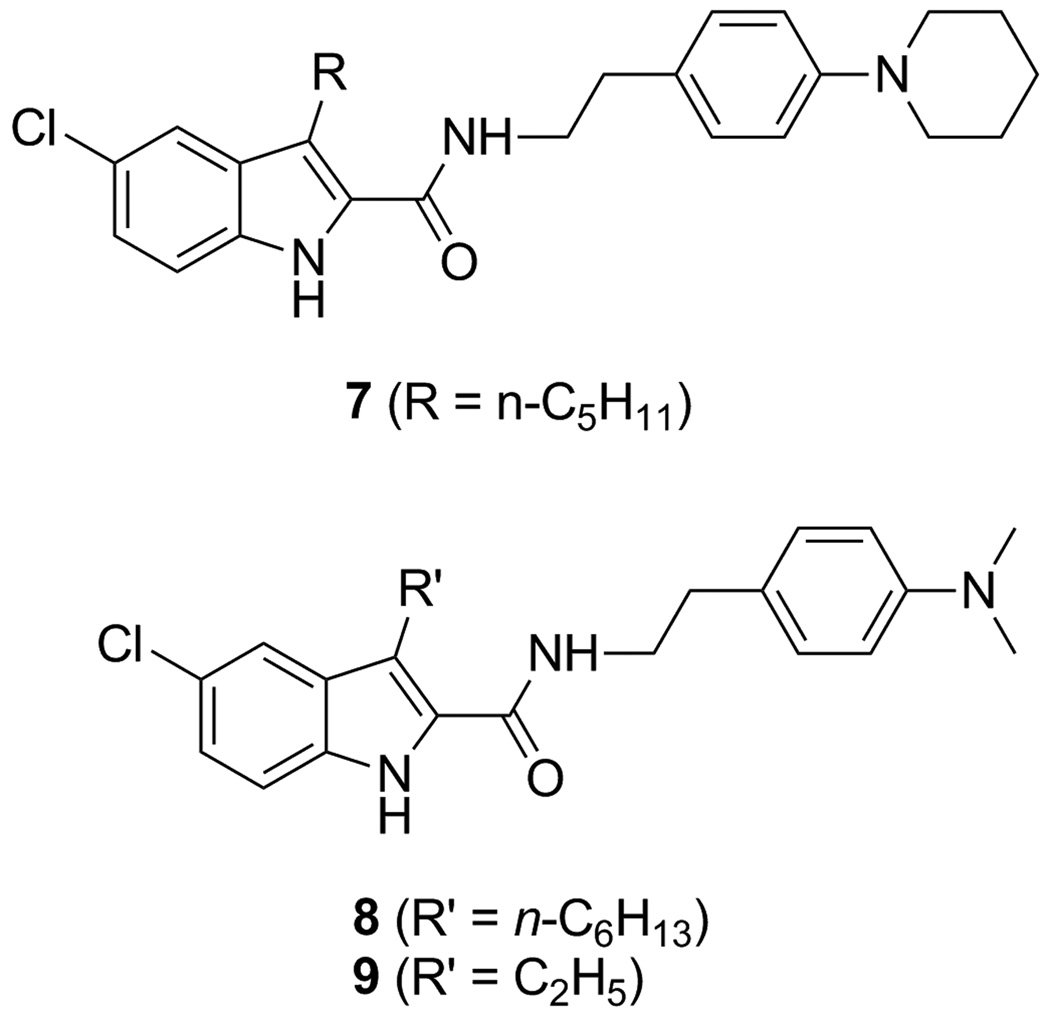
The indole-2-carboxamide 1 is the first and a prototypical allosteric modulator for the CB1 receptor [10]. It enhances specific binding of the CB1 agonist [3H]CP55,940 and activates the ERK1/2 pathway while it decreases the Gi coupling activity [10, 29]. Thus, depending on the signaling pathways under investigation, compound 1 can behave either as a positive CB1 allosteric modulator [29] or a negative allosteric modulator [10, 30]. Following the discovery of 1, several improved CB1 allosteric modulators have been identified from optimization of the indole-2-carboxamide scaffold [31–35]. However, the investigation of the precise location and the structure of their binding sites is still in its early stage [36–38]. On the other hand, the therapeutic potential of CB1 allosteric modulators is emerging. For instance: 1 was demonstrated as effective as a CB1 inverse agonist that inhibits reinstatement of drug-seeking behavior [39]; 2 was shown to have plausible acute hypophagic effects [11]; 4 was found to reduce β-amyloid-provoked neurotoxicity [13]. The positive CB1 allosteric modulator 5 was shown to generate antinociceptive effects without psychoactive effects [14]. Thus, identification of the binding motif of CB1 allosteric modulators and mapping the 3D structure of the CB1 allosteric sites are of great value for rational design of novel, more selective and potent CB1 allosteric modulators. To facilitate future proteomic study of allosteric sites on the CB1 receptor, we designed and synthesized several photoactivatable affinity ligands based on 1 and several other robust CB1 allosteric modulators (i.e. 7–9, Figure 2) reported previously [31–33]. Compounds 7 [31], 8 [33] and 9 [32, 35] have been demonstrated to have either comparable or improved allosteric effects to 1 [31–33]. Therefore, they were selected along with 1 as the parent compounds to introduce photoactivatable functionalities. Reasonable binding affinity, similar pharmacological properties to the parent compounds and sufficient photo-crosslinking efficiency of photoactivatable allosteric modulators can warrant selective and effective labeling of the allosteric binding sites.
Fig. 2.
Structures of robust CB1 allosteric modulators 7, 8 and 9, which served as parent compounds along with 1 for the development of photoactivatable ligands.
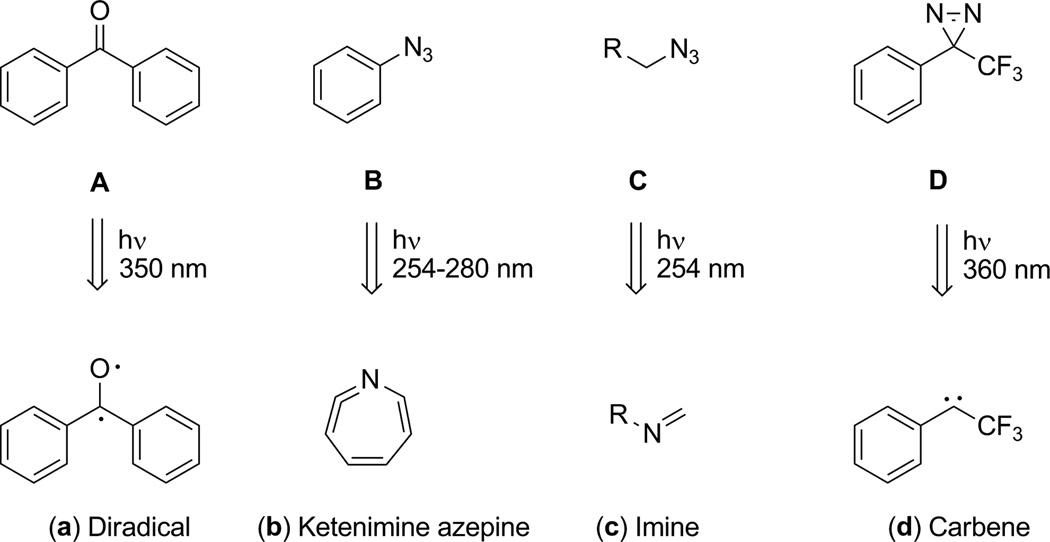
Generally, synthesis of photoaffinity ligands requires the introduction of photoactivatable functionalities (photophores) such as aryl or aliphatic azide, aryl or aliphatic diazirine, and benzophenone or other biaryl ketone derivatives into the parent molecules [40, 41]. Upon UV irradiation, these photophores can produce highly reactive species (Figure 3) that include: C-O diradical (a) derived from benzophenone (A), ketenimine azepine (b) derived from aryl azide (B), imine (c) derived from aliphatic azide (C) and carbene (d) derived from diazirine (D) [40–42]. Ligands producing these reactive species can form irreversible covalent bonding between the reversibly-bounded ligands and the amino acids within the ligand-binding site [40, 42].
Fig. 3.
Photoactivatable functionalities frequently employed in the synthesis of photoaffinity ligands and their corresponding reactive species produced from UV irradiation.
Among these photophores, benzophenone (BP) is known for highly efficient photoaffinity labeling of macromolecules because of its three distinct features: 1) BP is chemically more stable than other photophores; 2) it can be handled in an ambient light environment and activated at ultraviolet wavelengths (350–360 nm) that avoid protein damage; 3) upon UV irradiation, BP can preferentially react with unreactive C-H bonds of macromolecules, even in the presence of solvent water and bulk nucleophiles [42, 43]. Notably, the photoactivatable BP not only has a preference to label the carbon atom at the α-position of amino acids but also has higher cross-linking efficiency (often greater than 70%) than phenyltrifluoromethyldiazirine (PTFMD) and phenyl azide; both commonly exhibit cross-linking efficiency less than 30% [40]. In comparison with BP, PTFMD and phenyl azide (PAZ) can label NH2 and OH in addition to carbon atoms on amino acids [40, 42]. These two photophores generally have less steric bulk than BP and may result in more accurate representation of the parent ligands in binding to the receptor [21].
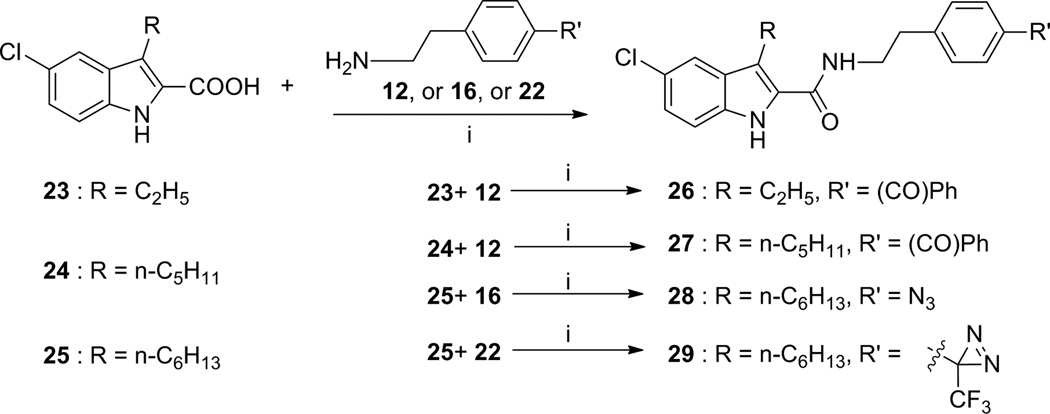
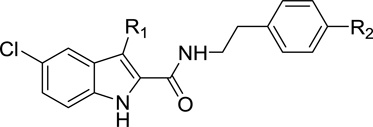
Earlier studies of the structure-activity relationship (SAR) of indole-2-carboxamides revealed that the substitution on the phenyl group of 1 can be structurally varied without significant reduction in allosteric modulatory activity [32–35]. Therefore, benzoyl, azido, and trifluoromethyldiazirinyl (TFMD) groups were respectively employed to replace the C4-substituents on the phenyl ring of compounds 1 7, 8 and 9 to introduce photoactivatable functionalities (i.e., benzophenone, phenyl azide, and phenyltrifluoromethyl diazirine). The phenyl ring of their parent molecules was utilized as a part of the photoactivatable functionalities. These modifications created photoactivatable compounds 26–29 (Table 1).
Table 1.
Allosteric binding parameters of compounds 26–29 in comparison with their corresponding parent compounds 1, 7, and 8.
 | |||||
|---|---|---|---|---|---|
| Type | Compd | R1 | R2 | KB (nM)a | αb |
| PARe | 1c | C2H5 | N-piperidinyl | 217 (170–277) | 6.9 |
| PhAf | 26 | C2H5 | (CO)Ph | 254 (124–518) | 8.4 |
| PARe | 7c | n-C5H11 | N-piperidinyl | 470 (126–1750) | 17.6 |
| PhAf | 27 | n-C5H11 | (CO)Ph | 559 (332–941) | 12.4 |
| PARe | 8c | n-C6H13 | N(CH3)2 | 89.1(47.1–168) | 5.1 |
| PhAf | 28 | n-C6H13 | N3 | 314 (85–1164) | 14.7 |
| PhAf | 29 | n-C6H13 | TFMDd | 3275 (1183–9070) | 16.6 |
KB: equilibrium dissociation constant of a potential allosteric ligand.
α: binding cooperativity factor for the tested allosteric modulator.
Data cited for parent compounds 1, 7, and 8 are from our earlier reports [32, 33] and are given for comparison.
TFMD: (trifluoromethyl)-3H–diazirin-3-yl.
PAR: parent compound.
PhA: photoactivatable compound.
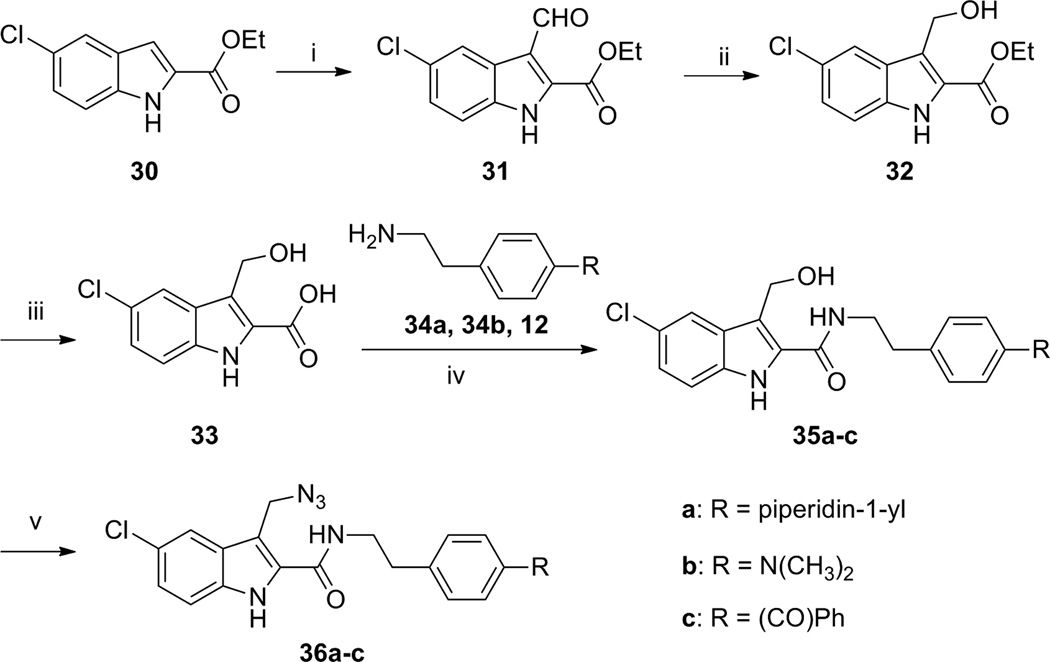
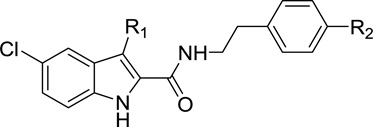
Compared to the three aryl photophores described above, the aliphatic azide moiety is less frequently used in developing photoactivatable ligands. However, successful cross-linking of aliphatic-azide-containing ligands with the CB1 receptor have been reported [44, 45]. To explore the possibility of developing photoactivatable ligands that bear two different photophores, we hypothesized that an aliphatic azido group at the C3-position of the parent indole-2-carboxamides might enable receptor binding and probably function as another photophore. This is supported by previous reports [44, 45] and prompted our synthesis of aliphatic-azide-containing compounds 36a-36c (Table 2).
Table 2.
Allosteric binding parameters of 36a–36c in comparison with their corresponding parent compounds 1 and 9.
 | |||||
|---|---|---|---|---|---|
| Type | Compd | R1 | R2 | KB (nM)a | αb |
| PARd | 1c | CH2CH3 | N-piperidinyl | 217(170–277) | 6.9 |
| PhAe | 36a | CH2-N3 | N-piperidinyl | 784(346–1776) | 13.7 |
| PARd | 9c | CH2CH3 | N(CH3)2 | 207(156–2759) | 19.7 |
| PhAe | 36b | CH2-N3 | N(CH3)2 | 200 (118–340) | 6.7 |
| PhAe | 36c | CH2-N3 | (CO)Ph | 6308 (557–71520) | 1.85 |
KB: equilibrium dissociation constant of a potential allosteric ligand.
α: binding cooperativity factor for the tested allosteric modulator.
Data cited for parent compounds 1 and 9 are from our earlier reports[32, 33] and are given for comparison.
PAR: parent compound.
PhA: photoactivatable compound.
Chemistry
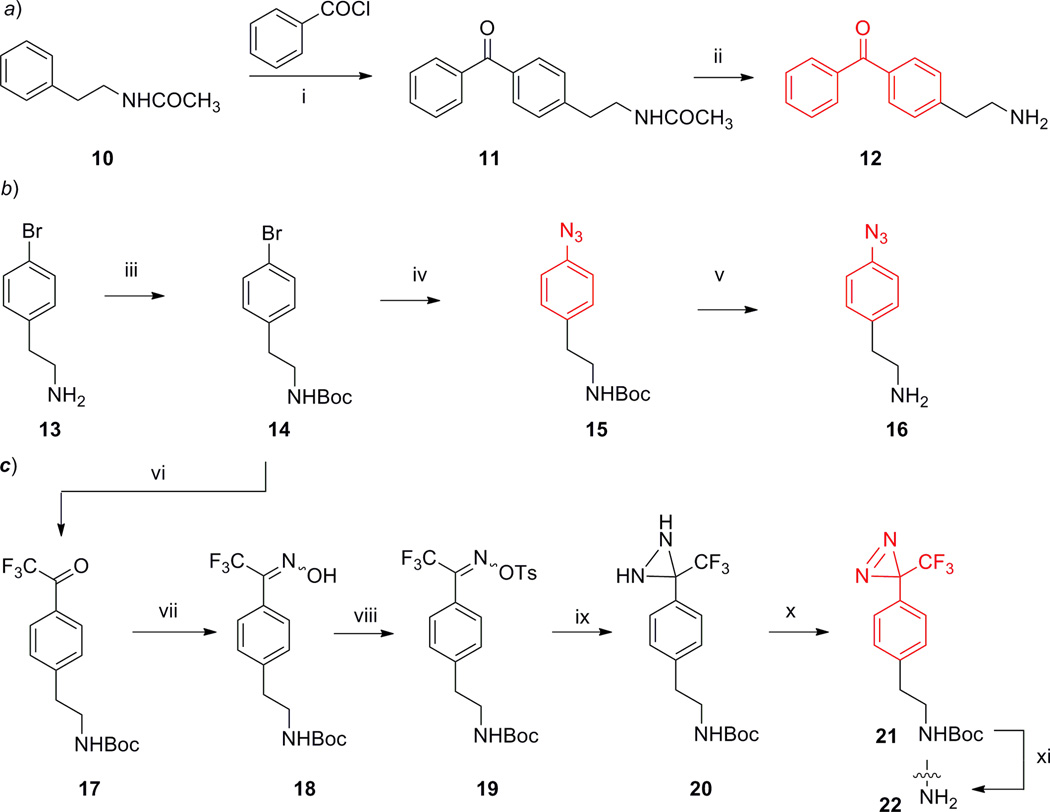
The designed photoactivatable ligands were synthesized according to the procedures described in Schemes 1, 2 and 3. The synthesis started from preparation of the required C2-photophore-substituted ethanamines 12, 16, and 22 as illustrated by Scheme 1 (i.e. route a, b and c respectively).
Scheme 1.
Reagents and conditions: (i) AlCl3, nitrobenzene, 50 °C, 8h, 41%; (ii) conc HCl, EtOH, reflux, 4h, 82%; (iii) Boc2O, NaOH, l,4-dioxane/H2O, 0 °C to rt, 5.5 h, 90%; (iv) NaN3, CuI, Na ascorbate, N,N’-dimethylethylenediamine, EtOH/ H2O, reflux, overnight, 55%; (v) TFA, CH2C12, 0 °C, 2 h, 89%; (vi) KH, 0 °C 1h, then t-BuLi, CF3COOEt, −78 °C, 4h, 35%; (vii) NH2OH·HCl, pyridine, 80 °C, 2h, 64%; (viii) TEA, TsCl, acetone, 0 °C, overnight, 99%; (ix) NH3(liquid), diethyl ether, 30 °C, 1.5 h; (x) MnO2, diethyl ether, rt, 3 h, 85% for two steps; (xi) TFA, CH2Cl2, 0 °C, 2 h, 74%. bPhotophores are highlighted in red.
Scheme 2.
Reagents and conditions: (i) BOP, DIPEA, DMF, rt, 4–12 h.
Scheme 3.
Reagents and conditions: (i) POCl3, DMF, 90 °C, 2h, 89%; (ii) NaBH4, THF, rt, 1h, 57%; (iii) 1N NaOH (aq), reflux, 30 min, 95%; (iv) BOP, DIPEA, DMF, rt, 4–12 h, 18–42%; (v) DPP A, DBU, THF, 0 °C-rt, 2–16 h, 26–37%.
The benzophenone-substituted ethanamine 12 was synthesized using the methods depicted in Scheme 1 (a) with slight modification from a reported protocol in the purification process [46]. Briefly, Friedel-Crafts benzoylation of the commercially available N-phenethylacetamide 10 in nitrobenzene provided the benzophenone-substituted ethylenyl acetamide 11 and, subsequently was purified by chromatography upon complete removal of nitrobenzene through steam distillation. Hydrolysis of 11 in concentrated hydrochloric acid yielded the benzophenone-substituted ethanamine 12.
To prepare the p-azidophenyl-substituted ethanamine 16, the commercially available 2-(4-bromophenyl)ethylamine 13 was firstly treated with di-tert-butyl dicarbonate to give its Boc-protected analogue 14. The conversion of its bromo group to an azido moiety was achieved by a CuI/diamine-catalyzed reaction and yielded 4-azidophenethylcarbamate 15 [47]. Subsequent acidic treatment of the 4-azidophenethylcarbamate 15 yielded 4-azidophenylethanamine 16.
To obtain the desired phenyltrifluoromethyldiazrine-substitued ethanamine 22, the bromo group of 14 was converted to a trifluoroacetyl group (17) by reacting with ethyl 2,2,2-trifluoroacetate in the presence of KH and t-BuLi. To construct the diazrine ring, compound 17 was transformed to the tosyloxime 19 in two steps by following a protocol described earlier [48]. Treating tosyloxime 19 with liquid ammonia in a pressure tube at 30 °C generated the trifluoromethyl diaziridine 20, which was then oxidized by MnO2 to produce (trifluoromethyl)diaziridinylphenethylcarbamate 21. Subsequent removal of the Boc-protective group yielded the desired phenyltrifluoromethyldiazrine-substituted ethanamine 22. It was reported that diazrines can be directly obtained by treating tosyloximes with liquid ammonia above 50 °C [49]. However, our attempt to obtain 21 directly from tosyloxime 19 by reacting with liquid ammonia at a temperature above 50 °C failed to deliver the desired product because of the substantial pressure built up by gaseous ammonia in the sealed reaction vessel.
The obtained photophore-bearing ethanamines 12, 16 and 22 were then employed in the synthesis of corresponding indole-2-carboxamides 26–29 through the methods (Scheme 2) as we described earlier[32]. The required indole-2-carboxylic acids 23–25 were prepared according to our previous report [33].
We envisioned that placing photoactivatable moieties at different positions on the indole-2-carboxamide scaffold could enhance the chances to map out the 3D structure of the allosteric binding site. This can be achieved either by individual labeling of amino acids located at different places in the binding site or through cross-linking by dual-photophore containing ligands. This led us to synthesize the C3-aliphatic azide analogue 36a-36c, of which 36c is a compound bearing two different photophores (i.e. aliphatic azide and benzophenone). The aliphatic-azide containing compounds were synthesized through the methods illustrated in Scheme 3. To introduce the azide group on the C3 alkyl group of indole-2-carboxamides, a formyl group was introduced at C3 under the Vilsmeier–Haack condition to generate the intermediate 31. Reduction of its formyl group with sodium borohydride led to the C3-hydroxymethyl indole-2-carboxylate 32. Upon hydrolysis of its ester group in aqueous NaOH solution, the key building block C3-hydroxymethyl indole-2-carboxylic acid 33 was obtained. Coupling the commercially available amines 34a, 34b and benzophenone-substituted ethylamine 12 individually with the indole-2-carboxylic acid 33 provided the C3-hydroxymethyl indole-2-carboxamides 35a-35c in yields ranging from 18–42%. Conversion of their hydroxyl group to an azido group by reacting with diphenyl phosphorazidate (DPPA) in the presence of DBU produced the desired 3-azidomethyl-carboxamide analogues (36a-36c) in yields ranging from 26 to 37%.
Results and Discussion
The synthesized photoactivatable indole-2-carboxamides (26–29 and 36a-36c) were evaluated for their CB1 allosteric properties. The allosteric modulatory effects were first assessed by their impact on the binding of the orthosteric agonist CP55,940. The binding affinities of 26–29 and 36a–c towards the allosteric site were quantified by their equilibrium dissociation constant KB which was determined according to the protocols reported earlier [29]. Their binding cooperativity factor, α, was analyzed according to the allosteric ternary complex model [10]. The data are presented in Tables 1 and 2 and referenced to their corresponding parent compounds 1 and 7–9, respectively.
In comparison with their parent compounds 1 and 7, the results of 26 and 27 showed that introducing the photophore benzophenone at the 4-position of the phenyl ring of the indole-2-carboxamide scaffold maintained the binding to the CB1 receptor and the allosteric effect on orthosteric ligand binding (Table 1). This suggested that using the phenyl ring of the parent compounds as part of the photophore structure (i.e., benzophenone) is an acceptable approach. The compound 8 has the highest binding affinity to the CB1 receptor in our earlier series of indole-2-carboxamide analogues [32, 33]. To introduce phenyl azide and PTFMD photophores into the indole-2-carboxamide scaffold, we selected compound 8 as the parent molecule because the three nitrogen atoms of azide forms a linear structure [50] and the diazrine is a cyclic moiety. These conformation-restricted moieties might cause steric interference when the ligands bearing these photophores interact with the CB1 allosteric site. In earlier SAR studies, it was also found that cyclic substituents on the 4-position of the phenyl ring of this scaffold was not as tolerated as acyclic substituents [32–34]. The results of 28 and 29 showed that azido and TFMD moieties located at the 4-position of the phenyl ring indeed reduced the binding capacity of the ligands to the allosteric site of CB1 receptor. However, although the azido-containing analogue 28 exhibited a reduced binding capacity to the CB1 receptor in comparison with its parent compound 8, it still showed a binding affinity similar to the prototypical allosteric modulator 1 with an enhanced binding cooperative factor (α=14.7). This result suggests that compound 28 is still a viable allosteric modulator of the CB1 receptor. In this work, benzophenone and phenyl azide were found to be acceptable photophores for incorporation into the indole-2-carboxamide scaffold.
Introducing aliphatic azide at the C3-position of parent compounds 1 and 9 led to photoactivatable ligands 36a and 36b, respectively (Table 2). When the piperidinylphenyl is present, introduction of aliphatic azide (36a) resulted in a significant reduction of binding affinity while its cooperativity factor α was increased. Interestingly, compound 36b showed binding affinity comparable to its parent molecule 9, while its cooperativity factor α was reduced. The results of 36a and 36b suggested that aliphatic azide can be introduced onto the C3 alkyl chain of the indole-2-carboxamides. The binding affinity and allosteric modulatory effects of resultant compounds depend on the C4-substitution of the phenyl ring (R2). Moving forward, simultaneous introduction of aliphatic azide and benzophenone into indole-2-carboxamide yielded the compound 36c whose binding affinity (KB) and allosteric effects on orthosteric ligand binding (α) were remarkably reduced. This probably resulted from the synergistic effects of the two conformation-restricted photophores onto the molecule such that the flexibility and the ligand conformation were limited. Consequently, the ligand could not adopt the proper conformation for binding to the allosteric site. Further investigation of this strategy of introducing two different photophores into indole-2-carboxamide derived CB1 allosteric modulators is required.
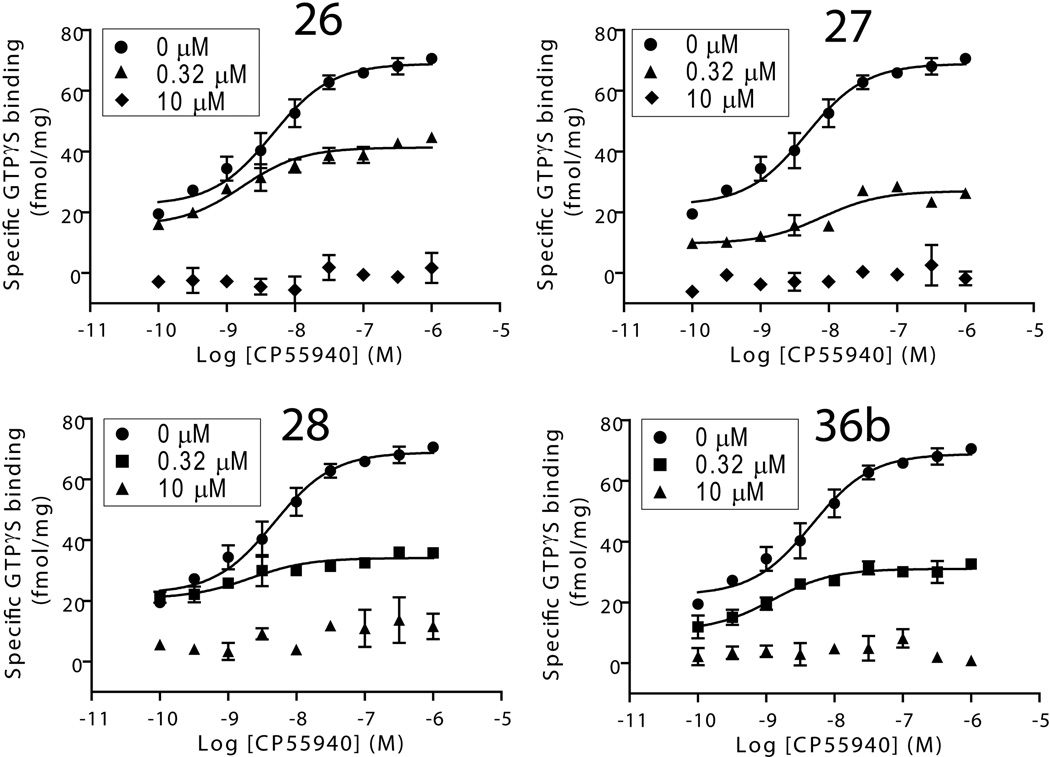
Given the allosteric binding parameters of the active compounds 26–28 and 36b, we further assessed these photoactivatable modulators for their impact on G-protein coupling activity using stimulation of GTPγS binding as an indicator. Similarly to their parent compounds, GTPγS binding data (Figure 4 and Table 3) indicated that these compounds antagonized the CB1 agonist CP55,940-induced G protein coupling in a concentration-dependent manner with a complete inhibition at 10 µM. The results demonstrated that these novel CB1 allosteric modulators behaved similarly to their parent compounds in terms of influencing the function of the CB1 receptor at the level of G-protein coupling. Interestingly, an analog of ORG27569 bearing an isocyanato group was recently reported with potent allosteric modulation of CB1 function and was predicted by computational simulation to be able to covalently label a cysteine residue (Cys382) on the CB1 receptor [51, 52]. This work supports the value of using covalent probes to identify the allosteric site. In contrast, our photoactivatable ligands can provide more specificity upon binding to the allosteric site reversibly and then be activated by UV irradiation to form covalent bonds with the amino acids within their binding site.
Fig. 4.
Effect of photoactivatable allosteric compounds on stimulation of agonist-induced [35S]GTPγS binding to the CB1 receptor. Dose-response curves for CP55,940-induced [35S]GTPγS binding to HEK293 cell membranes expressing the CB1 receptor in the absence and presence of 0.32 µM and 10 µM 26, 27, 28, or 36b. Data are presented as specific binding of GTPγS binding. Nonspecific binding was determined in the presence of 10 µM unlabeled GTPγS. The level of GTPγS binding of mock-transfected samples (non-CBl mediated) was subtracted from each specific binding data point. Each data point represents the mean ± S.E. (error bars) of at least three independent experiments performed in duplicated.
Table 3.
Effect of photoactivatable allosteric modulators on stimulation of [35S]GTPγS binding to HEK293 cell membranes expressing the CB1 wild-type receptor.
| Concentration of photoactivatable allosteric modulators |
||||||
|---|---|---|---|---|---|---|
| Compd | 0 µM | 3.2 µM | 10 µM | |||
| Emax (fmol/mg)a |
EC50 (nM) a |
Emax (fmol/mg) a |
EC50 (nM) a |
Emax (fmol/mg) a |
EC50 (nM) a |
|
| 26 | 68.9 ± 1.7 | 4.7 (2.7–8.0) | 41.3 ± 1.1 | 2.7 (0.9–4.6) | ND | ND |
| 27 | 68.9 ± 1.7 | 4.7 (2.7–8.0) | 27.0 ± 1.3 | 7.9 (2.8–22.0) | ND | ND |
| 28 | 68.9 ± 1.7 | 4.7 (2.7–8.0) | 34.1 ± 1.1 | 2.1 (0.5–8.3) | ND | ND |
| 36b | 68.9 ± 1.7 | 4.7 (2.7–8.0) | 31.1±0.9 | 1.2 (0.5–3.8) | ND | ND |
Emax and EC50 values were determined from the CP55,940 dose-response curves. The level of GTPγS binding of mock-transfected cell membrane (30.1 ± 1.6 fmol/mg) was subtracted from each Emax value. ND, no detectable specific [35S]GTPγS binding.
Our work provides evidence that introducing photoactivatable functionalities into the indole-2-carboxamide scaffold is feasible. The CB1 allosteric modulators bearing photoactivatable benzophenone (i.e. 26), phenyl azide (i.e. 28) and aliphatic azide (i.e. 36b) can interact with the CB1 allosteric site as the prototypical CB1 allosteric modulators do without significant alternation of their properties. Given that aliphatic azide has been found with low cross-linking efficiency (<3–7%) [40], the benzophenone and phenyl azide containing ligands 26 and 28 are more attractive for future application in photolabeling the allosteric site of CB1 receptor. Additionally, we found that all synthesized allosteric modulators bearing photoactivatable functionalities are stable at room temperature storage for at least 6 months.
Conclusion
In this work, we report the development of novel allosteric modulators possessing photoactivatable functionalities for the cannabinoid CB1 receptor. Four photoactivatable analogues of the CB1 allosteric modulator 1 were synthesized through incorporation of different photophores. Binding and functional assays for these ligands indicated that they preserved the pharmacological properties of the prototypical allosteric modulator 1 and have sufficient activity to warrant future development of radioactive and photoactivatable CB1 allosteric modulators. Obtaining such compounds will assist future proteomic investigation of the CB1 allosteric site. This will inform the key amino acids and architecture in the binding motif for allosteric modulators to the CB1 receptor. Obtaining structural information about the allosteric site will propel the structure-based rational design and development of novel CB1 allosteric modulators, which hold great promise in treating disease conditions that can be modified by altering CB1 activity.
Experimental Section
Pharmacology
CB1 Expression and Membrane Preparation
HEK 293 cells were transiently transfected by the calcium phosphate precipitation method. [53] The cells were harvested 24 hours post transfection and washed with phosphate buffered saline (PBS). The cells were re-suspended in PBS containing protease inhibitor cocktail ((4-2-aminoethyl) benzene-sulfonyl fluoride, pepstatin A, E-64, bestatin, leupeptin, and aprotinin) (Sigma-Aldrich, St. Louis, MO) and lysed by nitrogen cavitation at 750 psi for 5 min using a Parr cell disruption bomb. The lysate was clarified by centrifugation at 500 g for 10 min at 4°C, and the supernatant was subsequently spun at 100,000 g for 45 min at 4°C. The membrane-containing pellet was resuspended in TME buffer (25 mM Tris-HCl, 5 mM MgCl2, and 1 mM EDTA, pH 7.4) containing 7% w/v sucrose.
Radioligand Binding Assay
Ligand binding assays were performed as previously described to determine the cooperativity between the orthosteric and allosteric ligands.[29] Briefly, 4 µg of membrane preparation was incubated for 60 min with a fixed concentration of tracer [3H]CP55940 (141 Ci/mmol, PerkinElmer Life Sciences (Boston, MA)) typically at its Kd which was determined from a saturation binding isotherm in TME buffer containing 0.1% fatty acid-free BSA. Nonspecific binding was determined in the presence of unlabeled 1 µM CP55,940. The reaction was terminated by rapid filtration with a Brandell cell harvester through Whatman GF/C filter paper and radioactivity was measured.
GTPγS Binding Assay
GTPγS binding assays were performed as described previously [29]. Briefly, 6 µg of membranes were incubated for 60 min at 30°C in GTPγS binding assay buffer (50 mM Tris-HCl, pH 7.4, 3 mM MgCl2, 0.2 mM EGTA, and 100 mM NaCl) with unlabeled CP55,940 (at least nine different concentrations were used ranging between 100 pM and 1γM), 0.1 nM [35S]GTPγS (1250 Ci/mmol; PerkinElmer Life Sciences, Boston, MA), 10 µM GDP (Sigma, St. Louis, MO), and 0.1% (w/v) BSA in the absence and presence of varying concentrations of the test compounds as indicated. Non-specific binding was determined with 10 µM unlabeled GTPγS (Sigma, St. Louis, MO). The reaction was terminated by rapid filtration through Whatman GF/C filters and the trapped radioactivity determined.
Ligand and GTPγS Binding Data Analysis
Data are presented as the mean ± S.E. or the mean with the corresponding 95% confidence limits from at least three independent experiments each done in duplicate. To assess the level of CB1-mediated GTPγS binding, the level of GTPγS binding of mock-transfected samples (non-CB1 mediated) was subtracted from each data point. [3H]CP55,940 binding and agonist-induced GTPγS binding were analyzed by nonlinear regression using Prism 6.0 (Graphpad Software Inc., San Diego, CA) as previously described. [29]
Chemistry
All chemical reagents and solvents were purchased from Sigma-Aldrich Chemical Co., unless specified otherwise, and used without further purification. All anhydrous reactions were performed under a static argon atmosphere in dried glassware using anhydrous solvents. Organic phases in the work up were dried over anhydrous Na2SO4, and removed by evaporation under reduced pressure. The crude compounds were purified by a Combiflash Rf chromatography system (Teledyne Technologies, Inc, Thousand Oaks, CA) unless specified otherwise. Purities of the intermediates were established by thin-layer chromatography (TLC), melting point, 1H NMR, and mass spectrometry. Analytical Thin-layer Chromatography (TLC) was run on pre-coated silica gel TLC aluminum plates (Whatman®, UV254, layer thickness 250 µm), and the chromatograms were visualized under ultraviolet (UV) light. Melting points were determined on a capillary Electrothermal® melting point apparatus and are uncorrected. 1H NMR spectra of intermediates and 13C NMR of final compounds were recorded on a Bruker Avance DPX-300 spectrometer operating at 300 MHz. 1H NMR spectra of the final compounds were also recorded on a Bruker AV-500 spectrometer operating at 500 MHz. All NMR spectra were recorded using chloroform-d or DMSO-d6 as solvent unless otherwise stated and chemical shifts are reported in ppm (parts per million) relative to tetramethylsilane (TMS) as an internal standard. Multiplicities are indicated as br (broadened), s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), and bs (broadened singlet), and coupling constants (J) are reported in hertz (Hz). High resolution mass spectra were performed at the School of Chemical Sciences, University of Illinois at Urbana-Champaign. The purity of each tested compound was analyzed by combustion elemental analysis performed in Roberson Microlit laboratories (Madison, NJ). Analyses indicated by the symbols of the elements were within ±0.4%. The purity of final compounds were determined to be greater than 95% by elemental analyses (C, H, N).
Preparation of N-(4-benzoylphenethyl)acetamide (11)
The mixture of benzoyl chloride (4.64 g, 33.0 mmole) and N-phenethylacetamide (10; 4.9 g, 30 mmole) in 5 ml of dry nitrobenzene was added anhydrous aluminum chloride (6.0 g, 45 mmole) in small portions with stirring at 0°C. The reaction mixture was subsequently heated at 50 °C for 8 h, and then poured into concentrated hydrochloric acid mixed with ice. The nitrobenzene was completely removed by steam distillation. The resulted residue was cooled and extracted with ethyl acetate. The combined organic extracts were washed with 10% sodium hydroxide aqueous solution, water and brine, and dried over anhydrous sodium sulfate. Filtration and concentration gave the crude product, which was purified by Combiflash chromatography (10% methanol in ethyl acetate) to afford 3.64 g (41%) of light-yellow oil product. 1H NMR (500 MHz, chloroform-d): δ 7.82-7.76 (m, 4H), 7.60 (td, J = 7.5, 1.2 Hz, 1H), 7.50 (t, J = 7.5 Hz, 2H), 7.32 (d, J = 7.9 Hz, 2H), 5.51 (s, 1H), 3.57 (q, J = 7.0 Hz, 2H), 2.93 (t, J = 7.0 Hz, 2H), 1.97 (s, 3H).
(4-(2-Aminoethyl)phenyl)(phenyl)methanone (12)
The mixture of N-(4-benzoylphenethyl)acetamide (11; 2.0 g, 7.5 mmole), ethanol (10 mL) and concentrated HCl (10 mL) was stirred under reflux for 4 h. After cooling to room temperature, the reaction mixture was poured into water, then basified with sodium hydroxide aqueous solution and extracted with diethyl ether. The organic layer was separated and extracted several times with 10% HCl. The acidic aqueous extracts were combined and washed with ether, basified with dilute sodium hydroxide and extracted with diethyl ether. The ethereal extracts were combined, washed with water and brine, dried over sodium sulfate, and evaporated in vacuo to afford 1.38 g (82%) of oily product. 1H NMR (500 MHz, chloroform-d): δ 7.76 (d, J = 8.9 Hz, 2H), 7.58 (d, J = 7.6 Hz, 2H), 7.53-7.42 (m, 3H), 7.34 (d, J = 7.6 Hz, 2H), 3.51 (q, J = 6.5 Hz, 2H), 3.04 (t, J = 6.5 Hz, 2H), 1.10 (br s, 2H). MS (EI): m/z = 196.1 (M+ - CH2=NH).
tert-Butyl 4-bromophenethylcarbamate (14)
2-(4-Bromophenyl)-ethylamine (13, 10.0 g, 50 mmol) and NaOH (3.0 g, 75 mmol) were dissolved in the mixture of dioxane (150 mL) and H2O (150 mL). The reaction mixture was cooled to 0°C. The solution of di-tert-butyl dicarbonate (16.36 g, 75 mmol) in dioxane (72 mL) was added dropwise to the reaction mixture, which was stirred at room temperature for 5.5 h. Dioxane was removed in vacuo and the residue was extracted with ethyl acetate 3 times. The combined organic layer was washed with water and brine, and then dried over anhydrous Na2SO4. Filtration and removal of solvent provided the crude product, which was purified by Combiflash chromatography (5–10% of ethyl acetate in hexane). The title compound 5 (13.5 g, 90.0%) was obtained as a white solid; mp 59–60 °C. 1H NMR (300 MHz, chloroform-d): δ = 7.42 (d, J = 8.0 Hz, 2H), 7.07 (d, J = 8.0 Hz, 2H), 4.51 (br. s, 1H), 3.40-3.27 (m, 2H), 2.75 (t, J = 6.9 Hz, 2H), 1.43 (s, 9H). MS (EI): m/z = 300.1 (M++H).
tert-Butyl 4-azidophenethylcarbamate (15)
tert-4-Bromophenethylcarbamate (14, 2.0 g, 6.6 mmol), NaN3 (1.716 g, 26.4 mmol), sodium ascorbate (133 mg, 0.67 mmol) and N,N’-dimethylethylenediamine (232 mg, 2 mmol) in EtOH (14 mL) and water (6 mL) in a pressure tube were degassed by bubbling argon flow into the reaction mixture for 10 min. CuI (256 mg, 1.32 mmol) was added to the reaction mixture. It was heated at 100 °C for 12 h. The reaction mixture was then poured into 40 mL of water and extracted with ethyl acetate 3 times. The combined organic layer was washed with water and brine, and dried with anhydrous Na2SO4. Filtration and concentration provided the crude product, which was purified by Combiflash chromatography (0–10% of ethyl acetate in hexane) to yield the product 15 as a light yellow solid (960 mg, 54.9%); mp 60–61 °C. 1H NMR (300 MHz, chloroform-d): δ = 7.18 (d, J = 7.8 Hz, 2H), 6.97 (d, J = 7.8 Hz, 2H), 4.53 (br. s, 1H), 3.40-3.24 (m, 2H), 2.77 (t, J = 6.6 Hz, 2H), 1.43 (s, 9H). MS (EI): m/z = 263.2 (M++H).
2-(4-Azidophenyl)ethanamine (16)
Trifluoroacetic acid (TFA, 0.35 mL, 4.57 mmol) was added to the solution of tert-butyl-4-azidophenethylcarbamate (15, 200 mg, 0.762 mmol) in dichloromethane (3 mL) at 0 °C, and the reaction mixture was stirred at the same temperature for 2 h. After TFA and dichloromethane were removed in vacuo, the reaction mixture was added water (10 mL) and then basified with aqueous NaOH solution (2 N, 5 mL). The reaction mixture was stirred for 30 min at room temperature and then extracted with ethyl acetate. The combined organic layer was washed with water, brine and dried over anhydrous Na2SO4. Filtration and removal of solvent in vacuo gave product 7 (110 mg, 89%) as an oil. 1H NMR (300 MHz, chloroform-d): δ = 7.19 (d, J = 8.2 Hz, 2H), 6.97 (d, J = 8.2 Hz, 2H), 2.95 (t, J = 6.9 Hz, 2H), 2.73 (t, J = 6.9 Hz, 2H). MS (EI): m/z = 163.1 (M++H).
tert-Butyl 4-(2,2,2-trifluoroacetyl)phenethylcarbamate (17)
tert-Butyl 4-bromophenethylcarbamate 14 (3.0 g, 10 mmol) in anhydrous THF (5 mL) was added to the suspension of KH (30% in mineral oil 1.5 g, 11mmol) in 50 mL anhydrous THF at 0 °C and stirred for 1h under nitrogen atmosphere. The reaction mixture was cooled to −78 °C, then t-BuLi (1.7 M in heptanes, 14.7 mL, 25 mmol) was added dropwise. The reaction mixture was stirred at −78 °C for 1 h and ethyl trifluoroacetate (6 mL, 50 mmol) was added dropwise and stirred for 2h at same temperature. After the reaction mixture being warmed to room temperature, saturated ammonium chloride aqueous solution was added and stirred for 30 min. The mixture was extracted with ethyl acetate 3 times. The combined organic layer was washed with water and brine, and then dried over anhydrous Na2SO4. Filtration and removal of solvent in vacuo gave the crude product, which was purified by Combiflash chromatography (10–20% of ethyl acetate in hexane) to yield product 17 (1.1 g, 34.7%) as a colorless amorphous solid. 1H NMR (300 MHz, chloroform-d): δ = 8.02 (d, J = 8.1 Hz, 2H), 7.38 (d, J = 8.1 Hz, 2H), 4.57 (br. s, 1H), 3.46-3.38 (m, 2H), 2.91 (t, J = 6.9 Hz,2H), 1.43(s,9H). MS (EI): m/z = 316.1 (M-H)−.
tert-Butyl 4-(2,2,2-trifluoro-1-(hydroxyimino)ethyl)phenethylcarbamate (18)
tert-Butyl 4-(2,2,2-trifluoroacetyl)phenethylcarbamate (17, 400 mg, 1.26 mmol) and hydroxylamine hydrochloride (263 mg, 3.79 mmol) in pyridine (5 mL) were stirred at 80 °C for 2 h. The pyridine was removed in vacuo. The resulted residue was partitioned between water and diethyl ether. The aqueous layer was separated and extracted with diethyl ether 3 times. The combined organic layer was washed with HCl (1 N), water and brine, and dried over anhydrous Na2SO4. Filtration and removal of solvent by evaporation in vacuo gave the crude product, which was purified by Combiflash chromatography (10–20% of ethyl acetate in hexane) to yield the product 9 (268 mg, 64%) as a white solid of a mixture of two isomers; mp of mixture 134–136 °C. 1H NMR (300 MHz, chloroform-d): δ = 9.70 (s, 0.4H), 9.19 (s, 0.2H), 7.45 (d, J = 7.8 Hz, 0.7H), 7.36 (d, J = 7.5 Hz, 1.3H), 7.28 (d, J = 7.8 Hz, 0.7H), 7.20 (d, J = 7.5 Hz, 1.3H), 4.66 (br. s, 1H), 3.40 (br. s, 2H), 2.82 (br. s, 2H), 1.45 (s, 9H). MS (EI): m/z = 331.1 (M-H)−.
tert-Butyl 4-(2,2,2-trifluoro-1-((tosyloxy)imino)ethyl)phenethylcarbamate (19)
Triethylamine (0.57 mL, 4.06 mmol) and p-toluenesulfonyl chloride (515 mg, 2.7 mmol) were added to the solution of tert-butyl 4-(2,2,2-trifluoro-1-(hydroxyimino)ethyl)phenethylcarbamate (18, 450 mg, 1.35 mmol) in acetone (10 mL) at 0 °C. The reaction mixture was stirred at the same temperature for 1 h and allowed to warm up to room temperature. Solvent was evaporated in vacuo, and the residue was partitioned between water and ethyl acetate. The aqueous layer was extracted with ethyl acetate 3 times. The combined organic layer was washed with water and brine, and dried over anhydrous Na2SO4. Filtration and removal of solvent by evaporation in vacuo gave the crude product, which was purified by Combiflash chromatography (10–20% of ethyl acetate in hexane) to provide the product 19 in two fractions (650 mg, 98.7%). The first fraction (Rf = 0.52, hexane/ethyl acetate = 2/1) provided a pure isomer as a white solid; mp 129–130 °C. 1H NMR (300MHz, Chloroform-d): δ = 7.89 (d, J = 8.1 Hz, 2H), 7.42-7.27 (m, 6H), 4.59 (br. s, 1H), 3.46-3.32 (m, 2H), 2.85 (t, J = 6.9 Hz, 2H), 2.48 (s, 3H), 1.44 (s, 9H). The second fraction gave a mixture of two isomers (Rf = 0.52 and Rf = 0.46, hexane/ethyl acetate = 2/1); mp 90–96 °C. 1H NMR (300 MHz, chloroform-d): δ =7.90 (d, J = 8.4 Hz, 2H), 7.42-7.21 (m, 6H), 4.55 (br. s, 1H), 3.45-3.30 (m, 2H), 2.90-2.78 (m, 2H), 2.50-2.44 (m, 3H), 1.42 (s, 9H). MS (EI): m/z = 509.1 (M++Na).
tert-Butyl 4-(3-(trifluoromethyl)diaziridin-3-yl)phenethylcarbamate (20)
A solution of tert-butyl 4-(2,2,2-trifluoro-1-(tosylimino)ethyl)phenethylcarbamate (19, 300 mg, 0.62 mmol) in anhydrous diethyl ether (1 mL) was added into a pressure tube, liquid ammonia (~10 mL) was condensed at −78 °C in a three neck round bottom flask and transferred through cannula into the pressure tube that was also cooled to −78 °C. The reaction mixture was cautiously warmed up to 30 °C and stirred for 1.5 h in the tightly sealed pressure tube. Then it was cooled to room temperature and gradually allowing the residual liquid ammonia to evaporate into an ammonia-disposal container in a draft chamber. The residue containing the crude product 20 (320 mg) was sufficiently pure and was used directly in the next step without further purification. 1H NMR (300 MHz, chloroform-d): δ = 7.55 (d, J = 7.8 Hz, 2H), 7.25 (d, J = 7.8 Hz, 2H), 4.54 (br. s, 1H), 3.44-3.30 (m, 2H), 2.88-2.73 (m, 3H), 2.21 (d, J = 9.0 Hz, 1H), 1.43 (s, 9H). MS (EI): m/z = 332.1 (M++H).
tert-Butyl 4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenethylcarbamate (21)
The mixture of crude tert-butyl 4-(3-(trifluoromethyl)diaziridin-3-yl)phenethylcarbamate (20, 320 mg, 0.97 mmol) and MnO2 (756 mg, 8.7 mmol) in anhydrous diethyl ether (10 mL) was stirred at room temperature for 3 h. MnO2 was then removed by filtration. The residue was concentrated in vacuo and purified by Combiflash chromatography (10–20% of ethyl acetate in hexane) to yield product 21 (173 mg, 85.2% for two steps) as a white solid; mp 69–70 °C. 1H NMR (300 MHz, chloroform-d): δ = 7.23 (d, J = 8.1 Hz, 2H), 7.13 (d, J = 8.1 Hz, 2H), 4.52 (br. s, 1H), 3.42-3.28 (m, 2H), 2.81 (t, J = 6.9 Hz, 2H), 1.42 (s, 9H). MS (EI): m/z = 330.0 (M++H).
2-(4-(3-(Trifluoromethyl)-3H-diazirin-3-yl)phenyl)ethanamine (22)
Trifluoroacetic acid (TFA, 0.33 mL, 4.25 mmol) was added into the solution of tert-butyl 4-(3-(trifluoromethyl)-3H–diazirin-3-yl)phenethylcarbamate (21, 233 mg, 0.71 mmol) in anhydrous dichloromethane (3 mL) at 0 °C and was stirred at the same temperature for 2 h. The reaction was quenched with water (2 mL), followed by removal of TFA in vacuo. The residue was dissolved in ethyl acetate (2 mL) and treated with aqueous NaOH (2 N, 3 mL) to pH 8–9. The mixture was stirred for 1 h and extracted with ethyl acetate 3 times. The combined organic layer was washed with water and brine, dried over anhydrous Na2SO4. Filtration and concentration gave the crude product, which was purified by Combiflash chromatography (10–20% of methanol in dichloromethane) to yield the product 22 (120 mg, 74.0%) as a light-yellow solid; mp 73–77 °C. 1H NMR (300 MHz, chloroform-d): δ = 7.21 (d, J = 8.1 Hz, 2H), 7.12 (d, J = 8.1 Hz, 2H), 3.18-3.06 (m, 2H), 3.02-2.90 (m, 2H). MS (EI): m/z = 230.0 (M++H).
Ethyl 5-chloro-3-formyl-1H-indole-2-carboxylate (31)
The title compound was prepared by modification of a reported method [54]. The solution of ethyl 5-chloro-1H-indole-2-carboxylate (30, 5 g, 0.0224 mol) in dry DMF (10 mL) was cooled in an ice-bath for 30 min. Phosphorus oxychloride (3.9 mL, 0.042 mol) was added dropwise into the reaction mixture in about 5 min. The cooling bath was removed and the reaction mixture was then heated and stirred for 2 h at 90 °C. The colored solution obtained was cooled to room temperature and poured into crushed ice and then neutralized with ammonia. The resulting orange precipitate was filtered and washed with water and crystallized from ethanol to yield 4.88 g (87 %) of light-yellow solid; mp 232–234 °C. 1H NMR (300 MHz, DMSO-d6) δ 13.00 (bs, 1H), 10.56 (s, 1H), 8.21 (s, 1H), 7.58 (d, J = 8.3 Hz, 1H), 7.42 (d, J = 8.3 Hz, 1H), 4.46 (q, J = 6.9 Hz, 2H), 1.40 (t, J = 6.9 Hz, 3H). MS (EI): m/z = 252.04 (M++1).
Ethyl 5-chloro-3-(hydroxymethyl)-1H-indole-2-carboxylate (32)
To the solution of ethyl 5-chloro-3-formyl-1H-indole-2-carboxylate (31, 3.06 g, 0.0122 mol) in anhydrous THF (60 mL) was added NaBH4 (1.15 g, 0.03 mol) slowly at room temperature. The resultant suspension was stirred for 1 h under argon. The reaction was quenched slowly with saturated aqueous NH4Cl solution and extracted twice by THF (2 × 25 mL). The organic extract was washed with brine, separated and dried over anhydrous Na2SO4. Filtration and concentration afforded 2.86 g of yellow solid product, which was purified by Combiflash chromatography (0–20% of ethyl acetate in hexane) to yield 1.77 g (57 %) of colorless solid; mp 139–141°C. 1H NMR (300 MHz, DMSO-d6) δ 11.81 (bs, 1H; exchangeable with D2O), 7.91 (s, 1H), 7.43 (d, J = 8.9 Hz, 1H), 7.26 (d, J = 9.0 Hz, 1H), 4.97 (s, 3H), 4.35 (q, J = 7.0 Hz, 2H), 1.35 (t, J = 7.0 Hz, 3H). MS (EI): m/z = 252.9 (M+).
5-Chloro-3-(hydroxymethyl)-1H-indole-2-carboxylic acid (33)
Ethyl 5-chloro-3-(hydroxymethyl)-1H-indole-2-carboxylate (32, 4.3 g, 16.95 mmol) was added to 1 N sodium hydroxide aqueous solution (30 mL). The reaction mixture was stirred and refluxed to give a homogenous solution in 10 min. The reaction was monitored by TLC (50% ethyl acetate in hexane). Upon completion of the hydrolysis in 30 min, the solution was cooled to room temperature and treated with 1 N HCl to pH 2. The white solid precipitate was filtered, and the cake was washed with a minimal amount of cold water and hexane (3 mL) and then dried in a vacuum oven overnight to afford 3.65 g (95 %) of white solid product which was used in the next step without further purification. mp 206–208 °C. 1H NMR (300 MHz, DMSO-d6) δ 13.37 (bs, 1H), 11.71(s, 1H), 7.88 (s, 1H), 7.41 (d, J = 8.9 Hz, 1H), 7.23 (d, J = 8.9 Hz, 1H), 4.96 (s, 2H). MS (EI): m/z = 225.0 (M+).
General Procedure A: Preparation of indole-2-carboxamides 26–29 and 35a–35c
The solution of a C2-appropriately substituted ethanamine (0.82 mmol) in 1 mL of anhydrous DMF was added to the solution of selected C3-substituted 5-chloroindole-2-carboxylic acid (0.68 mmol), BOP (502 mg, 1.02 mmol), and N,N-diisopropylethylamine (0.71 mL, 4.08 mmol) in anhydrous DMF (4 mL). The reaction mixture was stirred at room temperature for 4h-6h. Upon completion of the coupling reaction, which was monitored by TLC (30% ethyl acetate in hexane), the mixture was poured into cold water (40 mL) and extracted with ethyl acetate twice. The organic layer was separated and washed with water, brine and dried over anhydrous sodium sulfate. Filtration and removal of solvent in vacuo provided the crude product, which was then purified either by trituration followed by recrystallization in the solvent as specified or by Combiflash chromatography to generate the indole-2-carboxamides 26–29 or 35a–35c respectively.
5-Chloro-3-ethyl-N-(4-ethylbenzophenone)-1H-indole-2-carboxamide (26)
The title compound was prepared from 5-chloro-3-ethyl-1H–indole-2-carboxylic acid (23, 200 mg, 0.894 mmol) and (4-(2-aminoethyl)phenyl)(phenyl)methanone (12; 242 mg, 1.07 mmol) according to the general procedure A. The crude product (545 mg) was triturated sequentially with acetone (5 mL) and diethyl ether (5 mL), which was then purified by Combiflash chromatography (0–30% of ethyl acetate in hexane) to yield 143 mg (37 %) of white solid; mp 182–184 °C. 1H NMR (500 MHz, chloroform-d) δ 9.12 (bs, 1H), 7.84-7.77 (m, 4H), 7.61(td, J = 7.3, 1.2 Hz, 1H), 7.57 (s, 1H), 7.48 (t, J = 8.7 Hz, 2H), 7.39 (d, J = 7.9 Hz, 2H), 7.32 (d, J = 8.7 Hz, 1H), 7.23 (dd, J = 8.7, 1.8 Hz, 1H), 6.03 (t, J = 5.3 Hz, 1H), 3.88 (q, J = 6.4 Hz, 2H), 3.09 (t, J = 6.4 Hz, 2H), 2.78 (q, J = 7.8 Hz, 2H), 1.15 (t, J = 7.8 Hz, 3H). 13C NMR (75 MHz, chloroform-d): δ 15.35, 18.35, 35.61, 40.58, 112.88, 118.09, 119.37, 125.13, 125.62, 127.56, 128.32 (2C), 128.72 (2C), 128.86, 129.95 (2C), 130.71 (2C), 132.47, 133.48, 136.23, 137.56, 143.54, 162.16, 196.23. HRMS (ESI) m/z [M+H]+ Calcd for C26H24ClN2O2: 431.1526. Found: 431.1530. Anal. Calcd for (C26H23ClN2O2): C, 72.47; H, 5.38; N, 6.50; found: C, 72.21; H, 5.16; N, 6.43.
5-Chloro-3-pentyl-N-(4-ethylbenzophenone)-1H-indole-2-carboxamide (27)
The title compound was prepared from 5-chloro-3-pentyl-1H-indole-2-carboxylic acid (24, 208 mg, 0.783 mmol) and (4-(2-aminoethyl)phenyl)(phenyl)methanone (12; 212 mg, 0.94 mmol) according to the general procedure A. The crude product (766 mg) was then purified by Combiflash chromatography (0–30% of ethyl acetate in hexane) to yield 339 mg (91.5%) of white solid; mp 149–151 °C. 1H NMR (500 MHz, chloroform-d): δ 9.13 (bs, 1H), 7.82 (d, J = 73 Hz, 2H), 7.79 (d, J = 7.3 Hz, 2H), 7.59 (td, J = 7.3, 1.0 Hz, 1H), 7.55 (s, 1H), 7.48 (t, J = 7.6 Hz, 2H), 7.38 (d, J = 7.6 Hz, 2H), 7.32 (d, J = 8.7 Hz, 1H), 7.23 (dd, J = 8.7, 2.1 Hz, 1H), 6.05 (t, J = 5.8 Hz, 1H), 3.88 (q, J = 6.4 Hz, 2H), 3.08 (t, J = 6.4 Hz, 2H), 2.75 (t, J = 7.8 Hz, 2H), 1.52 (quin, J = 7.8 Hz, 2H), 1.31-1.19 (m, 4H), 0.85 (t, J = 7.8 Hz, 3H). 13C NMR (75 MHz, chloroform-d): δ 13.98, 22.47, 25.12, 30.60, 31.75, 35.68, 40.58, 112.88, 116.85, 119.53, 125.10, 125.59, 127.82, 128.31 (2C), 128.69 (2C), 129.35, 129.91 (2C), 130.73 (2C), 132.42, 133.46, 136.14, 137.59, 143.59, 162.25, 196.12. HRMS (ESI) m/z [M+H]+ Calcd for (C29H29ClN2O2+H): 473.1996. Found: 473.1996. Anal. Calcd for (C29H29ClN2O2): C, 73.64; H, 6.18; N, 5.92; found: C, 73.35; H, 6.10; N, 5.91.
5-Chloro-3-hexyl-N-((4-azido)phenethyl)-1H-indole-2-carboxamide (28)
The title compound was prepared from 5-chloro-3-hexyl-1H-indole-2-carboxylic acid (25, 158 mg, 0.57 mmol) and 2-(4-azidophenyl)ethanamine (16, 110 mg, 0.68 mmol) according to the general procedure A. The crude product was purified by Combiflash chromatography (0–25% of ethyl acetate in hexane) to yield the product 28 (122 mg, 50%) as a white solid; mp 144–146 °C. 1H NMR (500 MHz, chloroform-d): δ = 9.13 (s, 1H), 7.54 (s, 1H), 7.30 (d, J = 8.5 Hz, 1H), 7.24 (d, J = 8.0 Hz, 2H), 7.22 (d, J = 8.5 Hz, 1H), 7.00 (d, J = 8.0 Hz, 2H), 5.98 (s, 1H), 3.81 (q, J = 6.0 Hz, 2H) 2.95 (t, J = 6.5 Hz, 2H), 2.71 (t, J = 7.5 Hz, 2H), 1.51-1.40 (m, 2H), 1.34-1.13 (m, 6H), 0.89 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, chloroform-d): δ = 14.06, 22.61, 25.17, 29.36, 30.87, 31.64, 34.93, 40.73, 112.86, 116.65, 119.42 (2C), 119.50, 125.03, 125.54, 127.90, 129.35, 130.09 (2C), 133.44, 135.20, 138.70, 162.17. HRMS (ESI) m/z [M+H]+ Calcd for (C23H26ClN5O+H): 424.1904. Found: 424.1898. Anal. Calcd for (C23H26ClN5O): C, 65.16; H, 6.18; N, 16.52. Found: C, 65.07; H, 6.26; N, 16.40.
5-Chloro-3-hexyl-N-(4-(3-trifluoromethyl-3H-diazirin-3-yl)phenethyl)-1H-indole-2-carboxamide (29)
The title compound was prepared from 5-chloro-3-hexyl-1H-indole-2-carboxylic acid (25, 101.8 mg, 0.364 mmol) and 2-(4-(3-(Trifluoromethyl)-3H-diazirin-3-yl)phenyl)ethanamine (22, 100 mg, 0.436 mmol) according to the general procedure A. the crude product was purified by Combiflash chromatography (10–25% of ethyl acetate in hexane) to give final product 29 (100 mg, 56%) as a white solid; mp 128–129 °C. 1H NMR (500 MHz, chloroform-d): δ = 9.08 (s, 1H), 7.54 (d, J = 3.0 Hz, 1H), 7.33-7.27 (m, 3H), 7.22 (dd, J = 3.5 Hz, 15 Hz, 1H), 7.17 (d, J = 13.5 Hz, 2H), 5.95 (t, J = 10 Hz, 1H), 3.80 (q, J = 10.5 Hz, 2H), 2.99 (t, J = 11.0Hz, 2H), 2.69 (t, J = 13.0 Hz, 2H), 1.46-1.39 (m, 2H), 1.27-1.13 (m, 6H), 0.89 (t, J = 11.5 Hz, 3H). 13C NMR (75 MHz, chloroform-d): δ = 14.03, 22.55, 25.08, 28.30 (q, 2JC,F = 40.1 Hz), 29.25, 30.81, 31.55, 35.27, 40.57, 112.86, 116.75, 119.53, 122.10 (q, 1JC,F = 272.9 Hz), 125.09, 125.59, 126.90 (2C), 127.74, 127.81, 129.25 (2C), 129.34, 133.45, 140.47, 162.20. HRMS (ESI) m/z [M+H]+ Calcd for (C25H26ClF3N4O+H): 491.1825. Found: 491.1823. Anal. Calcd for (C25H26ClF3N4O): C, 61.16; H, 5.34; N, 11.41. Found: C, 61.33; H, 5.53; N, 11.14.
5-Chloro-N-(4-(piperidin-1-yl)phenethyl)-3-(hydroxymethyl)-1H-indole-2-carboxamide (35a)
The title compound was prepared from ethyl-5-chloro-(3-hydroxymethyl)-1H-indole-2-carboxylic acid (33; 500 mg, 2.22 mmol) and commercially available 2-(4-(piperidin-1-yl)phenyl)ethanamine (34a; 543 mg, 2.66 mmol) according to the general procedure A. 160 mg (17.5 %) of white solid was isolated as product by Combiflash chromatography (0–30% of ethyl acetate in hexane); mp 202–205 °C. 1H NMR (300 MHz, DMSO-d6): δ 11.72 (bs, 1H; exchangeable with D2O), 8.86 (bs, 1H; exchangeable with D2O), 7.77 (s, 1H), 7.40 (d, J = 8.50 Hz, 1H), 7.18 (d, J = 9.40 Hz, 1H), 7.10 (d, J = 8.0 Hz, 2H), 6.85 (d, J = 8.0 Hz, 2H), 5.81 (bs,1H; exchangeable with D2O), 4.76 (bs, 2H), 3.52 (q, J = 5.3 Hz, 2H), 3.13-3.02 (m, 4H), 2.75 (t, J = 5.5 Hz, 2H), 1.69-1.44 (m, 6H). MS (EI): m/z = 411.1 (M+), 396.1 (M+-OH+2). Anal. Calcd for (C23H26ClN3O2): C, 67.06; H, 6.36; N, 10.20. Found: C, 67.34; H, 6.54; N, 10.04.
5-Chloro-N-(4-(dimethylamino)phenethyl)-3-(hydroxymethyl)-1H-indole-2-carboxamide (35b)
The title compound was prepared from ethyl-5-chloro-(3-hydroxymethyl)-1H-indole-2-carboxylic acid (33; 460 mg, 2.04 mmol) and commercially available 4-(2-aminoethyl)-N,N-dimethyl aniline (34b; ChemBridge, 402 mg, 2.45 mmol) according to the general procedure A. 227 mg (30 %) of white solid was isolated as product by Combiflash chromatography (0–30% of ethyl acetate in hexane); mp 199–202 °C. 1H NMR (300 MHz, DMSO-d6): δ 11.71 (bs, 1H; exchangeable with D2O), 8.84 (bs, 1H; exchangeable with D2O), 7.77 (s, 1H), 7.41 (d, J = 8.50 Hz, 1H), 7.18 (d, J = 8.50 Hz, 1H), 7.09 (d, J = 8.10 Hz, 2H), 6.66 (d, J = 8.10 Hz, 2H), 5.80 (t, J = 4.30 Hz, 1H; exchangeable with D2O), 4.77 (d, J = 4.30 Hz, 2H), 3.51 (q, J = 6.8 Hz, 2H), 2.84 (s, 6H), 2.74 (t, J = 6.8 Hz, 2H). MS (EI): m/z = 372.4 (M++1), 354.4 (M+-OH-1).
N-(4-Benzoylphenethyl)-5-chloro-3-(hydroxymethyl)-1H-indole-2-carboxamide (35c)
The title compound was prepared from ethyl-5-chloro-(3-hydroxymethyl)-1H-indole-2-carboxylic acid (33; 500 mg, 2.22 mmol) and (4-(2-aminoethyl)phenyl)(phenyl)methanone (12; 600 mg, 2.66 mmol) according to the general procedure A. 163 mg (41.8 %) of white solid was isolated as product by Combiflash chromatography (0–30% of ethyl acetate in hexane); mp 193–196 °C. 1H NMR (300 MHz, DMSO-d6): δ 11.72 (bs, 1H; exchangeable with D2O), 8.91 (bs, 1H; exchangeable with D2O), 7.77 (s, 1H), 7.75-7.60 (m, 5H), 7.60-7.51(m, 2H), 7.48 (d, J = 7.50 Hz, 2H), 7.41 (d, J = 8.50 Hz, 1H), 7.18 (d, J = 8.70 Hz, 1H), 5.79 (t, J = 5.0 Hz, 1H; exchangeable with D2O), 4.74 (d, J = 4.90 Hz, 2H), 3.67 (q, J = 6.7 Hz, 2H), 2.99 (t, J = 6.7 Hz, 2H). MS (EI): m/z = 432.1 (M+), 416.1 (M+-OH+1). Anal. Calcd for (C25H21ClN2O3): C, 69.36; H, 4.89; N, 6.47. Found: C, 69.24; H, 5.11; N, 6.25.
General procedure B: Preparation of 5-chloro-N-(4-substituted-phenethyl)-3-(azidomethyl)-1H-indole-2-carboxamides (36a–36c)
A mixture 5-chloro-N-(4-substituted phenethyl)-3-(hydroxymethyl)-1H–indole-2-carbox-amides (35, 0.5 mmol) and diphenylphosphoryl azide (DPPA) (166.5 mg, 0.605 mmol) was dissolved in anhydrous THF (15 mL). The mixture was cooled to 0 °C under nitrogen, and then DBU (92 mg, 0.605 mmol) was added. The reaction was stirred for 2 h at 0 °C and then at 20 °C for 16 h. Upon completion of the replacement reaction, which was monitored by TLC (30% ethyl acetate in hexane), the reaction mixture was poured into cold water (30 mL) and extracted with dichloromethane twice (2 × 20 mL). The organic layer was separated and washed twice with water (2 × 10 mL), 5% HCl (10 mL) and brine, and then dried over anhydrous sodium sulfate. Filtration and removal of solvent in vacuo provided the crude product, which was purified by Combiflash chromatography (0–30 % of ethyl acetate in hexane) to afford the products (36a–36c).
3-(Azidomethyl)-5-chloro-N-(4-(piperidin-1-yl)phenethyl)-1H-indole-2-carboxamide (36a)
The title compound was prepared from 5-chloro-N-(4-(piperidin-1-yl)phenethyl)-3-(hydroxymethyl)-1H–indole-2-carboxamide (35a; 206 mg, 0.50 mmol), DPPA (166.5 mg, 0.605 mmol), and DBU (92 mg, 0.605 mmol) according to the general procedure B. 56.5 mg (26 %) of white solid was isolated by Combiflash chromatography (0–30% of ethyl acetate in hexane); mp 148–151°C. 1H NMR (500 MHz, chloroform-d): δ 9.82 (bs, 1H), 7.61 (d, J = 1.9 Hz, 1H), 7.38 (d, J = 8.5 Hz, 1H), 7.25 (dd, J = 8.5, 1.9 Hz,1H), 7.14 (d, J = 7.6 Hz, 2H), 7.03 (t, J = 5.6 Hz, 1H), 6.91 (d, J = 7.6 Hz, 2H), 4.45 (s, 2H), 3.78 (q, J = 6.3 Hz, 2H), 3.15-3.09 (m. 4H), 2.90 (t, J = 63 Hz, 2H), 1.73-1.67 (m, 4H), 1.59-1.54 (m. 2H). 13C NMR (75 MHz, chloroform-d): δ 24.27, 25.83 (2C), 34.40, 41.43, 44.71, 50.83 (2C), 109.02, 113.43, 116.87 (2C), 118.31, 125.28, 126.74, 128.62, 128.81, 129.30 (2C), 131.25, 133.09, 151.13, 161.26. HRMS (ESI) m/z [M + H]+ Calcd for (C23H25ClN6O+H): 437.1857. Found: 437.1858. Anal. Calcd for (C23H25ClN6O): C, 63.22; H, 5.77; N, 19.23. Found: C, 62.99; H, 5.68; N, 18.95.
5-Chloro-N-(4-(dimethylamino)phenethyl)-3-(azidomethyl)-1H-indole-2-carboxamide (36b)
The title compound was prepared from 5-chloro-N-(4-(dimethylamino)phenethyl)-3-(hydroxymethyl)-1H-indole-2-carboxamide (35b; 186 mg, 0.5 mmol), DPPA (166.5 mg, 0.605 mmol), and DBU (92 mg, 0.605 mmol) according to the general procedure B. 74 mg (37 %) of white solid was isolated by Combiflash chromatography (0–30% of ethyl acetate in hexane); mp 154–157 °C. 1H NMR (500 MHz, chloroform-d): δ 9.66 (bs, 1H; exchangeable with D2O), 7.61 (d, J = 2.0 Hz,, 1H), 7.37 (d, J = 8.50 Hz, 1H), 7.25 (dd, J = 8.50, 2.0 Hz, 1H), 7.14 (d, J = 8.20 Hz, 2H), 7.01 (t, J = 4.8 Hz, 1H), 6.72 (d, J = 8.20 Hz, 2H), 4.47 (s, 2H), 3.77 (q, J = 6.3 Hz, 2H), 2.92 (s, 6H), 2.89 (t, J = 6.3 Hz, 2H). 13C NMR (75 MHz, chloroform-d): δ = 34.32, 40.75 (2C), 41.56, 44.74, 109.01, 113.04 (2C), 113.43, 118.32, 125.25, 126.13, 126.72, 128.62, 129.37 (2C), 131.30, 133.11, 149.61, 161.27. HRMS (ESI) m/z [M + H]+ Calcd for (C20H21ClN6O+H): 397.1544. Found: 397.1531. Anal. Calcd for (C20H21ClN6O): C, 60.53; H, 5.33; N, 21.18. Found: C, 60.57; H, 5.25; N, 20.91.
3-(Azidomethyl)-N-(4-benzoylphenethyl)-5-chloro-1H-indole-2-carboxamide (36c)
The title compound was prepared from N-(4-benzoylphenethyl)-5-chloro-3-(hydroxymethyl)-1H-indole-2-carboxamide (35c; 217 mg, 0.50 mmol), DPPA (166.5 mg, 0.605 mmol), and DBU (92 mg, 0.605 mmol) according to the general procedure B. 69 mg (30%) of white solid was isolated by Combiflash chromatography (0–30% of ethyl acetate in hexane); mp 151–153°C. 1H NMR (500 MHz, chloroform-d): δ 9.72 (bs, 1H), 7.83-7.72 (m, 4H), 7.62 (s, 1H), 7.59 (dt, J = 7.5, 1.2 Hz, 1H), 7.48 (t, J = 7.5 Hz, 2H), 7.41-7.36 (m, 3H), 7.27 (dd, J = 8.7, 1.8 Hz,1H), 7.17 (t, J = 5.4 Hz, 1H), 4.50 (s, 2H), 3.87 (q, J = 6.4 Hz, 2H), 3.09 (t, J = 6.4 Hz, 2H). 13 C NMR(75 MHz, chloroform-d): δ 35.50, 40.96, 44.78, 109.23, 113.40, 118.36, 125.50, 126.93, 128.30 (2C), 128.60, 128.71 (2C), 129.96 (2C), 130.61 (2C), 130.96, 132.41, 133.08, 136.14, 137.60, 143.55, 161.35, 196.30. MS (EI): m/z = 458.1 (M++1), 417.1 (M+-N3+1). HRMS (ESI) m/z [M + Na]+ Calcd for (C25H20ClN5O2+Na): 480.1203. Found: 480.1202. Anal. Calcd for (C25H20ClN5O2): C, 65.57; H, 4.40; N, 15.29. Found: C, 65.77; H, 4.31; N, 15.05.
Supplementary Material
Highlights.
Benzophenone, aryl azide, aliphatic azide and diazrine are selected as photophores.
Allosteric photoactivatable ligands were developed for the CB1 receptor.
The photophore-bearing CB1 allosteric modulators behave similarly as their parent ligands.
Allosteric modulation of these new ligands on the CB1 receptor was demonstrated.
Acknowledgments
This work was supported by National Institutes of Health (Grant DA039942 to D.A.K. and D.L.), and a scholarship from the China Scholarship Council (Grant 201408210107 to C.J.Q. and D.L.).
Abbreviations
- CB1
Cannabinoid type 1 receptor
- GPCR
G-protein coupled receptor
- nAChRs
nicotinic acetylcholine receptors
- BP
benzophenone
- PTFMD
phenyltrifluoromethyldiazirine
- PAZ
phenyl azide
- SAR
structure activity relationship
- TFMD
trifluoromethyldiazirinyl
- DIPEA
diisopropylethyl amine
- PAR
parent compound
- PhA
photoactivatable compound
- PBS
phosphate buffered saline
- TLC
thin-layer chromatography
- TMS
tetramethylsilane
- TFA
trifluoroacetic acid
- DPPA
diphenylphosphoryl azide
- [35S]GTPγS
guanosine 5′-O-(3-[35S]thio)triphosphate
- ERK
extracellular signal-regulated kinases
- cAMP
3'-5'-cyclic adenosine monophosphate
- HEK293
human embryonic kidney 293 cells
- BSA
bovine serum albumin
- TME
Tris-Mg2+-EDTA
- EGTA
ethylene glycol tetraacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORSHIP CONTRIBUTIONS AND AUTHOR DECLARATION
Participated in research design: Lu, Kendall, Ahn, Kolluru
Conducted experiments: Ali, Ahn, Qiao, Lu
Performed data analysis: Lu, Kendall, Ahn, Ali, Qiao
Wrote or contributed to the writing of the manuscript: Lu, Kendall, Ahn, Ali, Qiao, Kolluru
Authors disclosed no actual or potential conflict of interest, and have approved the article.
References
- 1.Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Cannabinoids: Springer; 2005. pp. 299–325. [DOI] [PubMed] [Google Scholar]
- 2.Pertwee RG. Pharmacology of cannabinoid CB 1 and CB 2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 3.Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47(Suppl 1):345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br. J. Pharmacol. 2006;147(Suppl 1):S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackie K. Cannabinoid receptors as therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- 6.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 8.Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47:345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology (Berl.) 2009;205:171–174. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, McLean A, McIntosh L, Goodwin G, Walker G, Westwood P, Marrs J, Thomson F, Cowley P, Christopoulos A, Pertwee RG, Ross RA. Allosteric modulation of the cannabinoid CB1 receptor. Mol. Pharmacol. 2005;68:1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- 11.Horswill JG, Bali U, Shaaban S, Keily JF, Jeevaratnam P, Babbs AJ, Reynet C, Wong Kai P. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br. J. Pharmacol. 2007;152:805–814. doi: 10.1038/sj.bjp.0707347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro HA, Howard JL, Pollard GT, Carroll FI. Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br. J. Pharmacol. 2009;156:1178–1184. doi: 10.1111/j.1476-5381.2009.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pamplona FA, Ferreira J, Menezes de Lima O, Jr, Duarte FS, Bento AF, Forner S, Villarinho JG, Bellochio L, Wotjak CT, Lerner R, Monory K, Lutz B, Canetti C, Matias I, Calixto JB, Marsicano G, Guimaraes MZ, Takahashi RN. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc. Natl. Acad. Sci. U. S. A. 2012;109:21134–21139. doi: 10.1073/pnas.1202906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ignatowska-Jankowska BM, Baillie GL, Kinsey S, Crowe M, Ghosh S, Owens RA, Damaj MI, Poklis J, Wiley JL, Zanda M. A Cannabinoid CB1 Receptor Positive Allosteric Modulator Reduces Neuropathic Pain in the Mouse with no Psychoactive Effects. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer M, Chicca A, Tamborrini M, Eisen D, Lerner R, Lutz B, Poetz O, Pluschke G, Gertsch J. Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J. Biol. Chem. 2012;287:36944–36967. doi: 10.1074/jbc.M112.382481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller CE, Schiedel AC, Baqi Y. Allosteric modulators of rhodopsin-like G protein-coupled receptors: opportunities in drug development. Pharmacol. Ther. 2012;135:292–315. doi: 10.1016/j.pharmthera.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinette D, Neamati N, Tomer KB, Borchers CH. Photoaffinity labeling combined with mass spectrometric approaches as a tool for structural proteomics. Expert Rev Proteomics. 2006;3:399–408. doi: 10.1586/14789450.3.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arevalo E, Chiara DC, Forman SA, Cohen JB, Miller KW. Gating-enhanced Accessibility of Hydrophobic Sites within the Transmembrane Region of the Nicotinic Acetylcholine Receptor’s δ-Subunit A TIME-RESOLVED PHOTOLABELING STUDY. J. Biol. Chem. 2005;280:13631–13640. doi: 10.1074/jbc.M413911200. [DOI] [PubMed] [Google Scholar]
- 20.Hamouda AK, Stewart DS, Husain SS, Cohen JB. Multiple transmembrane binding sites for p-trifluoromethyldiazirinyl-etomidate, a photoreactive Torpedo nicotinic acetylcholine receptor allosteric inhibitor. J. Biol. Chem. 2011;286:20466–20477. doi: 10.1074/jbc.M111.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davie BJ, Sexton PM, Capuano B, Christopoulos A, Scammells PJ. Development of a Photoactivatable Allosteric Ligand for the M1 Muscarinic Acetylcholine Receptor. ACS Chem. Neurosci. 2014;5:902–907. doi: 10.1021/cn500173x. [DOI] [PubMed] [Google Scholar]
- 22.Fisher A, Mann A, Verma V, Thomas N, Mishra RK, Johnson RL. Design and synthesis of photoaffinity-labeling ligands of the L-prolyl-L-leucylglycinamide binding site involved in the allosteric modulation of the dopamine receptor. J. Med. Chem. 2006;49:307–317. doi: 10.1021/jm050644n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigel E, Buhr A. The benzodiazepine binding site of GAB A A receptors. Trends Pharmacol. Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- 24.Pozdnyakov N, Murrey HE, Crump CJ, Pettersson M, Ballard TE, am Ende CW, Ahn K, Li Y-M, Bales KR, Johnson DS. γ-Secretase modulator (GSM) photoaffinity probes reveal distinct allosteric binding sites on presenilin. J. Biol. Chem. 2013;288:9710–9720. doi: 10.1074/jbc.M112.398602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eriksson S, Caras IW, Martin DW. Direct photoaffinity labeling of an allosteric site on subunit protein M1 of mouse ribonucleotide reductase by dTTP. Proc. Natl. Acad. Sci. U. S. A. 1982;79:81–85. doi: 10.1073/pnas.79.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson W, Hopkins JL, Jeanfavre DD, Barney KL, Kelly TA, Grygon CA. Characterization of the allosteric inhibition of a protein-protein interaction by mass spectrometry. J. Am. Soc. Mass Spectrom. 2003;14:8–13. doi: 10.1016/S1044-0305(02)00706-7. [DOI] [PubMed] [Google Scholar]
- 27.Zvonok N, Xu W, Williams J, Janero DR, Krishnan SC, Makriyannis A. Mass spectrometry-based GPCR proteomics: comprehensive characterization of the human cannabinoid 1 receptor. J. Proteome Res. 2010;9:1746–1753. doi: 10.1021/pr900870p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szymanski DW, Papanastasiou M, Melchior K, Zvonok N, Mercier RW, Janero DR, Thakur GA, Cha S, Wu B, Karger B, Makriyannis A. Mass spectrometry-based proteomics of human cannabinoid receptor 2: covalent cysteine 6.47(257)-ligand interaction affording megagonist receptor activation. J. Proteome Res. 2011;10:4789–4798. doi: 10.1021/pr2005583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn KH, Mahmoud MM, Kendall DA. Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J. Biol. Chem. 2012;287:12070–12082. doi: 10.1074/jbc.M111.316463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baillie GL, Horswill JG, Anavi-Goffer S, Reggio PH, Bolognini D, Abood ME, McAllister S, Strange PG, Stephens GJ, Pertwee RG, Ross RA. CB(1) receptor allosteric modulators display both agonist and signaling pathway specificity. Mol. Pharmacol. 2013;83:322–338. doi: 10.1124/mol.112.080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn KH, Mahmoud MM, Samala S, Lu D, DA K. Profiling two indole-2-carboxamides for allosteric modulation of the CB1 receptor. J. Neurochem. 2013;124:584–589. doi: 10.1111/jnc.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmoud MM, Ali HI, Ahn KH, Damaraju A, Samala S, Pulipati VK, Kolluru S, Kendall DA, Lu D. Structure-Activity Relationship Study of Indole-2-carboxamides Identifies a Potent Allosteric Modulator for the Cannabinoid Receptor 1 (CB1) J. Med. Chem. 2013;56:7965–7975. doi: 10.1021/jm4009828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khurana L, Ali HI, Olszewska T, Ahn KH, Damaraju A, Kendall DA, Lu D. Optimization of Chemical Functionalities of Indole-2-Carboxamides to Improve Allosteric Parameters for the Cannabinoid Receptor 1 (CB1) J. Med. Chem. 2014;57:3040–3052. doi: 10.1021/jm5000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen T, German N, Decker AM, Li J-X, Wiley JL, Thomas BF, Kenakin TP, Zhang Y. Structure-activity relationships of substituted 1H–indole-2-carboxamides as CB1 receptor allosteric modulators. Bioorganic & medicinal chemistry. 2015;23:2195–2203. doi: 10.1016/j.bmc.2015.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montecucco F, Di Marzo V, da Silva RF, Vuilleumier N, Capettini L, Lenglet S, Pagano S, Piscitelli F, Quintao S, Bertolotto M, Pelli G, Galan K, Pilet L, Kuzmanovic K, Burger F, Pane B, Spinella G, Braunersreuther V, Gayet-Ageron A, Pende A, Viviani GL, Palombo D, Dallegri F, Roux-Lombard P, Santos RA, Stergiopulos N, Steffens S, Mach F. The activation of the cannabinoid receptor type 2 reduces neutrophilic protease-mediated vulnerability in atherosclerotic plaques. Eur. Heart J. 2012;33:846–856. doi: 10.1093/eurheartj/ehr449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shore DM, Baillie GL, Hurst DH, Navas F, Seltzman HH, Marcu JP, Abood ME, Ross RA, Reggio PH. Allosteric Modulation of a Cannabinoid G Protein-coupled Receptor BINDING SITE ELUCIDATION AND RELATIONSHIP TO G PROTEIN SIGNALING. J. Biol. Chem. 2014;289:5828–5845. doi: 10.1074/jbc.M113.478495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fay JF, Farrens DL. A key agonist-induced conformational change in the cannabinoid receptor CB1 is blocked by the allosteric ligand Org 27569. J. Biol. Chem. 2012;287:33873–33882. doi: 10.1074/jbc.M112.352328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fay JF, Farrens DL. Structural dynamics and energetics underlying allosteric inactivation of the cannabinoid receptor CB1. Proc. Natl. Acad. Sci. U. S. A. 2015;112:8469–8474. doi: 10.1073/pnas.1500895112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jing L, Qiu Y, Zhang Y, Li J-X. Effects of the cannabinoid CB 1 receptor allosteric modulator ORG 27569 on reinstatement of cocaine-and methamphetamine-seeking behavior in rats. Drug Alcohol Depend. 2014;143:251–256. doi: 10.1016/j.drugalcdep.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleming SA. Chemical reagents in photoaffinity labeling. Tetrahedron. 1995;51:12479–12520. [Google Scholar]
- 41.Baslé E, Joubert N, Pucheault M. Protein chemical modification on endogenous amino acids. Chem. Biol. 2010;17:213–227. doi: 10.1016/j.chembiol.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Dorman G, Prestwich GD. Using photolabile ligands in drug discovery and development. Trends Biotechnol. 2000;18:64–77. doi: 10.1016/s0167-7799(99)01402-x. [DOI] [PubMed] [Google Scholar]
- 43.Dorman G, Prestwich GD. Benzophenone photophores in biochemistry. Biochemistry. 1994;33:5661–5673. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- 44.Charalambous A, Yan G, Houston DB, Howlett AC, Compton DR, Martin BR, Makriyannis A. 5'-Azido-.DELTA.8-THC: a novel photoaffinity label for the cannabinoid receptor. J. Med. Chem. 1992;35:3076–3079. doi: 10.1021/jm00094a023. [DOI] [PubMed] [Google Scholar]
- 45.Li C, Xu W, Vadivel SK, Fan P, Makriyannis A. High affinity electrophilic and photoactivatable covalent endocannabinoid probes for the CB1 receptor. J. Med. Chem. 2005;48:6423–6429. doi: 10.1021/jm050272i. [DOI] [PubMed] [Google Scholar]
- 46.Murai Y, Masuda K, Ogasawara Y, Wang L, Hashidoko Y, Hatanaka Y, Iwata S, Kobayashi T, Hashimoto M. Synthesis of Photoreactive 2-Phenethylamine Derivatives– Synthesis of Adenosine Derivatives Enabling Functional Analysis of Adenosine Receptors by Photoaffinity Labeling. European J. Org. Chem. 2013:2428–2433. [Google Scholar]
- 47.Andersen J, Madsen U, Björkling F, Liang X. Rapid synthesis of aryl azides from aryl halides under mild conditions. Synlett. 2005:2209–2213. [Google Scholar]
- 48.Murai Y, Masuda K, Sakihama Y, Hashidoko Y, Hatanaka Y, Hashimoto M. Comprehensive synthesis of photoreactive (3-trifluoromethyl) diazirinyl indole derivatives from 5-and 6-trifluoroacetylindoles for photoaffinity labeling. J. Org. Chem. 2012;77:8581–8587. doi: 10.1021/jo301552m. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Murai Y, Yoshida T, Ishida A, Masuda K, Sakihama Y, Hashidoko Y, Hatanaka Y, Hashimoto M. Alternative One-Pot Synthesis of (Trifluoromethyl) phenyldiazirines from Tosyloxime Derivatives: Application for New Synthesis of Optically Pure Diazirinylphenylalanines for Photoaffinity Labeling. Org. Lett. 2015;17:616–619. doi: 10.1021/ol503630z. [DOI] [PubMed] [Google Scholar]
- 50.Bräse S, Gil C, Knepper K, Zimmermann V. Organic azides: an exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. 2005;44:5188–5240. doi: 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]
- 51.Kulkarni PM, Kulkarni AR, Korde A, Tichkule RB, Laprairie RB, Denovan-Wright EM, Zhou H, Janero DR, Zvonok N, Makriyannis A. Novel electrophilic and photoaffinity covalent probes for mapping the cannabinoid 1 receptor allosteric site (s) J. Med. Chem. 2015;59:44–60. doi: 10.1021/acs.jmedchem.5b01303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laprairie RB, Kulkarni AR, Kulkarni PM, Hurst DP, Lynch DL, Reggio PH, Janero DR, Pertwee RG, Stevenson LA, Kelly M. Mapping Cannabinoid Receptor 1 Allosteric Site (S): Critical Molecular Determinant and Signaling Profile of GAT100, A Novel, Potent and Irreversibly Binding Probe. ACS Chem. Neurosci. 2016 doi: 10.1021/acschemneuro.6b00041. [Epub ahead of print, Nov.28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Gendy AA, Said MM, Ghareb N, Mostafa YM, El-Ashry ESH. Synthesis and Biological Activity of Functionalized Indole-2-carboxylates, Triazino- and Pyridazin-indoles. Arch. Pharm. 2008;341:294–300. doi: 10.1002/ardp.200700161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.