Abstract
Objective
To investigate the association between HIV, antiretroviral therapy (ART), and pregnancy-associated hypertension (PAH) in an HIV-endemic setting.
Methods
A retrospective cohort study was undertaken of pregnant women for whom information was recorded between February 2006 and December 2012 in the Zambia Electronic Perinatal Record System, which captures data from 25 facilities in Lusaka, Zambia. PAH was defined as eclampsia, pre-eclampsia, hypertension, or elevated blood pressure (>140/80 mm Hg) during delivery admission. Logistic regression estimated the odds of PAH among women by HIV serostatus, and by most recent CD4 T lymphocyte count and ART status among women with HIV infection.
Results
Among 249 771 women included in the analysis, 5354 (2.1%) had PAH. Compared with women without HIV infection, women with HIV infection not receiving ART had lower odds of PAH (adjusted odds ratio [AOR] 0.86, 95% confidence interval 0.78–0.95), whereas those with HIV infection who had initiated ART had higher odds of PAH (AOR 1.15, 95% CI 1.01–1.32). No association was found between PAH and timing of ART initiation or CD4 lymphocyte count.
Conclusion
In a large African urban cohort, women with untreated HIV infection had the lowest odds of PAH. Treatment with ART could increase PAH risk beyond that of women without HIV infection and those with untreated infection.
Keywords: Africa, Antiretroviral therapy, HIV, Hypertension
1. Introduction
Increased access to antiretroviral therapy (ART) has substantially reduced the risk of mother-to-child transmission of HIV worldwide. Nevertheless, there are growing concerns about the effects of long-term use of ART on adverse fetal, infant, and maternal outcomes, including hypertensive disorders of pregnancy such as gestational hypertension and pre-eclampsia [1–3]. The American College of Obstetricians and Gynecologists defines gestational hypertension as elevated blood pressure after 20 weeks of pregnancy in the absence of proteinuria or new signs of end-organ dysfunction [4]. Pre-eclampsia is defined as blood pressure elevation after 20 weeks of pregnancy with proteinuria or new signs of end-organ dysfunction [4].
Before the ART era, pregnant women with HIV infection were found to be at a decreased risk of pre-eclampsia compared with women without HIV infection [5,6]. However, with the increasing availability of ART, there has been a trend toward increased incidence of pre-eclampsia among women with HIV infection. In a study of 11 European countries, pre-eclampsia was the most common adverse outcome among pregnant women receiving ART [3]. A study in Spain [2] found that women living with HIV and receiving ART before pregnancy had a five-fold increase in the likelihood of pre-eclampsia compared with counterparts not receiving treatment or receiving fewer than three antiretroviral drugs (odds ratio [OR] 5.6, 95% confidence interval [CI] 1.7–18.1). However, the results have not been consistent; other studies have found no association [5,7–9] or a lower risk of pre-eclampsia among women receiving ART than among women without HIV infection [10,11]. The aim of the present study was to investigate the association between HIV status, ART, and development of pregnancy-associated hypertension (PAH) in a large cohort of women in Lusaka, Zambia.
2. Materials and methods
A retrospective cohort study was undertaken of pregnant women for whom information about prenatal care and delivery was recorded in the Zambia Electronic Perinatal Record System (ZEPRS) between February 1, 2006, and December 31, 2012. ZEPRS is an electronic medical record system that captures data on women receiving prenatal care and delivery services at 25 public health facilities, including the University Teaching Hospital (UTH), in Lusaka [12]. Use of these data was approved by the Biomedical Research Ethics Committee at the University of Zambia School of Medicine (Lusaka, Zambia) and by the Institutional Review Board of the University of North Carolina at Chapel Hill (Chapel Hill, NC, USA). ZEPRS was established for routine clinical care purposes and individual informed consent was not obtained directly from patients; however, all individual identifiers were removed before data analysis.
Lusaka has a population of 2 million and is served by the 25 district public health sector facilities covered by ZEPRS. The rate of HIV infection among pregnant women attending these facilities is estimated to be 22% [12]. Zambian national guidelines for the prevention of mother-to-child transmission of HIV have evolved since 2001, when provider-initiated testing and counseling, and maternal and infant antiretroviral prophylaxis became the standard of care [13]. The guidelines now recommend that all pregnant women with HIV infection start lifelong ART irrespective of their CD4 count or clinical stage [14]. Changes in policy have resulted in a large, and growing, number of women in Lusaka receiving ART during pregnancy.
In the study setting, many delivery locations lacked the laboratory capacity to diagnose proteinuria or other abnormalities necessary to meet the diagnostic criteria for pre-eclampsia as defined in the American College of Obstetricians and Gynecologists guidelines [4]. Women were diagnosed empirically, on the basis of blood pressure and symptoms, including neurological symptoms and/or seizures. Women with hypertension were diagnosed at the time of labor and delivery admission according to an a priori definition of PAH: (1) eclampsia as defined by local practitioners, (2) pre-eclampsia as defined by local practitioners, (3) diagnosis of hypertension (yes/no), or (4) a recorded systolic blood pressure of or above 140 mm Hg or diastolic blood pressure of or above 90 mm Hg during labor and delivery admission. All four diagnoses were established using a “problem labor” form completed by clinical or research staff when a woman was identified as having a high-risk pregnancy according to local practices. Women with chronic hypertension—i.e. a documented medical history of hypertension before pregnancy—were excluded from the analysis. Hypertension during prenatal care was defined as elevated blood pressure during any prenatal care visit; it was not included in the outcome definition because time of blood pressure reading was unknown.
HIV status and treatment were classified into three groups: (1) women without HIV infection; (2) women with HIV infection that was untreated, who initiated mother-to-child transmission prophylaxis or a non-suppressive regimen (i.e. peripartum nevirapine with or without prenatal zidovudine) during pregnancy and labor; and (3) women with HIV infection who had been receiving combination ART (i.e. ≥3 drugs) since before pregnancy or who initiated such a regimen during pregnancy. Timing of ART initiation was dichotomized as initiation during or before pregnancy. Length of pregnancy was based on the last menstrual period; routine obstetrical ultrasonography for confirmation of gestational age was not routinely available in the study setting.
Maternal and newborn characteristics were compared between women with and without PAH. Frequencies and percentages or medians with the interquartile range were reported and were compared using χ2 or Wilcoxon tests, as appropriate. A logistic regression model was used to estimate the OR for the association between HIV status and treatment, and PAH. Generalized estimating equations with an unstructured correlation structure were used to create 95% CIs that account for repeat deliveries over the study period. Potential confounders deemed clinically important and which were significantly associated with the outcome in univariable analysis at an alpha level of P<0.05 were included in the multivariable logistic regression model. Age was modeled using a quadratic term because results from a likelihood ratio test indicated that a model with the quadratic term was a better fit to the data compared with a model with linear age (P<0.001). Two subanalyses were performed, including a model restricted to women with HIV infection and one restricted to women receiving ART, to further explore the association between CD4 lymphocyte count, timing of treatment initiation, and PAH. Another subgroup analysis was performed to explore how excluding blood pressure from the outcome definition affected the results because only one measurement was recorded and the other three diagnoses (eclampsia, pre-eclampsia, and hypertension) were identified by local standards. All subanalyses were defined a priori. All analyses were conducted with SAS version 9.3 (SAS Institute, Cary, NC, USA).
3. Results
A prenatal care visit was recorded for 390 244 women during the study period. Final analyses included 249 771 women (Figure 1).
Figure 1.
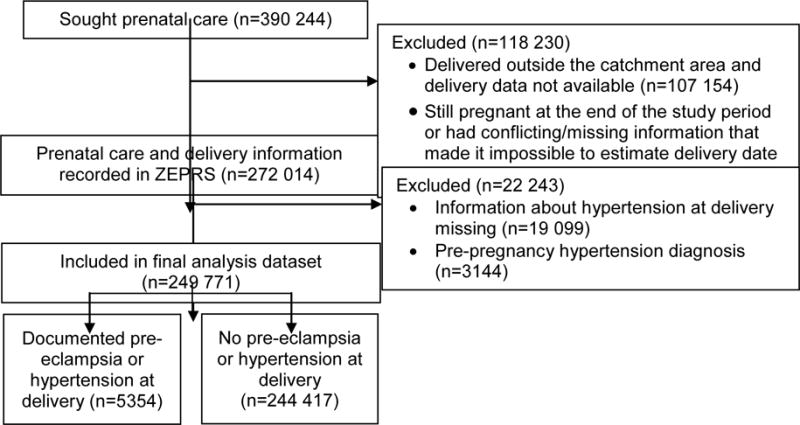
Flow of patients through the study. Abbreviation: ZEPRS, Zambia Electronic Perinatal System.
Among the 249 771 included women, 5354 (2.1%) met the criteria for PAH. Most (n=4671 [87.2%]) met the criteria on the basis of blood pressure measurements; a smaller number of women had a diagnosis of pre-eclampsia (n=701 [13.1%]), hypertension (n=372 [6.9%]), or eclampsia (n=394 [7.4%]), although these categories are not mutually exclusive. Compared with women without PAH, those with the disorder had a significantly higher body mass index (BMI, calculated as weight in kilograms divided by the square of height in meters) and more frequently had a multiple pregnancy, hypertension during prenatal care, and a neonate weighing 1500–2500 g at birth (P<0.001 for all) (Table 1). They were also more frequently in the youngest or oldest age groups (P<0.001) (Table 1). The proportion of women without HIV infection was higher among those with PAH than among those without this disorder, and more women in the PAH group had HIV infection and were receiving ART (Table 1).
Table 1.
Characteristics of participants by PAH status.a
| Characteristic | PAH
|
P value | |
|---|---|---|---|
| No (n=244 417) | Yes (n=5354) | ||
| HIV status and treatmentb | |||
| Negative | 184 042 (78.0) | 4115 (80.2) | <0.001c |
| Positive, untreated | 5869 (2.5) | 106 (2.1) | |
| Positive, given prophylaxis | 31 154 (13.2) | 545 (10.6) | |
| Positive, given antiretroviral therapy | 14 996 (6.4) | 362 (7.1) | |
| Maternal age, y | 25 (21–29) | 25 (20–31) | <0.001d |
| <20 | 44 942 (18.4) | 1069 (20.0) | <0.001c |
| 20–24 | 76 596 (31.3) | 1356 (25.3) | |
| 25–34 | 104 148 (42.6) | 2271 (42.4) | |
| ≥35 | 18 731 (7.7) | 658 (12.3) | |
| Parity | 1 (0–2) | 1 (0–2) | <0.001c |
| Length of pregnancy at first prenatal care visit (according to last menstrual period), wke | 22 (18–26) | 21 (17–25) | <0.001d |
| <14 | 20 892 (8.6) | 565 (10.6) | |
| 14–24 | 131 018 (53.8) | 3059 (57.2) | |
| ≥24 | 91 805 (37.7) | 1725 (32.2) | |
| Baseline body mass indexf | 23.4 (21.4–26.0) | 24.5 (22.1–28.0) | <0.001d |
| <18.5 | 5137 (3.1) | 73 (2.0) | <0.001c |
| 18.5–24 | 104 864 (64.3) | 1897 (52.1) | |
| 24–29 | 40 640 (24.9) | 1068 (29.3) | |
| ≥30 | 12 571 (7.7) | 602 (16.5) | |
| Multiple pregnancy | 3677 (1.5) | 196 (3.7) | <0.001c |
| Hypertension during prenatal careg | |||
| No | 209 314 (94.8) | 3981 (78.6) | <0.001c |
| Yes | 11 430 (5.2) | 1087 (21.4) | |
| Baseline hemoglobin, g/Lh | 116 (107–126) | 117 (109–126) | <0.001d |
| ≥100 | 104 311 (90.3) | 2356 (91.9) | 0.013c |
| 80–90 | 9878 (8.6) | 188 (7.3) | |
| <80 | 1323 (1.1) | 19 (0.7) | |
| Baseline syphilis by rapid plasma reagini | |||
| Non-reactive | 164 710 (96.5) | 3828 (97.0) | <0.001c |
| Reactive/no documentation of treatment | 1838 (1.1) | 30 (0.8) | |
| Reactive/documentation of treatment | 4135 (2.4) | 90 (2.3) | |
| Neonate birth weight, gj | 3000 (2700–3300) | 3000 (2600–3300) | <0.001d |
| <1000 | 702 (0.3) | 30 (0.6) | <0.001c |
| 1000–1500 | 2275 (0.9) | 141 (2.7) | |
| 1500–2500 | 23 235 (9.6) | 849 (16.2) | |
| >2500 | 215 456 (89.2) | 4227 (80.6) | |
| Most recent CD4 count (among women with HIV infection), cells/mm3 k | 421 (286–579) | 413 (278–568) | 0.600d |
| <200 | 4468 (11.9) | 76 (11.2) | 0.637c |
| 200–250 | 9159 (24.4) | 179 (26.4) | |
| 350–500 | 10 316 (27.5) | 189 (27.8) | |
| ≥500 | 13 613 (36.2) | 235 (34.6) | |
Abbreviation: PAH, pregnancy-induced hypertension
Values are given as number (percentage) or median (interquartile range), unless indicated otherwise.
Data available for 236 061 women without PAH and 5128 with PAH.
χ2 test.
Wilcoxon test.
Data available for 243 715 women without PAH and 5349 with PAH.
Calculated as weight in kilograms divided by the square of height in meters. Data available for 163 212 women without PAH and 3640 with PAH.
Data available for 220 744 women without PAH and 5068 with PAH.
Data available for 115 512 women without PAH and 2563 with PAH.
Data available for 170 683 women without PAH and 3948 with PAH.
Data available for 241 668 women without PAH and 5247 with PAH.
Data available for 37 556 women with HIV infection and without PAH and 679 with HIV infection and PAH.
In the primary analysis, PAH was associated with parity, BMI, multiple pregnancy, and HIV status and treatment (Table 2). PAH was also associated with age (data not shown). In unadjusted models, women with HIV infection who were untreated or given prophylaxis had a lower likelihood of PAH than did women without HIV infection, whereas women with HIV infection given ART had similar odds to women without the infection (Table 2). In multivariable analysis, women receiving ART had significantly increased odds of PAH when compared with HIV-negative women (Table 2). Women with HIV infection not receiving ART still had decreased odds. In a subanalysis that excluded blood pressure measurements from the outcome, similar patterns were noted in the odds of PAH among women with an untreated HIV infection and women living with HIV and receiving ART when compared with those who did not have HIV infection (Table 2).
Table 2.
| Factor | Crude modelc | Adjusted modeld | Adjusted model excluding systolic and diastolic blood pressurese |
|---|---|---|---|
| HIV status and treatment | |||
| Negative | 1 | 1 | 1 |
| Positive, untreated or given prophylaxis only | 0.78 (0.72–0.85) | 0.86 (0.78–0.95) | 0.75 (0.61–0.93) |
| Positive, given antiretroviral therapy | 1.07 (0.96–1.19) | 1.15 (1.01–1.32) | 1.05 (0.80–1.38) |
| Parity | |||
| 0 | 1 | 1 | 1 |
| ≥1 | 0.73 (0.69–0.77) | 0.66 (0.62–0.71) | 0.58 (0.50–0.66) |
| Multiple pregnancy | |||
| No | 1 | 1 | 1 |
| Yes | 2.49 (2.15–2.88) | 2.52 (2.11–3.01) | 4.53 (3.45–5.94) |
| Baseline body mass index f | |||
| <18.5 | 0.79 (0.62–0.99) | 0.77 (0.61–0.97) | 0.63 (0.37–1.07) |
| 18.5–24 | 1 | 1 | 1 |
| 25–29 | 1.45 (1.35–1.57) | 1.46 (1.35–1.58) | 1.63 (1.40–1.91) |
| ≥30 | 2.65 (2.41–2.91) | 2.60 (2.35–2.87) | 3.35 (2.79–4.03) |
Values are given as odds ratio (95% confidence interval).
Maternal age is also included as a confounder but is not shown because of the use of a quadratic term which is not easily interpretable.
Univariate associations.
Adjusted for maternal age, parity, multiple pregnancy, and body mass index.
Adjusted for maternal age, parity, multiple pregnancy, and body mass index. Outcome definition does not include a recorded systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.
Calculated as weight in kilograms divided by the square of height in meters.
Among the women with HIV infection, PAH was associated with parity, BMI, multiple pregnancy, and ART (Table 3). PAH was also associated with age (data not shown). The odds of PAH among women receiving ART were 1.27-fold those of PAH in untreated women or those receiving prophylaxis only. Most recent CD4 count was not associated with PAH.
Table 3.
Logistic regression of factors associated with pregnancy-associated hypertension among women with HIV infection.a,b
| Factor | Model adjusting for CD4 count among women with HIV infection (n=49 164)c | Model for timing of ART initiation among women taking ART (n=9918)d |
|---|---|---|
| HIV status and treatment | ||
| Positive, untreated or given prophylaxis only | 1 | NA |
| Positive, given ART | 1.27 (1.04–1.55) | NA |
| Parity ≥1 | 0.72 (0.59–0.87) | 1.20 (0.79–1.83) |
| Multiple pregnancy | 2.79 (1.84–4.24) | 2.99 (1.35–6.62) |
| Baseline body mass indexe | ||
| <18.5 | 1.16 (0.66–2.05) | 0.59 (0.14–2.45) |
| 18.5–24 | 1 | 1 |
| 25–29 | 1.47 (1.19–1.82) | 1.56 (1.02–2.40) |
| ≥30 | 3.73 (2.89–4.81) | 4.48 (2.69–7.47) |
| Most recent CD4 count, cells/mm3 | ||
| <200 | 0.97 (0.71–1.34) | 1.19 (0.68–2.08) |
| 200–250 | 1.14 (0.90–1.43) | 1.17 (0.74–1.84) |
| 350–500 | 1.02 (0.81–1.29) | 0.69 (0.39–1.19) |
| ≥500 | 1 | 1 |
| Timing of ART initiation | ||
| Before pregnancy | NA | 1 |
| During pregnancy | NA | 0.91 (0.47–1.76) |
Abbreviations: ART, antiretroviral therapy; NA, not available.
Values are given as odds ratio (95% confidence interval).
Maternal age is included in all models as a confounder but is not shown because of the use of a quadratic term which is not easily interpretable.
Adjusted for all maternal age, parity, multiple pregnancy, body mass index, CD4 T lymphocyte count, and ART.
Adjusted for all maternal age, parity, multiple pregnancy, BMI, and CD4 T lymphocyte count.
Calculated as weight in kilograms divided by the square of height in meters.
Among the 12 813 women with HIV infection receiving ART for whom timing of initiation was known, 11 581 (90.4%) initiated treatment during pregnancy. PAH was associated with BMI and multiple pregnancy (Table 3). In multivariable analysis, women initiating treatment during pregnancy had similar odds of PAH to those initiating treatment before pregnancy (Table 3). Most recent CD4 count was not associated with PAH.
4. Discussion
The present results suggest that women with an untreated HIV infection had lower odds of PAH than did women without HIV infection. However, on initiation of ART, the risk of PAH for women with HIV infection could return to baseline levels or even increase. An association between timing of ART initiation and PAH was not observed. These findings are consistent with previous studies and indicate that women initiating ART during pregnancy could require increased surveillance and treatment for hypertensive disorders of pregnancy [2,5].
Diagnosing hypertensive disorders of pregnancy in the study settings is a challenge. During prenatal care, blood pressure measurements are not consistently recorded and the timing of these measurements is uncertain. Information on proteinuria is not available because not all delivery locations have diagnostic laboratory capacity. Therefore, blood pressure elevation could not be reliably diagnosed after 20 weeks of pregnancy as indicated in the current definition of gestational hypertension and pre-eclampsia [4].
The outcome metric of PAH was developed to reflect these challenges. PAH was defined as a diagnosis of elevated blood pressure identified on admission to labor and delivery. Because of the structure of ZEPRS, in which a “problem form” is created only for recognized complications, women diagnosed with PAH in the analysis cohort probably represent severe cases. The use of hypertension diagnosed during labor and delivery admission and not at any prenatal care visit would have also resulted in an underestimate of the prevalence of gestational hypertension in this population. However, the results remained consistent when blood pressure measurements were excluded from the outcome definition.
The present findings support a reduced risk of hypertension among pregnant women with HIV infection when compared with uninfected women [5,6,15] and a higher risk among women receiving ART compared with those receiving no treatment or fewer than three antiretroviral drugs [2,5]. However, the estimates for the association between ART and hypertension are modest compared with previous studies, which have demonstrated more than a five-fold increase among women receiving ART [2,5]. The lower estimates observed in the present study could be a result of an under-representation of pre-eclampsia without severe features or mild gestational hypertension in the study sample. As far as we are aware, this is the first study to report higher odds of hypertension among women with HIV infection receiving ART than among women without HIV infection. Previous studies comparing treated women with women without HIV infection have found no association [5,7–9] or a lower risk among women taking ART [10,11].
Hypertension in pregnancy during ART use could be linked to immune reconstitution following HIV treatment [5]. A leading theory is that pre-eclampsia develops from an excessive generalized maternal inflammatory response during pregnancy [16]. Because women living with HIV have decreased CD4 T lymphocyte cell counts, their immune system might be less likely to have an immunological hyper-reactivity to the pregnancy, leading to a reduced incidence of pre-eclampsia. This reduced risk is restored back to pre-infection levels among women receiving ART as a result of increasing CD4 T lymphocyte levels [5]. The present findings are compatible with this theory, although a relationship between PAH and either CD4 T lymphocyte count or timing of ART initiation was not observed, as might have been expected under this hypothesis. Thus, additional research is needed to further clarify these relationships. Specifically, studies focusing on African countries are urgently needed given that the number of pregnant women being treated for HIV infection in the region continues to increase, and that access to obstetric care and the ability to recognize and safely manage pre-eclampsia remain limited [17,18].
Estimates of the incidence of pre-eclampsia from South Africa, Egypt, Tanzania, Ethiopia, and Nigeria range from 1.8% to 16.7% [19]. Given the broader definition for PAH used in the present study, the 2.1% frequency obtained is much lower than would be expected. The definition of PAH used in the present study includes hypertensive disorders diagnosed only during labor and delivery admission, but not during any prenatal care visit. This would have resulted in an underestimation of PAH given the exclusion of women with either new-onset hypertension detected in prenatal care or superimposed pre-eclampsia. Additionally, women for whom the “problem labor” form used to diagnose PAH was not completed were defined as having a normal pregnancy; if these women indeed had undiagnosed PAH, this would have contributed to the low recorded incidence. Finally, data on proteinuria is not reliably captured in the ZEPRS database and it was not possible to distinguish gestational hypertension from the more precise definition of pre-eclampsia. However, these diagnostic challenges do reflect the reality of care in the study settings, where only women with severe cases of PAH might be identified. It is likely that women with more severe symptoms were identified and that the risk factors associated with PAH are similar to those found in previous studies [20].
Several additional limitations are noted. First, only one measure of blood pressure was recorded. However, a subanalysis removing systolic and diastolic blood pressure from the outcome definition showed similar results. Second, the most recent CD4 cell count was used, but it is recognized that changes with time could have been a more appropriate measure of immune reconstitution. Finally, no information related to viral load or antiretroviral drug adherence was available in ZEPRS. Thus, poor adherence to ART, for example, would have biased the results toward the null.
In summary, the present findings highlight the importance of monitoring and treating hypertensive disorders during pregnancy, particularly among women with HIV infection taking ART. However, given the lack of data to appropriately diagnose pre-eclampsia, studies that can more precisely identify pre-eclampsia in African settings are needed. Although the database is large and indicates that PAH could be an issue in this population, pre-eclampsia specifically can lead to serious complications during pregnancy and should be better understood. Monitoring of hypertension during pregnancy is critical given recent policy changes in Sub-Saharan African countries—including Zambia—that will dramatically increase the number of women with HIV infection initiating ART.
Synopsis.
In Lusaka, Zambia, women with HIV infection receiving antiretroviral therapy have higher odds of pregnancy-associated hypertension than women without HIV infection or with untreated infection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicts of interest.
References
- 1.Short CE, Taylor GP. Antiretroviral therapy and preterm birth in HIV-infected women. Expert Rev Anti Infect Ther. 2014;12(3):293–306. doi: 10.1586/14787210.2014.885837. [DOI] [PubMed] [Google Scholar]
- 2.Suy A, Martínez E, Coll O, Lonca M, Palacio M, de Lazzari E, et al. Increased risk of pre-eclampsia and fetal death in HIV-infected pregnant women receiving highly active antiretroviral therapy. AIDS. 2006;20(1):59–66. doi: 10.1097/01.aids.0000198090.70325.bd. [DOI] [PubMed] [Google Scholar]
- 3.European Collaborative Study. Pregnancy-related changes in the longer-term management of HIV-infected women in Europe. Eur J Obstet Gynecol Reprod Biol. 2003;111(1):3–8. doi: 10.1016/s0301-2115(03)00153-2. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 5.Wimalasundera RC, Larbalestier N, Smith JH, de Ruiter A, McG Thom SA, Hughes AD, et al. Pre-eclampsia, antiretroviral therapy, and immune reconstitution. Lancet. 2002;360(9340):1152–4. doi: 10.1016/s0140-6736(02)11195-0. [DOI] [PubMed] [Google Scholar]
- 6.Stratton P, Tuomala RE, Abboud R, Rodriguez E, Rich K, Pitt J, et al. Obstetric and newborn outcomes in a cohort of HIV-infected pregnant women: a report of the women and infants transmission study. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(2):179–86. doi: 10.1097/00042560-199902010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Kourtis AP, Bansil P, McPheeters M, Meikle SF, Posner SF, Jamieson DJ. Hospitalizations of pregnant HIV-infected women in the USA prior to and during the era of HAART, 1994–2003. AIDS. 2006;20(14):1823–31. doi: 10.1097/01.aids.0000244201.11006.1c. [DOI] [PubMed] [Google Scholar]
- 8.Boer K, Nellen JF, Patel D, Timmermans S, Tempelman C, Wibaut M, et al. The AmRo study: pregnancy outcome in HIV-1-infected women under effective highly active antiretroviral therapy and a policy of vaginal delivery. BJOG. 2007;114(2):148–55. doi: 10.1111/j.1471-0528.2006.01183.x. [DOI] [PubMed] [Google Scholar]
- 9.Boyajian T, Shah PS, Murphy KE. Risk of preeclampsia in HIV-positive pregnant women receiving HAART: a matched cohort study. J Obstet Gynaecol Can. 2012;34(2):136–41. doi: 10.1016/S1701-2163(16)35156-8. [DOI] [PubMed] [Google Scholar]
- 10.Haeri S, Shauer M, Dale M, Leslie J, Baker AM, Saddlemire S, et al. Obstetric and newborn infant outcomes in human immunodeficiency virus-infected women who receive highly active antiretroviral therapy. Am J Obstet Gynecol. 2009;201(3):315.e1–5. doi: 10.1016/j.ajog.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Mattar R, Amed AM, Lindsey PC, Sass N, Daher S. Preeclampsia and HIV infection. Eur J Obstet Gynecol Reprod Biol. 2004;117(2):240–1. doi: 10.1016/j.ejogrb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Chi BH, Vwalika B, Killam WP, Wamalume C, Giganti MJ, Mbewe R, et al. Implementation of the Zambia electronic perinatal record system for comprehensive prenatal and delivery care. Int J Gynecol Obstet. 2011;113(2):131–6. doi: 10.1016/j.ijgo.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stringer EM, Sinkala M, Stringer JS, Mzyece E, Makuka I, Goldenberg RL, et al. Prevention of mother-to-child transmission of HIV in Africa: successes and challenges in scaling-up a nevirapine-based program in Lusaka, Zambia. AIDS. 2003;17(9):1377–82. doi: 10.1097/01.aids.0000060395.18106.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zambian Ministry of Health and Ministry of Community Development. Zambia Consolidated Guidelines for Treatment and Prevention of HIV Infection Lusaka. Zambia: Zambian Ministry of Health and Ministry of Community Development; 2014. [Google Scholar]
- 15.Kalumba VM, Moodley J, Naidoo TD. Is the prevalence of pre-eclampsia affected by HIV/AIDS? A retrospective case-control study. Cardiovasc J Afr. 2013;24(2):24–7. doi: 10.5830/CVJA-2012-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall DR. Is pre-eclampsia less common in patients with HIV/AIDS? J Reprod Immunol. 2007;76(1–2):75–7. doi: 10.1016/j.jri.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Firoz T, Sanghvi H, Merialdi M, von Dadelszen P. Pre-eclampsia in low and middle income countries. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):537–48. doi: 10.1016/j.bpobgyn.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization HIV/AIDS Programme. Antiretroviral medicines in low- and middle-income countries: forecasts of global and regional demand for 2012–2015. http://apps.who.int/iris/bitstream/10665/83148/1/9789241505468_eng.pdf. Published May 2013. Accessed January 17, 2016.
- 19.Osungbade KO, Ige OK. Public health perspectives of preeclampsia in developing countries: implication for health system strengthening. J Pregnancy. 2011;2011:481095. doi: 10.1155/2011/481095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conde-Agudelo A, Belizan JM. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG. 2000;107(1):75–83. doi: 10.1111/j.1471-0528.2000.tb11582.x. [DOI] [PubMed] [Google Scholar]


