Abstract
Skilled motor training results in reorganization of contralateral motor cortex movement representations. The ipsilateral motor cortex is believed to play a role in skilled motor control, but little is known about how training influences reorganization of ipsilateral motor representations of the trained limb. To determine whether training results in reorganization of ipsilateral motor cortex maps, rats were trained to perform the isometric pull task, an automated motor task that requires skilled forelimb use. After either 3 or 6 months of training, intracortical microstimulation (ICMS) mapping was performed to document motor representations of the trained forelimb in the hemisphere ipsilateral to that limb. Motor training for 3 months resulted in a robust expansion of right forelimb representation in the right motor cortex, demonstrating that skilled motor training drives map plasticity ipsilateral to the trained limb. After 6 months of training, the right forelimb representation in the right motor cortex was significantly smaller than the representation observed in rats trained for 3 months and similar to untrained controls, consistent with a normalization of motor cortex maps. Forelimb map area was not correlated with performance on the trained task, suggesting that task performance is maintained despite normalization of cortical maps. This study provides new insights into how the ipsilateral cortex changes in response to skilled learning and may inform rehabilitative strategies to enhance cortical plasticity to support recovery after brain injury.
Keywords: motor training, map plasticity, ipsilateral hemisphere
1. Introduction
Skilled motor training causes reorganization of movement representation maps in the motor cortex contralateral to the trained limb [1–4]. This reorganization is specific to the trained movement, and repetitive unskilled movements do not result in map reorganization [1,2].
Neural circuits in ipsilateral motor cortex also contribute to motor function in both healthy [5–8] and injured subjects [9,10]. Unilateral motor cortex injury has been shown to result in ipsilateral motor dysfunction [11,12]. Additionally, after unilateral brain injury, significant reorganization occurs in the undamaged hemisphere (the hemisphere ipsilateral to the affected limb) [13]. This ipsilateral reorganization is believed to contribute to recovery of motor function [14,15]. Despite the presence of ipsilateral motor control in both healthy and injured conditions, little is known about how motor training affects plasticity in the ipsilateral hemisphere.
We hypothesized that unilateral skilled motor training would increase the representation of the trained forelimb in the ipsilateral motor cortex. To test this, we trained rats to perform the isometric pull task, which involves skilled usage of the right forelimb. After several weeks of training, standard ICMS was used to derive motor cortex organization in the right hemisphere (ipsilateral to the trained limb) in trained rats and untrained controls.
We found that training increases the area representing the trained forelimb in ipsilateral motor cortex. Additionally, we observed that these map changes are transient despite continued training, similar to reports examining the motor cortex contralateral to the trained limb [16]. This study documents novel cortical map plasticity of trained forelimb representations in ipsilateral motor cortex and may inform strategies to enhance ipsilateral reorganization to support recovery after unilateral brain injury.
2. Methods
2.1 Subjects
Seventeen adult female Sprague-Dawley rats were used. All rats were 4 – 5 months old and weighed at least 250 grams at the beginning of the experiment. Rats were food deprived to no less than 85% of their normal body weight throughout the experiment. 85% of normal body weight was calculated using each rat’s weight at the beginning of the experiment. The rats were housed in a reverse 12:12 hour light cycle. All handling, housing, surgical procedures, and behavioral training of the rats were approved by the University of Texas Institutional Animal Care and Use Committee.
2.2 Experimental Design
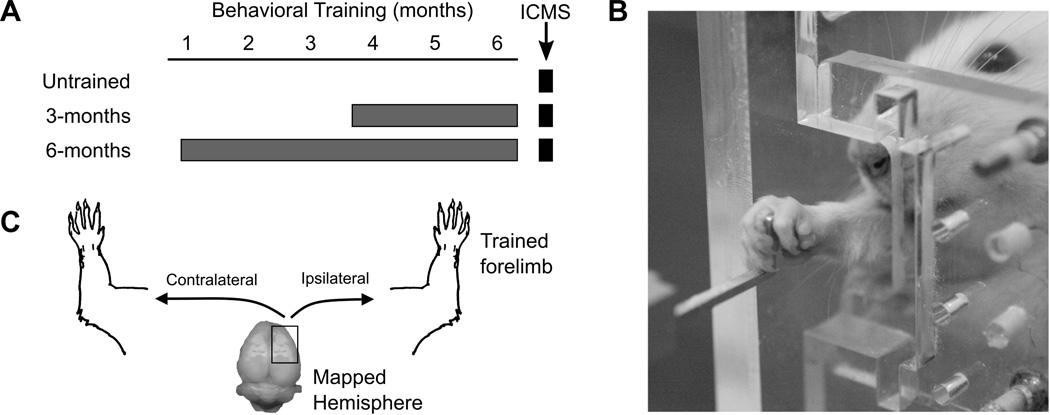
The rats were separated into three experimental groups: untrained controls (n = 6), 3-months training (n = 6), and 6-months training (n = 5). Each of the training groups performed the isometric pull task for two 30 minute sessions each day, 5 days per week (Figure 1A and 1B). Rats in the 3-month training group were trained for 11 – 13 weeks, and rats in the 6-month training group were trained for 22 – 24 weeks. Untrained rats underwent ICMS mapping to document baseline cortical representations. After completion of the prescribed duration of training, all rats underwent ICMS to investigate right-sided movement representations in the ipsilateral (right) hemisphere (Figure 1C). Both untrained and trained rats were between 7 and 10 months of age at the time of ICMS.
Figure 1.
Experimental Design. (A) Rats were trained on the isometric pull task for 3 or 6 months and then underwent ICMS mapping. An additional group of untrained controls rats was mapped. (B) Example of a rat performing the isometric pull task. (C) ICMS was performed in the right hemisphere in all rats. The primary outcome measure was the number of ipsilateral (right) forelimb responses observed during ICMS. Contralateral (left) movements evoked by ICMS in the right hemisphere were also recorded.
2.3 Behavioral Apparatus
The behavioral apparatus and software were used as described in previous studies [17–20]. The isometric pull task is designed to assess overall skilled forelimb function and volitional forelimb strength. The apparatus consisted of an acrylic box (25.4 × 30.5 × 12.1 cm) (MotoTrak Base Cage, Vulintus, Inc., Dallas, TX). The box contained a slot in the front right corner that the rat could reach through to access an aluminum pull handle. The slot was sized and positioned such that rats could only reach and pull using their right forelimb. For fully trained rats, the pull handle was centered in the slot at a height of 6.4 cm from the cage floor and 1.9 cm outside the cage relative to the inner cage wall. The aluminum handle was connected to a force transducer that could measure pull force with sub-gram accuracy (Pull Behavior Module, Vulintus, Inc., Dallas, TX). The force transducers were inspected daily and recalibrated when necessary. Matlab software was used to control the behavioral apparatus. A microprocessor controller (Controller, Vulintus, Inc., Dallas, TX) sampled the force transducer at 100 Hz and sent the information to the Matlab software which displayed the data on screen, controlled the behavior session, and saved the data to permanent files.
2.4 Behavioral Training
Rats underwent training for two 30-minute sessions per day, five days per week, with at least 2 hours between daily sessions. In initial training sessions, shaping procedures were similar to those previously described [17–20]. Rats were trained to pull on the handle with at least 120 g of force, and single reward pellets were dispensed following successful trials (45mg dustless precision pellet, BioServ, Frenchtown, NJ). If rats did not receive at least 50 pellets per day, they were given 10 g of additional pellets after daily training sessions were completed.
A trial was initiated when at least 10 g of force were applied to the pull handle. After initiation, the force on the pull handle was sampled for 4 seconds. If the 120 g force threshold was met within a 2 second window after force initiation, the trial was recorded as a success and a reward pellet was delivered. If the force threshold was not reached within 2 seconds, the trial was recorded as a failure and no reward was delivered.
2.5 Intracortical Microstimulation Procedure
After the completion of training, standard short-duration ICMS [21,22] was performed in the right hemisphere, and both left-side (contralateral) and right-sided (ipsilateral) movements were evaluated by an experimenter blinded to the rat’s experimental condition. Rats were anesthetized with a cocktail of ketamine hydrochloride (50 mg/kg), xylazine (20 mg/kg), and acepromazine (5 mg/kg) injected intramuscularly. Supplemental doses were administered as required to maintain a constant level of anesthesia based on observations of whisking, toe-pinch response, and oxygen saturation.
After placing the rat in the stereotaxic frame, a craniotomy and duratomy were performed to expose motor cortex in the right hemisphere (4 mm to −3 mm AP, and 0.25 mm to 5 mm ML). A tungsten electrode (impedance less than 1 MΩ) was inserted to a depth of 1.8 mm into the cortex. Electrode penetrations were performed along a grid with each site spaced 500 µm apart. Stimulation sites were chosen randomly, with an effort made to ensure that each site was at least 1 mm in distance from the immediately previous penetration. Each stimulation consisted of a 40 ms pulse train of ten 200-µs monophasic cathodal pulses delivered at 286 Hz [21,22]. A maximum stimulation intensity of 300 µA was used to determine the presence of any ipsilateral (right) forelimb response to stimulation. The maximum stimulation intensity was chosen based on previous studies evaluating ipsilateral motor responses [5,9]. The current was then lowered to find the threshold current at which movement was observed. After determining the presence of an ipsilateral forelimb response, the current was once again lowered until a threshold could be found for any contralateral (left) motor movements associated with the stimulation site. If no ipsilateral or contralateral movement was observed at the maximal stimulation intensity, then the site was deemed nonresponsive. The borders of motor cortex were defined based on nonresponsive sites on all sides.
Blind motor mapping procedures were performed with two experimenters, such that the experimenter classifying the movement was blind to treatment group and electrode location. The first experimenter would place the electrode and record data; the second experimenter would deliver stimulation and classify responses. Each site was classified based on the part of the body that moved at the threshold stimulation current. Movements were divided into the following categories: head, forelimb, and hindlimb. Jaw, vibrissae, and neck movement were recorded as head movement. Movement of the digits, wrist, elbow, and shoulder were classified as forelimb movement. Cortical area was calculated by multiplying the number of sites eliciting a response by 0.25 mm2 (0.5 mm × 0.5 mm). Individual map data for all subjects can be found in the online supplement.
2.6 Statistical Analysis
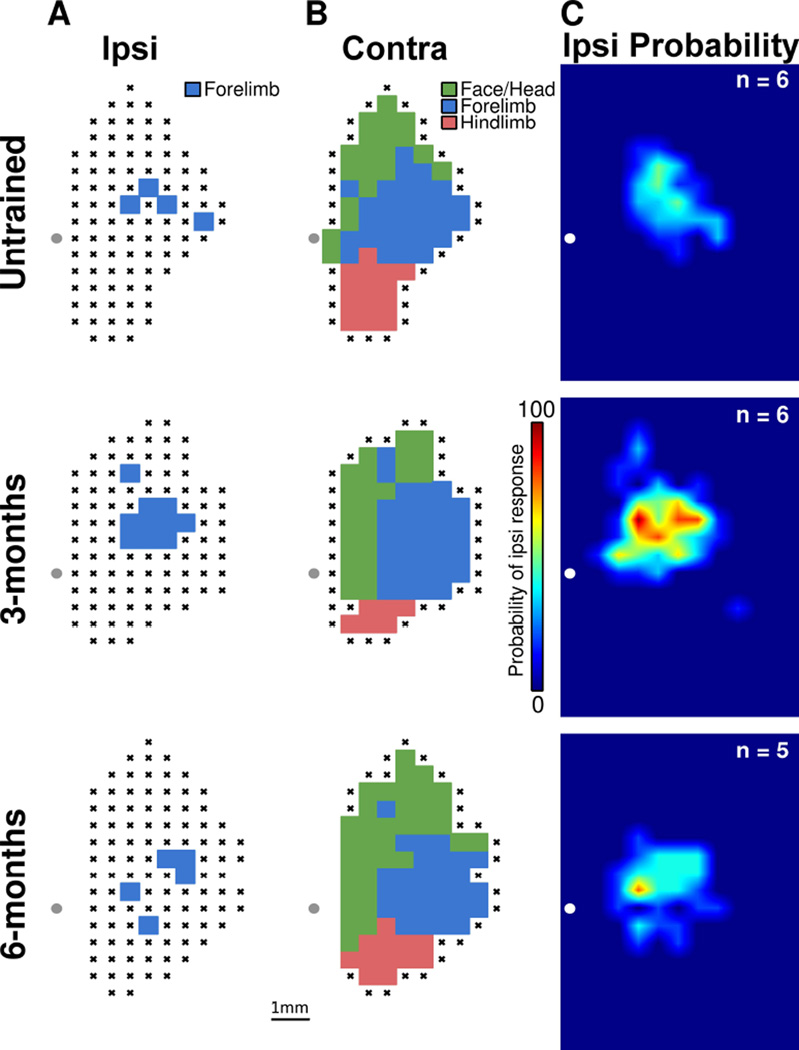
Behavioral data obtained with the isometric pull task was analyzed using Matlab software similar to previous descriptions [17–20]. Training groups were compared across time using a repeated-measures ANOVA. Mapping data was compared across groups using one-way ANOVA followed by post hoc unpaired t-tests, Bonferroni corrected for multiple comparisons to an adjusted cut-off value of α = 0.0167. In Figure 3, heat maps indicate the probability of observing an ipsilateral forelimb response at each site in motor cortex within each group. These heat maps were generated by summing the total number of within-group ipsilateral forelimb responses at each site and then dividing by the total number of rats in each group. This yielded a probability of observing a within-group response at each site, and these were plotted onto the heat map with warmer colors indicating greater probabilities. Two-dimensional interpolation was performed to smooth the heat map, with 5 interpolation points being positioned between each penetration site.
Figure 3.
Skilled motor training results in a transient expansion of ipsilateral forelimb movement representation. (A) Representative ipsilateral motor maps for each group. This data represents right-side forelimb movements evoked by stimulation of the right motor cortex. Note the expansion in forelimb representation in the rats trained for 3 months. (B) Representative contralateral movement representations from the same subjects shown in panel A. This data represents left-side movements evoked by stimulation of the right motor cortex. No differences were observed in contralateral movement representations between groups. (C) Group heat plots demonstrating the probability that any individual location in the motor cortex will evoke an ipsilateral forelimb response. After 3 months of training, stimulation has a higher probability to evoke an ipsilateral forelimb movement. The grey dot in each representative map, and the white dot in each heat map, represent the location of bregma. Each location marked with an X panels A and B represents an unresponsive site.
3 Results
3.1 Rats rapidly acquired and consistently performed the pull task
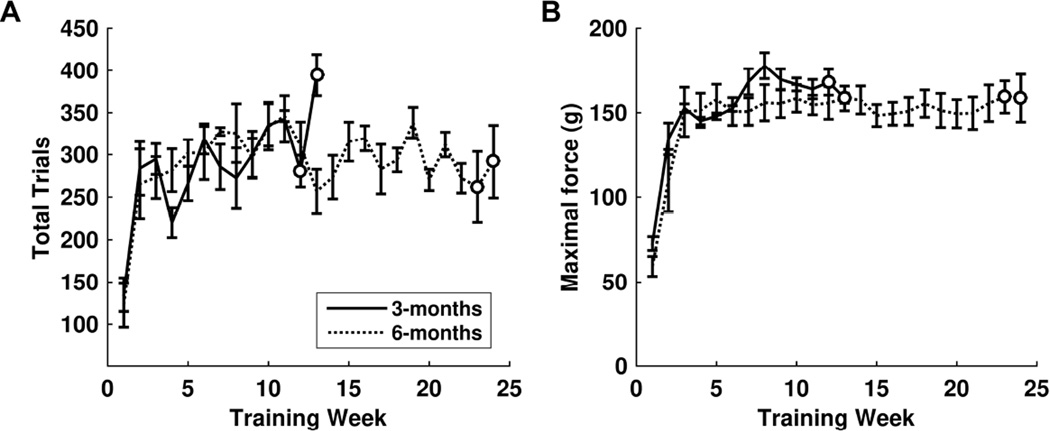
Rats in both training groups acquired the pull task quickly. By the end of the first week of training, rats were performing an average of 141 ± 19 trials each day. By the second week, rats were performing 273 ± 21 trials each day. From that point forward, the number of daily trials remained consistent at this level for the duration of training. Rats were randomly assigned groups to undergo training for either 3 months or 6 months (Figure 1A). No significant differences in total trials were observed between the two different groups of trained rats during the first 3 months of training (repeated-measures ANOVA, F(1, 63) = 2.56, p = 0.11; Figure 2A).
Figure 2.
Rats demonstrate stable forelimb performance on the isometric pull task. (A) Within 2 weeks of beginning training, rats consistently performed over 250 trials per day. No difference in number of trials per day was observed between the 3-months and 6-months training groups. (B) By the third week of training, both groups demonstrated stable performance, consistently reaching forces higher than the 120 g threshold. No differences in pull force were observed between training groups. Open circles denote weeks during which rats were removed for ICMS, resulting in a lower n at these time points. The 3-months training group had n = 5 at week 12 and n = 2 at week 13. The 6-months training group had n = 4 at week 23 and n = 2 at week 24.
3.2 Rats maintained high pull forces throughout training
Pull force steadily increased during the first three weeks of training across both groups. After the third week, pull force reached an asymptote and remained stable throughout the rest of the training period. By the third week of training, daily maximal pull force of rats averaged 152.2 ± 6.3 g. There were no significant differences in pull force between the two training groups during the initial 3 months of training (repeated-measures ANOVA, F(1, 63) = 3.02, p = 0.087; Figure 2B). Additionally, between the initial 3 months and the end of training, rats in the 6-months training group experienced no change in pull force (repeated-measures ANOVA from week 13 to week 24 of the 6-months training rats, F(11, 44) = 0.22, p = 0.99). This indicates that performance was not different between groups and remained highly stable over long periods of time.
3.3 Pull training results in increased ipsilateral cortical area representing the trained forelimb
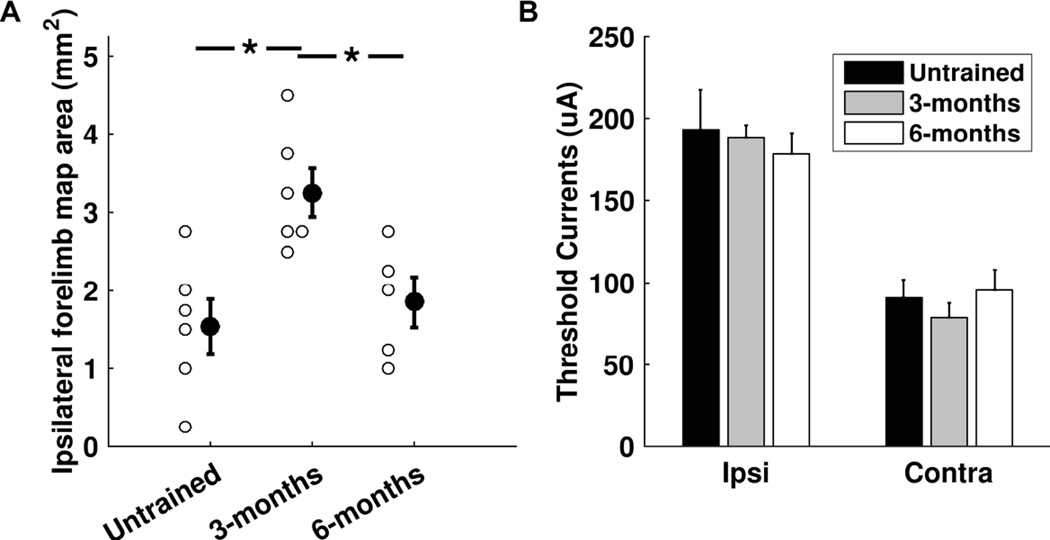
At the completion of the prescribed duration of training, all rats underwent ICMS to determine whether training results in expansion of trained forelimb representation in the ipsilateral hemisphere (Figure 1C). An additional group of untrained control rats were mapped to derive baseline cortical movement representations. Figures 3A and 3B illustrate example ipsilateral and contralateral maps from each group. Individual map data for each subject can be found in the online supplement. An analysis of variance revealed a significant effect of pull training on ipsilateral cortical area representing the trained forelimb (F(2, 14) = 7.95, p = 0.0049). Post-hoc tests demonstrated that rats in the 3-month training group had significantly greater cortical representation of the right forelimb than untrained control rats (untrained controls = 1.54 ± 0.35 mm2, 3-months training = 3.25 ± 0.31 mm2, unpaired t-test, p = 0.0044). Figure 3C shows heat maps that illustrate the respective probabilities that any site in motor cortex would elicit an ipsilateral forelimb response in the experimental groups. The group of untrained controls had 0.75 mm2 of cortical area with at least 50% probability of evoking an ipsilateral forelimb movement, while the 3-months training group had 3.25 mm2 of cortical area with at least 50% probability of evoking an ipsilateral forelimb movement. These results indicate that training on the pull task drives map plasticity in motor cortex ipsilateral to the trained limb.
Previous studies in the contralateral hemisphere indicate that training-dependent map plasticity is transient and renormalizes despite continued training [16]. We examined whether the ipsilateral motor cortex exhibited a similar normalization of map representations. Ipsilateral forelimb map area was significantly smaller in rats trained for 6 months than in rats trained for 3 months (6-months training = 1.85 ± 0.32 mm2, unpaired t-test vs 3-months training, p = 0.012; Figure 4A). Moreover, ipsilateral cortical representation of the trained forelimb in the 6-month training group was comparable to untrained controls (unpaired t-test, p = 0.54). The 6 month training group had 0.25 mm2 of cortical area with at least 50% probability of evoking ipsilateral movement, similar to levels observed in untrained controls (Figure 3C). These results suggest that normalization of trained forelimb maps within ipsilateral cortex occurs despite continued training.
Figure 4.
Summary of movement representations over the course of training. (A) Subjects that undergo forelimb training for 3 months show an expansion of the trained forelimb representation in ipsilateral motor cortex. At six months of training, this expansion in no longer observed. Open circles denote map area in individual subjects. Solid circles with error bars denote the group mean ± SEM. * denotes p < 0.0167. (B) Ipsilateral forelimb response thresholds were consistently significantly higher than corresponding contralateral forelimb response thresholds.
Although there was an expansion of cortical representation of the trained forelimb in the ipsilateral hemisphere, no changes were observed in movement representations corresponding to the untrained, contralateral limb within that same hemisphere (untrained controls = 8.0 ± 0.6 mm2, 3-months training = 8.1 ± 0.5 mm2, 6-months training = 8.2 ± 0.5 mm2; F(2, 14) = 0.03, p = 0.97). Additionally, we did not observe any significant differences in contralateral hindlimb representations (F(2, 14) = 0.84, p = 0.45) or total area representing all contralateral movements (F(2, 14) = 1.92, p = 0.18). Figure 3B shows representative motor maps of contralateral movements derived from the same subjects and same hemisphere shown in Figure 3A. This further indicates that cortical organization of the untrained, contralateral limbs was not affected by training of the ipsilateral forelimb. These findings demonstrate that cortical reorganization within the right hemisphere (ipsilateral to the trained limb) was specific to the trained right forelimb.
To assess whether ipsilateral shoulder, proximal, or distal forelimb map representations were differentially affected by skilled motor training, we analyzed these separate responses as a percentage of total ipsilateral forelimb responses. We did not observe any significant changes in the percentage of ipsilateral responses that were distal forelimb, proximal forelimb, or shoulder responses, respectively (distal: F(2, 14) = 1.99, p = 0.17; proximal: F(2,14) = 2.83, p = 0.093; shoulder: F(2, 14) = 0.67, p = 0.53). No significant differences were found in threshold currents to evoke ipsilateral forelimb responses between groups (untrained controls = 193.15 ± 24.2 µA, 3-months training = 188.46 ± 7.4 µA, 6-months training = 178.11 ± 12.89 µA; F(2, 14) = 0.2, p = 0.82; Figure 4B). Similar to previous studies [5], ipsilateral forelimb response thresholds were significantly greater than contralateral forelimb response thresholds across corresponding stimulation sites (All ipsilateral response thresholds = 192.30 ± 5.0 µA, corresponding contralateral response thresholds = 86.74 ± 3.3 µA; paired t-test, p = 5.6×10−51). Figure 4B shows threshold currents of ipsilateral and contralateral forelimb responses at corresponding sites and between groups. No significant differences between groups were observed in threshold amplitudes of contralateral forelimb responses at sites that also had ipsilateral forelimb responses (untrained controls = 90.87 ± 11.1 µA, 3-months training = 78.75 ± 8.9 µA, 6-months training = 95.45 ± 12.6 µA; F(2, 14) = 0.64, p = 0.54; Figure 4B). Additionally, no significant differences between groups were observed in threshold amplitudes evoking responses of the contralateral, untrained forelimb (untrained controls = 134.25 ± 5.6 µA, 3-months training = 123.81 ± 5.8 µA, 6-months training = 125.11 ± 13.1 µA; F(2, 14) = 0.5, p = 0.62). These results indicate that training did not result in significant changes to cortical response threshold within the ipsilateral hemisphere.
To determine whether performance on the pull task was associated with trained forelimb cortical map area, a Pearson correlation analysis was used to compare total ipsilateral cortical forelimb area and maximal force used during the last five days of training. No correlation was observed in either group between maximal pull force and total cortical area representing the trained forelimb (3-months training: r = 0.15, p = 0.76, 6-months training: r = 0.04, p = 0.94), nor in all trained rats as a whole (r = 0.31, p = 0.36). Additionally, there was also no correlation in all rats and in each training group between total ipsilateral forelimb map area and variance in pull force (all rats: r = −0.08, p = 0.81; 3-months training: r = −0.22, p = 0.68, 6-months training: r = −0.14, p = 0.82). This suggests that performance on the isometric pull task is maintained despite normalization of motor map representations.
4 Discussion
In this study, we demonstrate that skilled forelimb training results in expansion of forelimb movement representation maps in the ipsilateral motor cortex. This reorganization is present even after three months of training, several weeks after the task was initially learned. After six months of training, movement representations were indistinguishable from untrained controls, suggesting a normalization of this movement representation. Ipsilateral cortical area representing the trained forelimb was not correlated with motor performance. Similar to previous studies, organization of the untrained limb within the ipsilateral hemisphere was not affected by training [23].
The cortical reorganization observed in this study corroborates what has previously been found to occur in the contralateral hemisphere. Skilled motor training results in reorganization of movement representations in the contralateral motor cortex [1,4,16,24,25]. This reorganization is specific to the trained limb [1,2]. Training-dependent expansion of movement representations is transient, and map sizes renormalize after a period of time in spite of continued training [16]. Previous studies have investigated map reorganization during the learning phase of task training, however, in this study we chose to investigate map reorganization after the task was already well-learned and rats had achieved a stable level of performance on the task. The results of this study, show expansion and normalization at different time-points than what has been observed in previous studies [1,4,16]. It is possible that the rate of expansion and normalization is influenced by many factors including hemispheric differences, task difficulty, or motivation. Indeed, previous studies indicate that skilled learning-induced potentiation, which is associated with an increase in map area, is not induced within the ipsilateral motor cortex in the first two weeks of training [26,27]. Therefore, it is likely that these changes in ipsilateral representations occur after the initial learning phase of the motor task, and are accompanied by a similar process of potentiation at this later phase. Regardless of the factors that influence the timing of map dynamics, we observed transient, training-specific expansion and normalization of movement representations in the motor cortex ipsilateral to the trained forelimb similar to what has been previously observed in the contralateral motor cortex.
Although the majority of forelimb motor studies have focused on control of the contralateral limb, previous studies have indicated the presence of functionally-relevant motor control from the ipsilateral motor cortex. Brus-Ramer et al. (2009) demonstrated the presence of ipsilateral cortical motor maps in untrained rats. Based on their study, these ipsilateral motor responses seem primarily dependent on the presence of interhemispheric connections, because inactivation of contralateral pathways inhibits the capacity of the ipsilateral hemisphere to elicit forelimb movement. Despite this, evidence suggests that latent ipsilateral corticospinal connections exist that may be important for direct ipsilateral control [5]. The present study builds on these findings and indicates that movement representations in ipsilateral motor cortex undergo training-dependent plasticity. This study is limited in that we cannot disambiguate whether this training-dependent plasticity represents strengthened interhemispheric connections, altered connectivity within latent ipsilateral efferents, or a combination of both mechanisms. Future studies may use a combination of intracortical microstimulation and tract tracing techniques to evaluate whether ipsilateral cortical reorganization is due to strengthening direct corticospinal projections or interhemispheric connections. Regardless, this study demonstrates that ipsilateral motor cortex displays transient, training-dependent expansion of movement representations.
Cortical plasticity is important for supporting recovery after brain injury. It is well documented that there is significant reorganization in both the ipsilesional and the contralesional hemispheres after brain injury [9,13,28–31]. This study indicates that skilled training can influence plasticity in the ipsilateral hemisphere; this training-dependent ipsilateral plasticity may interact with contralesional plasticity after unilateral brain injury to influence functional recovery. Further studies are needed to document the interaction of these effects.
This study supports previous findings that indicate task performance is maintained after map normalization, and performance is not dependent on total cortical map area [16,32], but it does not rule out that map reorganization plays a role in task acquisition or motor rehabilitation. Because map expansion is often observed earlier in training, the extent of reorganization may initially be related to performance while still actively learning a skilled task. Additionally, motor rehabilitation after brain injury also promotes cortical reorganization [33], and some previous studies have demonstrated that the amount of post-injury training-dependent reorganization is associated with motor recovery [31,34]. The Expansion-Renormalization hypothesis [35] theorizes that both expansion and normalization of cortical maps occur normally with learning, and that this process allows for the selection of optimal neural circuits to performed trained tasks. Our results indicate that a similar selection process may occur in the motor cortex ipsilateral to the trained limb, although our results indicate that the timing of expansion and normalization may differ in the ipsilateral hemisphere than in the contralateral hemisphere, and may not be directly coincident with the learning process.
In this study, we report that skilled motor training results in transient map plasticity in the ipsilateral hemisphere. Map expansion within the ipsilateral hemisphere occurs within 3 months of the onset of skilled training. At 6 months, forelimb map representations have renormalized to control levels in spite of continued training. Other movement representations and representations of the untrained contralateral limb were unchanged. Motor performance was not correlated with map area. This study provides new insight into ipsilateral motor cortex map plasticity in response to training on a skilled motor task. These results may have important implications for future studies that investigate training-dependent plasticity and motor recovery after brain injury.
Supplementary Material
Highlights.
Motor training results in trained forelimb map plasticity in ipsilateral cortex.
Map expansion was observed after three months of training.
Map normalization occurs within six months of continued training.
Task performance is retained despite map normalization.
Acknowledgments
We would like to acknowledge the help of Bernadette Baker, Bruno Ifebi, Caroline Abe, Megan Lutchman, and Jenny Trieu for their additional assistance with training rats. We would also like acknowledge Priyanka Das for her assistance with mapping procedures.
Funding Sources
This study was supported by the following funding sources: NIH NINDS (R01 NS085167), NIH NIDCD (R01 DC010433), Defense Advanced Research Projects Agency (DARPA, HR0011-15-2-0017), and the Texas Biomedical Device Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
RLR is the owner of Vulintus, Inc.
References
- 1.Kleim JA, Barbay S, Nudo RJ. Functional Reorganization of the Rat Motor Cortex Following Motor Skill Learning. [accessed December 28, 2015];J Neurophysiol. 1998 80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. http://jn.physiology.org/content/80/6/3321. [DOI] [PubMed] [Google Scholar]
- 2.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol. Learn. Mem. 2000;74:27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- 3.Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol. Learn. Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 4.Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J. Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J. Neurosci. 2009;29:6196–6206. doi: 10.1523/JNEUROSCI.5852-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cramer SC, Finklestein SP, Schaechter JD, Bush G, Rosen BR. Activation of Distinct Motor Cortex Regions During Ipsilateral and Contralateral Finger Movements. [accessed January 11, 2016];J Neurophysiol. 1999 81:383–387. doi: 10.1152/jn.1999.81.1.383. http://jn.physiology.org/content/81/1/383.short. [DOI] [PubMed] [Google Scholar]
- 7.Chen R, Gerloff C, Hallett M, Cohen LG. Involvement of the ipsilateral motor cortex in finger movements of different complexities. Ann. Neurol. 1997;41:247–254. doi: 10.1002/ana.410410216. [DOI] [PubMed] [Google Scholar]
- 8.Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J. Neurophysiol. 2005;93:1209–1222. doi: 10.1152/jn.00720.2004. [DOI] [PubMed] [Google Scholar]
- 9.Axelson HW, Winkler T, Flygt J, Djupsjö A, Hånell A, Marklund N. Plasticity of the contralateral motor cortex following focal traumatic brain injury in the rat. Restor. Neurol. Neurosci. 2013;31:73–85. doi: 10.3233/RNN-2012-120242. [DOI] [PubMed] [Google Scholar]
- 10.Caramia MD, Palmieri MG, Giacomini P, Iani C, Dally L, Silvestrini M. Ipsilateral activation of the unaffected motor cortex in patients with hemiparetic stroke. Clin. Neurophysiol. 2000;111:1990–1996. doi: 10.1016/s1388-2457(00)00430-2. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez CLR, Gharbawie OA, Williams PT, Kleim JA, Kolb B, Whishaw IQ. Evidence for bilateral control of skilled movements: ipsilateral skilled forelimb reaching deficits and functional recovery in rats follow motor cortex and lateral frontal cortex lesions. Eur. J. Neurosci. 2004;20:3442–3452. doi: 10.1111/j.1460-9568.2004.03751.x. [DOI] [PubMed] [Google Scholar]
- 12.Hsu J, Jones T. Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Exp. Neurol. 2006;201:479–494. doi: 10.1016/j.expneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Harris NG, Chen S-F, Pickard JD. Cortical Reorganization after Experimental Traumatic Brain Injury: A Functional Autoradiography Study. J. Neurotrauma. 2013;30:1137–1146. doi: 10.1089/neu.2012.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur. J. Neurosci. 2005;21:989–999. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- 15.Johansen-Berg H, Rushworth MFS, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc. Natl. Acad. Sci. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina-Luna K, Hertler B, Buitrago MM, Luft AR. Motor learning transiently changes cortical somatotopy. Neuroimage. 2008;40:1748–1754. doi: 10.1016/j.neuroimage.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Hays SA, Khodaparast N, Sloan AM, Hulsey DR, Pantoja M, Ruiz AD, Kilgard MP, Rennaker RL. The isometric pull task: a novel automated method for quantifying forelimb force generation in rats. J. Neurosci. Methods. 2013;212:329–337. doi: 10.1016/j.jneumeth.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, Rennaker RL, Kilgard MP. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol. Dis. 2013;60:80–88. doi: 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Pruitt D, Hays S, Schmid A, Choua C, Kim L, Trieu J, Kilgard MP, Rennaker RL. Controlled-cortical impact reduces volitional forelimb strength in rats. Brain Res. 2014;1582:91–98. doi: 10.1016/j.brainres.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 20.Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, Hays SA, Kilgard MP, Rennaker RL. Vagus Nerve Stimulation Delivered with Motor Training Enhances Recovery of Function after Traumatic Brain Injury. J. Neurotrauma. 2015 doi: 10.1089/neu.2015.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, Rennaker RL, Kilgard MP. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb. Cortex. 2012;22:2365–2374. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- 22.Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, Kilgard MP. Reorganization of Motor Cortex by Vagus Nerve Stimulation Requires Cholinergic Innervation. Brain Stimul. 2016 doi: 10.1016/j.brs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young NA, Vuong J, Teskey GC. Development of motor maps in rats and their modulation by experience. J. Neurophysiol. 2012;108:1309–1317. doi: 10.1152/jn.01045.2011. [DOI] [PubMed] [Google Scholar]
- 24.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. [accessed November 14, 2015];J. Neurosci. 1996 16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. http://www.ncbi.nlm.nih.gov/pubmed/8551360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liepert J, Terborg C, Weiller C. Motor plasticity induced by synchronized thumb and foot movements. Exp. Brain Res. 1999;125:435–439. doi: 10.1007/s002210050700. [DOI] [PubMed] [Google Scholar]
- 26.Monfils MH, Teskey GC. Skilled-learning-induced potentiation in rat sensorimotor cortex: a transient form of behavioural long-term potentiation. Neuroscience. 2004;125:329–336. doi: 10.1016/j.neuroscience.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 27.Monfils MH, VandenBerg PM, Kleim JA, Teskey GC. Long-term potentiation induces expanded movement representations and dendritic hypertrophy in layer V of rat sensorimotor neocortex. Cereb. Cortex. 2004;14:586–593. doi: 10.1093/cercor/bhh020. [DOI] [PubMed] [Google Scholar]
- 28.Castro-Alamancos MA, Borrell J. Functional recovery of forelimb response capacity after forelimb primary motor cortex damage in the rat is due to the reorganization of adjacent areas of cortex. Neuroscience. 1995;68:793–805. doi: 10.1016/0306-4522(95)00178-l. [DOI] [PubMed] [Google Scholar]
- 29.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. [accessed February 1, 2016];J Neurophysiol. 1996 75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. http://jn.physiology.org/content/75/5/2144.short. [DOI] [PubMed] [Google Scholar]
- 30.Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J. Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 31.Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11370–11375. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–131. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 33.Jones TA, Chu CJ, Grande LA, Gregory AD. Motor Skills Training Enhances Lesion-Induced Structural Plasticity in the Motor Cortex of Adult Rats. [accessed December 28, 2015];J. Neurosci. 1999 19:10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. http://www.jneurosci.org/content/19/22/10153.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawaki L, Butler AJ, Leng X, Wassenaar PA, Mohammad YM, Blanton S, Sathian K, Nichols-Larsen DS, Wolf SL, Good DC, Wittenberg GF. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil. Neural Repair. 2008;22:505–513. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends Neurosci. 2012;35:715–722. doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.