Abstract
Obesity and metabolic disorders are a major health concern in all developed countries and a primary focus of current medical research is to improve our understanding treatment of metabolic diseases. One avenue of research that has attracted a great deal of recent interest focuses upon understanding the role of miRNAs in the development of metabolic diseases. miRNAs have been shown to be dysregulated in a number of different tissues under conditions of obesity and insulin resistance, and have been demonstrated to be important regulators of a number of critical metabolic functions, including insulin secretion in the pancreas, lipid and glucose metabolism in the liver, and nutrient signaling in the hypothalymus. In this review we will focus on the important role of miRNAs in regulating the differentiation and function of white and brown adipose tissue and the potential importance of this for maintaining metabolic function and treating metabolic diseases.
Keywords: miRNA, WAT, BAT, Adipogenesis, Obesity, Metabolic Syndrome
Introduction
White adipose tissue (WAT) is critical for maintaining energy homeostasis, especially under conditions of obesity. An inability of WAT to take up and store circulating lipids can lead to the accumulation of lipid droplets in non-adipose tissues, thereby promoting diseases, such as type II diabetes and atherosclerosis [1, 2]. Recently, WAT has also been shown to play an additional role in the maintenance of metabolic function through the release of signaling molecules, such as adiponectin and leptin, that regulate appetite and insulin sensitivity [3, 4]. Prolonged nutrient overload stimulates adipocyte hypertrophy and hyperplasia leading to the development of obesity, which is commonly associated with the development of insulin resistance, dyslipidemia and hypertension [5]. However, expansion of adipose tissue does not always promote metabolic syndrome, and mutations that impair the expansion or differentiation of WAT (lipodystrophy) can cause severe insulin resistance and metabolic dysfunction [6]. In short, impaired WAT function, whether due to excessive lipid accumulation or genetic abnormalities, can lead to the development of metabolic syndrome.
Unlike WAT, the primary function of BAT is not lipid storage, but the generation of heat for the purpose of thermoregulation. To accomplish this, brown adipocytes contain a large number of mitochondria and have high expression of mitochondrial uncoupling protein 1 (UCP1). UCP1, and other mitochondrial uncoupling proteins, facilitate the transfer of protons across the mitochondrial membrane independently of ATP synthase, thus separating oxidative phosphorylation from ATP production, and dissipating energy in the form of heat. Adult humans have been shown to have thermogenically active adipose tissue depots that can be induced by exposure to cold [7]. Regulation of BAT has garnered a great deal of interest due to its ability to mediate whole body energy expenditure. Additionally, cold exposure or genetic modifications can induce WAT browning, a process whereby expression of genes involved in BAT function are induced in WAT resulting in “bright” or “beige” adipocytes. Importantly, induction of thermogenesis in BAT or WAT has been demonstrated to increase energy expenditure, and impede the development of diet induced obesity and metabolic dysfunction.
Recently, miRNAs have been demonstrated to play a critical role in regulating differentiation and function in both WAT and BAT. Indeed, adipocyte specific disruption of miRNA processing, by knockout of DGCR8 or DICER, has been demonstrated to both alter WAT accumulation and promote the whitening of BAT, leading to impaired metabolic function [8, 9]. Additionally, numerous studies have identified individual miRNAs that play critical roles in the regulation of adipogenesis in WAT and BAT. In this review we will attempt to summarize the work that has been done on miRNAs regulating adipogenesis, with a specific emphasis on those miRNAs that have been demonstrated to regulate WAT or BAT in vivo, be dysregulated under conditions of obesity and metabolic syndrome, or target critical regulators of adipocyte differentiation.
miRNAs regulating differentiation and function of white adipose tissue
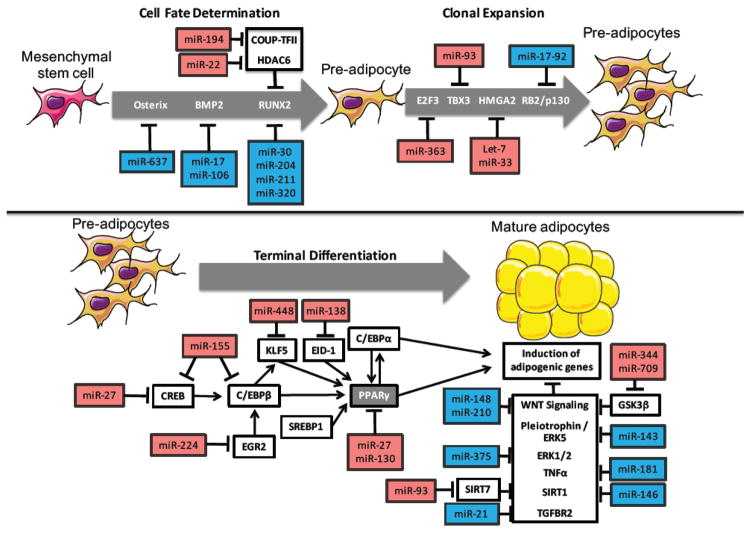
Differentiation of pre-adipocytes into mature adipocytes is a multi-stage process involving cell fate determination, clonal expansion, and terminal differentiation/lipid accumulation. Over the years a great deal of work has gone into identifying those factors involved in regulating the different stages of adipogenesis, and recently miRNAs have been shown to target many of the key factors involved in mediating these processes. As such, numerous miRNAs have been identified whose expression is altered during adipocyte differentiation, and many of these have been demonstrated to be important regulators of adipogenesis. Specifically, the miRNA cluster miR-17–92, miR-21, miR-26b, miR-30, miR-103, miR-143, miR-146b, miR-148a, miR-181, miR-199a, miR-204/211, miR-210, miR-320, miR-371, miR-375, miR-378, and miR-637 have been shown to promote adipogenesis [10–28], while let-7, miR-15a, miR-22, miR-27a/b, miR-33b, miR-93, miR-125a, miR-130, miR-138, miR-145, miR-155, mirR-193a/b, miR-194, miR-205, miR-221, miR-222, miR-224, miR-344, miR-363, miR-365, miR-369, miR-448, and miR-709 have been reported to impair adipocyte differentiation [26, 29–51]. Importantly, miRNA expression analysis in WAT of human patients has identified numerous miRNAs that are dysregulated during conditions of obesity and metabolic syndrome, including many of those known to be involved in regulation of adipogenesis [52–54]. While the exact mechanisms by which many miRNAs regulate adipogenesis are still under investigation, this is largely due to the ability of miRNAs to target numerous mRNAs, including multiple targets within the same or similar pathways. Nevertheless, it has been clearly demonstrated that miRNAs play a critical role in regulating adipogenesis through direct targeting of key factors involved in all phases of adipocyte differentiation (Figure 1).
Figure 1.
miRNAs regulating differentiation of White Adipose Tissue (WAT)
miRNAs regulate cell fate determination toward adipocyte lineage
One of the first reported roles of miRNAs was in the regulation of cellular plasticity and cell fate determination during development. Since then, specific miRNAs have been identified that are important regulators of cell fate determination in a variety different cell types. Importantly, pre-adipocytes are derived from mesenchymal stem cells (MSCs), which can also differentiate into other mesenchymal lineages including osteoblasts, and miRNAs have been identified as important regulators of this cell fate determination. Runt-related transcription factor 2 (RUNX2) is considered the master regulator of osteoblast differentiation and is one of the main sites of miRNA-mediated adipocyte/osteoblast cell fate determination. Indeed, miR-30a/d, miR-204, miR-211, and miR-320 have all been found to target RUNX2, resulting in impaired osteogenesis and improved adipogenesis [15, 16, 28]. Similarly, miR-17 and miR-106a have been shown to inhibit osteoblast differentiation and promote adipogenesis by targeting BMP2 [55]. Conversely, miR-22 and miR-194 have been shown to promote osteoblast differentiation and reduce adipogenesis by targeting histone deacetylase 6 (HDAC6) [37], which acts as a co-repressor of RUNX2, and chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) [47], respectively. Osterix also plays an important role in promoting osteoblast differentiation, and miR-637 has been found to disrupt osteogenesis by targeting osterix, thereby increasing differentiation into adipocytes [19]. Importantly, following injection into nude mice, miR-637-transduced human MSCs showed enhanced adipose tissue expansion and impaired osteoblast differentiation. MSCs are found in multiple tissues, including bone marrow, and accumulation of bone marrow fat may contribute to the development of age-related bone loss. Taken together, these findings highlight the potential for novel miRNA-based therapeutics for the treatment of both metabolic syndrome and osteoporosis.
miRNAs regulating pre-adipocyte clonal expansion
Prior to terminal differentiation, stimulated pre-adipocytes undergo clonal expansion, a necessary step in adipocyte differentiation. miRNAs have been demonstrated to be key regulators of cellular proliferation under a variety of different conditions, including during development and tumor growth. Consistent with this, miRNAs have also been shown to regulate pre-adipocyte proliferation during the clonal expansion phase of adipogenesis. In addition to their role in regulating cell fate determination between osteoblast and adipocyte lineages, the miR-17–92 miRNA cluster was previously found to be increased upon induction of adipocyte differentiation, and to promote clonal expansion and adipocyte differentiation by targeting the tumor suppressor RB2/p130 [11]. Interestingly, miR-17 expression has been shown to be reduced in WAT under conditions of obesity and insulin resistance in humans [52, 54] and circulating levels of miR-17 have been shown to be altered in a number of metabolic diseases [52, 56, 57]. Alternatively, the targeting of E2F3 and T-Box 3 (TBX3), positive regulators of clonal expansion, by miR-363 and miR-93, respectively, has been demonstrated to impair pre-adipocyte proliferation and differentiation [46, 50].
High-mobility group AT-hook 2 (HMGA2) has also been shown to play an important role in the clonal expansion phase of adipogenesis, and has been reported to be the primary target for let-7-mediated inhibition of adipocyte differentiation [30]. Interestingly, let-7-mediated repression of adipogenesis was also found to promote osteogenesis [58]. HMGA2 and let-7 have been found be important regulators of tumor growth and are known to be dysregulated in lipomas and liposarcomas from human patients [59, 60]. Recently, miR-33b was also shown to regulate HMGA2 in human pre-adipocytes, leading to impaired proliferation and differentiation. Moreover, miR-33b expression was also highly induced during adipocyte differentiation, consistent with its co-transcription with its host gene, sterol regulatory element-binding protein 1 (SREBP1) [51]. While miR-33b is lost in mice and other small mammals, knockout mice for the other family member, miR-33a, results in increased susceptibility to diet induced obesity and metabolic dysfunction [61]. These findings suggest that miR-33 may be an important regulator of WAT in vivo. Additionally, miR-33b may be an important factor in the development of metabolic diseases in humans, as transcriptional regulation of SREBP1 is known to be repressed under conditions of insulin resistance [62, 63].
As the clonal expansion phase of adipogenesis is transient, it has also been demonstrated that miRNAs, which inhibit clonal expansion, can promote adipocyte differentiation. This is the case for miR-146b, which has been demonstrated to impair pre-adipocyte proliferation, thus promoting the transition to terminal differentiation and enhancing adipogenesis through direct targeting of KLF7 [25]. These findings demonstrate that miRNAs play an important role in both the induction and conclusion of the clonal expansion phase of adipogenesis as part of a complex and tightly regulated series of events required for proper adipocyte differentiation.
miRNAs regulating adipocyte terminal differentiation and lipid metabolism
The final and most studied phase of adipocyte differentiation involves terminal differentiation and the induction of a signaling cascade to promote the expression of genes necessary for adipocyte function. These gene expression changes are mediated by a number of key transcription factors including, peroxisome proliferator-activated receptor gamma (PPARγ), SREBP1, and CCAAT/enhancer-binding protein alpha/beta (C/EBPα/β). miRNAs have been demonstrated to regulate adipocyte differentiation through both direct and indirect targeting of these critical transcription factors, as well as their downstream targets. PPARγ is considered the master regulator of adipocyte differentiation and is a direct target of miR-27a/b [29, 42, 43] and miR-130 [38]. Overexpression of miR-130 has been demonstrated to inhibit differentiation of human pre-adipocytes by targeting PPARγ, and expression of miR-130b was found to be reduced in obese patients compared to lean controls. Interestingly, it was recently reported that treatment of porcine pre-adipocytes with microvesicles produced from cells overexpressing miR-130b caused impaired adipocyte differentiation and repression of PPARγ [64]. Moreover, intravenous injection of these miR-130b-laden microvesicles into mice was sufficient to elevate miR-130b levels in adipose tissue resulting in decreased expression of PPARγ and protection from diet induced obesity [65].
miR-27a and mir-27b have also been demonstrated to be important negative regulators of both mouse and human adipogenesis [29, 42, 43] and levels of miR-27a/b were found to be decreased in adipocytes of obese mice compared to lean controls [66]. While these effects are primarily attributed to the ability of miR-27 to target PPARγ, additional targets including prohibitin (PHB) [67], and cAMP response element binding protein (CREB) [66] have also been suggested to be at least partially involved in mediating these effects. These findings highlight the capacity of a single miRNA to target numerous factors involved in similar processes, with the ultimate outcome being impairment of many facets of adipogenesis. Similarly, miR-155 has been shown to directly target CREB, as well as C/EBPβ, a critical transcription factor induced early in the process of adipogenesis [35]. Induction of C/EBPβ has also been shown to be regulated in part by early growth response 2 (EGR2), which can be disrupted by targeting of EGR2 by miR-224 [41]. C/EBPβ in turn regulates the expression of PPARγ both directly and through induction of another transcription factor, Kruppel-like factor-5 (KLF5), which is a direct target of miR-448 [32]. Similarly, miR-138 was also found to impair adipocyte differentiation by disrupting activation of PPARγ through targeting E1A-like inhibitor of differentiation (EID-1) [36]. miR-27, miR-130 and miR-155 have all been reported to be induced in response to tumor necrosis factor alpha (TNF-α), suggesting that induction of specific anti-adipogenic miRNAs may contribute to TNF-α induced inhibition of adipogenesis [35, 66, 68]. Conversely, miR-181a has been shown to promote adipogenesis by directly targeting TNF-α [20].
Similar to the targeting of TNF-α by miR-181a, many miRNAs that promote adipogenesis do so by targeting factors and pathways known to suppress adipocyte differentiation. Transforming growth factor beta (TGF-β) is known to inhibit adipogenesis both in vitro and in vivo. miR-21 has been shown to disrupt this negative regulatory signal by targeting TGF-β receptor type II (TGFBR2) [14]. In addition to promoting adipogenesis in vitro, miR-21 was positively correlated with body mass index (BMI) in subcutaneous adipose tissue [53]. Similarly, pleiotrophin (PTN), a growth factor known to impair adipocyte differentiation, was recently shown to be targeted by miR-143 [22]. miR-143 expression was also found to be reduced in mice fed a high fat diet, and in response to treatment with TNF-α [12]. Despite these in vitro findings, whole body overexpression of miR-143 was found to promote high fat diet induced insulin resistance, while knockout of the miR-143/145 locus improved insulin sensitivity [69]. PTN regulates adipogenesis through the PI3K/AKT pathway, which has been shown to crosstalk with wingless-type MMTV integration site family (WNT) signaling to impair adipogenesis. WNT signaling has also been demonstrated to be a target for miRNA mediated regulation of adipogenesis. Expression of miR-148 was elevated with obesity in mice and humans, and miR-148a was found to promote adipogenesis and impair WNT-mediated repression of adipogenesis by targeting WNT1 [24]. miR-210 has also been shown to help promote adipocyte differentiation by targeting transcription factor 7 like 2 (Tcf7l2), which acts downstream of WNT signaling [13]. Conversely, miR-344 and miR-709 directly target glycogen synthase kinase 3 beta (GSK3β), leading to activation of WNT signaling and impaired adipogenesis [39, 48].
In addition to targeting PTN, miR-143 has previously been shown to promote adipocyte differentiation through targeting of extracellular signal regulated kinase 5 (ERK5) [10]. ERK5, and other members of the ERK family are known to directly regulate PPARγ phosphorylation, leading to its inactivation. Similarly, miR-375 was found to promote adipogenesis by ERK1/2 signaling [18]. The protein deacetylase sirtuin 1 (SIRT1) has also been shown to impair differentiation and promote lipolysis in adipocytes [70]. In addition to its role in mediating the transition between clonal expansion and terminal differentiation, miR-146b has previously been shown to promote adipogenesis by targeting SIRT1. This led to a decrease in the active (deacetylated) form of the SIRT1 target forkhead box O1 (FOXO1) [27], which has also previously been shown to impair adipocyte differentiation [71]. Knockdown of miR-146b in vivo led to upregulation of SIRT1, decreased body weight and fat mass, and protection from high fat diet induced metabolic dysfunction [27]. Conversely, miR-93 was recently demonstrated to impair adipogenesis by promoting SIRT1 activity through targeting of sirtuin 7 (SIRT7). As such, loss of miR-93 was found to promote weight gain and insulin resistance [50].
In addition to regulating adipocyte differentiation, miRNAs have also been demonstrated to target downstream factors necessary for WAT function. These include miR-224, which can disrupt fatty acid metabolism in differentiating adipocytes by targeting acyl-CoA synthetase long-chain family member 4 (ACSL4) [41] and miR-369, which disrupts fatty acid trafficking by targeting fatty acid binding protein 4 (FABP4) [26]. Additionally, a number of miRNAs have been identified that regulate the ability of WAT to respond to insulin. Circulating factors including free fatty acids, adipokines and cytokines have been shown to induce insulin resistance in WAT. Treatment with these factors caused a reduction in miR-26b, miR-103 and miR-143 and an increase in miR-335 and miR-378, suggesting that these miRNAs may be involved in propagating the effects of insulin resistance in WAT [12, 72–75]. Similarly, expression of miR-222 was found to be elevated in patients with gestational diabetes, and in vitro studies indicate that miR-222 may promote estrogen induced insulin resistance by targeting estrogen receptor alpha (ER-α) [76]. The expression of miR-222 was also found to be elevated in diabetic mice and in the plasma of obese human patients [77–80]. Expression of miR-103 and miR-107 were increased in obese mice leading to downregulation of their target gene caveolin-1 (CAV-1), destabilization of the insulin receptor, and impaired insulin stimulated glucose uptake [81]. Similarly, miR-29 was found to disrupt insulin stimulated glucose uptake [82], while miR-320 regulates induction of the insulin receptor [83], and miR-145 targets insulin receptor substrate 1 (IRS1) [44]. Overall miRNAs have been demonstrated to be key regulators of adipocyte differentiation and function (Figure 1). Considering WAT’s critical role in regulating fuel availability in response to changes in insulin and other cues, these miRNA therapies may provide promising options for treatment of metabolic diseases.
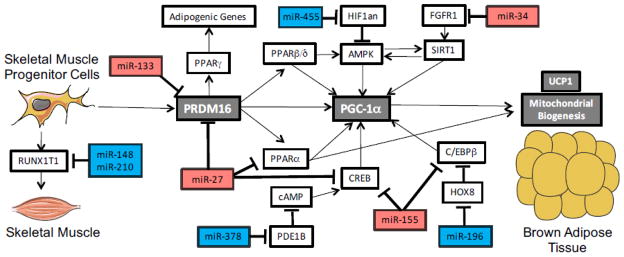
miRNAs regulating differentiation and function of brown adipose tissue
Unlike WAT, the primary function of BAT is to carry out uncoupled respiration to produce heat to regulate body temperature. Stimulation of BAT, or induction of browning in WAT, has been shown to increase whole body energy expenditure and protect against diet induced metabolic dysfunction [84–86]. Differentiation of BAT involves many of the same signaling pathways as WAT differentiation, and not surprisingly, most changes in miRNA expression were also found to be similar during differentiation of WAT and BAT [87]. As such, some of the miRNAs previously identified as regulators of adipogenesis in WAT have also recently been demonstrated to be involved in the regulation of BAT, including miR-27, miR-155, miR-193, miR-365, and miR-378 [21, 88–90]. Alternatively, expression of miR-143 was specific to WAT, while other miRNAs including the myogenic miR-1, miR-133 and miR-206, were expressed only in BAT [87].
The pre-cursor cells that give rise to classical BAT are derived from skeletal muscle progenitor cells. As such, miR-193 and miR-365 have been shown to promote BAT differentiation, through targeting of runt-related transcription factor 1; translocated to, 1 (Runx1t1), which promotes myogenesis [21]. Importantly, introduction of miR-365 was sufficient to promote expression of UCP1 in DICER knockout mice [9]. Alternatively, the transition of muscle progenitor cells to brown adipocytes is mediated by the transcription factor PR domain containing 16 (Prdm16), which has also been shown to play an important role in promoting the browning of WAT [86]. Interestingly, recent work has shown that muscle-enriched miR-133 directly targets Prdm16 to prevent differentiation of BAT and that expression of miR-133 in both muscle satellite cells and WAT is decreased in response to cold exposure [91, 92]. Consistent with this, inhibition or knockout of miR-133 results in an induction of genes involved in BAT function and improved metabolic regulation [93].
In response to cold exposure or pharmacologic stimulation of browning, miR-196a expression is induced and is involved in promoting the induction of BAT genes in WAT. In vivo, fat specific overexpression of miR-196a results in browning of WAT, increased energy expenditure, and protection from diet induced obesity and metabolic dysfunction [94]. These effects have been shown to be mediated through direct targeting of homeotic gene 8 (HOX8), a negative regulator of C/EBPβ. C/EBPβ is a positive regulator of BAT differentiation, and this function is enhanced by miR-196a-mediated repression of HOX8 [94]. Conversely, miR-155 has been demonstrated to directly target C/EBPβ and is induced upon treatment with TGFβ, indicating it may help mediate TGFβ induced repression of adipogenesis in BAT. Consistent with these findings, the size of BAT depots was found to be reduced in miR-155 transgenic mice, while loss of miR-155 was found to enhance BAT differentiation and improve responsiveness to cold exposure in WAT [90].
Similarly, miR-27 has been shown to be induced by glucocorticoids, and may be responsible for the ability of glucocorticoids to impair browning. Indeed, inhibition of miR-27 in glucocorticoid treated mice was found to increase energy expenditure, reduce body weight and improve regulation of glucose homeostasis [95]. These effects are likely mediated through targeting of PRDM16, although numerous other factors involved in promoting BAT function including CREB, PPARα, and peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC-1β) have also been identified as targets of miR-27 [88]. Interestingly, miR-378 is an intronic miRNA encoded within the PGC-1β gene, which has also been shown to be an important regulator of BAT. miR-378 has been shown to target phosphodiesterase 1b (PDE1B), thus elevating cAMP levels and promoting BAT activity. Consistent with this, fat specific overexpression of miR-378 resulted in increased BAT mass and activity, and improved metabolic function [89].
PGC-1β and PGC-1α are the primary regulators of mitochondrial biogenesis and function, a critical process in BAT. Recently, miRNAs have been shown to regulate browning of WAT through regulation of PGC-1α activity. Full activation of PGC-1α requires both phosphorylation by AMP-activated protein kinase (AMPK) and deacetylation by SIRT1. Recently, miR-455 was shown to be induced upon cold exposure, and to increase browning of WAT both in vitro and in vivo when overexpressed exclusively in adipose tissue. This was attributed to the ability of miR-455 to target hypoxia-inducible factor 1 alpha inhibitor (HIF1an), a negative regulator of AMPK [96]. Conversely, miR-34a has been shown to prevent browning of WAT by preventing activation of PGC-1α. Fibroblast growth factor 1 (FGF21) is a circulating factor that has been shown to induce browning and improve metabolic function by inducing SIRT1-mediated deacetylation of PGC-1α. However, these effects can be attenuated by targeting of FGF receptor 1 (FGFR1) by miR-34a. miR-34a expression is decreased in response to cold exposure, and inhibition of miR-34a increased browning of WAT and resulted in an improved metabolic profile. Together these results establish the important role of miRNAs in regulating BAT differentiation and function and the browning of WAT, and demonstrate that dysregulation of these miRNAs can have dramatic effects on whole body metabolic function (Figure 2).
Figure 2.
miRNAs regulating differentiation of Brown Adipose Tissue (BAT)
Concluding Remarks
Overall miRNAs have been shown to play a critical role in regulating the differentiation and function of both WAT and BAT, and therapeutic regulation of individual miRNAs can have dramatic effects on lipid storage, thermoregulation and overall metabolic function. Because miRNAs are dysregulated under conditions of obesity and metabolic dysfunction and can be stably transported in plasma, they have the potential to serve as important mediators of metabolic crosstalk between different organs. Indeed, circulating levels of miR-130b have been reported to regulate crosstalk between adipose tissue and muscle during conditions of obesity [97], and more recent work has demonstrated that administration of miR-130b laden microvesicles can prevent high fat diet induced obesity and metabolic dysfunction [65]. Circulating miRNAs may also serve as useful biomarkers of metabolic disease states. While many miRNAs have been identified that are dysregulated in the plasma of patients with obesity or metabolic syndrome [98], the amount of variability between studies raises questions about the use of miRNAs as biomarkers for metabolic diseases. Overall, the dysregulation of miRNAs during metabolic disease, and their ability to regulate critical genes involved in the differentiation and function of WAT and BAT highlight the promise of miRNAs as therapeutic targets for obesity and metabolic syndrome.
Highlights.
miRNAs are critical regulators of all phases of adipogenesis in WAT
miRNAs regulate the differentiation and function of BAT and browning of WAT
Therapeutic targeting of miRNAs in WAT and BAT may prevent obesity and metabolic dysfunction
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01HL107953 and R01HL106063 to CF-H and 5F32DK103489-03 to NP) and The Leducq Transatlantic Network in Vascular microRNAs (MIRVAD). Figures were made using the Servier Medical Art illustration resources (http://www.servier.com). We apologize to those whose work could not be cited due to space limitations.
Footnotes
CONFLICTS OF INTEREST
CF-H has patents on the use of miR-33, miR-27b, and miR-148a inhibitors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 2.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coll AP, Farooqi IS, O’Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremer AA, Jialal I. Adipose tissue dysfunction in nascent metabolic syndrome. J Obes. 2013;2013:393192. doi: 10.1155/2013/393192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 6.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 7.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Cho H, Alexander R, Patterson HC, Gu M, Lo KA, Xu D, Goh VJ, Nguyen LN, Chai X, Huang CX, Kovalik JP, Ghosh S, Trajkovski M, Silver DL, Lodish H, Sun L. MicroRNAs are required for the feature maintenance and differentiation of brown adipocytes. Diabetes. 2014;63:4045–4056. doi: 10.2337/db14-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori MA, Thomou T, Boucher J, Lee KY, Lallukka S, Kim JK, Torriani M, Yki-Jarvinen H, Grinspoon SK, Cypess AM, Kahn CR. Altered miRNA processing disrupts brown/white adipocyte determination and associates with lipodystrophy. J Clin Invest. 2014;124:3339–3351. doi: 10.1172/JCI73468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, Sun Y, Koo S, Perera RJ, Jain R, Dean NM, Freier SM, Bennett CF, Lollo B, Griffey R. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem. 2004;279:52361–52365. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ, Li X. miR-17–92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci U S A. 2008;105:2889–2894. doi: 10.1073/pnas.0800178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie H, Lim B, Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes. 2009;58:1050–1057. doi: 10.2337/db08-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin L, Chen Y, Niu Y, Chen W, Wang Q, Xiao S, Li A, Xie Y, Li J, Zhao X, He Z, Mo D. A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genomics. 2010;11:320. doi: 10.1186/1471-2164-11-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YJ, Hwang SJ, Bae YC, Jung JS. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells. 2009;27:3093–3102. doi: 10.1002/stem.235. [DOI] [PubMed] [Google Scholar]
- 15.Zaragosi LE, Wdziekonski B, Brigand KL, Villageois P, Mari B, Waldmann R, Dani C, Barbry P. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 2011;12:R64. doi: 10.1186/gb-2011-12-7-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–364. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerin I, Bommer GT, McCoin CS, Sousa KM, Krishnan V, MacDougald OA. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. Am J Physiol Endocrinol Metab. 2010;299:E198–206. doi: 10.1152/ajpendo.00179.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling HY, Wen GB, Feng SD, Tuo QH, Ou HS, Yao CH, Zhu BY, Gao ZP, Zhang L, Liao DF. MicroRNA-375 promotes 3T3-L1 adipocyte differentiation through modulation of extracellular signal-regulated kinase signalling. Clin Exp Pharmacol Physiol. 2011;38:239–246. doi: 10.1111/j.1440-1681.2011.05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JF, Fu WM, He ML, Wang H, Wang WM, Yu SC, Bian XW, Zhou J, Lin MC, Lu G, Poon WS, Kung HF. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol Biol Cell. 2011;22:3955–3961. doi: 10.1091/mbc.E11-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Chen X, Guan L, Qi Q, Shu G, Jiang Q, Yuan L, Xi Q, Zhang Y. MiRNA-181a regulates adipogenesis by targeting tumor necrosis factor-alpha (TNF-alpha) in the porcine model. PLoS One. 2013;8:e71568. doi: 10.1371/journal.pone.0071568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L, Xie H, Mori MA, Alexander R, Yuan B, Hattangadi SM, Liu Q, Kahn CR, Lodish HF. Mir193b-365 is essential for brown fat differentiation. Nat Cell Biol. 2011;13:958–965. doi: 10.1038/ncb2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi C, Xie WD, Li F, Lv Q, He J, Wu J, Gu D, Xu N, Zhang Y. MiR-143 enhances adipogenic differentiation of 3T3-L1 cells through targeting the coding region of mouse pleiotrophin. FEBS Lett. 2011;585:3303–3309. doi: 10.1016/j.febslet.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Shi XE, Li YF, Jia L, Ji HL, Song ZY, Cheng J, Wu GF, Song CC, Zhang QL, Zhu JY, Yang GS. MicroRNA-199a-5p affects porcine preadipocyte proliferation and differentiation. Int J Mol Sci. 2014;15:8526–8538. doi: 10.3390/ijms15058526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi C, Zhang M, Tong M, Yang L, Pang L, Chen L, Xu G, Chi X, Hong Q, Ni Y, Ji C, Guo X. miR-148a is Associated with Obesity and Modulates Adipocyte Differentiation of Mesenchymal Stem Cells through Wnt Signaling. Sci Rep. 2015;5:9930. doi: 10.1038/srep09930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Dai YM, Ji CB, Yang L, Shi CM, Xu GF, Pang LX, Huang FY, Zhang CM, Guo XR. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity. Mol Cell Endocrinol. 2014;393:65–74. doi: 10.1016/j.mce.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Bork S, Horn P, Castoldi M, Hellwig I, Ho AD, Wagner W. Adipogenic differentiation of human mesenchymal stromal cells is down-regulated by microRNA-369–5p and up-regulated by microRNA-371. J Cell Physiol. 2011;226:2226–2234. doi: 10.1002/jcp.22557. [DOI] [PubMed] [Google Scholar]
- 27.Ahn J, Lee H, Jung CH, Jeon TI, Ha TY. MicroRNA-146b promotes adipogenesis by suppressing the SIRT1-FOXO1 cascade. EMBO Mol Med. 2013;5:1602–1612. doi: 10.1002/emmm.201302647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamam D, Ali D, Vishnubalaji R, Hamam R, Al-Nbaheen M, Chen L, Kassem M, Aldahmash A, Alajez NM. microRNA-320/RUNX2 axis regulates adipocytic differentiation of human mesenchymal (skeletal) stem cells. Cell Death Dis. 2014;5:e1499. doi: 10.1038/cddis.2014.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, Dani C, Amri EZ, Scheideler M. microRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem Biophys Res Commun. 2009;390:247–251. doi: 10.1016/j.bbrc.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 30.Sun T, Fu M, Bookout AL, Kliewer SA, Mangelsdorf DJ. MicroRNA let-7 regulates 3T3-L1 adipogenesis. Mol Endocrinol. 2009;23:925–931. doi: 10.1210/me.2008-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang MR, Lee SW, Um E, Kang HT, Hwang ES, Kim EJ, Um SJ. Reciprocal roles of SIRT1 and SKIP in the regulation of RAR activity: implication in the retinoic acid-induced neuronal differentiation of P19 cells. Nucleic Acids Res. 2010;38:822–831. doi: 10.1093/nar/gkp1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinoshita M, Ono K, Horie T, Nagao K, Nishi H, Kuwabara Y, Takanabe-Mori R, Hasegawa K, Kita T, Kimura T. Regulation of adipocyte differentiation by activation of serotonin (5-HT) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448-mediated repression of KLF5. Mol Endocrinol. 2010;24:1978–1987. doi: 10.1210/me.2010-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen DC, Jensen CH, Schneider M, Nossent AY, Eskildsen T, Hansen JL, Teisner B, Sheikh SP. MicroRNA-15a fine-tunes the level of Delta-like 1 homolog (DLK1) in proliferating 3T3-L1 preadipocytes. Exp Cell Res. 2010;316:1681–1691. doi: 10.1016/j.yexcr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Skarn M, Namlos HM, Noordhuis P, Wang MY, Meza-Zepeda LA, Myklebost O. Adipocyte differentiation of human bone marrow-derived stromal cells is modulated by microRNA-155, microRNA-221, and microRNA-222. Stem Cells Dev. 2012;21:873–883. doi: 10.1089/scd.2010.0503. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Yang Y, Wu J. TNFalpha-induced up-regulation of miR-155 inhibits adipogenesis by down-regulating early adipogenic transcription factors. Biochem Biophys Res Commun. 2011;414:618–624. doi: 10.1016/j.bbrc.2011.09.131. [DOI] [PubMed] [Google Scholar]
- 36.Yang Z, Bian C, Zhou H, Huang S, Wang S, Liao L, Zhao RC. MicroRNA hsa-miR-138 inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells through adenovirus EID-1. Stem Cells Dev. 2011;20:259–267. doi: 10.1089/scd.2010.0072. [DOI] [PubMed] [Google Scholar]
- 37.Huang S, Wang S, Bian C, Yang Z, Zhou H, Zeng Y, Li H, Han Q, Zhao RC. Upregulation of miR-22 promotes osteogenic differentiation and inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells by repressing HDAC6 protein expression. Stem Cells Dev. 2012;21:2531–2540. doi: 10.1089/scd.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim MM, Srikantan S, Martindale JL, Hutchison ER, Kim HH, Marasa BS, Selimyan R, Egan JM, Smith SR, Fried SK, Gorospe M. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol. 2011;31:626–638. doi: 10.1128/MCB.00894-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H, Wang S, Chen L, Chen Y, Wu M, Zhang Y, Yu K, Huang Z, Qin L, Mo D. MicroRNA-344 inhibits. 3T3-L1 cell differentiation via targeting GSK3beta of Wnt/beta-catenin signaling pathway. FEBS Lett. 2013 doi: 10.1016/j.febslet.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Song G, Xu G, Ji C, Shi C, Shen Y, Chen L, Zhu L, Yang L, Zhao Y, Guo X. The role of microRNA-26b in human adipocyte differentiation and proliferation. Gene. 2014;533:481–487. doi: 10.1016/j.gene.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Peng Y, Xiang H, Chen C, Zheng R, Chai J, Peng J, Jiang S. MiR-224 impairs adipocyte early differentiation and regulates fatty acid metabolism. Int J Biochem Cell Biol. 2013;45:1585–1593. doi: 10.1016/j.biocel.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 42.Kim SY, Kim AY, Lee HW, Son YH, Lee GY, Lee JW, Lee YS, Kim JB. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem Biophys Res Commun. 2010;392:323–328. doi: 10.1016/j.bbrc.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Lin Q, Gao Z, Alarcon RM, Ye J, Yun Z. A role of miR-27 in the regulation of adipogenesis. Febs J. 2009;276:2348–2358. doi: 10.1111/j.1742-4658.2009.06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo Y, Chen Y, Zhang Y, Chen L, Mo D. Up-regulated miR-145 expression inhibits porcine preadipocytes differentiation by targeting IRS1. Int J Biol Sci. 2012;8:1408–1417. doi: 10.7150/ijbs.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji HL, Song CC, Li YF, He JJ, Li YL, Zheng XL, Yang GS. miR-125a inhibits porcine preadipocytes differentiation by targeting ERRalpha. Mol Cell Biochem. 2014;395:155–165. doi: 10.1007/s11010-014-2121-4. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, Cui J, Hou J, Long J, Li C, Liu L. A novel negative regulator of adipogenesis: microRNA-363. Stem Cells. 2014;32:510–520. doi: 10.1002/stem.1549. [DOI] [PubMed] [Google Scholar]
- 47.Jeong BC, Kang IH, Hwang YC, Kim SH, Koh JT. MicroRNA-194 reciprocally stimulates osteogenesis and inhibits adipogenesis via regulating COUP-TFII expression. Cell Death Dis. 2014;5:e1532. doi: 10.1038/cddis.2014.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, Mo D, Li M, Zhang Y, Chen L, Zhang X, Zhou X, Chen Y. miR-709 inhibits 3T3-L1 cell differentiation by targeting GSK3beta of Wnt/beta-catenin signaling. Cell Signal. 2014;26:2583–2589. doi: 10.1016/j.cellsig.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Taniguchi M, Nakajima I, Chikuni K, Kojima M, Awata T, Mikawa S. MicroRNA-33b downregulates the differentiation and development of porcine preadipocytes. Mol Biol Rep. 2014;41:1081–1090. doi: 10.1007/s11033-013-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cioffi M, Vallespinos-Serrano M, Trabulo SM, Fernandez-Marcos PJ, Firment AN, Vazquez BN, Vieira CR, Mulero F, Camara JA, Cronin UP, Perez M, Soriano J, GGB, Castells-Garcia A, Haage V, Raj D, Megias D, Hahn S, Serrano L, Moon A, Aicher A, Heeschen C. MiR-93 Controls Adiposity via Inhibition of Sirt7 and Tbx3. Cell Rep. 2015;12:1594–1605. doi: 10.1016/j.celrep.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Price NL, Holtrup B, Kwei SL, Wabitsh M, Rodeheffer MS, Bianchini L, Suarez Y, Fernandez-Hernando C. SREBP-1c/miR-33b genomic loci controls adipocyte differentiation. Mol Cell Biol. 2016 doi: 10.1128/MCB.00745-15. Accepted, awaiting publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heneghan HM, Miller N, McAnena OJ, O’Brien T, Kerin MJ. Differential miRNA expression in omental adipose tissue and in the circulation of obese patients identifies novel metabolic biomarkers. J Clin Endocrinol Metab. 2011;96:E846–850. doi: 10.1210/jc.2010-2701. [DOI] [PubMed] [Google Scholar]
- 53.Keller P, Gburcik V, Petrovic N, Gallagher IJ, Nedergaard J, Cannon B, Timmons JA. Gene-chip studies of adipogenesis-regulated microRNAs in mouse primary adipocytes and human obesity. BMC Endocr Disord. 2011;11:7. doi: 10.1186/1472-6823-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kloting N, Berthold S, Kovacs P, Schon MR, Fasshauer M, Ruschke K, Stumvoll M, Bluher M. MicroRNA expression in human omental and subcutaneous adipose tissue. PLoS One. 2009;4:e4699. doi: 10.1371/journal.pone.0004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H, Li T, Wang S, Wei J, Fan J, Li J, Han Q, Liao L, Shao C, Zhao RC. miR-17–5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Res. 2013;10:313–324. doi: 10.1016/j.scr.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 57.Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res. 2013;100:7–18. doi: 10.1093/cvr/cvt161. [DOI] [PubMed] [Google Scholar]
- 58.Wei J, Li H, Wang S, Li T, Fan J, Liang X, Li J, Han Q, Zhu L, Fan L, Zhao RC. let-7 enhances osteogenesis and bone formation while repressing adipogenesis of human stromal/mesenchymal stem cells by regulating HMGA2. Stem Cells Dev. 2014;23:1452–1463. doi: 10.1089/scd.2013.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bianchini L, Saada E, Gjernes E, Marty M, Haudebourg J, Birtwisle-Peyrottes I, Keslair F, Chignon-Sicard B, Chamorey E, Pedeutour F. Let-7 microRNA and HMGA2 levels of expression are not inversely linked in adipocytic tumors: analysis of 56 lipomas and liposarcomas with molecular cytogenetic data. Genes Chromosomes Cancer. 2011;50:442–455. doi: 10.1002/gcc.20869. [DOI] [PubMed] [Google Scholar]
- 60.Mandahl N, Bartuma H, Magnusson L, Isaksson M, Macchia G, Mertens F. HMGA2 and MDM2 expression in lipomatous tumors with partial, low-level amplification of sequences from the long arm of chromosome 12. Cancer Genet. 2011;204:550–556. doi: 10.1016/j.cancergen.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Horie T, Nishino T, Baba O, Kuwabara Y, Nakao T, Nishiga M, Usami S, Izuhara M, Sowa N, Yahagi N, Shimano H, Matsumura S, Inoue K, Marusawa H, Nakamura T, Hasegawa K, Kume N, Yokode M, Kita T, Kimura T, Ono K. MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. Nat Commun. 2013;4:2883. doi: 10.1038/ncomms3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sewter C, Berger D, Considine RV, Medina G, Rochford J, Ciaraldi T, Henry R, Dohm L, Flier JS, O’Rahilly S, Vidal-Puig AJ. Human obesity and type 2 diabetes are associated with alterations in SREBP1 isoform expression that are reproduced ex vivo by tumor necrosis factor-alpha. Diabetes. 2002;51:1035–1041. doi: 10.2337/diabetes.51.4.1035. [DOI] [PubMed] [Google Scholar]
- 63.Carobbio S, Hagen RM, Lelliott CJ, Slawik M, Medina-Gomez G, Tan CY, Sicard A, Atherton HJ, Barbarroja N, Bjursell M, Bohlooly YM, Virtue S, Tuthill A, Lefai E, Laville M, Wu T, Considine RV, Vidal H, Langin D, Oresic M, Tinahones FJ, Fernandez-Real JM, Griffin JL, Sethi JK, Lopez M, Vidal-Puig A. Adaptive changes of the Insig1/SREBP1/SCD1 set point help adipose tissue to cope with increased storage demands of obesity. Diabetes. 2013;62:3697–3708. doi: 10.2337/db12-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan S, Yang X, Jia Y, Li R, Zhao R. Microvesicle-shuttled miR-130b reduces fat deposition in recipient primary cultured porcine adipocytes by inhibiting PPAR-gamma expression. J Cell Physiol. 2013 doi: 10.1002/jcp.24486. [DOI] [PubMed]
- 65.Pan S, Yang X, Jia Y, Li Y, Chen R, Wang M, Cai D, Zhao R. Intravenous injection of microvesicle-delivery miR-130b alleviates high-fat diet-induced obesity in C57BL/6 mice through translational repression of PPAR-gamma. J Biomed Sci. 2015;22:86. doi: 10.1186/s12929-015-0193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu Y, Zhang X, Ding X, Wang H, Chen X, Zhao H, Jia Y, Liu S, Liu Y. miR-27 inhibits adipocyte differentiation via suppressing CREB expression. Acta Biochim Biophys Sin (Shanghai) 2014;46:590–596. doi: 10.1093/abbs/gmu036. [DOI] [PubMed] [Google Scholar]
- 67.Kang T, Lu W, Xu W, Anderson L, Bacanamwo M, Thompson W, Chen YE, Liu D. MicroRNA-27 (miR-27) targets prohibitin and impairs adipocyte differentiation and mitochondrial function in human adipose-derived stem cells. J Biol Chem. 2013;288:34394–34402. doi: 10.1074/jbc.M113.514372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim C, Lee H, Cho YM, Kwon OJ, Kim W, Lee EK. TNFalpha-induced miR-130 resulted in adipocyte dysfunction during obesity-related inflammation. FEBS Lett. 2013;587:3853–3858. [PubMed] [Google Scholar]
- 69.Jordan SD, Kruger M, Willmes DM, Redemann N, Wunderlich FT, Bronneke HS, Merkwirth C, Kashkar H, Olkkonen VM, Bottger T, Braun T, Seibler J, Bruning JC. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol. 2011;13:434–446. doi: 10.1038/ncb2211. [DOI] [PubMed] [Google Scholar]
- 70.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, Quon MJ, Karnieli E. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J Biol Chem. 2006;281:19881–19891. doi: 10.1074/jbc.M600320200. [DOI] [PubMed] [Google Scholar]
- 72.Zhu L, Shi C, Ji C, Xu G, Chen L, Yang L, Fu Z, Cui X, Lu Y, Guo X. FFAs and adipokine-mediated regulation of hsa-miR-143 expression in human adipocytes. Mol Biol Rep. 2013;40:5669–5675. doi: 10.1007/s11033-013-2668-2. [DOI] [PubMed] [Google Scholar]
- 73.Zhu L, Chen L, Shi CM, Xu GF, Xu LL, Zhu LL, Guo XR, Ni Y, Cui Y, Ji C. MiR-335, an Adipogenesis-Related MicroRNA, is Involved in Adipose Tissue Inflammation. Cell Biochem Biophys. 2013 doi: 10.1007/s12013-013-9708-3. [DOI] [PubMed] [Google Scholar]
- 74.Xu G, Ji C, Shi C, Fu H, Zhu L, Xu L, Chen L, Feng Y, Zhao Y, Guo X. Modulation of hsa-miR-26b levels following adipokine stimulation. Mol Biol Rep. 2013;40:3577–3582. doi: 10.1007/s11033-012-2431-0. [DOI] [PubMed] [Google Scholar]
- 75.Xu LL, Shi CM, Xu GF, Chen L, Zhu LL, Zhu L, Guo XR, Xu MY, Ji CB. TNF-alpha, IL-6, and leptin increase the expression of miR-378, an adipogenesis-related microRNA in human adipocytes. Cell Biochem Biophys. 2014;70:771–776. doi: 10.1007/s12013-014-9980-x. [DOI] [PubMed] [Google Scholar]
- 76.Shi Z, Zhao C, Guo X, Ding H, Cui Y, Shen R, Liu J. Differential expression of microRNAs in omental adipose tissue from gestational diabetes mellitus subjects reveals miR-222 as a regulator of ERalpha expression in estrogen-induced insulin resistance. Endocrinology. 2014;155:1982–1990. doi: 10.1210/en.2013-2046. [DOI] [PubMed] [Google Scholar]
- 77.Herrera BM, Lockstone HE, Taylor JM, Ria M, Barrett A, Collins S, Kaisaki P, Argoud K, Fernandez C, Travers ME, Grew JP, Randall JC, Gloyn AL, Gauguier D, McCarthy MI, Lindgren CM. Global microRNA expression profiles in insulin target tissues in a spontaneous rat model of type 2 diabetes. Diabetologia. 2010;53:1099–1109. doi: 10.1007/s00125-010-1667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chartoumpekis DV, Zaravinos A, Ziros PG, Iskrenova RP, Psyrogiannis AI, Kyriazopoulou VE, Habeos IG. Differential expression of microRNAs in adipose tissue after long-term high-fat diet-induced obesity in mice. PLoS One. 2012;7:e34872. doi: 10.1371/journal.pone.0034872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ortega FJ, Mercader JM, Moreno-Navarrete JM, Rovira O, Guerra E, Esteve E, Xifra G, Martinez C, Ricart W, Rieusset J, Rome S, Karczewska-Kupczewska M, Straczkowski M, Fernandez-Real JM. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care. 2014;37:1375–1383. doi: 10.2337/dc13-1847. [DOI] [PubMed] [Google Scholar]
- 80.Ortega FJ, Mercader JM, Catalan V, Moreno-Navarrete JM, Pueyo N, Sabater M, Gomez-Ambrosi J, Anglada R, Fernandez-Formoso JA, Ricart W, Fruhbeck G, Fernandez-Real JM. Targeting the circulating microRNA signature of obesity. Clin Chem. 2013;59:781–792. doi: 10.1373/clinchem.2012.195776. [DOI] [PubMed] [Google Scholar]
- 81.Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, Heim MH, Stoffel M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474:649–653. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- 82.He A, Zhu L, Gupta N, Chang Y, Fang F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol. 2007;21:2785–2794. doi: 10.1210/me.2007-0167. [DOI] [PubMed] [Google Scholar]
- 83.Ling HY, Ou HS, Feng SD, Zhang XY, Tuo QH, Chen LX, Zhu BY, Gao ZP, Tang CK, Yin WD, Zhang L, Liao DF. CHANGES IN microRNA (miR) profile and effects of miR-320 in insulin-resistant 3T3-L1 adipocytes. Clin Exp Pharmacol Physiol. 2009;36:e32–39. doi: 10.1111/j.1440-1681.2009.05207.x. [DOI] [PubMed] [Google Scholar]
- 84.Cannon B, Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans) Int J Obes (Lond) 2010;34(Suppl 1):S7–16. doi: 10.1038/ijo.2010.177. [DOI] [PubMed] [Google Scholar]
- 85.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walden TB, Timmons JA, Keller P, Nedergaard J, Cannon B. Distinct expression of muscle-specific microRNAs (myomirs) in brown adipocytes. J Cell Physiol. 2009;218:444–449. doi: 10.1002/jcp.21621. [DOI] [PubMed] [Google Scholar]
- 88.Sun L, Trajkovski M. MiR-27 orchestrates the transcriptional regulation of brown adipogenesis. Metabolism. 2014;63:272–282. doi: 10.1016/j.metabol.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 89.Pan D, Mao C, Quattrochi B, Friedline RH, Zhu LJ, Jung DY, Kim JK, Lewis B, Wang YX. MicroRNA-378 controls classical brown fat expansion to counteract obesity. Nat Commun. 2014;5:4725. doi: 10.1038/ncomms5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, Siegel F, Kipschull S, Haas B, Frohlich H, Meister G, Pfeifer A. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun. 2013;4:1769. doi: 10.1038/ncomms2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trajkovski M, Ahmed K, Esau CC, Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol. 2012;14:1330–1335. doi: 10.1038/ncb2612. [DOI] [PubMed] [Google Scholar]
- 92.Liu W, Bi P, Shan T, Yang X, Yin H, Wang YX, Liu N, Rudnicki MA, Kuang S. miR-133a regulates adipocyte browning in vivo. PLoS Genet. 2013;9:e1003626. doi: 10.1371/journal.pgen.1003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, Thorn S, Seale P, Fernando P, van Ijcken W, Grosveld F, Dekemp RA, Boushel R, Harper ME, Rudnicki MA. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab. 2013;17:210–224. doi: 10.1016/j.cmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 2012;10:e1001314. doi: 10.1371/journal.pbio.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kong X, Yu J, Bi J, Qi H, Di W, Wu L, Wang L, Zha J, Lv S, Zhang F, Li Y, Hu F, Liu F, Zhou H, Liu J, Ding G. Glucocorticoids transcriptionally regulate miR-27b expression promoting body fat accumulation via suppressing the browning of white adipose tissue. Diabetes. 2015;64:393–404. doi: 10.2337/db14-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang H, Guan M, Townsend KL, Huang TL, An D, Yan X, Xue R, Schulz TJ, Winnay J, Mori M, Hirshman MF, Kristiansen K, Tsang JS, White AP, Cypess AM, Goodyear LJ, Tseng YH. MicroRNA-455 regulates brown adipogenesis via a novel HIF1an-AMPK-PGC1alpha signaling network. EMBO Rep. 2015;16:1378–1393. doi: 10.15252/embr.201540837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang YC, Li Y, Wang XY, Zhang D, Zhang H, Wu Q, He YQ, Wang JY, Zhang L, Xia H, Yan J, Li X, Ying H. Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity. Diabetologia. 2013;56:2275–2285. doi: 10.1007/s00125-013-2996-8. [DOI] [PubMed] [Google Scholar]
- 98.Price NL, Ramirez CM, Fernandez-Hernando C. Relevance of microRNA in metabolic diseases. Crit Rev Clin Lab Sci. 2014;51:305–320. doi: 10.3109/10408363.2014.937522. [DOI] [PubMed] [Google Scholar]