Abstract
Inhibition of hypoxia-induced stress signaling through JNK potentiates the effects of oxaliplatin. The JNK pathway plays a role in both autophagy and apoptosis; therefore, it was determined how much of the effect of JNK inhibition on oxaliplatin sensitivity is dependent on its effect on autophagy. We studied the impact of JNK isoform down-regulation in the HT29 colon adenocarcinoma cell line on hypoxia- and oxaliplatin-induced responses. Electron microscopic analyses demonstrated that both oxaliplatin- and hypoxia-induced formation of autophagosomes were reduced significantly in HT29 cells treated with the JNK inhibitor SP600125. The role of specific JNK isoforms was defined using HT29-derived cell lines stably expressing dominant negative constructs for JNK1 and JNK2 (HTJ1.3 and HTJ2.2, respectively). These cell lines demonstrated that functional JNK1 is required for hypoxia-induced autophagy and that JNK2 does not substitute for it. Inhibition of autophagy in HTJ1.3 cells also coincided with enhancement of intrinsic apoptosis. Analysis of Bcl2-family proteins revealed hyper-phosphorylation of Bcl-XL in the HTJ1.3 cell line, but this did not lead to the expected dissociation from Beclin-1. Consistent with this, knockdown of Bcl-XL in HT29 cells did not significantly affect the induction of autophagy, but abrogated hypoxic resistance to oxaliplatin due to the faster and more robust activation of apoptosis.
Implications
These data suggest that balance between autophagy and apoptosis are shifted toward apoptosis by down-regulation of JNK1, contributing to oxaliplatin sensitization. These findings further support the investigation of JNK inhibition in colorectal cancer treatment.
Keywords: hypoxia, JNK1, autophagy, oxaliplatin, colon cancer
INTRODUCTION
One of the characteristic of solid tumors is the occurrence of regions with low oxygen tension as a result of uncontrolled cell growth and disordered angiogenesis, to which oxygen delivery can be restored through angiogenesis inhibition (1, 2). This phenomenon contributes to a complicated microenvironment, where both continuous and cycling hypoxia are able to significantly affect tumor behavior, and are associated with poor prognosis due in part to a more aggressive tumor phenotype, resistance to chemotherapy and radiation, and increased genetic instability (3). Therefore, targeting of hypoxia is considered a promising approach to augment the efficacy of cancer treatment (4, 5). Hypoxia induces multiple signaling pathways, resulting in activation of major transcription factors, including hypoxia-induced factors (HIFs), NF-κB, p53 and AP-1 (6, 7), which in turn regulate cellular responses to the lack of oxygen, such as metabolic adaptations, angiogenesis, cell death and autophagy, among others.
Autophagy is a catabolic process, involving “packing” of various cytoplasmic components into double-membrane vehicles (autophagosomes), followed by their fusion with lysosomes and formation of autolysosomes, where degradation of the autophagic cargo takes place (8). It was first extensively studied as an adaptive response to starvation, but later was acknowledged as a key process in maintenance of cellular homeostasis, damage responses and progression of various diseases, including cancer (9). There is ample evidence for both oncosuppressive and tumor-supportive roles of autophagy, depending on cellular and tissue context (9), which makes targeting autophagy a controversial issue. It is a highly regulated process, depending on activation and interaction of multiple molecular components, including close to 30 proteins [so called ATG proteins, products of autophagy related genes (ATG)]. One of these, Beclin1, is crucial for initiation of autophagy, as a part of multi-protein complex, regulating nucleation and isolation of autophagosome membrane (10). Beclin 1 is inactive when bound though its BH3-domain to pro-survival members of Bcl2 family. It is commonly accepted that release of Beclin 1 from this inhibitory complex can be achieved by phosphorylation of Bcl2 by JNK, phosphorylation of Beclin1 by DAPK, or competitive displacement of Beclin 1 by pro-apoptotic BH3-only proteins or BH3 mimetics (10,11). Involvement of Bcl2-family proteins in autophagy regulation indicates the existence of cross-talk between autophagy and apoptosis, which together can influence cell fate. Indeed, the same signals often can induce both processes (11) with final outcome (survival or cell death) depending on severity of the stress. Among key autophagy markers are LC3B, an integral part of the autophagosome membrane, and p62 (also known as SQSTM1), which targets cargo to the autophagosome through interaction with LC3. Accumulation of LC3B and its redistribution to autophagosomes allows assessment of autophagy initiation, whereas degradation of p62 marks the final stages of the process. When autophagy proceeds to completion, degradation of macromolecules and organelles in autolysosomes provides renewed supply of “building blocks” (sugars, amino acids, nucleotides, etc) necessary for cellular survival in adverse conditions (8, 11).
Both hypoxia and chemotherapeutic agents have been shown to induce autophagy, and both treatments result in activation of stress signaling through JNK (12,13). JNK belongs to the family of MAP kinases, distal members of tri-tiered signal transduction cascades, which are activated by a plethora of external and internal stimuli, and mediate appropriate cellular responses (14). There are three isoforms of JNK (JNK1, JNK2 and JNK3), expressed ubiquitously, except JNK3, which is expressed mostly in brain. JNK1 and JNK2 share the majority of targets, and were considered redundant, but evidence for distinct functions of the isoforms has accumulated (15). Signaling through JNK is critical for normal cell function, but in cancer JNKs can demonstrate both oncogenic and cancer suppressive features (16). Involvement of JNK in the regulation of autophagy in general, and of hypoxia-induced autophagy in particular, is thought to be implemented on both transcriptional and post-transcriptional levels. Phosphorylation of Bcl2 and Bcl-XL by JNK results in release of Beclin1, as mentioned above, and allows autophagy to proceed (10, 11). JNK can also influence autophagy through activation of transcription factors of the AP-1 and FOXO families, which are implicated in driving the expression of several ATG proteins (17). As a key mediator of stress signaling, induced by hypoxia or DNA-damaging drugs, JNK also participates in regulating the DNA-damage response and apoptosis (18,19). Therefore, its multi-faceted functions in hypoxia, DNA-damage, autophagy and apoptosis underlie our interest in JNK as a target to reverse hypoxic resistance to DNA-damaging drugs (primarily oxaliplatin) in colon cancer model.
We have shown (20), that in hypoxic HT29 colon adenocarcinoma cells, down-regulation of JNK-activating kinases, MKK4 or MKK7, exerts differing effects on oxaliplatin cytotoxicity: cells with impaired MKK4 demonstrate higher sensitivity, whereas down regulation of MKK7, which leads to more profound inhibition of JNK activation, causes increase in resistance. Next, we expanded our studies to evaluate effects of JNK down-regulation on chemotherapeutic drug resistance in colon cancer cell lines, and showed that inhibition by the small molecule JNK inhibitor CC-401 enhances sensitivity to oxaliplatin in a panel of 6 cell lines (21). In addition, we demonstrated that down-regulation of JNK1, but not JNK2, abrogates hypoxia-induced resistance to oxaliplatin in HT29 cells most effectively. We also demonstrated protective function of hypoxia- and oxaliplatin-induced autophagy in colon cancer cell lines by showing synergism of chloroquine and chemotherapy in vitro and in vivo (22). Here we set out to define the role of each JNK isoform (in HTJ1.3 and HTJ2.2 cell lines, stably expressing dominant negative constructs for JNK1 and JNK2, respectively) in the induction of autophagy in HT29 colon adenocarcinoma in response to hypoxia and chemotherapy. We hoped in this way to focus inhibitory strategies toward the relevant isoform, so as to minimize adverse effect from inhibiting such an ubiquitous pathway.
MATERIALS AND METHODS
Cells and reagents
The HT 29 human adenocarcinoma cell line was purchased from ATCC (Manassas, VA). Immediately after receiving cells were thawed, propagated and frozen in multiple aliquots. For experiments, cells were used within 2 months or resuscitation. HT29-derived cell lines stably expressing empty vector (HTLX), dominant negative constructs for JNK1 (HTJ1.3) or JNK2 (HTJ2.2) were described in (21). Cells were grown in DMEM medium supplemented with 10% FBS and antibiotic-antimycotic reagent (Invitrogen, Carlsbad, CA). Cultures were maintained in a humidified incubator at 37°C in 5%CO2-95% air. Chemical inhibitor for JNK (SP600125) was purchased from Biomol (Plymouth Meeting, PA), CC-401 was from ChemScene (Monmouth Junction, NJ). Chloroquine diphosphate and puromycin were from Sigma-Aldrich (St. Louis, MO), and oxaliplatin - from LKT Labs (St. Paul, MN). Acridine orange (AO) was purchased from Sigma, Apoptosis and Necrosis Quantification Kit was from Biotium (Hayward, CA).
Viral constructs and infections
For stable delivery of shRNA against JNK1 and JNK2 into HT29-derived cell lines, we constructed retroviral vectors as described in Supplemental Figure S1. After transfection into Phoenix-Ampho packaging cell line viruses were collected, purified, aliquoted and stored at −80°C. For infections, cells plated into 6 well plates were incubated with retroviruses in the presence of polybrene (8 μg/ml, Chemicon, Temecula, CA), and 48 hours later selective media containing 1 μg/ml of puromycin was added. Puromycin-resistant cells were pooled, evaluated for JNK1/JNK2 levels and used in further experiments.
For Bcl-XL knock-down, ready-to-use lentiviral particles (control and shRNA-encoding) were purchased from Santa Cruz (sc-77361-V), and used according to manufacturer’s recommendations. Since in pooled cultures knock-down of the target protein was incomplete, individual monoclonal sub-lines were isolated, assessed for Bcl-XL expression and used in further experiments.
Hypoxic treatment
Exposure of cells to acute hypoxia was achieved by incubation in an anaerobic chamber (Forma Scientific, Inc., Marietta, OH) filled with gas mixture consisting of 5% CO2, 9% H2 and 86% N2. Oxygen content (0.1− 0.5%) was monitored by PROOX 110 oxygen sensor (BioSpherix, Redfield, NY). Cells were plated in 100 mm glass Petri dishes to a density of 2 × 106 cells per dish and subjected to hypoxia within 36 hours. The cells were harvested at various time points for further experiments.
Protein extract preparation
Total protein extracts were prepared as follows: after hypoxia, cells were washed twice with PBS and lyzed inside the chamber in cell lysis buffer (Cell Signaling Technology, Beverly, MA), supplemented with complete protease inhibitor cocktail (Roche) and 1mM PMSF (Sigma). The contents of scraped dishes were transferred into microcentrifuge tubes, taken out of hypoxia chamber and placed in a shaker for 30 min at 4°C. Lysates were then centrifuged for 10 min at 10,000 rpm (4°C) and the protein concentration of cleared extracts was measured using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA).
Western blotting
For protein electrophoresis, protein extracts were used in amounts of 10 μg per lane. Western blotting was carried out according to standard procedures, using horseradish peroxidase-conjugated secondary antibodies purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and the ECL+Plus detection system (Amersham, Arlington Heights, IL). Results were analyzed with BioSpectrum 810 Imaging System using VisionWorksLS Image Acquisition and Analysis Software (UVP, Upland CA). The antibodies used were: antibodies against p-Bcl-XL, p62 and actin were from Santa Cruz Biotechnology; antibodies against caspase 3 and caspase 7 - from BD Pharmingen. The remaining antibodies were purchased from Cell Signaling Technology. LC3 content was assessed with an antibody against LC3B isoform.
Immunoprecipitation
Cells were subjected to hypoxia for 6 hours, followed by isolation of cellular extracts as above. From each sample, containing 2 mg of total protein, Beclin1 was immunoprecipitated overnight with goat antibodies (Santa Cruz, 20 μl) using ExactoCruz system (Santa Cruz Biotechnology) according to manufacturer’s recommendations. Immunoprecipitates were lysed in 60 μl of 2x gel loading buffer, and subjected to electrophoresis (15 μl per lane) followed by Western blot analysis, using rabbit primary antibodies from Cell Signaling Technology.
Cytotoxicity assays and calculation of combination indices
For assessment of cytotoxicity, cells were plated in 96-well plates (2000 cells per well), and 24 hours later various amounts of oxaliplatin and chloroquine alone or in combination were added, immediately before hypoxic exposure for 24 hours, followed by cultivation in normal condition for additional 48 hours. Cytotoxicity was measured using a standard MTT assay. Combination indices (CI) were calculated based on Chou-Talalay methods using CompuSinq software (as in 21), CI=1 indicates additivity, CI<1 indicates synergism, CI>1 indicates antagonism.
Transmission electron microscopy (TEM)
For TEM quantitation of autophagosomes, HT29 cells were subjected to hypoxia and/or oxaliplatin (IC50 dose) with or without 10 μM of SP600125. Cell pellets were collected, fixed in 2.5% glutaraldehyde/2% formaldehyde with 0.1 M sodium cacodylate and stored at 4°C until embedding. Embedded samples were processed for TEM as described earlier (22). Images were examined with a JEOL-1010 electron microscope (JOEL) at 80 kV. For quantitation of cells using electron microscopy, high-powered micrographs (x12, 000-20,000) of 25 single cells from multiple distinct low-powered fields in each sample were obtained. Cells with more than three to four double-membrane vesicles were scored as positive for autophagosomes.
Fluorescent staining procedures
To assess autophagy and apoptosis induction we employed fluorescent microscopy: i) autophagy induction (as LC3II puncta formation) was monitored in HT29 cells infected with GFP-LC3-encoding retrovirus (Addgene plasmid # 22405, pBABEpuro GFP-LC3 from Dr. Jayanta Debnath was used to generate retrovirus), ii) apoptotic cells were identified using Apoptosis and Necrosis Quantification Kit, iii) staining of live cells with acridine orange was carried out to assess accumulation of acidic vesicles (Supplemental Material). Results were observed with EVOSfl fluorescent microscope (Advanced Microscopy Group (AMG), Bothell, WA). Cells were plated on 2 well glass slides (Lab-Teck II, Thermo Fisher Scientific, Rochester, NY) at the density of 50x103 per well and next day were subjected to hypoxia for various time, with or without oxaliplatin (5 μM, 5xIC50) or chloroquine (3μM, 1xIC50), followed immediately by staining procedures. Throughout this study we used equimolar drug concentrations, based on IC50 values for control cell line (HTLX) in oxic conditions (derived from MTT assays), which allow to compare responses between cell lines. Higher concentrations were used for Western analyses than for more sensitive fluorescent staining procedures.
GFP-LC3-expressing cells were fixed for 15 min in 1% formaldehyde before being observed in PBS. Staining for apoptosis and necrosis was carried out according manufacturers recommendations, using FITS-Annexin V and Ethidium Homodimer III to identify apoptotic and necrotic cells, respectively.
Colony-forming assays
For clonogenic assays, cells were plated in 6-well plates at a density of 300 cells per well; after 24 hours oxaliplatin was added at specified concentrations immediately prior to transfer of the plates to the hypoxia chamber. After 24 hours of hypoxia, plates were returned to normal conditions for 48 hours, and, following the addition of fresh media, cultivated for 10-14 days; colonies were then fixed in 75% ethanol, stained with Coomassie Blue (Sigma) and counted manually. All experiments were performed at least two times in duplicate.
Caspase assays
For evaluation of caspases’ activation, colorimetric assay kits for Caspase 8, Caspase 9 and Caspase 3 were used (Enzo Life Sciences, Farmingdale, NY). Cells were subjected to hypoxia with or without oxaliplatin (5 μM) for 24 hours, and caspase activation assays were performed according to manufacturer’s recommendation. Results were quantified using a plate reader at 405nm, and presented as fold increase in caspase activity compared to untreated control in normal conditions.
Statistical analysis
Data were analyzed with unpaired Student’s test: P < 0.05 was accepted as a statistically significant difference compared with corresponding control. In figures: *, P < 0.05; **, P<0.01; ***, P<0.001.
RESULTS
Pharmacological inhibition of JNK diminishes hypoxia- and oxaliplatin-induced autophagy in HT29 colon adenocarcinoma cells
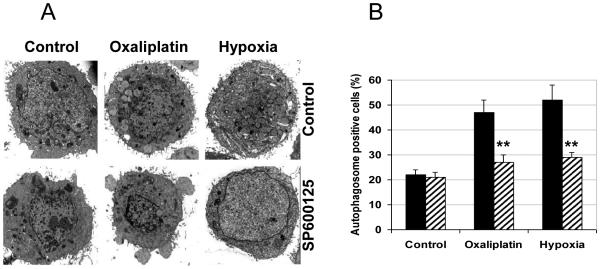
Earlier we examined the development of autophagy in hypoxia- and oxaliplatin-treated HT29 colon cancer cells, and demonstrated its induction by both treatments through fluorescent (GFP-LC3 puncta) and electron microscopic (EM) ultra-structural analyses (22). To assess JNK involvement in autophagy induction, we treated HT29 cells with the JNK inhibitor SP600125 (10 μM), under hypoxia or with oxaliplatin (1xIC50 dose) for 24 hours , followed by EM analysis. Our data demonstrate that both oxaliplatin- and hypoxia-induced formation of autophagosomes were reduced by SP600125, which confirms that JNK pathway is involved in the early steps of vacuolar formation during autophagy (Figure 1A and 1B). We then expanded our studies to assess the role for each of major JNK isoform, JNK1 and JNK2, in autophagy induction, to delineate further molecular mechanisms underlying sensitization of HT29 cells to hypoxia and oxaliplatin in the context of JNK inhibition.
Figure 1.
JNK inhibition attenuates autophagy in HT29 cell line. A, Cells on glass slides were subjected to hypoxia or oxaliplatin for 24 hours with or without SP600125 (10 μM ) and observed under electron microscopy to assess autophagy by appearance of autophagosomes. B, Quantitative analysis was carried out: cells with more than 5 autophagosomes were considered “positive” for induction of autophagy. Graph represents average values from at least three slides for each condition, bars represent standard deviation; **, P<0.01.
Down-regulation of JNK1 inhibits hypoxia-induced autophagy in HT29 cells
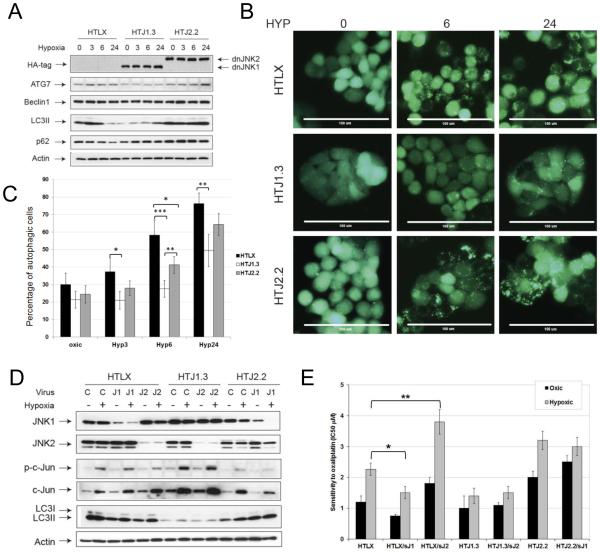
To assess autophagy induction under hypoxia we evaluated expression of molecular markers of autophagy by Western blot analysis and found diminished levels of ATG 7 and LC3II in HTJ1.3 cell line, then compared to control or HTJ2.2 cells (Figure 2A). The inhibitory effect on autophagy induction in JNK1-deficient cells also was confirmed by assessment of GFP-LC3 puncta formation following hypoxia treatment (Figure 2B and 2C), which demonstrated most significant effect at 6 hours of hypoxia. Acridine orange staining (Figure S2) also showed lower content and smaller size of acidic vesicles (including autolysosomes) in HTJ1.3 cell line, which supports inhibition of autophagy. To evaluate possible compensatory effects of JNK1 and JNK2 in this circumstance, we infected HTJ1.3 and HTJ2.2 cell lines with retrovirus encoding for shRNA of JNK2 or JNK1, respectively. We were able to achieve significant down-regulation of target proteins in pooled puromycin-resistant cells (Figure 2D). However, our data show, that under hypoxic conditions down-regulation of second JNK isoform did not cause significant changes in levels of either phospho-cJun or LC3II (Figure 2D). Clonogenic assays after hypoxia and oxaliplatin treatment, showed the higher sensitivity to oxaliplatin in hypoxic lines with impaired JNK1, both in control (HTLX) and modified cells (IC50 of 1.3 μM for HTJ1.3 vs. 3.2 μM for HTJ2.2; and IC50 of 1.5 μM in HTJ1.3/shJNK2 vs. 3 μM for HTJ2.2/shJNK1 (Figure 2E)). Results of MTT assays were similar (Supplemental Figure S3), with differences in IC50 values most likely reflecting both cell death and a growth arrest. Notably, control cell lines treated with shRNA for JNK1 or JNK2 differed in oxaliplatin sensitivity as described. However in the cell lines already expressing the dominant-negative counterpart of JNK1 or JNK2, the introduction of shRNA against the alternative isoform, though effective in ablating its target, did not further modify profiles of sensitivity. This observation likely derives from residual isoform activity in the dominant-negative modified cells over-riding the impact of ablation of the opposing isoform. Thus, our data suggest that under hypoxic conditions JNK2 does not compensate for JNK1 in mediating sensitivity to chemotherapy, and indicate the primary role of JNK1 in hypoxic induction of autophagy in HT29 cells.
Figure 2.
Functional JNK1 in necessary for induction of autophagy in HT29 cells. A, HT29-derived cell lines were subjected to hypoxia, and the levels of autophagy markers assessed by Western blot analysis. B, Lower GFP-LC3 puncta formation was observed in HTJ1.3 cells, as compared to control and HTJ2.2 lines. Scale bar – 100μm. C, Autophagic cells were counted in multiple fields (n= 6-12) for each condition, and their content (average percentage values of total cell count) is presented as a graph. Cells which lost even cytoplasmic fluorescence and the ones containing more than 15 clearly visible puncta were considered positive for autophagy induction. Bars represent standard deviation, *, P < 0.05; **, P<0.01; ***, P<0.001. D, In HTJ1.3 and HTJ2.2 lines the counterpart JNK isoform was down-regulated by control or shRNA-encoding retroviruses (sJ2 or sJ1, respectively), puromycin-resistant cells were pooled, subjected to hypoxia and assessed for c-Jun and LC3B by western blot analysis. E, Results of clonogenic assays in HT29-derived cell lines after down-regulation of each, or both of the JNK. Graph shows average values from at least three independent experiments in duplicates, bars represent standard deviation. *, P<0.05; **, P<0.01.
The JNK1-deficient cell line is more sensitive to autophagy inhibitor CQ, and displays enhanced apoptosis induction under hypoxia
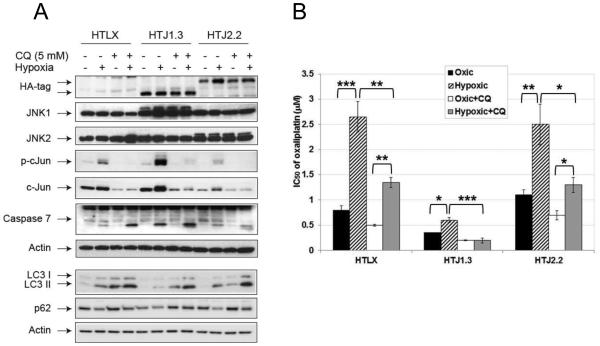
Since we have shown previously that inhibitor of autophagy chloroquine (CQ) enhances oxaliplatin cytotoxicity in vitro and in vivo (22), we set to find out if modulation of JNK activity will affect the outcome of the treatment. First, in a panel of colon cancer cell lines we established synergism, both in normal and hypoxic conditions, of CQ and small molecule JNK inhibitor CC-401, with CI50 ranging from 0.43 to 0.88 (Supplemental Table S1). We then continued with our studies in HT29-derived panel and observed increased sensitivity to CQ under hypoxia in all cell lines, with highest ratio of sensitization in HTJ1.3 and lowest in HTJ2.2 cells (Table 1). In the presence of CQ (5 μM, 24 hours) the most significant enhancement of apoptosis was detected in hypoxic HTJ1.3 cells (Figure 3A), as judged by processing of caspase 7. Protein markers confirmed inhibition of autophagy in all CQ-treated cell lines, both in normal and hypoxic conditions, whereas only in JNK1-deficient cells autophagy was less pronounced in the absence of CQ (in accordance with Figure 2A). More robust apoptosis in HTJ1.3 cells was also confirmed by fluorescent staining with FITC-Annexin V (Supplemental Figure S4A and S4B). Use of CQ ( 2.5 μM, corresponding to IC30 of oxic control) in combination with oxaliplatin sensitized all cell lines under both oxic and hypoxic conditions, but the strongest effect was observed, again, in HTJ1.3 cell line (Figure 3B), in which hypoxic resistance to oxaliplatin was completely abolished. Finally, when combining CQ and oxaliplatin in MTT assays, we established synergism of the combination in all three cell lines, with lowest CI50 in HTJ2.2 cells (Table 1), which suggests that inhibition of autophagy in its late stage can partially reverse oxaliplatin resistance in JNK2-deficient cells under hypoxia. We also have noticed that in CQ-treated cells hypoxia-induced expression and activation of c-Jun is diminished, which suggests the inhibition of JNK signaling. It was shown, that CQ can cause ATM-dependent decrease in JNK activity in macrophages (23), which, if true for colon cancer cell lines, could have additional deleterious effect on cell survival in hypoxic conditions.
Table 1.
Combination of CQ and oxaliplatin is synergistic in HT29-derived cell lines with altered JNK status
| Oxaliplatin | Ratio | CQ | Ratio | Oxali/CQ | |
|---|---|---|---|---|---|
|
|
|
|
|||
| IC50 (μM) | Hyp/Oxic | IC50 (μM) | Hyp/Oxic | CI at IC50 | |
| HTLX oxic | 1.05 | 3.4 | 0.93 ± 0.13 | ||
| HTLX hyp | 2.2 | 2.1 | 2.1 | 0.62 | 0.93 ± 0.16 |
| HTJ1.3 oxic | 0.9 | 5.7 | 0.91 ± 0.15 | ||
| HTJ1.3 hyp | 0.8 | 0.89 | 2.1 | 0.37 | 0.86 ± 0.19 |
| HTJ2.2 oxic | 1.25 | 5.3 | 0.77 ± 0.08 | ||
| HTJ2.2 hyp | 3 | 2.4 | 3.4 | 0.64 | 0.66 ± 0.13 |
Cytotoxicity was established in MTT-based assays. Combination indices (CI) were calculated based on Chou-Talalay methods using CompuSinq software.
CI=1 indicates additivity, CI<1 indicates synergism, CI>1 indicates antagonism.
Figure 3.
Chloroquine enhances cell death in hypoxic HT29-derived cell lines. A, Western blot analysis shows inhibition of JNK signaling, down-regulation of autophagy and enhancement of apoptosis in the presence of CQ, especially in HTJ1.3 cell line. B, Results of MTT assays demonstrate strongest enhancement of oxaliplatin cytotoxicity in hypoxic HTJ1.3 cells, when combined with 2.5 μM of CQ. Graph shows average values of IC50 derived from at least three independent experiments in triplicate, bars represent standard deviation. *, P < 0.05; **, P<0.01; ***, P<0.001.
Sensitization to oxaliplatin in JNK1-deficient HT29 cells is associated with enhancement of intrinsic apoptosis
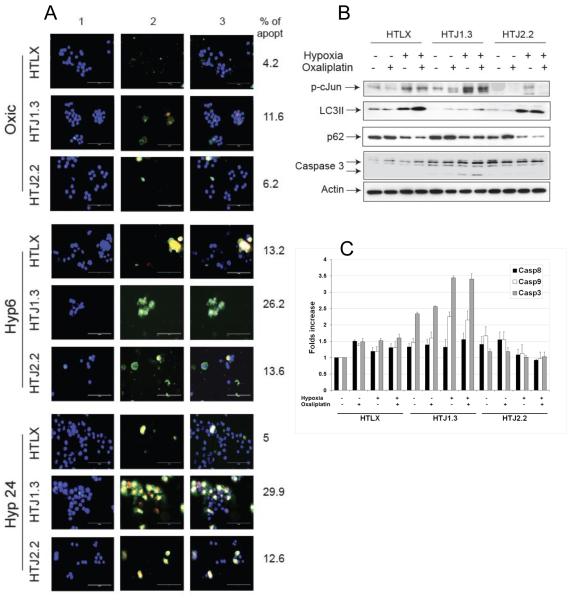
To assess the cell death pathways in our panel, we evaluated induction of apoptosis and necrosis in hypoxic cells by fluorescent staining (Figure 4A). Stronger and faster induction of apoptosis was evident in HTJ1.3 cells. The percentage of apoptotic cells for each condition (n=3) from three independent experiments was calculated and is shown next to the corresponding image. Western blot analysis of protein extracts from cells treated with oxaliplatin under hypoxia (5 μM for 24 hours) demonstrated inhibition of hypoxia-induced autophagy in the JNK1-deficient line, which was accompanied by enhanced apoptosis, as evident by processing of caspase 3 (Figure 4B). When hypoxia was followed by reoxygenation for additional 24 hours, we could still detect differential activation of autophagy, but apoptosis was activated equivalently in all three cell lines, suggesting that the severity of the stress could not be counteracted by autophagy in HTLX and HTJ2.2 cells (Supplemental Figure S5). Finally, caspase activation assays demonstrated activation of intrinsic apoptotic pathway in HTJ1.3 cells (Figure 4C), especially under hypoxia, whereas in HTLX and HTJ2.2 cell lines it was much less pronounced. These data is in accord with our previous findings, pointing to low level of apoptotic cell death in hypoxic HT29 treated with oxaliplatin (24), as compared to necrosis.
Figure 4.
JNK1 deficiency results in enhancement of apoptosis in HT29 cell line. A, Cells were seeded on glass slides, subjected to hypoxia for 6 or 24 hours, and stained using Apoptosis and Necrosis Quantification kit. Representative images from three independent experiments are shown, column 1- Hoechst 33342, column 2 – FITC-Annexin V+ Ethidium Homodimer III, column 3 - merged images; scale bar – 100μm. Apoptotic cells were counted from 3 different fields in each experiment, average values are shown. B, Cells were subjected to hypoxia with or without oxaliplatin (5 μM) for 24 hours followed by Western blot analysis. C, Colorimetric caspase activation assays were carried out as described in Material and Methods. Results are presented as folds increase in caspase activity when compared to untreated control. Graph shows average values from 2 independent experiments in duplicate, bars represent standard deviation.
Bcl-X knock-down enhances apoptosis in HT29 cell without affecting induction of autophagy
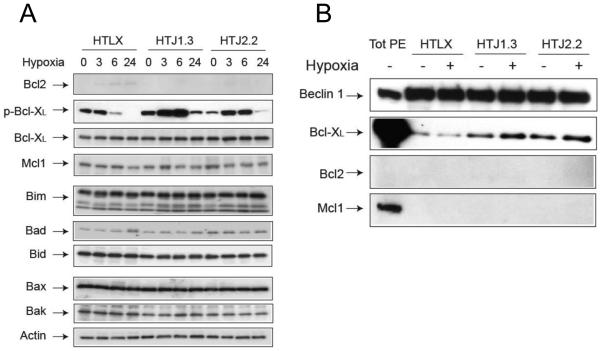
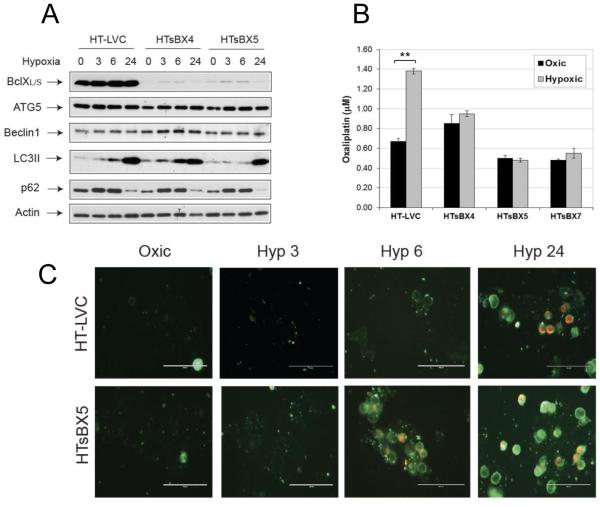
Since for intrinsic apoptosis the interactions between Bcl2-family members are crucial in setting cell death threshold, we wished to find out if down-regulation of JNK1 affects Bcl2 proteins in hypoxic HT29 cells. Our data show, that expression of majority of proteins does not differ significantly between HTLX, HTJ1.3 and HTJ2.2 cell lines under hypoxia. The only difference was the higher level of Bcl-XL phosphorylation in JNK1-deficient cell line (Figure 5A). Also, levels of Bcl2 were barely detectable in all cell lines. In immunoprecipitation experiments we identified Bcl-XL, but not Bcl2, as the binding partner of Beclin1 in HTLX, HTJ1.3 and HTJ2.2 cells is (Figure 5B). Notably, despite the differing levels of phosphorylation, Bcl-XL stayed bound to Beclin 1 in both HTJ1.3 and HTJ2.2 cells, at least at six hours of hypoxic exposure, whereas effects on autophagy induction varied (Figure 2). These conflicting data prompted us to investigate if down-regulation of Bcl-XL would affect autophagy and oxaliplatin sensitivity in HT29 cells. To do so, we isolated several HT29-derived monoclonal sub-lines, in which Bcl-XL expression was down-regulated by lentiviral delivery of shRNA constructs (HTsBX4 and HTxBX5, with control HT-LVC line). Western blotting did not reveal significant changes in autophagy induction under hypoxia in these cell lines (Figure 6A), whereas MTT analysis clearly demonstrated abrogation of hypoxic resistance to the drug (Figure 6B). The acridine orange staining also demonstrated comparatively equal accumulation of acidic vesicles by 24 hours in hypoxic control and HTsBX5 cells, with the latter showing slightly lesser staining at 6 hours of hypoxia (Supplemental Figure S6). At the same time, HTsBX5 cells exhibit faster and stronger induction of apoptosis under hypoxia, when compared to control, which is likely to be the basis of increased cytotoxicity of oxaliplatin in this cell line (Figure 6C).
Figure 5.
Bcl-XL is a key mediator of hypoxia-induced autophagy in HT29 cells. A, Cells were subjected to hypoxia, followed by Western blot analysis of Bcl2-family proteins, to assess possible impact of JNK inhibition in this setting. B, Immunoprecipitation experiments with Beclin 1 antibody revealed Bcl-XL as its binding partner in HT29-derived cell lines. In the first lane total protein extract (TotPE) was loaded at the concentration of 10 μg; figure is a representative blot from two independent experiments.
Figure 6.
Bcl-XL mediates hypoxic resistance to oxaliplatin in the HT29 colon adenocarcinoma model. A, Bcl-XL was down-regulated in HT29 cells by lentiviral introduction of scrambled (HT-LVC) or Bcl-X-targeting shRNA constructs, followed by isolation of monoclonal sub-lines. Cells were subjected to hypoxia and assessed for expression of target protein and of autophagy markers. B, MTT assays revealed abrogation of hypoxia-induced oxaliplatin resistance in HT29 cells lacking Bcl-XL. Graph shows average values of IC50 in three monoclonal sub-lines derived from at least three independent experiments in triplicates. Bars represent standard deviation; **, P<0.01. C, Staining for apoptosis and necrosis demonstrate faster induction of apoptosis under hypoxia in HT29 cells lacking Bcl-XL.
DISCUSSION
Involvement of JNK in the regulation of autophagy has been implicated in multiple studies, some of which relied on pan-inhibition of JNK, whereas others employed genetic approaches to investigate input of specific isoforms. In their pioneer paper (25) Wei and co-authors showed that in HeLa and MCF7 cells, under starvation, Bcl2 undergoes multisite phosphorylation by JNK, leading to its dissociation from Beclin1, thus blocking the anti-autophagy function of Bcl2. Further, using MEFs with targeted disruption of jnk1 or jnk2, they demonstrated essential role of JNK1 in Bcl2 phosphorylation, and inability of JNK2 to compensate for it in these conditions. Finally, they also proposed the model, linking the switch from autophagy to apoptosis in starved HeLa cells with the duration of signal and the differing affinity of Bcl-2 to its binding partners, Beclin 1 and BAX, dependent on the level of multi-site Bcl-2 phosphorylation (26). Although since then multiple studies have implicated JNK in induction of protective autophagy in various models, data on its impact on cytotoxicity of chemotherapeutic drugs in solid hypoxic tumors are scarce.
This project was initiated to broaden our study of the impact of hypoxia-induced signaling to AP-1 transcription factor on sensitivity of hypoxic colon cancer cell lines to oxaliplatin. After showing synergism of JNK inhibition with oxaliplatin in a panel of cell lines, we demonstrated increased sensitivity to the drug in HT29 cell line with impaired JNK1 function (21). Coupling that with data showing synergism of CQ and oxaliplatin under hypoxia (22) led us focus on autophagy and its effects on outcome of chemotherapeutic treatment in HT29-derived cell lines in which either JNK1 or JNK2 activities were down-regulated by overexpression of corresponding dominant-negative construct.
The majority of JNK isoform activities are considered redundant, but specific functions of different isoforms in various cellular processes have been described (15, 16), and autophagy is not an exception: several recent studies showed central role of JNK2 in autophagy induced by different stressors (27-29), including hypoxia-induced mitophagy (30). Our results confirm inhibition of autophagy by SP600125 in both hypoxia- and oxaliplatin-treated HT29 cells. We also show that JNK1 is essential for hypoxia-induced autophagy, and cannot be substituted by JNK2 (Figure 2), suggesting that higher sensitivity of HTJ1.3 cells to oxaliplatin could be, at least in part, based on autophagy down-regulation in this cell line. We have observed increased levels of c-Jun and phospho-cJun in a JNK1-deficient cell line (21), but our experiments with shJNK2 constructs show that it cannot be attributed solely to JNK2 overcompensation, suggesting either the activity of another mediator of hypoxia-induced signaling to c-Jun or disruption of this protein’s turnover. The latter is suggested by highest c-Jun levels in HTJ1.3 cells, especially under hypoxia. Previous studies in JNK1−/− and JNK2−/− MEFs reported down-regulation of c-Jun in the absence of JNK1 and its up-regulation in JNK2-deficient fibroblasts (31,32). In our cells both endogenous JNK1 and dominant negative JNK1 are present, resulting in competitive inhibition of signaling to c-Jun, which creates more complex environment. Since JNK1 was shown to be most effective activating kinase of the E3 ligase Itch, which is responsible for c-Jun ubiquitination and degradation (33), it is plausible to assume that JNK1 inhibition in HT29 cells could lead to increase in c-Jun levels. And although this phenomenon could be investigated further, we did not pursue it in this study, since it does not seem to affect autophagy inhibition in HTJ1.3 cells, as judged by multiple autophagy markers.
Autophagy induction, as a cellular response to stress, is dependent on genetic background. Thus, certain features of HT29 cells able to affect this process should be mentioned. It was shown that in multiple cell lines hypoxia-induced autophagy is mediated through the HIF-1 transcriptional target BH3-only protein BNIP3, which can displace Bcl2 from Beclin1, prompting autophagy activation (34, 35). However, in colon cancer cell lines, including HT29, expression of BNIP3 is extremely low, due to epigenetic silencing of the promoter (36). On the other hand, we have shown earlier high constitutive activity of ERK pathway in HT29 cells, both in normal conditions and upon hypoxia or oxaliplatin treatments (20, 37), which could enhance autophagy (38) and underlie low apoptotic cell death in our model when treated with oxaliplatin, as compared to necrosis (24). Deregulation of intrinsic apoptosis could also be a consequence of the p53 mutation in HT29, since majority of BCL2-family members are p53 transcriptional targets (39). When Benard et al (40) investigated activity of apoptotic genes in a panel of colon cancer cell lines, they designated the status of apoptotic pathways in HT29 as “inconclusive”, with the cell line demonstrating very low induction of intrinsic apoptosis upon cisplatin and radiation treatments. However, when autophagy was inhibited either by CQ or JNK1 down regulation in our experiments, we observed significant enhancement of apoptosis (Figures 3A, S4 and 4C) under hypoxic conditions. Thus our data strongly suggest that hypoxia-induced autophagy is a barrier to apoptosis in HT29 cells treated with oxaliplatin.
Bcl2-family proteins are indispensable for an intrinsic apoptosis induced by multiple stimuli (41), DNA damage in particular. Reversible phosphorylation and dephosphorylation of both anti- and pro-apoptotic members of the family plays central role in their regulation. Nevertheless, in general, consequences of Bcl2 proteins’ phosphorylation in direct regulation of cell death are still somewhat controversial, since phosphorylation of the same protein on different amino acid residues can cause activation or inhibition of apoptosis (42). It is accepted that phosphorylation of Bcl-XL mostly leads to inhibition of its anti-apoptotic function, with JNK1 being responsible in a majority of circumstances (42). In contrast, study by Du et al (43) suggested that JNK does not phosphorylate Bcl-XL after vinblastine treatment, and in this model apoptosis induction correlates with dephosphorylation of the protein. Kinases other than JNK1 were reported to phosphorylate Bcl-XL, JNK2 among others (44-46). Our data show that increased phosphorylation of Bcl-XL (with no change in the protein content) in JNK1-deficient cells does not affect autophagy significantly, but is pro-apoptotic under these conditions. We have not attempted to elucidate mechanism of hyper-phosphorylation of Bcl-XL in this study, but are planning to do so after expansion of our model to a panel of colon cancer cell lines representative of human disease.
The complexity of cellular responses to stress, including hypoxia and DNA-damaging drugs, is staggering. Engagement of the same signaling pathways and molecular mechanisms with differing outcomes underlies the terminology often used when discussing the topic: double-edged sword, balance, switch, crosstalk. Crosstalks between autophagy, apoptosis, necrosis and necroptosis were described for various models (11), leading to the search for approaches to exploit these phenomena in targeted treatment of cancer (47). Inhibitors of autophagy are currently tested in multiple clinical trials (48), and combination of hydroxychloroquine with a standard chemotherapy was shown to be beneficial for the treatment of solid tumors (49, 50). In our studies inhibiting autophagy with CQ (22) or JNK activity (through pan-JNK pharmacological inhibition or molecular JNK1 down-regulation (21)) lead to enhanced cytotoxicity of oxaliplatin and 5-FU in hypoxic colon cancer cell lines, which strongly supports studies of the JNK inhibitors in clinical setting to improve chemotherapy efficacy both alone and in combination with CQ.
Supplementary Material
Acknowledgments
Financial support: Supported in part by R01CA139003 and RO1CA158377 from NCI, NIH
Footnotes
The authors disclose no potential conflicts of interest.
REFERENCES
- 1.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 2.Span PN, Bussink J. Biology of hypoxia. Semin Nucl Med. 2015;45:101–9. doi: 10.1053/j.semnuclmed.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Rowher N, Cramer T. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of HIFs and cell death pathways. Drug Res Updates. 2011;14:191–201. doi: 10.1016/j.drup.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Karakashev SV, Reginato MJ. Progress toward overcoming hypoxia-induced resistance to solid tumor therapy. Cancer Manag Res. 2015;7:253–64. doi: 10.2147/CMAR.S58285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Q, Li X. Targeting cyclic hypoxia to prevent malignant progression and therapeutic resistance of cancers. Histol Histopathol. 2015;30:51–60. doi: 10.14670/HH-30.51. [DOI] [PubMed] [Google Scholar]
- 6.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;9:3664–71. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450:363–71. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–31. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856–80. doi: 10.15252/embj.201490784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He C, Levine B. The Beclin1 interactome. Curr Opin Cell Biol. 2010;22:140–9. doi: 10.1016/j.ceb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouschop KMA, Wouters BG. Regulating of autophagy through multiple independent hypoxic signaling pathways. Curr Mol Med. 2009;9:417–24. doi: 10.2174/156652409788167131. [DOI] [PubMed] [Google Scholar]
- 13.Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, He C, Pan H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344:174–9. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 15.Bode AM, Dong Z. The functional contrariety of JNK. Mol Carcinogenesis. 2007;46:591–8. doi: 10.1002/mc.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tournier C. The 2 Faces of JNK Signaling in Cancer. Genes &Cancer. 2013;4:397–400. doi: 10.1177/1947601913486349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pietrocola F, Izzo V, Niso-Santano M, Vacchelli E, Galluzzi L, Maiuri MC, Kroemer G. Regulation of autophagy by stress-responsive transcription factors. Semin Cancer Biol. 2013;23:310–22. doi: 10.1016/j.semcancer.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Vasilevskaya IA, O’Dwyer PJ. Role of Jun and Jun kinase in resistance of cancer cell to therapy. Drug Res Updates. 2003;6:147–56. doi: 10.1016/s1368-7646(03)00043-8. [DOI] [PubMed] [Google Scholar]
- 19.Picco V, Pages G. Linking JNK activity to the DNA damage response. Genes & Cancer. 2013;4:360–8. doi: 10.1177/1947601913486347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasilevskaya IA, Selvakumaran M, O'Dwyer PJ. Disruption of signaling through SEK1 and MKK7 yields differential responses in hypoxic colon cancer cells treated with oxaliplatin. Mol Pharmacol. 2008;74:246–54. doi: 10.1124/mol.107.044644. [DOI] [PubMed] [Google Scholar]
- 21.Vasilevskaya IA, Selvakumaran M, Cabal-Hierro L, Goldstein SR, Winkler JD, O'Dwyer PJ. Inhibition of JNK Sensitizes Hypoxic Colon Cancer Cells to DNA-Damaging Agents. Clin Cancer Res. 2015;21:4143–52. doi: 10.1158/1078-0432.CCR-15-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selvakumaran M, Amaravadi RK, Vasilevskaya IA, O’Dwyer PJ. Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin Cancer Res. 2013;19:2995–3007. doi: 10.1158/1078-0432.CCR-12-1542. [DOI] [PubMed] [Google Scholar]
- 23.Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, Bernal-Mizrachi C, Muslin AJ, Kastan MB, Semenkovich CF. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006;4:377–89. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Rakitina TV, Vasilevskaya IA, O'Dwyer PJ. Inhibition of G1/S transition potentiates oxaliplatin-induced cell death in colon cancer cell lines. Biochem Pharmacol. 2007;73:1715–26. doi: 10.1016/j.bcp.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–88. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei Y, Sinha S, Levine B. Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy. 2008;4:949–51. doi: 10.4161/auto.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raciti M, Lotti LV, Valia S, Pulcinelli FM, Di Renzo L. JNK2 is activated during ER stress and promotes cell survival. Cell Death Dis. 2012;3:e429. doi: 10.1038/cddis.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Z, Liu T, Kamp DW, Wang Y, He H, Zhou X, Li D, Yang L, Zhao B, Liu G. AKT/mTOR and c-Jun N-terminal kinase signaling pathways are required for chrysotile asbestos-induced autophagy. Free Radic Biol Med. 2014;72:296–307. doi: 10.1016/j.freeradbiomed.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu QQ, Zheng RY, Li J, Hu L, Chang YX, Li L, Li MH, Wang RY, Huang DD, Wu MC, Hu HP, Chen L, Wang HY. Palmitic acid induces autophagy in hepatocytes via JNK2 activation. Acta Pharmacol Sin. 2014;35:504–12. doi: 10.1038/aps.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q, Kuang H, Chen C, Yan J, Do-Umehara HC, Liu XY, Dada L, Ridge KM, Chandel NS, Liu J. The kinase Jnk2 promotes stress-induced mitophagy by targeting the small mitochondrial form of the tumor suppressor ARF for degradation. Nat Immunol. 2015;16:458–66. doi: 10.1038/ni.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell. 2004;15:713–25. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Jaeschke A, Karasarides M, Ventura JJ, Ehrhardt A, Zhang C, Flavell RA, Shokat KM, Davis RJ. JNK2 is a positive regulator of the cJun transcription factor. Mol Cell. 2006;23:899–911. doi: 10.1016/j.molcel.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Gao M, Labuda T, Xia Y, Gallagher E, Fang D, Liu YC, Karin M. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science. 2004;306:271–5. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
- 34.Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, et al. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;16:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;10:2570–81. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacon AL, Fox S, Turley H, Harris AL. Selective silencing of the hypoxia-inducible 1 target gene BNIP3 by histone deacetylation and methylation in colorectal cancer. Oncogene. 2007;26:132–41. doi: 10.1038/sj.onc.1209761. [DOI] [PubMed] [Google Scholar]
- 37.Rakitina TV, Vasilevskaya IA, O'Dwyer PJ. Additive interaction of oxaliplatin and 17-allylamino-17-demethoxygeldanamycin in colon cancer cell lines results from inhibition of nuclear factor kappaB signaling. Cancer Res. 2003;63:8600–5. [PubMed] [Google Scholar]
- 38.Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 39.Galluzzi L, Morselli E, Kepp O, Vitale I, Pinti M, Kroemer G. Mitochondrial liaisons of p53. Antioxid Redox Signal. 2011;15:1691–714. doi: 10.1089/ars.2010.3504. [DOI] [PubMed] [Google Scholar]
- 40.Benard A, Janssen CM, van den Elsen PJ, van Eggermond MC, Hoon DS, van de Velde CJ, Kuppen PJ. Chromatin status of apoptosis genes correlates with sensitivity to chemo-, immune- and radiation therapy in colorectal cancer cell lines. Apoptosis. 2014;19:1769–78. doi: 10.1007/s10495-014-1042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 42.Kutik O, Letai A. Regulation of Bcl-2 family proteins by posttranslational modifications. Cur Mol Med. 2008;8 doi: 10.2174/156652408783769599. [DOI] [PubMed] [Google Scholar]
- 43.Du L, Lyle CS, Chambers TC. Characterization of vinblastine-induced Bcl-xL and Bcl-2 phosphorylation: evidence for a novel protein kinase and a coordinated phosphorylation/dephosphorylation cycle associated with apoptosis induction. Oncogene. 2005;24:107–17. doi: 10.1038/sj.onc.1208189. [DOI] [PubMed] [Google Scholar]
- 44.Arena G, Gelmetti V, Torosantucci L, Vignone D, Lamorte G, De Rosa P, Cilia E, Jonas EA, Valente EM. PINK1 protects against cell death induced by mitochondrial depolarization, by phosphorylating Bcl-xL and impairing its pro-apoptotic cleavage. Cell Death Differ. 2013;20:920–30. doi: 10.1038/cdd.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamura Y, Simizu S, Muroi M, Takagi S, Kawatani M, Watanabe N, Osada H. Polo-like kinase 1 phosphorylates and regulates Bcl-x(L) during pironetin induced apoptosis. Oncogene. 2009;28:107–16. doi: 10.1038/onc.2008.368. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Beauchemin M, Bertrand R. Phospho-Bcl-xL(Ser62) plays a key role at DNA damage-induced G2 checkpoint. Cell Cycle. 2012;11:2159–69. doi: 10.4161/cc.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radogna F, Dicato M, Diederich M. Cancer-type-specific crosstalk between autophagy, necroptosis and apoptosis as a pharmacological target. Biochem Pharmacol. 2015;94:1–11. doi: 10.1016/j.bcp.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 48.Duffy A1, Le J, Sausville E, Emadi A. Autophagy modulation: a target for cancer treatment development. Cancer Chemother Pharmacol. 2015;75:439–47. doi: 10.1007/s00280-014-2637-z. [DOI] [PubMed] [Google Scholar]
- 49.Poklepovic A, Gewirtz DA. Outcome of early clinical trials of the combination of hydroxychloroquine with chemotherapy in cancer. Autophagy. 2014;10:1478–80. doi: 10.4161/auto.29428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rebecca VW, Amaravadi RK. Emerging strategies to effectively target autophagy in cancer. Oncogene. 2016;35:1–11. doi: 10.1038/onc.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.