Abstract
New neurons are generated throughout adulthood in two regions of the brain, the olfactory bulb and dentate gyrus of the hippocampus, and are incorporated into the hippocampal network circuitry; disruption of this process has been postulated to contribute to neurodegenerative diseases including Alzheimer’s disease and Parkinson’s disease. Known modulators of adult neurogenesis include signal transduction pathways, the vascular and immune systems, metabolic factors, and epigenetic regulation. Multiple intrinsic and extrinsic factors such as neurotrophic factors, transcription factors, and cell cycle regulators control neural stem cell proliferation, maintenance in the adult neurogenic niche, and differentiation into mature neurons; these factors act in networks of signaling molecules that influence each other during construction and maintenance of neural circuits, and in turn contribute to learning and memory. The immune system and vascular system are necessary for neuronal formation and neural stem cell fate determination. Inflammatory cytokines regulate adult neurogenesis in response to immune system activation, whereas the vasculature regulates the neural stem cell niche. Vasculature, immune/support cell populations (microglia/astrocytes), adhesion molecules, growth factors, and the extracellular matrix also provide a homing environment for neural stem cells. Epigenetic changes during hippocampal neurogenesis also impact memory and learning. Some genetic variations in neurogenesis related genes may play important roles in the alteration of neural stem cells differentiation into new born neurons during adult neurogenesis, with important therapeutic implications. In this review, we discuss mechanisms of and interactions between these modulators of adult neurogenesis, as well as implications for neurodegenerative disease and current therapeutic research.
Keywords: neural stem cells, modulators, hippocampus, memory, therapeutic research
INTRODUCTION
After the discovery of neurogenesis in the adult human brain, there were many studies of adult neurogenesis over two decades to identify the underlying genetic and environmental mechanisms. In 1998, the presence of adult-born neurons in the dentate gyrus of the human hippocampus had been identified by using cancer patients who had received the labelled 5-bromo-2′-deoxyuridine (BrdU) in hippocampal neurons [Eriksson et al., 1998]. By measuring the concentration of nuclear bomb-test-derived 14C in genomic DNA in the adult human brain, Spalding et al. [2013] found that neurons are added in the hippocampus per day corresponding to an annual turnover of 1.75% of the neurons within the renewing fraction, with a modest decline during aging. Alterations in adult neurogenesis have been associated with neurological and psychiatric disorders. Enhanced understanding of the contribution of biological processes and genetic factors related to neurogenesis could lead to novel therapeutic strategies for neurodegenerative disease progression as well as many other conditions.
Numerous intrinsic and extrinsic factors affect the processes of adult neurogenesis, including the proliferation of neural progenitor cells, fate determination of neural progenitor cell progenies, and the differentiation, migration, and maturation of adult neurons. Following these processes, adult–born neurons integrate into the complex circuitry of the olfactory bulb and hippocampus [Zhao et al., 2008; Suh et al., 2009]. Although the hippocampus plays a crucial role in the formation of episodic and spatial memory and is associated with many neurodegenerative diseases [Squire, 1992; Sahay and Hen, 2008; Deng et al., 2010], we focus on hippocampal adult neurogenesis in this review.
NEUROGENESIS ROLE IN COGNITION
In the mammalian brain, adult neurogenesis occurs in two main regions: the dentate gyrus of the hippocampus, which is important for memory formation and cognitive functions, and the subventricular zone (SVZ) of the olfactory bulb, which is important for the sense of smell [Oomen et al., 2014; Ernst and Frisen, 2015]. Newborn neurons added to hippocampal circuitry during adult neurogenesis are important for the stimulation of spatial memory and learning [Barnea and Nottebohm, 1994; Gould et al., 1999]. Spatial memory is defined as pattern separation, the ability to discriminate among similar experiences [Yassa and Stark, 2011]. The dentate gyrus and CA3 regions of the hippocampus are implicated in spatial memory function and the capacity for pattern separation and are associated with learning new information. These cognitive functions have all been shown to decrease with age. Integration of new neurons into the existing hippocampal neural circuitry and environmental and behavioral factors modulating adult neurogenesis play important roles in hippocampal-dependent learning and memory [Yu et al., 2014].
Although there are a number of studies showing adult neurogenesis involvement in the formation of spatial memory and learning, the results are somewhat controversial. Genetic ablation of GFAP-expressing (GFAP+) neural progenitor cells did not impair a hippocampal-dependent learning or memory task, while it did inhibit contextual fear conditioning [Saxe et al., 2006]. In contrast, rats treated with the DNA methylating agent methylazoxymethanol acetate (MAM) toxin for proliferating cells showed a reduction in the formation of newborn neurons in the dentate gyrus, which was associated with impaired hippocampal-dependent memory formation, but not contextual fear conditioning [Shors et al., 2001].
New neurons generated by adult neurogenesis in the granule layer of the dentate gyrus within the hippocampus play a crucial role in the development of memory and learning [Lopez-Virgen et al., 2015]. There is delayed maturation of the adult-generated granule cells in the dentate gyrus [Overstreet-Wadiche et al., 2006]. After 1 week of differentiation, newborn neurons’ apical dendrites reach the molecular layer and their axonal projections reach to the CA3 region, and spines form 16 days after division. Dendritic spines express glutamate receptors and the TrkB receptor for BDNF on their surface to regulate the survival of the spines [Ngo-Anh et al., 2005; Kaneko et al., 2012]. Dendritic and axonal outgrowth accompany the maturation of the neurons. Spine density increases in the 4th week post-division. The hippocampal-dependent Morris water maze test showed that 4–28-days-old newborn neurons are required for the formation of long-term hippocampus-dependent spatial memory [Snyder et al., 2005]. Differential synaptic connectivity of the hippocampus along the septo-temporal axis disrupts the septal and temporal connections of the hippocampus and negatively effects learning and memory [Bannerman et al., 2004]. By 2 months, structurally modified highly dynamic and plastic spines are regulated by neuronal activity [Zhao et al., 2006]. Synaptic connectivity and excitability of new neurons in the adult hippocampus are stimulated by bHLH transcription factors such as Neurod1 and Neurod2 during adult neurogenesis and memory formation. A recent study showed that Neurod1 overexpression in dividing neural stem cells (NSCs) leads to differentiation of the new neurons, and inhibits memory deficits and rescues memory impairment in APP/PS1 mice [Richetin et al., 2015]. Adult hippocampal neurogenesis is highly involved in memory formation and learning which has important implications for neurodegenerative disease.
CELL TYPES IN ADULT NEUROGENESIS
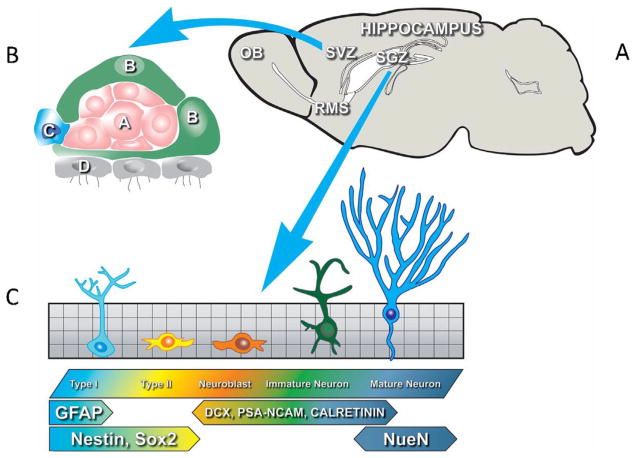
In the adult brain, two important regions where neural stem cells are retained are responsible for adult neurogenesis, the SVZ and the subgranular zone (SGZ) of the hippocampal dentate gyrus. In the SVZ, B1 cell residues line the border between the striatum and lateral ventricle; this subpopulation of cells possesses astroglial properties and acts as NSCs. B1 cells give rise to intermediate progenitors, which divide and generate the neurons destined for the olfactory bulb [Kriegstein and Alvarez-Buylla, 2009]. Unlike B1 cells in the SVZ, which lie in the ventricular wall and have processes that contact the cerebrospinal fluid (CSF), radial astrocytes in the SGZ reside deeper in the brain parenchyma in the granule cell layer of the dentate gyrus, which does not have contact with the ventricular system, and act as NSCs, also known as type 1 cells. Radial astrocytes generate intermediate progenitors (type2a and type 2b cells), which differentiate into type 3 cells, or immature granule cells. Radial astrocytes have three main domains. The side of the radial astrocytes known as the proximal domain faces the hilus and has contact with blood vessels and lateral processes, which also contact the other radial astrocytes. The second domain is the intermediate domain, which includes the cell body and main shaft. The intermediate domain of the radial astrocytes interacts with intermediate progenitor cells and granule cells. This second domain is important for cell–cell interactions of NSCs with their progeny and for detection of local neural activity and signaling from granule cells. The third domain is the distal domain, which is highly branched and contacts with other granule cells, axons, and synapses in the molecular layer [Seri et al., 2004; Merkle et al., 2014]. Briefly, the primary progenitor cells are known as Type-I or B-type cells. They divide to produce intermediate stage progenitors (Type-II, Type-III, or D-type cells), which then undergo further rounds of cell division to generate post-mitotic immature granule neurons. These glutamatergic neurons then integrate into the dentate gyrus as functional components of the hippocampal circuitry [van Praag et al., 2002; Ge et al., 2006; Hevner et al., 2006]. Regulatory signals from the peripheral environment play a crucial role in identifying neural progenitor fate to become neurons or astrocytes [Musaelyan et al., 2014] (Fig. 1).
FIG. 1.
A: Adult neurogenesis occurs in two regions: the subgranular zone (SGZ) and the subventricular zone (SVZ). B: In the SVZ, neural progenitor cells (type B cells) give rise to type C cells, which differentiate to neuroblasts (type A cells). Type A cells migrate via the rostral migratory stream (RMS) and differentiate into neurons in the olfactory bulb (OB). Neuroblasts migrate via the RMS to the olfactory bulb and generate new neurons. C: In the SGZ, glial-like radial stem cells known as Type-I cells express glial fibrillary acidic protein (GFAP) and nestin. They divide to produce intermediate stage progenitors (Type-II cells), which then undergo further rounds of cell division to generate neuroblasts and post-mitotic immature granule neurons. Type II cells express Sox2, while neuroblast and immature neurons express doublecortin (DCX), PSA-NCAM, and calretinin. Mature neurons are defined by expression of NeuN. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ajmgb].
Neural progenitor cells in the SVZ of the lateral ventricle migrate through the rostral migratory stream and become granule and perigranular neurons of the olfactory bulb, whereas the neural progenitor cells in the SGZ differentiate and integrate into the local neural network as granule cells of the hippocampus in the adult human brain [Eriksson et al., 1998; Deng et al., 2010; Spalding et al., 2013; Ernst et al., 2014]. NSCs in the SGZ and SVZ undergo self-renewal to provide a lifelong supply for the mature hippocampal dentate gyrus granule neurons and olfactory bulb, respectively. In both regions, NSCs lead to neural progenitor cells, which are limited in proliferation and differentiate into neurons or glia [Zhao et al., 2006].
Although there are many differences between adult SVZ and SGZ neurogenic niches, NSCs in both, like glial radial stem cells in the embryo, express GFAP, nestin, and Sox2, and they directly contact blood vessels. There is overlap between expression of these markers from different neural cell types and they might be responsible for the generation of the heterogeneous neural cell types [Zhao et al., 2008; Morrens et al., 2012]. The NSC population in the SVZ generates neurons and oligodendrocytes, whereas the NSC population in the SGZ generates neurons and astrocytes. Adult progenitors line the hilar side of the granule cell layer of the dentate gyrus where SGZ astrocytes lead to intermediate progenitors. These progenitors mature into granule neurons of the dentate gyrus and send axonal projections to the CA3 region [Markakis and Gage, 1999; Lledo et al., 2006]. GABA plays an important role in progenitor differentiation into newborn neurons and regulation of neuronal network activity and synaptic integration of proliferation in the SGZ; GABA-releasing neuroblast progeny of the SVZ stimulate differentiation through GABAergic synaptic input into progenitor cells [Liu et al., 2005]. After these progenitors differentiate to immature neurons, the dendrites of the newborn dentate gyrus cells become more complex and spread deeper into the molecular cell layer [Zhao et al., 2006; Van Deerlin et al., 2010].
Adult hippocampal neurogenesis begins with the proliferation of neural progenitor cells in the SGZ zone. Although a small population of neural progenitor cells differentiate into glia, most of the neural progenitor cells differentiate into dentate granule cells within the hippocampus and undergo the process of morphological and physiological maturation [Zhao et al., 2006; Sahay and Hen, 2008].
NEUROGENESIS ROLE IN THE HIPPOCAMPUS
The hippocampus is the most important region for learning and episodic/spatial memory. Within the hippocampus, the dentate gyrus provides a steady level of neurogenesis throughout life. Intrinsic and extrinsic regulatory factors affect the proliferation, differentiation, maturation, fate determination, and survival of newborn cells in the dentate gyrus [Bruel-Jungerman et al., 2007]. Pyramidal and granule cells comprise most of the cells in the hippocampus. The hippocampus receives a large number of sensory inputs uni-directionally from neocortical regions, which leads to the regulation of hippocampal formation. The granule cell population in the dentate gyrus is smaller than the pyramidal cell population in the CA3 region of the hippocampus, encodes the information from the entorhinal cortex, and projects to the CA3 region [Jung and McNaughton, 1993; O’Reilly and McClelland, 1994; Leutgeb and Leutgeb, 2007]. CA3 projects a signal to CA1 that changes the inputs in a more linear fashion from the entorhinal cortex to the CA3 region. CA1 subsequently projects the signal into the subiculum and entorhinal cortex [Guzowski et al., 2004]. This neuronal signaling flow plays an important role in the formation of memory and learning [Williamson and Bilbo, 2013]. NSCs in the dentate gyrus also differentiate into the astrocytes between the granule cell layer and the hilus, which support hippocampal-dependent memory function [Williamson and Bilbo, 2013].
MODULATORS IN ADULT NEUROGENESIS
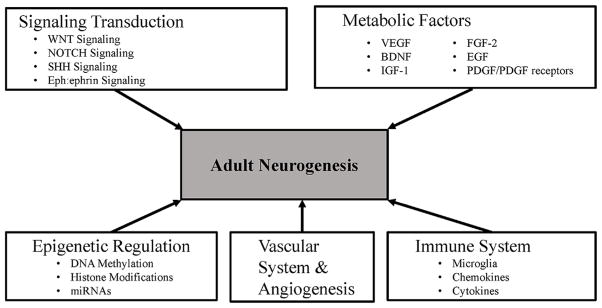
Recent studies have highlighted five important modulators of neurogenesis, including signaling transduction pathways, the vascular and immune systems, metabolic factors, and epigenetic regulation (Fig. 2) [Faigle and Song, 2013; Hussaini et al., 2014; Laussu et al., 2014]. The alteration in these modulators during adult neurogenesis may be related to the development of neurodegenerative diseases. A more complete understanding of the role and function of each modulator in regulating NSC fate and integration as neurons in the SGZ and olfactory bulb may provide crucial insights leading to new therapies for neurological diseases in humans.
FIG. 2.
Schematic illustration of adult neurogenesis related pathways: there are five crucial modulators controlling neural stem cell (NSC) proliferation, differentiation, migration, and maintenance during adult neurogenesis: signaling transduction pathways, the vascular and immune systems, metabolic factors, and epigenetic regulation. These five modulators are composed of diverse molecules and biological pathways and mechanisms acting to control neurogenesis.
MODULATORS OF ADULT NEUROGENESIS
Signaling Transduction in Adult Neurogenesis
Several essential signaling transduction pathways regulate proliferation and differentiation of NSCs, as well as migration and survival of the new-born neurons and their integration into the neuronal circuitry network in the adult brain. In this section, we discuss key signaling pathways Wnt, Notch, Sonic hedgehog (Shh), and Eph:ephrin, which modulate different stages of adult neurogenesis.
Wnt signaling
Wnt signaling regulates adult hippocampal neurogenesis on molecular, cellular, and behavioral levels. It has been shown that stem cells in the adult hippocampus express receptors and signaling molecules that correspond to Wnt proteins. Astrocytes and the hilar cells of the dentate gyrus express Wnt-3, and promote neuroblast proliferation and neuronal differentiation into hippocampal granule neurons through Wnt signaling-mediated activation of NeuroD1 [Garrido et al., 2002; Kuwabara et al., 2009]. NeuroD1 is required for the survival and the maturation of adult neurons. NSC proliferation and cell fate determination is controlled by Wnt signaling in hippocampal neurogenesis [Lie et al., 2005]. In the Wnt signaling pathway, β-catenin is necessary for the expression of Wnt target genes. The expression level of β-catenin by NSCs in the dentate gyrus of the hippocampus affects proliferation of stem cells, as well as axonal and dendritic development. Wnt signaling protects neurons from injury by neurotoxins such as amyloid-β accumulation in AD. For example, Wnt activation of Protein Kinase C (PKC) inhibits GSK-3β activity, which plays an important role in the phosphorylation and degradation of β–catenin and inhibits apoptosis of the progenitor cells [Garrido et al., 2002; Toledo et al., 2008; Zhang et al., 2011a]. Another Wnt signaling pathway component, survivin, increases adult neurogenesis in the dentate gyrus of the hippocampus during traumatic brain injury [Zhang et al., 2013]. On the other hand, it has been shown that the Wnt/β-catenin signaling pathway promotes neuronal proliferation, but not differentiation, via Disrupted in Schizophrenia 1 (DISC1), which is an inhibitor of GSK-3β, in the adult hippocampus [Mao et al., 2009]. However, another Wnt family member, Wnt-7A, is activated by the nuclear orphan receptor Tlx and enhances proliferation and self-renewal of NSCs via the canonical Wnt signaling pathway in adult neurogenesis [Qu et al., 2010]. To sum up, the canonical Wnt signaling pathway, including Wnt-3, Wnt-7A, GSK-3β, and Tlx, may induce progenitor cell proliferation and survival in the dentate gyrus of the hippocampus.
Notch signaling
Notch signaling is important for the maintenance and differentiation of NSCs in adult neurogenesis [Louvi and Artavanis-Tsakonas, 2006; Faigle and Song, 2013]. Notch signaling activates the expression of bHLH transcription factors such as Hes1, Hes3, and Hes5, which suppresses proneuronal gene expression and promotes maintenance of NSCs by inhibiting differentiation of NSCs in the SVZ [Imayoshi et al., 2010]. Likewise, Notch1 is necessary for the self-renewal and maintenance of neural stem and progenitor cells in the dentate gyrus of the hippocampus [Ables et al., 2010]. Notch signaling and EGFR signaling regulate the balanced interaction between NSCs’ and progenitor cells’ number and self-renewal in the SVZ region [Aguirre et al., 2010]. Notch signaling is necessary for the maintenance of undifferentiated cells, whereas EGFR promotes the proliferation and migration of progenitor cells [Hitoshi et al., 2002]. Although Notch1 and EGFR are substrates of ADAM 10, which prevents amyloid-β production, the well-known hallmark of AD, by proteolysis of the alpha site of the amyloid precursor protein (APP), there is strong link between Notch signaling and AD pathology as well as hippocampal neurogenesis [Haass and Selkoe, 1993; Hartmann et al., 2002; Woo et al., 2009].
There are also connections between Wnt signaling and Notch signaling during neurogenesis. FGF2 causes accumulation of β-catenin by inhibiting GSK-3β. β–catenin accumulation leads to proliferation and maintenance of NSCs by activating LEF/TCF transcription factors. Then, B-catenin and Notch1 make a complex with anti-neurogenic Hes1 and enhance Hes1 expression, which inhibits the differentiation of the progenitor cell population [Shimizu et al., 2008].
Sequential signaling through Notch1 and erbB receptors plays an important role in glial cell differentiation [Patten et al., 2003]. ErbB4 and neuregulin-1 and -2 receptors are expressed by immature neuroblasts as well as astrocytes and ependymal cells. When ErbB4 binds to neuregulin ligands, it drives progenitor cells proliferation and migration of neuroblasts in the SVZ. However, ErbB4 controls the formation and maintenance of glial cells and differentiation of glial cells via activation of Notch1 signaling from granule cells in the astroglia [Patten et al., 2003]. As a result, Notch signaling is involved in the regulation of NSC proliferation, maintenance, and cell fate determination.
Sonic hedgehog signaling
Sonic hedgehog signaling (Shh) plays a crucial role in differentiation of cell types and the formation of neurons during adult neurogenesis [Ericson et al., 1995]. Shh receptor Patch is expressed by the progenitor cells of the dentate gyrus as well as pyramidal cells in the CA1 through CA3, and drives hippocampal formation during adult neurogenesis. Shh controls the proliferation of progenitor cells in the dentate gyrus during hippocampal neurogenesis [Traiffort et al., 1999; Lai et al., 2003]. Smoothen (Smo), the other Shh receptor, is expressed in the adult hippocampus [Traiffort et al., 1998]. Smo knockout mice show a significant reduction of proliferation of NSCs and progenitor cells in the dentate gyrus of the hippocampus and SVZ [Machold et al., 2003; Han et al., 2008]. Both Patch1 and Smo are located on the primary cilia on the GFAP+ stem/progenitor cells, and primary cilia control the proliferation of Type2a progenitor cells without affecting the amplification of Type 1 radial NSCs in adult hippocampal neurogenesis, which is important for spatial learning [Amador-Arjona et al., 2011].
Eph:ephrin signaling
Eph:ephrin signaling, especially via receptor EphB1-B3 and ligand Ephrin-B1-B3, plays an important role in the migration of neuroblasts to the olfactory bulb and increases the proliferation of NSCs in the SVZ in adult neurogenesis [Conover et al., 2000] EphB1 is expressed in NSCs in the SGZ and controls proliferation, migration, and polarity of the neural/progenitor cells during hippocampal neurogenesis [Chumley et al.,2007]. A knockout mice study showed that Ephrin-A2 and Ephrin-A3 inhibit progenitor cell proliferation by activating Ephrin-A7-mediated signaling in the SVZ and SGZ regions [Jiao et al., 2008]. In addition, Ephrin-B2 is expressed in astrocytes, while corresponding EphB4 receptors are expressed in NSCs, and Ephrin-B2:EphB4 signaling activates β-catenin independently of Wnt signaling and increases the regulation of proneuronal transcription factors as well as inducing neuronal differentiation of progenitor cells in the dentate gyrus of the SGZ [Pasquale, 2005; Ashton et al., 2012]. EphB receptors are expressed in dendrites and play roles in the maturation and plasticity of synapses, regulating spine and synapse formation [Klein, 2009; Laussu et al., 2014]. Epha1 has also been identified as a risk gene for Alzheimer’s disease (AD) [Lambert et al., 2013]. Alteration in EphA4 and EphB2 have been shown to cause memory impairment in an AD mouse model [Simon et al., 2009], highlighting the importance of this signaling pathway in neurogenesis processes and its relevance to neurodegenerative disease.
Several signaling pathways including Wnt, Notch, Shh, and Eph: ephrin, regulate self-renewal, proliferation, and differentiation of NSCs, as well as migration of neuroblasts and integration of newborn neurons in the adult brain. Addressing the roles of these crucial signaling mechanisms in NSC regulation and integration into hippocampal network circuitry may lead to targeted new therapies for currently intractable human neurological disorders.
Vascular System and Angiogenesis
The vasculature plays an important role in stem cell niche regulation and maintenance in neurogenesis. Angiogenesis, the development of new vascular networks with existing vessels, is linked with neurogenesis since angiogenesis is stimulated by angiogenic genes expressed in neural progenitor cells [Teng et al., 2008]. NSCs are deliberately distributed around blood cells in the brain to facilitate access to circulating signaling molecules, growth factors, and nutrients. The vascular beds of the SVZ and SGZ support adult neurogenesis. Although a three-dimensional niche including NSC and transit-amplifying cells resides close to SVZ blood vessels and increases the vascular contact to other cells in the SVZ, radial astrocyte residues in areas near blood vessels and endothelial cells promote neural progenitor stem cell proliferation, neuronal differentiation, and survival by expressing pro-angiogenic factors such as FGF, VEGF, IGF-1, which enhance neurogenesis and neuroprotection in the SGZ [Cameron et al., 1998; Palmer et al., 2000; Goldberg and Hirschi, 2013]. Angiogenesis and neurogenesis are combined in the hippocampus; up to 37% of the cells in the SGZ are endothelial precursors. Neural progenitors and angioblasts proliferate together in the clusters associated with the microvasculature of the SGZ. The cells in this cluster express VEGF receptors, while the tissue surrounding this cluster expresses VEGF. Furthermore, newly generated capillaries express BDNF, which promotes the recruitment and migration of newborn neurons [Monje et al., 2002]. Exercise induces angiogenesis as well as neurogenesis in the hippocampus by increasing the expression of NGF and BDNF [Kempermann et al., 1998]. However, more research is needed to elucidate the factors modulating endogenous stem cell mobilization out of the stem cell niche. Some studies show that activated microglia and astrocytes express cytokines such as MCP-1, SCF, CXCL12, and VEGFA to attract NSC migration; NSCs express the compatible receptors CCR2, SCF receptor c-kit, CXCR4, and VEGFR, respectively, during disease processes such as stroke and brain tumor [Imitola et al., 2004; Sun et al., 2004; Schmidt et al., 2005; Yan et al., 2007]. Additionally, microvessel entorhinal cortex cytokine expression profiles show that many growth factors, chemokines, adhesion molecules, and extracellular matrix proteins such as PDGF-BB, RANTES, I-TAC, NAP-2, GROα, Ang-2, and M-CSF are secreted by the entorhinal cortex to promote chemo-attraction of NSCs [Schmidt et al., 2009]. Many vascular and angiogenic factors regulate neurogenesis in the adult brain; better understanding of the vascular compartment of the NSC niche may provide therapeutic insights for neuronal diseases.
Metabolic Factors and Their Role in Adult Neurogenesis
Adult neurogenesis is regulated by metabolic growth factors such as VEGF, BDNF, IGF-1, FGF2, IGF, and PDGF. As it is mentioned below, these growth factors play important roles in neural stem/progenitor cell proliferation, migration, cell fate determination, and maturation into new neurons.
VEGF
VEGF signaling plays an important role in NSC proliferation, survival, and neural progenitor migration and maturation [Wittko et al., 2009]. VEGF is a glycoprotein and is necessary for angiogenesis and vascularization. There are four isoforms belonging to the VEGF gene: VEGF-A, VEGF-B, VEGF-C, and VEGF-D. VEGFA is expressed by endothelial cells and the choroid plexus, and regulates NSC renewal and progenitor cell migration by binding to the VEGF receptors (VEGFR1 and VEGFR2/Flk-1) secreted by the neural stem cell niche [Maurer et al., 2003; Cao et al., 2004; Shen et al., 2004]. VEGFA expressed by astrocytes diffuses to the lateral ventricle and has a trophic effect on neural progenitor cells, which promotes their survival as well as increasing neurogenesis after cerebral ischemia [Schanzer et al., 2004]. Therefore, VEGFA/VEGFR signaling modulates vascular permeability and angiogenesis [Carmeliet, 2005; Wittko et al., 2009]. Lastly, VEGF-C/VEGFR3 signaling is required for NSC renewal and regulates adult neurogenesis. Inhibition of VEGR3 from NSCs causes reduction of neurogenesis [Calvo et al., 2011].
BDNF
BDNF is secreted by endothelial cells to promote neural progenitor cell proliferation, differentiation, and survival in adult neurogenesis [Leventhal et al., 1999]. NSCs express the neurotrophin receptor TrkB, which binds to BDNF and has multiple roles in NSC survival and neuronal plasticity [Lewin and Barde, 1996]. In the SVZ, neuroblasts secrete GABA, which induces TrkB expression in astrocytes to catch extracellular BDNF, which in turn stimulates neuroblast migration in the rostral migratory stream [Snapyan et al., 2009]. Besides BDNF, multiple neurotrophic factors such as FGF and EGF determine the NSC transition between proliferation and differentiation [Vescovi et al., 1993; Caldwell et al., 2001]. BDNF/TrkB signaling regulates differentiation and survival of neurons and synaptic plasticity by activating Ras/MAP kinase, Phospholipase C, and PI3K pathways. BDNF/TrkB signaling also enhances hyperphosphorylation of the tau protein, which contributes to AD pathology, whereas BDNF gene expression is elevated in transgenic AD mice via environmental enrichment, demonstrating the importance of this neurotrophic factor in neurodegenerative disease processes as well as neurogenesis [Zhang et al., 2012].
IGF-1
IGF-1 upregulation in the bloodstream, induced by exercise, promotes BDNF expression in the dentate gyrus, which modulates synaptic plasticity and cognitive enhancement [Ding et al., 2006]. IGF-1 induces the proliferation and differentiation of NSCs via MEK/ERK pathway signaling and the PI3K/Akt pathway signaling in the SGZ and SVZ, respectively [Yuan et al., 2015].
FGF-2
FGF-2 is the other important endothelial-derived effector of adult neurogenesis. FGF-2 modulates NSC renewal and proliferation of the granule cell precursors in the dentate gyrus of the hippocampus and the SVZ [Woodbury and Ikezu, 2014]. Although GFAP+ cells, astrocytes, and GFAP+ radial glia stem cells express FGF-2 in the SVZ, astrocytes express FGF-2 to promote neurogenesis by inducing neural progenitor proliferation in the SGZ during neurodegenerative disorders such as AD, Parkinson’s disease (PD), and traumatic brain injury [Newman et al., 2000; Kirby et al., 2013]. In addition to FGF-2, its receptor FGFR1 is expressed in NSCs in the SVZ and dentate gyrus of the hippocampus to enhance NSC proliferation [Woodbury and Ikezu, 2014].
EGF
EGF is expressed by the microvascular entorhinal cortex, while EGF receptors are expressed by type C transit-amplifying cells in the SVZ region. SDF-1 (CXCL12) stimulates EGFR to increase Type A neuroblast mobility to migrate from the SVZ to the olfactory bulb [Kokovay et al., 2010]. Another study showed the association between BDNF and EGF; BDNF treatment of EGF-induced cultured human stem cells in the SVZ promoted cell proliferation and migration [Zhang et al., 2011b]. The combination of EGF and FGF-2 neurotrophic factors induced progenitor cell proliferation in both the dentate gyrus of the hippocampus and the SVZ region in ischemia-induced rat brains [Tureyen et al., 2005].
PDGF
PDGF and PDGF receptors are important for the maintenance of neural progenitor cells in adult neurogenesis [Funa and Sasahara, 2014]. GFAP+ B cells in the SVZ express PDGFRA, which is necessary for oligodendrocyte formation, though not for neurogenesis. PDGF alone has mitogenic effects on B cells, but inhibits neuroblast production. PDGF/PDGFRA signaling modulates the balance between oligodendrogenesis and neurogenesis [Jackson et al., 2006].
As a result, neurotrophic factors mentioned above promote neural stem/progenitor cell proliferation, differentiation, and migration through different signaling cascades as well as the inhibition of neurodegenerative mechanisms for neuronal protection [Laske et al., 2009; Sopova et al., 2014].
Immune System and Neurogenesis
Immune mechanisms modulate neural plasticity and behavioral processes in the human brain. Physiological and psychological factors are crucial to promote immune mechanisms. The alterations of signals between immune, nervous, and hormonal systems such as elevation of adrenaline and norepinephrine levels in blood modulate synaptic plasticity and neurogenesis [Besedovsky and Rey, 2007; Yirmiya and Goshen, 2011]. The immune system controls communication between the environment and the neurogenic niche [Musaelyan et al., 2014]. Activated microglia and cytokine release as an immune response affects adult hippocampal neurogenesis as well as learning and memory [Musaelyan et al., 2014].
The hippocampus and the immune system are interconnected during injury. Non-neuronal glial cells such as astrocytes and microglia and the cells around the choroid plexus such as T cells and B cells play important roles in immune-derived remodeling by controlling interactions with the environment, such as exchange of nutrients and other compounds, between the brain and the rest of the body, and modulate neural progenitor proliferation and differentiation in the adult hippocampus [Musaelyan et al., 2014]. Microglia cell populations are distributed in the dentate hilus and granule cell layer and regulate the apoptosis of newborn cells via phagocytosis during hippocampal neurogenesis [Wirenfeldt et al., 2003; Sierra et al., 2010]. Besides non-neuronal cells and immune cells, identification of the MHC class I molecules in uninjured neural cell population confirmed that these molecules play significant roles in neurogenesis such as neuronal differentiation and synaptic plasticity [Boulanger and Shatz, 2004; Yirmiya and Goshen, 2011].
It has been shown in an immune-deficient AD mouse model that T cells are involved in the regeneration of neural precursor cells in the mature hippocampus and maintenance of neuronal plasticity [Liu et al., 2014]. In contrast, during early stages of human development, maternal infections change immune activation by intensely increasing inflammatory cytokine levels in the fetus, which leads to behavioral abnormalities such as autism, schizophrenia and depression in adulthood [Meyer et al., 2006; Rook, 2013]. Furthermore, environmental disruption has been shown to affect immune modulators and lead to hippocampal dysfunction by the activation of microglia and astrocytes, the accumulation of peripheral leukocytes (i.e., T cells) into injured brain region(s), and secretion of cytokines, chemokines, and prostaglandins to the site of brain pathology [Cunningham and De Souza, 1993; Yenari et al., 2010; Yirmiya and Goshen, 2011; Williamson and Bilbo, 2013]. Inflammatory and immune molecules such as cytokines and chemokines have important roles in hippocampal neurogenesis and synaptic plasticity throughout the human lifespan [Jankowsky et al., 2000; Bhattacharyya et al., 2008], as well as an having an important impact on neurological disease processes, and further study of this modulator of adult neurogenesis may provide important future direction to therapeutic efforts.
The Role of Chemokines in Adult Neurogenesis
Chemokines modulate brain plasticity and vulnerability in the hippocampus [Williamson and Bilbo, 2013]. There are various central nervous system cells such as microglia, astrocytes, endothelial cells, oligodendrocytes, perivascular macrophages, and neurons which produce cytokines and chemokines under different circumstances[Mantovani et al., 1992; Tyor et al., 1992; Sawada et al., 1993]. Chemokines induce chemotaxis, which facilitates the migration of leukocytes to injured areas [Mackay, 2001; Charo and Ransohoff, 2006]. During injury, endothelial cells, astrocytes, and microglia cells secrete chemokines across the blood-brain-barrier (BBB), which facilitates neuroimmune signaling and recruits immune cells to the injury area [Verma et al., 2006; Wiese et al., 2012].
Chemokines play significant roles in the migration and development of progenitor cells, which express chemokine receptors such as CCR1, CCR2, CCR5, CXCR2, CXCR3, and CXCR4 in the dentate gyrus of the hippocampus in the postnatal brain [Tran et al., 2007]. In CXCR4 receptor knockout mice, the number of dividing cells in the migratory stream of the hippocampus and the dentate gyrus was dramatically reduced because CXCR4 is a receptor for CXCL12 chemokine, which is expressed in dividing progenitor cells, and CXCR4/CXCL12 signaling is necessary for progenitor cell population proliferation and movement via the migratory stream [Lu et al., 2002]. In addition, as a neurotransmitter, CXCL12 promotes GABAergic transmission by a postsynaptic mechanism. GABAergic transmission depends on the synergic release of CXCL12 from neural progenitors in the dentate gyrus. As a result, neural progenitor cells receive GABAergic inputs, and CXCL12 is necessary for transmission of the GABAergic signal to the dividing progenitors [Bhattacharyya et al., 2008]. CX(3)CR1 and CX(3)CL1 regulate microglial responses modulating glutamate toxicity for neuroprotection and glutamatergic synaptic input (transmission) on the neurons in the hippocampus [Lauro et al., 2008]. Chemokines are important modulators in hippocampal signaling and synaptic plasticity, which is important for learning and memory [Alkon and Nelson, 1990; Jones et al., 2001].
The Role of Inflammatory Cytokines in Adult Neurogenesis
Inflammation adversely affects hippocampal neurogenesis and proper function of the hippocampus. IL-1β, IL-6, and TNF-α are the most important pro-inflammatory cytokines, which play key roles in hippocampal neurogenesis as well as memory function.
IL-1β
IL-1β, expressed in many immune cells and glia cells as well as neurons, impairs proliferation and differentiation of neural precursor cells and decreases NSC survival rate in hippocampal neurogenesis. IL-1β is the predominant pro-inflammatory cytokine in the brain, and negatively correlates with the proliferation of NSCs in the dentate gyrus of the hippocampus [Ryan et al., 2013]. The nuclear factor kappa B (NFκB) cascades and mitogen-activated protein kinase (MAPK) as well as several transcription factors in the nucleus induce the activation of IL-1β/IL-1β receptor signaling [Zhu et al., 1998; McCulloch et al., 2006]. Serum level of IL-1β increases with depressive symptoms and impairs learning and memory function [Depino et al., 2004; van den Biggelaar et al., 2007].
IL-6
IL-6 plays an important role in progenitor cell survival in the dentate gyrus of the hippocampus[Williamson and Bilbo, 2013]. Overexpression of IL-6 from astrocytes reduces neurogenesis in the dentate gyrus of the hippocampus by influencing proliferation, survival, and differentiation of progenitor cells [Ryan et al., 2013].
TNF-α
TNF-α inhibits neural progenitor cell proliferation during brain injury, whereas a normal level of TNF-α increases neurogenesis by affecting expression of neurotrophic factors such as NGF and BDNF [Golan et al., 2004; Iosif et al., 2006; Takei and Laskey, 2008]. Although ischemic stroke causes neuronal death in the striatum and the cerebral cortex, neurogenesis increases after this insult in the dentate gyrus of the SGZ and the rostral SVZ by migration of neuroblasts originating from the SVZ into the damaged striatum [Jin et al., 2001; Jin et al., 2003]. In response to ischemia, TNF-α produced by the microglia, astrocytes, and choroid plexus ependymal cells is upregulated. Similarly, TNF receptors such as TNF1 and TNF2 are expressed by glial and neuronal cells in response to ischemia. Depending on the TNF receptors, cell death or cell proliferation is activated [Botchkina et al., 1997; Shen et al., 1997]. Proinflammatory cytokines such as IL-1β, IL-6, and TNF-α alter hippocampal structural plasticity and neuronal structure via alteration in morphology of the granule cells differentiated from NSCs in the dentate gyrus and CA1 during inflammation.
Epigenetic Modulators
Epigenetics refers to changes in gene activity not resulting from changes in DNA sequence. Changes in DNA methylation, histone modifications and regulation by non-coding RNAs have important effects on different neuronal phenotypes [Bird, 2007; Fitzsimons et al., 2014]. Proteins with methyl-CpG-binding domains (MBDs) bind methylated DNA and regulate gene expression by blocking the binding site for transcription factors, while DNA methyltransferases (DNTMs) are directly responsible for methylating DNA and silencing gene expression. MBD-1 has been shown to play a crucial role in NSC differentiation [Singh et al., 2009]. The specific binding of MBD1 to the FGF2 promoter decreases FGF2 expression and inhibits the differentiation of proliferative neural stem cells during adult neurogenesis [Zheng et al., 2004]. Similarly, overexpression of DNA methyltransferases DNMT1 and DNMT3a decreases the differentiation and migration of NSCs. Methyl-CpG-binding protein 2 (MeCP2) regulates gene expression similarly to MBD1. MeCP2 binds to GFAP and inhibits its expression, thus controlling neuronal differentiation and maturation, as well as cell fate [Tsujimura et al., 2009; Fitzsimons et al., 2014]. DNA-damage-inducible protein 45 beta (Gadd45b) plays a role in DNA demethylation and DNA repair during neurogenesis. Gadd45b demethylates neurotrophic factors such as BDNF and FGF-1, which affects self-renewal and proliferation of NSCs and promotes neuronal maturation and dendritic growth [Alam et al., 1996; Aid et al., 2007; Ma et al., 2009].
Histone acetylation is a crucial process for the proliferation and differentiation of neural stem cells. HDAC3, HDAC5, and HDAC7 interact with the orphan nuclear receptor homologue of the Drosophila tailless gene (Tlx or NR2E1) and manipulate NSC self-renewal and proliferation [Sun et al., 2007]. Other epigenetic mechanisms involve non-coding RNAs such as microRNAs. MicroRNAs such as Let-7b, miR-9, miR-34a, and miR-184 regulate proliferation of NSCs and neuronal differentiation. MiR-137 and miR-132 also regulate synaptogenesis and the neuronal network, while miR-34a and miR-125b regulate dendritogenesis and spine morphology [Schouten et al., 2012; Volvert et al., 2012]. All of these epigenetic mechanisms highlight the importance of looking beyond the genome to understand the biological underpinnings of neurogenesis, which will be crucial to advance the state of research in therapeutic efforts to address neurogenesis in neurodegenerative disease. Epigenetic changes during neurogenesis have an important impact on memory and learning, and can play significant roles in neuropsychiatric disorders as well such as depression and schizophrenia [Sharma, 2005; Renthal et al., 2007; Hsieh and Eisch, 2010].
Role of Genetic Variation in Adult Neurogenesis
Many gene expression level changes have been observed during adult neurogenesis, as presented in the previous sections; these changes affect NSC and progenitor proliferation, maintenance in the adult neurogenic niche, and differentiation into mature neurons. Although most of the studies focused on the alteration of gene expression during adult neurogenesis, some studies showed that genetic variations in adult neurogenesis-related genes affect hippocampal structure and memory impairment. For instance, the REST gene, a known transcriptional repressor, negatively regulates neuronal differentiation during neurogenesis, and nonsynonymous variation in this gene is associated with less hippocampal loss and greater cortical thickness in individuals who carry at least one minor allele [Lu et al., 2014; Nho et al., 2015; Thiel et al., 2015]. Another important gene related to adult neurogenesis is G-coupled protein receptor adenosine receptor A2A (ADORA2A) which plays a role in neurite growth. Alteration of the expression level of ADORA2A during adult neurogenesis affected neuronal differentiation, migration and maturation of new neurons [Sun et al., 2010; Shetty et al., 2013]. Variants in the ADORA2A gene differentially influence the transfer of information into working memory in homozygous rare genotype groups due to alteration of glutamergic neural transmission [Ferre et al., 2011; Beste et al., 2012]. Moreover, it has been shown that an ADORA2A antagonist reduced cognitive decline and resulted in a protective effect on memory formation in Parkinson’s disease, Huntington’s disease, and Alzheimer’s disease models. [Chen, 2014; Rieck et al., 2015]. An additional Schizophrenia susceptibility gene, DISC-1, regulates neuronal integration of new neurons from neural progenitors into the adult brain and promotes structural plasticity [Duan et al., 2007). DISC-1 missense variation leads to a reduction of the proliferation of progenitor cells, which alters the balance between quiescent and proliferative neural stem cells in a transgenic mouse model [Chandran et al., 2014]. A missense mutation in the DISC-1 gene is related to alteration of the hippocampal structure by reducing gray matter volume and increases the risk for schizophrenia [Callicott et al., 2005]. As previously discussed, BDNF plays an important role in neural progenitor cell proliferation, differentiation and survival; additionally, overexpression of BDNF enhances adult neurogenesis by increasing dendritic spine density on granule cells. BDNF polymorphism Val66Met modulates integration of neurons in vivo and regulates episodic memory and hippocampal physiological activation in humans [Egan et al., 2003; McDole et al., 2015]. Moreover, genetic variation in BDNF associated with hippocampal atrophy and cognitive decline have been identified using neuroimaging-genetics methods [Honea et al., 2013]. Pro-inflammatory cytokine IL-6 plays an important role in the formation of new neurons and glial cells from neural progenitor cells during adult neurogenesis, and IL-6 variations have been associated with AD, multiple sclerosis, and severe traumatic brain injury [Schmidt et al., 2003; He et al., 2010; Dalla Libera et al., 2011; Erta et al., 2012]. A single nucleotide polymorphism (SNP) within the GRIN2B gene, which is an N-Methyl-D-Aspartate (NMDA) glutamate receptor and enhances synapse maturation and survival of new-born neurons, is strongly associated with temporal lobe volume in patients with AD and mild cognitive impairment (MCI) [Stein et al., 2010; Kelsch et al., 2012]. Finally, variation within genes strongly related to adult neurogenesis processes in AD such as CHRFAM7A, REST, RELN, BCHE, NCAM1, and ADORA2A have been identified by our colleagues in our laboratory using neuroimaging-genetic methods [Swaminathan et al., 2011, 2012; Ramanan et al., 2012, 2014; Horgusluoglu et al., 2015; Nho et al., 2015].
To sum up, both expression differences and allelic variations in neurogenesis-related genes in the human genome may have compensatory advantages or confer impairment of biological processes during adult neurogenesis.
ADULT NEUROGENESIS AND NEURODEGENERATIVE DISEASES
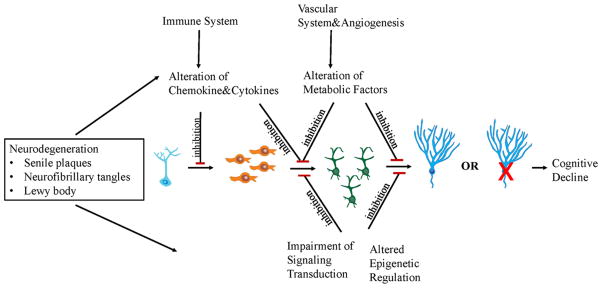
Over 50 years ago, it was reported for the first time that new neurons are generated in the dentate gyrus and SVZ of several species during the adult period, and this observation launched a new era of research to understand the mechanism of adult neurogenesis in humans [Altman and Das, 1965]. The important feature of adult neurogenesis is that there are many modulators playing roles during NSC proliferation, differentiation, migration and survival including genetic, transcriptional, and epigenetic factors as well as environmental factors and disease status [Mu et al., 2010]. Neurogenesis is a very important process for synaptic transmission and is associated with axonal and dendritic formation. Impaired adult neurogenesis in neurodegenerative diseases results in loss of existing neurons and reduced capacity for NSC renewal; the putative function of new neurons eventually is compromised or lost (Fig. 3). The crucial question is how neurodegenerative diseases affect adult neurogenesis and in turn how alterations in neurogenesis impact pathophysiological mechanisms of neurodegenerative disease. We consider five diseases that display symptoms related to hippocampal and olfactory dysfunction, the two main brain regions where adult neurogenesis occurs. Parkinson’s disease (PD), AD, Huntington disease (HD), and amyotrophic lateral sclerosis (ALS) are neurodegenerative diseases, whereas schizophrenia is a condition with both neurodevelopmental and neurodegenerative aspects.
FIG. 3.
Impaired neurogenesis in neurodegenerative diseases. Neurodegeneration negatively affects the adult neurogenesis process due to alteration of chemokines and cytokines, metabolic factors, and epigenetic regulation, as well as impairment of signaling transduction. Alteration of chemokines and cytokines impairs neuronal stem cell self-renewal and differentiation. Alteration of metabolic factors and epigenetic regulators, and defects in signaling transduction molecules, inhibits the proliferation of progenitor cells and their differentiation into neuroblasts. The inhibition of newborn neuron formation may contribute to cognitive decline. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/ajmgb].
Parkinson’s Disease
PD is a movement disorder with frequent psychiatric complications as well as a high prevalence of cognitive impairment [Weintraub and Burn, 2011]. The accumulation of α-synuclein as the major component of Lewy bodies and Lewy neurites is the pathological hallmark of PD [Schulz-Schaeffer, 2010]. Even though there are some conflicting findings regarding how adult neurogenesis affects PD processes, some studies in humans show that there is reduced proliferation of NSC progenitors in the SVZ [Hoglinger et al., 2004; van den Berge et al., 2011]. Postmortem human PD brain studies and transgenic PD animal models showed that the number of EGF and EGF2 receptor positive cells decrease in the adult SVZ, causing impairment of olfactory bulb neurogenesis associated with dopaminergic deafferentation [O’Keeffe et al., 2009]. In addition, transgenic PD animal model studies revealed that stimulation with neurotrophic factors such as EGF and FGF-2 cause massive proliferation and migration of neuroblasts into the SVZ region [Kuhn et al., 1997; Iwakura et al., 2005; Winner et al., 2008]. α-synuclein accumulation in neurogenic regions where adult neurogenesis occurs impairs olfactory bulb formation and hippocampal neurogenesis. Selective serotonin uptake inhibitor fluoxetine treatment of PD increases levels of neurotrophic factors such as BDNF and GDNF, and rescues impaired hippocampal neurogenesis in transgenic PD mice [Kohl et al., 2012].
Alzheimer’s Disease
AD is the most common form of adult-onset dementia. Patients display hippocampal atrophy, memory impairment, and other cognitive and olfactory deficits. There are two crucial hallmarks of AD: neurofibrillary tangles caused by hyperphosphorylation of the tau protein and amyloid-β plaque deposition [Hardy and Selkoe, 2002]. Currently, there is no approved disease modifying treatment for AD. It has been shown in numerous genetic studies that APP, presenilin 1 (PSEN1) and PSEN2 missense mutations cause familial AD [Philippsen et al., 1997]. These mutations result in increased amyloid-β production and intracellular and extracellular accumulation. Presenilin is a catalytic component of γ-secretase, which cuts APP, and also regulates Notch and Wnt signaling mechanisms by sequentially cleaving the Notch receptor to generate the Notch Intracellular Domain (NICD) [De Strooper and Woodgett, 2003; Kojro and Fahrenholz, 2005]. α-secretase (ADAM protease) cleaves APP to make sAPPα, and also cleaves Notch-1 and components of EGFR, which, as previously discussed, play important roles in adult neurogenesis [Hartmann et al., 2002]. Many of the molecular players in AD are also modulators of adult neurogenesis. The most important of these, PSEN1, modulates NSC differentiation in the adult brain, while sAPPα regulates the proliferation of NSCs [Gakhar-Koppole et al., 2008; Gadadhar et al., 2011]. BDNF/TrkB signaling enhances dephosphorylation of the tau protein in AD pathology, while BDNF gene expression is elevated in transgenic AD mice via environmental enrichment.
It is not completely understood how hippocampal neurogenesisis affected by AD pathology. However, alterations in the early stage of AD progression such as amyloid-β deposition and inflammation impair the maturation of newborn neurons and inhibit hippocampal neurogenesis [Mu and Gage, 2011]. Although abnormal accumulation of amyloid-β activates microglia and astrocytes to secrete more inflammatory cytokines such as IL-1β, IL-6, and TNF-α, which regulate the inflammatory response, AD has been proposed as a chronic inflammatory disorder of the central nervous system [Heneka et al., 2010; Thompson et al., 2014; Dursun et al., 2015]. Meta-analysis of proinflammatory cytokine levels in cerebrospinal fluid and peripheral serum have shown significant elevation of TNF-α and IL-1β in AD patients; this elevation might reflect activated neural progenitor cell proliferation as a compensatory mechanism during neurodegeneration [Swardfager et al., 2010]. Additional cytokine signaling processes have also been linked to neurodegenerative processes; for example, CXCL12/CXCR4 signaling causes astrocytes in the CA1 to release glutamate neurotransmitter, which regulates synaptic integration and neuronal excitability and decreases amyloid-β deposition [Bezzi et al., 2001]. Another signaling process involves MCP-1 and CCR2, which are both strongly upregulated in AD patients. Expression of MCP-1 secreted by macrophages, monocytes, and astrocytes is induced by amyloid-β; MCP-1 is known to regulate the migration of neural cells in the dentate gyrus toward damaged areas in the brain, suggesting an important function in neurodegenerative disease [Smits et al., 2002; Belmadani et al., 2006]. Furthermore, amyloid-β accumulation in the brain increases IL-1β, IL-6, and TNF-α as well as transforming growth factor (TGF)-β secretion, which has a negative effect on NSC proliferation and survival and inhibits hippocampal neurogenesis, emphasizing the importance of cytokines as a modulator of neurogenesis in neurodegenerative disease [Vallieres et al., 2002; Monje et al., 2003; Iosif et al., 2006; Kaneko et al., 2006].
Huntington’s Disease
HD is an autosomal dominant neurodegenerative disorder caused by tri-nucleotide repeat expansion within the Huntington gene [Pringsheim et al., 2012; Winner and Winkler, 2015]. There is no reported dysfunction of hippocampal neurogenesis in HD patients. Although there is potential migration of neural stem cells progenitors to the degenerating striatum, they do not differentiate to mature neurons, indicating a significant downstream effect of this process [Phillips et al., 2005; Low et al., 2011; Winner and Winkler, 2015]. Transgenic HD mouse model studies showed that reduction of the hippocampal progenitor cells was associated with diminished CREB signaling and elevated TGF-β1 signaling [Kandasamy et al., 2010]. Moreover, it has been found that a D2R antagonist partially enhance adult neurogenesis by increasing the proliferation of the neural precursor cells and immature neurons in the SGZ region of the HD mice model while a D2R antagonist did not have any effect on motor performance [Choi et al., 2014]. The other study showed that mutant HD mice had decreased NeuroD1 in neural progenitors in the dentate gyrus of the hippocampus and doublecortin and calretinin expression in newborn neurons, as well as impairment of spatial memory. NeuroD1 has a crucial effect on proliferation, differentiation, and maturation of the neural progenitor cells; HD pathology adversely affects the function of NeuroD1 [Gao et al., 2009; Fedele et al., 2011]. Alterations of proteins in HD pathology may impair effective adult neurogenesis and cause cognitive impairment.
Amyotrophic Lateral Sclerosis
ALS is a progressive neurodegenerative disorder caused by the degeneration of motor neurons, leading to muscle wasting, paralysis, and difficulty breathing [Popescu et al., 2013]. Neural stem cell culture from transgenic ALS mice showed that late stage ALS microenvironment impairs the functional capacity of NSCs [Lee et al., 2011]. There reduction in number of proliferative GFAP+ cells in SOD-1 transgenic mice, causing dominant form of ALS, in the olfactory bulb of the SVZ, and dentate gyrus of the hippocampus. However, in early stages of ALS, neurogenesis was preserved and there were no alterations of the NSCs [Liu and Martin, 2006]. ALS onset and progression promotes de novo neurogenesis with NSC proliferation and migration to the spinal cord increasing concomitantly with motor neuron degeneration. During disease onset and progression, an ALS-like mouse model showed increased expression of CXCR4, which is directly associated with NSC migration into the spinal cord, compared to age-matched control mice [Chi et al., 2006]. In addition to several ALS studies, immunohistochemical and histological studies of FTLD-ALS patients showed that the number of proliferative neural cells in the SVZ increased corresponding to disease progression as a compensatory mechanism for neurodegeneration [Galan et al., 2011].
Schizophrenia
Schizophrenia is a complex mental disorder with a strong genetic background. Schizophrenia is associated with impairment of adult neurogenesis by disrupting NSC proliferation and migration to the SGZ and SVZ [Reif et al., 2006; Muraki and Tanigaki, 2015]. Cognitive impairment in schizophrenia might be related to impairment of adult neurogenesis [Kurtz, 2005]. Several candidate genes have been suggested to play roles in adult neurogenesis as well as schizophrenia. Neuregulin (Nrg1)-ERBB signaling, altered in schizophrenia, normally promotes the maintenance of radial glial cells and their migration to the cerebral cortex by movement of cerebellar granule cells, which express neuregulin, along radial glial fibers, which express ErbB4 [Anton et al., 1997; Rio et al., 1997; Fisahn et al., 2009]. The other important gene significantly associated with schizophrenia as well as adult neurogenesis is the disrupted-in schizophrenia 1 (DISC1) gene [Ekelund et al., 2001; Lee et al., 2015]. DISC1 knockout rats showed aberrant positioning and impaired morphogenesis of newborn neurons in the dentate gyrus of the hippocampus [Lee et al., 2015]. Some genes, such as Wnt, GSK-3β, and Reelin, play roles in neuronal development and proliferation, cell fate determination, cell adhesion, and migration of NSCs, and are also known to have abnormal function in schizophrenia, which suggests that there is a strong link between expression of developmental genes with abnormal mechanisms of neurogenesis in schizophrenia [D’Arcangelo et al., 1995; Dale, 1998; Beasley et al., 2001; Goldberger et al., 2005].
SUMMARY OF ADULT NEUROGENESIS AND NEURODEGENERATIVE DISEASES
Each neurodegenerative condition has different effects on NSC fate during adult neurogenesis by controlling synaptic plasticity, spine morphology, and axonal pathology. Newly generated neurons play critical roles in brain development and maintenance in the adult brain. Several genes including PSEN1, MAPT, α-synuclein, SOD-1, and Huntingtin, are the main players in modulating synaptic plasticity and brain morphology. Alterations in these genes are linked to neurodegenerative diseases and changes in neurogenesis in specific areas such as the dentate gyrus of the hippocampus and the SVZ in early stages of neurodegenerative diseases. Discovery of the genetic mechanisms underlying adult neurogenesis and how neurodegenerative disorders affect new neuron formation could significantly inform therapeutic approaches to treat neurodegenerative diseases in early stages, when intervention has the most potential to prevent long-term dysfunction.
THERAPEUTIC RESEARCH FOCUSED ON ADULT NEUROGENESIS
Adult neurogenesis is defined by the formation of new functional neurons from NSCs and their integration into the neuronal circuitry to promote structural plasticity. Even though the complete mechanism underlying this process is not yet known, hippocampal neurogenesis appears to be critical for the formation and maintenance of hippocampal structure as well as memory and learning. Although modulators including signaling transduction, the vascular and immune systems, and epigenetic regulation enhance structural and synaptic plasticity during maturation of the newly generated neurons from their progenitors, many recent studies focus on novel pharmacologic strategies targeting adult neurogenesis and identification of biomarkers for human neurogenesis.
One novel therapeutic approach targets inflammatory molecules such as chemokines and cytokines known to be related to adult neurogenesis. Activated microglia and cytokines released as an immune response enhance neural progenitor proliferation, differentiation, and maturation into new neurons during adult hippocampal neurogenesis as well as facilitating memory formation and learning [Musaelyan et al., 2014]. Moreover, some cytokines such as IL-6, IL-β, and TNF-α modulate neural progenitor cell fate and contribute to neural repair mechanisms during neuroinflammation [Mueller et al., 2005]. The development of anti-inflammatory drugs targeting inflammatory molecules to preserve adult neurogenesis during chronic inflammation may provide novel insights into early stages of neurodegenerative diseases. Since neuroinflammation both a key component of AD and other neurodegenerative disorders and known to affect adult neurogenesis, it has been the focus of several therapeutic approaches. Studies have focused on anti-inflammatory drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs) and glucocorticoidsteroids, which target expression of proinflammatory cytokines and their complimentary molecules, as treatments for AD patients [Mackenzie, 2001; Fuster-Matanzo et al., 2013]. These approaches have not been successful to date although it is suspected that this may reflect their introduction in later stages of disease after substantial degenerative changes have already occurred.
Another therapeutic approach to modulate neurogenesis targets neurotrophic factors in AD pathology since there is an imbalance between neurotrophic factors in the AD brain. For example, the level of neurotrophic factors such as FGF-2 and NGF increases to compensate for increasing AD pathology, whereas the level of BDNF decreases in the hippocampus [Stopa et al., 1990; Hock et al., 2000]. NGF gene therapy causes NGF secretion from autologous fibroblasts into the basal forebrain, leading to expression of the neurotrophin receptors by cholinergic neurons and cholinergic axonal sprouting in the patients with AD for 10 years after gene transfer [Tuszynski et al., 2015]. Also, it has been shown that neuroproliferation of the immature neurons in the dentate gyrus of the SGZ increases in AD patients, although it is not clear whether these immature neurons are differentiating into mature neurons [Jin et al., 2004]. VEGF levels significantly increase in cerebrospinal fluid while there is decrease of them in serum level in AD patients [Tarkowski et al., 2002; Mateo et al., 2007]. Keilhoff et al. [2010] found that antipsychotics haloperidol and risperidone increased neuronal precursor cell proliferation and survival of the new granule cells via VEGF and MMP2 in adult schizophrenic rat brain. These factors play crucial roles in the determination of cell fate and new neuron formation, and an imbalance of these factors affects neurogenesis. The environmental changes in the NSC niche due to the altered levels of neurotrophic and neuroinflammatory factors in the hippocampus may adversely affect the differentiation of progenitor cells into mature neurons. Further studies are needed to address these factors with regard to possible therapeutic strategies.
CONCLUSIONS AND FUTURE DIRECTIONS
Adult neurogenesis is important for structural plasticity of the brain through turnover of neural stem cells/precursors to new functional neurons. Even though the molecular mechanisms underlying this process remain unclear, adult hippocampal neurogenesis plays a significant role in learning and memory formation, and it is affected by environmental changes and disease conditions [Costa et al., 2015]. There are multiple modulators that affect the formation of newborn neurons such as neurotrophic factors, cytokines and chemokines, epigenetic factors, and signaling pathways. Each modulator drives NSC proliferation, differentiation, migration, and survival in different ways. The alteration of these modulators under disease conditions negatively affects cognition and hippocampal structure and function. Future studies should also focus on identification of genetic variation contributing to neurogenesis in healthy adults as well as in neurodegenerative disease; such research has strong translational potential to identify novel therapeutic targets. It is crucial for future research to continue to investigate the functional role of adult neurogenesis in the normal human brain as well as alterations in neurodegenerative diseases. Loss of NSC populations and impairment of neuron formation are common hallmarks in neurodegenerative diseases such as AD, PD, HD, and ALS. Future therapeutic strategies hold promise for stimulation of neuronal plasticity and maintenance of newborn neurons in early stages of neurodegenerative diseases, potentially halting or reversing clinical symptoms in these common, devastating diseases.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant numbers: R01 AG19771, P30 AG10133, R00 LM011384, R01 LM011360, U01 AG024904; Grant sponsor: Indiana CTSI; Grant numbers: U54 RR025761, RR027710, RR020128.
The authors’ research is supported, in part, by the following grants from the National Institutes of Health: R01 AG19771, P30 AG10133, R00 LM011384, R01 LM011360, and U01 AG024904, as well as the Indiana CTSI (U54 RR025761, RR027710, and RR020128). The authors would like to thank Robert Lee Moloch for his technical assistance in generating the figures for publication.
Footnotes
Conflicts of interest: The authors declare no potential conflicts of interest.
References
- Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci. 2010;30(31):10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467(7313):323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85(3):525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam KY, Frostholm A, Hackshaw KV, Evans JE, Rotter A, Chiu IM. Characterization of the 1B promoter of fibroblast growth factor 1 and its expression in the adult and developing mouse brain. J Biol Chem. 1996;271(47):30263–30271. doi: 10.1074/jbc.271.47.30263. [DOI] [PubMed] [Google Scholar]
- Alkon DL, Nelson TJ. Specificity of molecular changes in neurons involved in memory storage. FASEB J. 1990;4(6):1567–1576. doi: 10.1096/fasebj.4.6.2108074. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Amador-Arjona A, Elliott J, Miller A, Ginbey A, Pazour GJ, Enikolopov G, Roberts AJ, Terskikh AV. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: Implications for learning and memory. J Neurosci. 2011;31(27):9933–9944. doi: 10.1523/JNEUROSCI.1062-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124(18):3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- Ashton RS, Conway A, Pangarkar C, Bergen J, Lim KI, Shah P, Bissell M, Schaffer DV. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat Neurosci. 2012;15(10):1399–1406. doi: 10.1038/nn.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus-memory and anxiety. Neurosci Biobehav Rev. 2004;28(3):273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc Natl Acad Sci USA. 1994;91(23):11217–11221. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley C, Cotter D, Khan N, Pollard C, Sheppard P, Varndell I, Lovestone S, Anderton B, Everall I. Glycogen synthase kinase-3beta immunoreactivity is reduced in the prefrontal cortex in schizophrenia. Neurosci Lett. 2001;302(2–3):117–120. doi: 10.1016/s0304-3940(01)01688-3. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci. 2006;26(12):3182–3191. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky HO, Rey AD. Physiology of psychoneuroimmunology: A personal view. Brain Behav Immun. 2007;21(1):34–44. doi: 10.1016/j.bbi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Beste C, Stock AK, Ness V, Epplen JT, Arning L. Differential effects of ADORA2A gene variations in pre-attentive visual sensory memory subprocesses. Eur Neuropsychopharmacol. 2012;22(8):555–561. doi: 10.1016/j.euroneuro.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: Amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4(7):702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya BJ, Banisadr G, Jung H, Ren D, Cronshaw DG, Zou Y, Miller RJ. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J Neurosci. 2008;28(26):6720–6730. doi: 10.1523/JNEUROSCI.1677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Botchkina GI, Meistrell ME, 3rd, Botchkina IL, Tracey KJ. Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med. 1997;3(11):765–781. [PMC free article] [PubMed] [Google Scholar]
- Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5(7):521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: Facts and hypotheses. Rev Neurosci. 2007;18(2):93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- Caldwell MA, He X, Wilkie N, Pollack S, Marshall G, Wafford KA, Svendsen CN. Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat Biotechnol. 2001;19(5):475–479. doi: 10.1038/88158. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, Verchinski BA, Meyer-Lindenberg A, Balkissoon R, Kolachana B, Goldberg TE, Weinberger DR. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci USA. 2005;102(24):8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo CF, Fontaine RH, Soueid J, Tammela T, Makinen T, Alfaro-Cervello C, Bonnaud F, Miguez A, Benhaim L, Xu Y, Barallobre MJ, Moutkine I, Lyytikka J, Tatlisumak T, Pytowski B, Zalc B, Richardson W, Kessaris N, Garcia-Verdugo JM, Alitalo K, Eichmann A, Thomas JL. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev. 2011;25(8):831–844. doi: 10.1101/gad.615311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36(2):287–306. [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36(8):827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Chandran JS, Kazanis I, Clapcote SJ, Ogawa F, Millar JK, Porteous DJ, Ffrench-Constant C. Disc1 variation leads to specific alterations in adult neurogenesis. PLoS ONE. 2014;9(10):e108088. doi: 10.1371/journal.pone.0108088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Chen JF. Adenosine receptor control of cognition in normal and disease. Int Rev Neurobiol. 2014;119:257–307. doi: 10.1016/B978-0-12-801022-8.00012-X. [DOI] [PubMed] [Google Scholar]
- Chi L, Ke Y, Luo C, Li B, Gozal D, Kalyanaraman B, Liu R. Motor neuron degeneration promotes neural progenitor cell proliferation, migration, and neurogenesis in the spinal cords of amyotrophic lateral sclerosis mice. Stem Cells. 2006;24(1):34–43. doi: 10.1634/stemcells.2005-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi ML, Begeti F, Oh JH, Lee SY, O’Keeffe GC, Clelland CD, Tyers P, Cho ZH, Kim YB, Barker RA. Dopaminergic manipulations and its effects on neurogenesis and motor function in a transgenic mouse model of Huntington’s disease. Neurobiol Dis. 2014;66:19–27. doi: 10.1016/j.nbd.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Chumley MJ, Catchpole T, Silvany RE, Kernie SG, Henkemeyer M. EphB receptors regulate stem/progenitor cell proliferation, migration, and polarity during hippocampal neurogenesis. J Neurosci. 2007;27(49):13481–13490. doi: 10.1523/JNEUROSCI.4158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3(11):1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- Costa V, Lugert S, Jagasia R. Role of adult hippocampal neurogenesis in cognition in physiology and disease: Pharmacological targets and biomarkers. Handb Exp Pharmacol. 2015;228:99–155. doi: 10.1007/978-3-319-16522-6_4. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, De Souza EB. Interleukin 1 receptors in the brain and endocrine tissues. Immunol Today. 1993;14(4):171–176. doi: 10.1016/0167-5699(93)90281-o. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374(6524):719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Dale TC. Signal transduction by the Wnt family of ligands. Biochem J. 1998;329(Pt 2):209–223. doi: 10.1042/bj3290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Libera AL, Regner A, de Paoli J, Centenaro L, Martins TT, Simon D. IL-6 polymorphism associated with fatal outcome in patients with severe traumatic brain injury. Brain Inj. 2011;25(4):365–369. doi: 10.3109/02699052.2011.556107. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Woodgett J. Alzheimer’s disease: Mental plaque removal. Nature. 2003;423(6938):392–393. doi: 10.1038/423392a. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depino AM, Alonso M, Ferrari C, del Rey A, Anthony D, Besedovsky H, Medina JH, Pitossi F. Learning modulation by endogenous hippocampal IL-1: Blockade of endogenous IL-1 facilitates memory formation. Hippocampus. 2004;14(4):526–535. doi: 10.1002/hipo.10164. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140(3):823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130(6):1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun E, Gezen-Ak D, Hanagasi H, Bilgic B, Lohmann E, Ertan S, Atasoy IL, Alaylioglu M, Araz OS, Onal B, Gunduz A, Apaydin H, Kiziltan G, Ulutin T, Gurvit H, Yilmazer S. The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s disease, mild cognitive impairment or Parkinson’s disease. J Neuroimmunol. 2015;283:50–57. doi: 10.1016/j.jneuroim.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ekelund J, Hovatta I, Parker A, Paunio T, Varilo T, Martin R, Suhonen J, Ellonen P, Chan G, Sinsheimer JS, Sobel E, Juvonen H, Arajarvi R, Partonen T, Suvisaari J, Lonnqvist J, Meyer J, Peltonen L. Chromosome 1 loci in Finnish schizophrenia families. Hum Mol Genet. 2001;10(15):1611–1617. doi: 10.1093/hmg/10.15.1611. [DOI] [PubMed] [Google Scholar]
- Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: A common signal for ventral patterning within the neural tube. Cell. 1995;81(5):747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156(5):1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Ernst A, Frisen J. Adult neurogenesis in humans—Common and unique traits in mammals. PLoS Biol. 2015;13(1):e1002045. doi: 10.1371/journal.pbio.1002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8(9):1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830(2):2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele V, Roybon L, Nordstrom U, Li JY, Brundin P. Neurogenesis in the R6/2 mouse model of Huntington’s disease is impaired at the level of Neuro D1. Neuroscience. 2011;173:76–81. doi: 10.1016/j.neuroscience.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Ferre S, Quiroz C, Orru M, Guitart X, Navarro G, Cortes A, Casado V, Canela EI, Lluis C, Franco R. Adenosine A(2A) receptors and A(2A) receptor heteromers as key players in striatal function. Front Neuroanat. 2011;5:36. doi: 10.3389/fnana.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: Implications for schizophrenia. Cereb Cortex. 2009;19(3):612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons CP, van Bodegraven E, Schouten M, Lardenoije R, Kompotis K, Kenis G, van den Hurk M, Boks MP, Biojone C, Joca S, Steinbusch HW, Lunnon K, Mastroeni DF, Mill J, Lucassen PJ, Coleman PD, van den Hove DL, Rutten BP. Epigenetic regulation of adult neural stem cells: Implications for Alzheimer’s disease. Mol Neurodegener. 2014;9:25. doi: 10.1186/1750-1326-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funa K, Sasahara M. The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J Neuroimmune Pharmacol. 2014;9(2):168–181. doi: 10.1007/s11481-013-9479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster-Matanzo A, Llorens-Martin M, Hernandez F, Avila J. Role of neuroinflammation in adult neurogenesis and Alzheimer disease: Therapeutic approaches. Mediators Inflamm. 2013;2013:260925. doi: 10.1155/2013/260925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadadhar A, Marr R, Lazarov O. Presenilin-1 regulates neural progenitor cell differentiation in the adult brain. J Neurosci. 2011;31(7):2615–2623. doi: 10.1523/JNEUROSCI.4767-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakhar-Koppole N, Hundeshagen P, Mandl C, Weyer SW, Allinquant B, Muller U, Ciccolini F. Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur J Neurosci. 2008;28(5):871–882. doi: 10.1111/j.1460-9568.2008.06398.x. [DOI] [PubMed] [Google Scholar]
- Galan L, Gomez-Pinedo U, Vela-Souto A, Guerrero-Sola A, Barcia JA, Gutierrez AR, Martinez-Martinez A, Jimenez MS, Garcia-Verdugo JM, Matias-Guiu J. Subventricular zone in motor neuron disease with frontotemporal dementia. Neurosci Lett. 2011;499(1):9–13. doi: 10.1016/j.neulet.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12(9):1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido JL, Godoy JA, Alvarez A, Bronfman M, Inestrosa NC. Protein kinase C inhibits amyloid beta peptide neurotoxicity by acting on members of the Wnt pathway. FASEB J. 2002;16(14):1982–1984. doi: 10.1096/fj.02-0327fje. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan H, Levav T, Mendelsohn A, Huleihel M. Involvement of tumor necrosis factor alpha in hippocampal development and function. Cereb Cortex. 2004;14(1):97–105. doi: 10.1093/cercor/bhg108. [DOI] [PubMed] [Google Scholar]
- Goldberg JS, Hirschi KK. A Vascular Perspective on Neurogenesis, Neural Stem Cells - New Perspectives. In: Bonfanti Luca., editor. InTech; 2013. Available from: http://www.intechopen.com/books/neural-stem-cells-new-perspectives/a-vascular-perspective-on-neurogenesis. [DOI] [Google Scholar]
- Goldberger C, Gourion D, Leroy S, Schurhoff F, Bourdel MC, Leboyer M, Krebs MO. Population-based and family-based association study of 5’UTR polymorphism of the reelin gene and schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2005;137B(1):51–55. doi: 10.1002/ajmg.b.30191. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2(3):260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44(4):581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993;75(6):1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11(3):277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, LenaIllert A, von Figura K, Saftig P. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet. 2002;11(21):2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- He MX, Yang WL, Zhang MM, Lian YJ, Hua HY, Zeng JS, Zhang LR. Association between interleukin-6 gene promoter -572C/G polymorphism and the risk of sporadic Alzheimer’s disease. Neurol Sci. 2010;31(2):165–168. doi: 10.1007/s10072-009-0199-3. [DOI] [PubMed] [Google Scholar]
- Heneka MT, O’Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer’s disease. J Neural Transm. 2010;117(8):919–947. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Hodge RD, Daza RA, Englund C. Transcription factors in glutamatergic neurogenesis: Conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res. 2006;55(3):223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16(7):846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57(6):846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7(7):726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Honea RA, Cruchaga C, Perea RD, Saykin AJ, Burns JM, Weinberger DR, Goate AM I Alzheimer’s Disease Neuroimaging. Characterizing the role of brain derived neurotrophic factor genetic variation in Alzheimer’s disease neurodegeneration. PLoS ONE. 2013;8(9):e76001. doi: 10.1371/journal.pone.0076001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgusluoglu E, Nho K, Risacher SL, Saykin AJ. Targeted neurogenesis pathway-based gene analysis identifies ADORA2A associated with hippocampal volume in mild cognitive impairment and Alzheimer’s disease. The American Society of Human Genetics Conference (ASHG); October 6–10, 2015; Baltimore, USA. 2015. Poster presentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Eisch AJ. Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: Unraveling the genome to understand the mind. Neurobiol Dis. 2010;39(1):73–84. doi: 10.1016/j.nbd.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussaini SM, Choi CI, Cho CH, Kim HJ, Jun H, Jang MH. Wnt signaling in neuropsychiatric disorders: Ties with adult hippocampal neurogenesis and behavior. Neurosci Biobehav Rev. 2014;47:369–383. doi: 10.1016/j.neubiorev.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30(9):3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101(52):18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26(38):9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Piao YS, Mizuno M, Takei N, Kakita A, Takahashi H, Nawa H. Influences of dopaminergic lesion on epidermal growth factor-ErbB signals in Parkinson’s disease and its model: Neurotrophic implication in nigrostriatal neurons. J Neurochem. 2005;93(4):974–983. doi: 10.1111/j.1471-4159.2005.03073.x. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51(2):187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Derrick BE, Patterson PH. Cytokine responses to LTP induction in the rat hippocampus: A comparison of in vitro and in vivo techniques. Learn Mem. 2000;7(6):400–412. doi: 10.1101/lm.32600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao JW, Feldheim DA, Chen DF. Ephrins as negative regulators of adult neurogenesis in diverse regions of the central nervous system. Proc Natl Acad Sci USA. 2008;105(25):8778–8783. doi: 10.1073/pnas.0708861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98(8):4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101(1):343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24(1):171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Jones MW, Peckham HM, Errington ML, Bliss TV, Routtenberg A. Synaptic plasticity in the hippocampus of awake C57BL/6 and DBA/2 mice: Interstrain differences and parallels with behavior. Hippocampus. 2001;11(4):391–396. doi: 10.1002/hipo.1053. [DOI] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3(2):165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Kandasamy M, Couillard-Despres S, Raber KA, Stephan M, Lehner B, Winner B, Kohl Z, Rivera FJ, Nguyen HP, Riess O, Bogdahn U, Winkler J, von Horsten S, Aigner L. Stem cell quiescence in the hippocampal neurogenic niche is associated with elevated transforming growth factor-beta signaling in an animal model of Huntington disease. J Neuropathol Exp Neurol. 2010;69(7):717–728. doi: 10.1097/NEN.0b013e3181e4f733. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Kudo K, Mabuchi T, Takemoto K, Fujimaki K, Wati H, Iguchi H, Tezuka H, Kanba S. Suppression of cell proliferation by interferon-alpha through interleukin-1 production in adult rat dentate gyrus. Neuropsychopharmacology. 2006;31(12):2619–2626. doi: 10.1038/sj.npp.1301137. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Xie Y, An JJ, Stryker MP, Xu B. Dendritic BDNF synthesis is required for late-phase spine maturation and recovery of cortical responses following sensory deprivation. J Neurosci. 2012;32(14):4790–4802. doi: 10.1523/JNEUROSCI.4462-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhoff G, Grecksch G, Bernstein HG, Roskoden T, Becker A. Risperidone and haloperidol promote survival of stem cells in the rat hippocampus. Eur Arch Psychiatry Clin Neurosci. 2010;260(2):151–162. doi: 10.1007/s00406-009-0033-1. [DOI] [PubMed] [Google Scholar]
- Kelsch W, Li Z, Eliava M, Goengrich C, Monyer H. GluN2B-containing NMDA receptors promote wiring of adult-born neurons into olfactory bulb circuits. J Neurosci. 2012;32(36):12603–12611. doi: 10.1523/JNEUROSCI.1459-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18(9):3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ED, Muroy SE, Sun WG, Covarrubias D, Leong MJ, Barchas LA, Kaufer D. Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. Elife. 2013;2:e00362. doi: 10.7554/eLife.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12(1):15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- Kohl Z, Winner B, Ubhi K, Rockenstein E, Mante M, Munch M, Barlow C, Carter T, Masliah E, Winkler J. Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. Eur J Neurosci. 2012;35(1):10–19. doi: 10.1111/j.1460-9568.2011.07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojro E, Fahrenholz F. The non-amyloidogenic pathway: Structure and function of alpha-secretases. Subcell Biochem. 2005;38:105–127. doi: 10.1007/0-387-23226-5_5. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7(2):163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17(15):5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM. Neurocognitive impairment across the lifespan in schizophrenia: An update. Schizophr Res. 2005;74(1):15–26. doi: 10.1016/j.schres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12(9):1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6(1):21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, De Stafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Moron FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fievet N, Huentelman MW, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuiness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossu P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Deniz Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews FI, Moebus S, Mecocci P, DelZompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannefelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O’Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH, Jr, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltuenen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nothen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P European Alzheimer’s Disease, Genetic D Environmental Risk in Alzheimer’s, C Alzheimer’s Disease Genetic, H Cohorts for, E Aging Research in Genomic. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laske C, Stellos K, Stransky E, Leyhe T, Gawaz M. Decreased plasma levels of granulocyte-colony stimulating factor (G-CSF) in patients with early Alzheimer’s disease. J Alzheimers Dis. 2009;17(1):115–123. doi: 10.3233/JAD-2009-1017. [DOI] [PubMed] [Google Scholar]
- Lauro C, Di Angelantonio S, Cipriani R, Sobrero F, Antonilli L, Brusadin V, Ragozzino D, Limatola C. Activity of adenosine receptors type 1 is required for CX3CL1-mediated neuroprotection and neuromodulation in hippocampal neurons. J Immunol. 2008;180(11):7590–7596. doi: 10.4049/jimmunol.180.11.7590. [DOI] [PubMed] [Google Scholar]
- Laussu J, Khuong A, Gautrais J, Davy A. Beyond boundaries—Eph: ephrin signaling in neurogenesis. Cell Adh Migr. 2014;8(4):349–359. doi: 10.4161/19336918.2014.969990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kang E, GoodSmith D, Yoon do Y, Song H, Knierim JJ, Ming GL, Christian KM. DISC1-mediated dysregulation of adult hippocampal neurogenesis in rats. Front Syst Neurosci. 2015;9:93. doi: 10.3389/fnsys.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Jin Y, Jin J, Kang BG, Nam DH, Joo KM, Cha CI. Functional neural stem cell isolation from brains of adult mutant SOD1 (SOD1 (G93A)) transgenic amyotrophic lateral sclerosis (ALS) mice. Neurol Res. 2011;33(1):33–37. doi: 10.1179/016164110X12807570509899. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 2007;14(11):745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13(6):450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Liu J, Ma Y, Tian S, Zhang L, Zhao M, Zhang Y, Xu D. T cells promote the regeneration of neural precursor cells in the hippocampus of Alzheimer’s disease mice. Neural Regen Res. 2014;9(16):1541–1547. doi: 10.4103/1673-5374.139481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8(9):1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Martin LJ. The adult neural stem and progenitor cell niche is altered in amyotrophic lateral sclerosis mouse brain. J Comp Neurol. 2006;497(3):468–488. doi: 10.1002/cne.21012. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7(3):179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lopez-Virgen V, Zarate-Lopez D, Adirsch FL, Collas-Aguilar J, Gonzalez-Perez O. Effects of sleep deprivation in hippocampal neurogenesis. Gac Med Mex. 2015;151(1):99–104. [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7(2):93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Low VF, Dragunow M, Tippett LJ, Faull RL, Curtis MA. No change in progenitor cell proliferation in the hippocampus in Huntington’s disease. Neuroscience. 2011;199:577–588. doi: 10.1016/j.neuroscience.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci USA. 2002;99(10):7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, Yang TH, Kim HM, Drake D, Liu XS, Bennett DA, Colaiacovo MP, Yankner BA. REST and stress resistance in ageing and Alzheimer’s disease. Nature. 2014;507(7493):448–454. doi: 10.1038/nature13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323(5917):1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39(6):937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Mackay CR. Chemokines: Immunology’s high impact factors. Nat Immunol. 2001;2(2):95–101. doi: 10.1038/84298. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR. Postmortem studies of the effect of anti-inflammatory drugs on Alzheimer-type pathology and associated inflammation. Neurobiol Aging. 2001;22(6):819–822. doi: 10.1016/s0197-4580(01)00304-9. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992;6(8):2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136(6):1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406(4):449–460. [PubMed] [Google Scholar]
- Mateo I, Llorca J, Infante J, Rodriguez-Rodriguez E, Fernandez-Viadero C, Pena N, Berciano J, Combarros O. Low serum VEGF levels are associated with Alzheimer’s disease. Acta Neurol Scand. 2007;116(1):56–58. doi: 10.1111/j.1600-0404.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- Maurer MH, Tripps WK, Feldmann RE, Jr, Kuschinsky W. Expression of vascular endothelial growth factor and its receptors in rat neural stem cells. Neurosci Lett. 2003;344(3):165–168. doi: 10.1016/s0304-3940(03)00407-5. [DOI] [PubMed] [Google Scholar]
- McCulloch CA, Downey GP, El-Gabalawy H. Signalling platforms that modulate the inflammatory response: New targets for drug development. Nat Rev Drug Discov. 2006;5(10):864–876. doi: 10.1038/nrd2109. [DOI] [PubMed] [Google Scholar]
- McDole B, Isgor C, Pare C, Guthrie K. BDNF over-expression increases olfactory bulb granule cell dendritic spine density in vivo. Neuroscience. 2015;304:146–160. doi: 10.1016/j.neuroscience.2015.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Fuentealba LC, Sanders TA, Magno L, Kessaris N, Alvarez-Buylla A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat Neurosci. 2014;17(2):207–214. doi: 10.1038/nn.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26(18):4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morrens J, Van Den Broeck W, Kempermann G. Glial cells in adult neurogenesis. Glia. 2012;60(2):159–174. doi: 10.1002/glia.21247. [DOI] [PubMed] [Google Scholar]
- Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener. 2011;6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Lee SW, Gage FH. Signaling in adult neurogenesis. Curr Opin Neurobiol. 2010;20(4):416–423. doi: 10.1016/j.conb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller FJ, McKercher SR, Imitola J, Loring JF, Yip S, Khoury SJ, Snyder EY. At the interface of the immune system and the nervous system: How neuroinflammation modulates the fate of neural progenitors in vivo. Ernst Schering Res Found Workshop. 2005;53:83–114. doi: 10.1007/3-540-27626-2_6. [DOI] [PubMed] [Google Scholar]
- Muraki K, Tanigaki K. Neuronal migration abnormalities and its possible implications for schizophrenia. Front Neurosci. 2015;9:74. doi: 10.3389/fnins.2015.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musaelyan K, Egeland M, Fernandes C, Pariante CM, Zunszain PA, Thuret S. Modulation of adult hippocampal neurogenesis by early-life environmental challenges triggering immune activation. Neural Plast. 2014;2014:194396. doi: 10.1155/2014/194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MP, Feron F, Mackay-Sim A. Growth factor regulation of neurogenesis in adult olfactory epithelium. Neuroscience. 2000;99(2):343–350. doi: 10.1016/s0306-4522(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8(5):642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Nho K, Kim S, Risacher SL, Shen L, Corneveaux JJ, Swaminathan S, Lin H, Ramanan VK, Liu Y, Foroud TM, Inlow MH, Siniard AL, Reiman RA, Aisen PS, Petersen RC, Green RC, Jack CR, Jr, Weiner MW, Baldwin CT, Lunetta KL, Farrer LA, Study M, Furney SJ, Lovestone S, Simmons A, Mecocci P, Vellas B, Tsolaki M, Kloszewska I, Soininen H, AddNeuroMed C, McDonald BC, Farlow MR, Ghetti B, Indiana M, Aging S, Huentelman MJ, Saykin AJ I Alzheimer’s Disease Neuroimaging. Protective variant for hippocampal atrophy identified by whole exome sequencing. Ann Neurol. 2015;77(3):547–552. doi: 10.1002/ana.24349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA. Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci USA. 2009;106(21):8754–8759. doi: 10.1073/pnas.0803955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: Avoiding a trade-off. Hippocampus. 1994;4(6):661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Bekinschtein P, Kent BA, Saksida LM, Bussey TJ. Adult hippocampal neurogenesis and its role in cognition. Wiley Interdiscip Rev Cogn Sci. 2014;5(5):573–587. doi: 10.1002/wcs.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26(8):2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6(6):462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Patten BA, Peyrin JM, Weinmaster G, Corfas G. Sequential signaling through Notch1 and erbB receptors mediates radial glia differentiation. J Neurosci. 2003;23(14):6132–6140. doi: 10.1523/JNEUROSCI.23-14-06132.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippsen P, Kleine K, Pohlmann R, Dusterhoft A, Hamberg K, Hegemann JH, Obermaier B, Urrestarazu LA, Aert R, Albermann K, Altmann R, Andre B, Baladron V, Ballesta JP, Becam AM, Beinhauer J, Boskovic J, Buitrago MJ, Bussereau F, Coster F, Crouzet M, D’Angelo M, Dal Pero F, De Antoni A, Del Rey F, Doignon F, Domdey H, Dubois E, Fiedler T, Fleig U, Floeth M, Fritz C, Gaillardin C, Garcia-Cantalejo JM, Glansdorff NN, Goffeau A, Gueldener U, Herbert C, Heumann K, Heuss-Neitzel D, Hilbert H, Hinni K, Iraqui Houssaini I, Jacquet M, Jimenez A, Jonniaux JL, Karpfinger L, Lanfranchi G, Lepingle A, Levesque H, Lyck R, Maftahi M, Mallet L, Maurer KC, Messenguy F, Mewes HW, Mosti D, Nasr F, Nicaud JM, Niedenthal RK, Pandolfo D, Pierard A, Piravandi E, Planta RJ, Pohl TM, Purnelle B, Rebischung C, Remacha M, Revuelta JL, Rinke M, Saiz JE, Sartorello F, Scherens B, Sen-Gupta M, Soler-Mira A, Urbanus JH, Valle G, Van Dyck L, Verhasselt P, Vierendeels F, Vissers S, Voet M, Volckaert G, Wach A, Wambutt R, Wedler H, Zollner A, Hani J. The nucleotide sequence of Saccharomyces cerevisiae chromosome XIV and its evolutionary implications. Nature. 1997;387(6632 Suppl):93–98. [PubMed] [Google Scholar]
- Phillips W, Morton AJ, Barker RA. Abnormalities of neurogenesis in the R6/2 mouse model of Huntington’s disease are attributable to the in vivo microenvironment. J Neurosci. 2005;25(50):11564–11576. doi: 10.1523/JNEUROSCI.3796-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu IR, Nicaise C, Liu S, Bisch G, Knippenberg S, Daubie V, Bohl D, Pochet R. Neural progenitors derived from human induced pluripotent stem cells survive and differentiate upon transplantation into a rat model of amyotrophic lateral sclerosis. Stem Cells Transl Med. 2013;2(3):167–174. doi: 10.5966/sctm.2012-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N. The incidence and prevalence of Huntington’s disease: A systematic review and meta-analysis. Mov Disord. 2012;27(9):1083–1091. doi: 10.1002/mds.25075. [DOI] [PubMed] [Google Scholar]
- Qu Q, Sun G, Li W, Yang S, Ye P, Zhao C, Yu RT, Gage FH, Evans RM, Shi Y. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol. 2010;12(1):31–40. 31–39. doi: 10.1038/ncb2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan VK, Kim S, Holohan K, Shen L, Nho K, Risacher SL, Foroud TM, Mukherjee S, Crane PK, Aisen PS, Petersen RC, Weiner MW, Saykin AJ I Alzheimer’s Disease Neuroimaging. Genome-wide pathway analysis of memory impairment in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort implicates gene candidates, canonical pathways, and networks. Brain Imaging Behav. 2012;6(4):634–648. doi: 10.1007/s11682-012-9196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan VK, Risacher SL, Nho K, Kim S, Swaminathan S, Shen L, Foroud TM, Hakonarson H, Huentelman MJ, Aisen PS, Petersen RC, Green RC, Jack CR, Koeppe RA, Jagust WJ, Weiner MW, Saykin AJ I Alzheimer’s Disease Neuroimaging. APOE and BCHE as modulators of cerebral amyloid deposition: A florbetapir PET genome-wide association study. Mol Psychiatry. 2014;19(3):351–357. doi: 10.1038/mp.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11(5):514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, Kerstetter KA, Neve RL, Haggarty SJ, McKinsey TA, Bassel-Duby R, Olson EN, Nestler EJ. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56(3):517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Richetin K, Leclerc C, Toni N, Gallopin T, Pech S, Roybon L, Rampon C. Genetic manipulation of adult-born hippocampal neurons rescues memory in a mouse model of Alzheimer’s disease. Brain. 2015;138(Pt 2):440–455. doi: 10.1093/brain/awu354. [DOI] [PubMed] [Google Scholar]
- Rieck M, Schumacher-Schuh AF, Callegari-Jacques SM, Altmann V, Schneider Medeiros M, Rieder CR, Hutz MH. Is there a role for ADORA2A polymorphisms in levodopa-induced dyskinesia in Parkinson’s disease patients? Pharmacogenomics. 2015;16(6):573–582. doi: 10.2217/pgs.15.23. [DOI] [PubMed] [Google Scholar]
- Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19(1):39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- Rook GA. Regulation of the immune system by biodiversity from the natural environment: An ecosystem service essential to health. Proc Natl Acad Sci USA. 2013;110(46):18360–18367. doi: 10.1073/pnas.1313731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SM, O’Keeffe GW, O’Connor C, Keeshan K, Nolan YM. Negative regulation of TLX by IL-1beta correlates with an inhibition of adult hippocampal neural precursor cell proliferation. Brain Behav Immun. 2013;33:7–13. doi: 10.1016/j.bbi.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Hippocampal neurogenesis and depression. Novartis Found Symp. 2008;289:152–160. doi: 10.1002/9780470751251.ch12. discussion 160–154, 193–155. [DOI] [PubMed] [Google Scholar]
- Sawada M, Itoh Y, Suzumura A, Marunouchi T. Expression of cytokine receptors in cultured neuronal and glial cells. Neurosci Lett. 1993;160(2):131–134. doi: 10.1016/0304-3940(93)90396-3. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103(46):17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14(3):237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NO, Koeder D, Messing M, Mueller FJ, Aboody KS, Kim SU, Black PM, Carroll RS, Westphal M, Lamszus K. Vascular endothelial growth factor-stimulated cerebral microvascular endothelial cells mediate the recruitment of neural stem cells to the neurovascular niche. Brain Res. 2009;1268:24–37. doi: 10.1016/j.brainres.2009.02.065. [DOI] [PubMed] [Google Scholar]
- Schmidt NO, Przylecki W, Yang W, Ziu M, Teng Y, Kim SU, Black PM, Aboody KS, Carroll RS. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;7(6):623–629. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Papassotiropoulos A, Sotgiu S, Kolsch H, Arru G, Fois ML, Haase CG, Schmitz S, Konig N, Harzheim M, Heun R, Klockgether T. Investigation of a genetic variation of a variable number tandem repeat polymorphism of interleukin-6 gene in patients with multiple sclerosis. J Neurol. 2003;250(5):607–611. doi: 10.1007/s00415-003-1051-y. [DOI] [PubMed] [Google Scholar]
- Schouten M, Buijink MR, Lucassen PJ, Fitzsimons CP. New neurons in aging brains: Molecular control by small non-coding RNAs. Front Neurosci. 2012;6:25. doi: 10.3389/fnins.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120(2):131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478(4):359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Sharma RP. Schizophrenia, epigenetics and ligand-activated nuclear receptors: A framework for chromatin therapeutics. Schizophr Res. 2005;72(2–3):79–90. doi: 10.1016/j.schres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Y, Li R, Shiosaki K. Inhibition of p75 tumor necrosis factor receptor by antisense oligonucleotides increases hypoxic injury and beta-amyloid toxicity in human neuronal cell line. J Biol Chem. 1997;272(6):3550–3553. [PubMed] [Google Scholar]
- Shetty GA, Hattiangady B, Shetty AK. Neural stem cell- and neurogenesis-related gene expression profiles in the young and aged dentate gyrus. Age (Dordr) 2013;35(6):2165–2176. doi: 10.1007/s11357-012-9507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Kagawa T, Inoue T, Nonaka A, Takada S, Aburatani H, Taga T. Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-J kappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol Cell Biol. 2008;28(24):7427–7441. doi: 10.1128/MCB.01962-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AM, de Maturana RL, Ricobaraza A, Escribano L, Schiapparelli L, Cuadrado-Tejedor M, Perez-Mediavilla A, Avila J, Del Rio J, Frechilla D. Early changes in hippocampal Eph receptors precede the onset of memory decline in mouse models of Alzheimer’s disease. J Alzheimers Dis. 2009;17(4):773–786. doi: 10.3233/JAD-2009-1096. [DOI] [PubMed] [Google Scholar]
- Singh RP, Shiue K, Schomberg D, Zhou FC. Cellular epigenetic modifications of neural stem cell differentiation. Cell Transplant. 2009;18(10):1197–1211. doi: 10.3727/096368909X12483162197204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits HA, Rijsmus A, van Loon JH, Wat JW, Verhoef J, Boven LA, Nottet HS. Amyloid-beta-induced chemokine production in primary human macrophages and astrocytes. J Neuroimmunol. 2002;127(1–2):160–168. doi: 10.1016/s0165-5728(02)00112-1. [DOI] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, Parent A, Saghatelyan A. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29(13):4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130(4):843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Sopova K, Gatsiou K, Stellos K, Laske C. Dysregulation of neurotrophic and haematopoietic growth factors in Alzheimer’s disease: From pathophysiology to novel treatment strategies. Curr Alzheimer Res. 2014;11(1):27–39. doi: 10.2174/1567205010666131120100743. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stein JL, Hua X, Morra JH, Lee S, Hibar DP, Ho AJ, Leow AD, Toga AW, Sul JH, Kang HM, Eskin E, Saykin AJ, Shen L, Foroud T, Pankratz N, Huentelman MJ, Craig DW, Gerber JD, Allen AN, Corneveaux JJ, Stephan DA, Webster J, De Chairo BM, Potkin SG, Jack CR, Jr, Weiner MW, Thompson PM I Alzheimer’s Disease Neuroimaging. Genome-wide analysis reveals novel genes influencing temporal lobe structure with relevance to neurodegeneration in Alzheimer’s disease. Neuroimage. 2010;51(2):542–554. doi: 10.1016/j.neuroimage.2010.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopa EG, Gonzalez AM, Chorsky R, Corona RJ, Alvarez J, Bird ED, Baird A. Basic fibroblast growth factor in Alzheimer’s disease. Biochem Biophys Res Commun. 1990;171(2):690–696. doi: 10.1016/0006-291x(90)91201-3. [DOI] [PubMed] [Google Scholar]
- Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- Sun CN, Chuang HC, Wang JY, Chen SY, Cheng YY, Lee CF, Chern Y. The A2A adenosine receptor rescues neuritogenesis impaired by p53 blockage via KIF2A, a kinesin family member. Dev Neurobiol. 2010;70(8):604–621. doi: 10.1002/dneu.20802. [DOI] [PubMed] [Google Scholar]
- Sun G, Yu RT, Evans RM, Shi Y. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc Natl Acad Sci USA. 2007;104(39):15282–15287. doi: 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest. 2004;113(9):1364–1374. doi: 10.1172/JCI20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S, Huentelman MJ, Corneveaux JJ, Myers AJ, Faber KM, Foroud T, Mayeux R, Shen L, Kim S, Turk M, Hardy J, Reiman EM, Saykin AJ I Alzheimer’s Disease Neuroimaging, NLNFS Group. Analysis of copy number variation in Alzheimer’s disease in a cohort of clinically characterized and neuropathologically verified individuals. PLoS ONE. 2012;7(12):e50640. doi: 10.1371/journal.pone.0050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S, Kim S, Shen L, Risacher SL, Foroud T, Pankratz N, Potkin SG, Huentelman MJ, Craig DW, Weiner MW, Saykin AJA The Alzheimer’s Disease Neuroimaging Initiative. Genomic copy number analysis in Alzheimer’s disease and mild cognitive impairment: An ADNI study. Int J Alzheimers Dis. 2011;2011:729478. doi: 10.4061/2011/729478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2010;68(10):930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Takei Y, Laskey R. Interpreting crosstalk between TNF-alpha and NGF: Potential implications for disease. Trends Mol Med. 2008;14(9):381–388. doi: 10.1016/j.molmed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, Kumar P. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer’s disease and vascular dementia. Neurobiol Aging. 2002;23(2):237–243. doi: 10.1016/s0197-4580(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M, Zlokovic BV, Chopp M. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28(4):764–771. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G, Ekici M, Rossler OG. RE-1 silencing transcription factor (REST): A regulator of neuronal development and neuronal/endocrine function. Cell Tissue Res. 2015;359(1):99–109. doi: 10.1007/s00441-014-1963-0. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, Toro R, Jahanshad N, Schumann G, Franke B, Wright MJ, Martin NG, Agartz I, Alda M, Alhusaini S, Almasy L, Almeida J, Alpert K, Andreasen NC, Andreassen OA, Apostolova LG, Appel K, Armstrong NJ, Aribisala B, Bastin ME, Bauer M, Bearden CE, Bergmann O, Binder EB, Blangero J, Bockholt HJ, Boen E, Bois C, Boomsma DI, Booth T, Bowman IJ, Bralten J, Brouwer RM, Brunner HG, Brohawn DG, Buckner RL, Buitelaar J, Bulayeva K, Bustillo JR, Calhoun VD, Cannon DM, Cantor RM, Carless MA, Caseras X, Cavalleri GL, Chakravarty MM, Chang KD, Ching CR, Christoforou A, Cichon S, Clark VP, Conrod P, Coppola G, Crespo-Facorro B, Curran JE, Czisch M, Deary IJ, de Geus EJ, den Braber A, Delvecchio G, Depondt C, de Haan L, de Zubicaray GI, Dima D, Dimitrova R, Djurovic S, Dong H, Donohoe G, Duggirala R, Dyer TD, Ehrlich S, Ekman CJ, Elvsashagen T, Emsell L, Erk S, Espeseth T, Fagerness J, Fears S, Fedko I, Fernandez G, Fisher SE, Foroud T, Fox PT, Francks C, Frangou S, Frey EM, Frodl T, Frouin V, Garavan H, Giddaluru S, Glahn DC, Godlewska B, Goldstein RZ, Gollub RL, Grabe HJ, Grimm O, Gruber O, Guadalupe T, Gur RE, Gur RC, Goring HH, Hagenaars S, Hajek T, Hall GB, Hall J, Hardy J, Hartman CA, Hass J, Hatton SN, Haukvik UK, Hegenscheid K, Heinz A, Hickie IB, Ho BC, Hoehn D, Hoekstra PJ, Hollinshead M, Holmes AJ, Homuth G, Hoogman M, Hong LE, Hosten N, Hottenga JJ, Hulshoff Pol HE, Hwang KS, Jack CR, Jenkinson M, Johnston C, Jonsson EG, Kahn RS, Kasperaviciute D, Kelly S, Kim S, Kochunov P, Koenders L, Kramer B, Kwok JB, Lagopoulos J, Laje G, Landen M, Landman BA, Lauriello J, Lawrie SM, Lee PH, Le Hellard S, Lemaitre H, Leonardo CD, Li CS, Liberg B, Liewald DC, Liu X, Lopez LM, Loth E, Lourdusamy A, Luciano M, Macciardi F, Machielsen MW, Macqueen GM, Malt UF, Mandl R, Manoach DS, Martinot JL, Matarin M, Mather KA, Mattheisen M, Mattingsdal M, Meyer-Lindenberg A, McDonald C, McIntosh AM, McMahon FJ, McMahon KL, Meisenzahl E, Melle I, Milaneschi Y, Mohnke S, Montgomery GW, Morris DW, Moses EK, Mueller BA, Munoz Maniega S, Muhleisen TW, Muller-Myhsok B, Mwangi B, Nauck M, Nho K, Nichols TE, Nilsson LG, Nugent AC, Nyberg L, Olvera RL, Oosterlaan J, Ophoff RA, Pandolfo M, Papalampropoulou-Tsiridou M, Papmeyer M, Paus T, Pausova Z, Pearlson GD, Penninx BW, Peterson CP, Pfennig A, Phillips M, Pike GB, Poline JB, Potkin SG, Putz B, Ramasamy A, Rasmussen J, Rietschel M, Rijpkema M, Risacher SL, Roffman JL, Roiz-Santianez R, Romanczuk-Seiferth N, Rose EJ, Royle NA, Rujescu D, Ryten M, Sachdev PS, Salami A, Satterthwaite TD, Savitz J, Saykin AJ, Scanlon C, Schmaal L, Schnack HG, Schork AJ, Schulz SC, Schur R, Seidman L, Shen L, Shoemaker JM, Simmons A, Sisodiya SM, Smith C, Smoller JW, Soares JC, Sponheim SR, Sprooten E, Starr JM, Steen VM, Strakowski S, Strike L, Sussmann J, Samann PG, Teumer A, Toga AW, Tordesillas-Gutierrez D, Trabzuni D, Trost S, Turner J, Van den Heuvel M, van der Wee NJ, van Eijk K, van Erp TG, van Haren NE, van ’t Ent D, van Tol MJ, Valdes Hernandez MC, Veltman DJ, Versace A, Volzke H, Walker R, Walter H, Wang L, Wardlaw JM, Weale ME, Weiner MW, Wen W, Westlye LT, Whalley HC, Whelan CD, White T, Winkler AM, Wittfeld K, Woldehawariat G, Wolf C, Zilles D, Zwiers MP, Thalamuthu A, Schofield PR, Freimer NB, Lawrence NS, Drevets W ECICSYSG Alzheimer’s Disease Neuroimaging Initiative. The ENIGMA Consortium: Large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8(2):153–182. doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo EM, Colombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol. 2008;86(3):281–296. doi: 10.1016/j.pneurobio.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Charytoniuk D, Watroba L, Faure H, Sales N, Ruat M. Discrete localizations of hedgehog signalling components in the developing and adult rat nervous system. Eur J Neurosci. 1999;11(9):3199–3214. doi: 10.1046/j.1460-9568.1999.00777.x. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Charytoniuk DA, Faure H, Ruat M. Regional distribution of sonic hedgehog, patched, and smoothened mRNA in the adult rat brain. J Neurochem. 1998;70(3):1327–1330. doi: 10.1046/j.1471-4159.1998.70031327.x. [DOI] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500(6):1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura K, Abematsu M, Kohyama J, Namihira M, Nakashima K. Neuronal differentiation of neural precursor cells is promoted by the methyl-CpG-binding protein Me CP2. Exp Neurol. 2009;219(1):104–111. doi: 10.1016/j.expneurol.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Vemuganti R, Bowen KK, Sailor KA, Dempsey RJ. EGF and FGF-2 infusion increases post-ischemic neural progenitor cell proliferation in the adult rat brain. Neurosurgery. 2005;57(6):1254–1263. doi: 10.1227/01.neu.0000186040.96929.8a. discussion 1254–1263. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Yang JH, Barba D, HSU, Bakay RA, Pay MM, Masliah E, Conner JM, Kobalka P, Roy S, Nagahara AH. Nerve growth factor gene therapy: Activation of neuronal responses in Alzheimer disease. JAMA Neurol. 2015;72(10):1139–1147. doi: 10.1001/jamaneurol.2015.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31(4):349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci. 2002;22(2):486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M, Arnold SE, Mann DM, Pickering-Brown SM, Seelaar H, Heutink P, van Swieten JC, Murrell JR, Ghetti B, Spina S, Grafman J, Hodges J, Spillantini MG, Gilman S, Lieberman AP, Kaye JA, Woltjer RL, Bigio EH, Mesulam M, Al-Sarraj S, Troakes C, Rosenberg RN, White CL, Ferrer I, Llado A, Neumann M, Kretzschmar HA, Hulette CM, Welsh-Bohmer KA, Miller BL, Alzualde A, Lopez de Munain A, McKee AC, Gearing M, Levey AI, Lah JJ, Hardy J, Rohrer JD, Lashley T, Mackenzie IR, Feldman HH, Hamilton RL, Dekosky ST, van der Zee J, Kumar-Singh S, Van Broeckhoven C, Mayeux R, Vonsattel JP, Troncoso JC, Kril JJ, Kwok JB, Halliday GM, Bird TD, Ince PG, Shaw PJ, Cairns NJ, Morris JC, McLean CA, De Carli C, Ellis WG, Freeman SH, Frosch MP, Growdon JH, Perl DP, Sano M, Bennett DA, Schneider JA, Beach TG, Reiman EM, Woodruff BK, Cummings J, Vinters HV, Miller CA, Chui HC, Alafuzoff I, Hartikainen P, Seilhean D, Galasko D, Masliah E, Cotman CW, Tunon MT, Martinez MC, Munoz DG, Carroll SL, Marson D, Riederer PF, Bogdanovic N, Schellenberg GD, Hakonarson H, Trojanowski JQ, Lee VM. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42(3):234–239. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berge SA, van Strien ME, Korecka JA, Dijkstra AA, Sluijs JA, Kooijman L, Eggers R, De Filippis L, Vescovi AL, Verhaagen J, van de Berg WD, Hol EM. The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain. 2011;134(Pt 11):3249–3263. doi: 10.1093/brain/awr256. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar AH, Gussekloo J, de Craen AJ, Frolich M, Stek ML, van der Mast RC, Westendorp RG. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42(7):693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain Behav Immun. 2006;20(5):449–455. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11(5):951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- Volvert ML, Rogister F, Moonen G, Malgrange B, Nguyen L. Micro-RNAs tune cerebral cortical neurogenesis. Cell Death Differ. 2012;19(10):1573–1581. doi: 10.1038/cdd.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Burn DJ. Parkinson’s disease: The quintessential neuropsychiatric disorder. Mov Disord. 2011;26(6):1022–1031. doi: 10.1002/mds.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese S, Karus M, Faissner A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharmacol. 2012;3:120. doi: 10.3389/fphar.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, Bilbo SD. Chemokines and the hippocampus: A new perspective on hippocampal plasticity and vulnerability. Brain Behav Immun. 2013;30:186–194. doi: 10.1016/j.bbi.2013.01.077. [DOI] [PubMed] [Google Scholar]
- Winner B, Couillard-Despres S, Geyer M, Aigner R, Bogdahn U, Aigner L, Kuhn HG, Winkler J. Dopaminergic lesion enhances growth factor-induced striatal neuroblast migration. J Neuropathol Exp Neurol. 2008;67(2):105–116. doi: 10.1097/nen.0b013e3181630cff. [DOI] [PubMed] [Google Scholar]
- Winner B, Winkler J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2015;7(4):a021287. doi: 10.1101/cshperspect.a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirenfeldt M, Dalmau I, Finsen B. Estimation of absolute microglial cell numbers in mouse fascia dentata using unbiased and efficient stereological cell counting principles. Glia. 2003;44(2):129–139. doi: 10.1002/glia.10277. [DOI] [PubMed] [Google Scholar]
- Wittko IM, Schanzer A, Kuzmichev A, Schneider FT, Shibuya M, Raab S, Plate KH. VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo. J Neurosci. 2009;29(27):8704–8714. doi: 10.1523/JNEUROSCI.5527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HN, Park JS, Gwon AR, Arumugam TV, Jo DG. Alzheimer’s disease and notch signaling. Biochem Biophys Res Commun. 2009;390(4):1093–1097. doi: 10.1016/j.bbrc.2009.10.093. [DOI] [PubMed] [Google Scholar]
- Woodbury ME, Ikezu T. Fibroblast growth factor-2 signaling in neurogenesis and neurodegeneration. J Neuroimmune Pharmacol. 2014;9(2):92–101. doi: 10.1007/s11481-013-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27(6):1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: Therapeutic targets. Neurotherapeutics. 2010;7(4):378–391. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Yu DX, Marchetto MC, Gage FH. How to make a hippocampal dentate gyrus granule neuron. Development. 2014;141(12):2366–2375. doi: 10.1242/dev.096776. [DOI] [PubMed] [Google Scholar]
- Yuan H, Chen R, Wu L, Chen Q, Hu A, Zhang T, Wang Z, Zhu X. The regulatory mechanism of neurogenesis by IGF-1 in adult mice. Mol Neurobiol. 2015;51(2):512–522. doi: 10.1007/s12035-014-8717-6. [DOI] [PubMed] [Google Scholar]
- Zhang F, Kang Z, Li W, Xiao Z, Zhou X. Roles of brain-derived neurotrophic factor/tropomyosin-related kinase B (BDNF/TrkB) signalling in Alzheimer’s disease. J Clin Neurosci. 2012;19(7):946–949. doi: 10.1016/j.jocn.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yan R, Zhang Q, Wang H, Kang X, Li J, Yang S, Zhang J, Liu Z, Yang X. Survivin, a key component of the Wnt/beta-catenin signaling pathway, contributes to traumatic brain injury-induced adult neurogenesis in the mouse dentate gyrus. Int J Mol Med. 2013;32(4):867–875. doi: 10.3892/ijmm.2013.1456. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yang X, Yang S, Zhang J. The Wnt/beta-catenin signaling pathway in the adult neurogenesis. Eur J Neurosci. 2011a;33(1):1–8. doi: 10.1111/j.1460-9568.2010.7483.x. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Liu G, Wu Y, Sha H, Zhang P, Jia J. BDNF promotes EGF-induced proliferation and migration of human fetal neural stem/progenitor cells via the PI3K/Akt pathway. Molecules. 2011b;16(12):10146–10156. doi: 10.3390/molecules161210146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci. 2004;26(2–4):181–196. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]
- Zhu P, Xiong W, Rodgers G, Qwarnstrom EE. Regulation of interleukin 1 signalling through integrin binding and actin reorganization: Disparate effects on NF-kappaB and stress kinase pathways. Biochem J. 1998;330(Pt 2):975–981. doi: 10.1042/bj3300975. [DOI] [PMC free article] [PubMed] [Google Scholar]