Abstract
Arsenic (+3 oxidation state) methyltransferase (As3mt) is the key enzyme in the pathway for methylation of inorganic arsenic (iAs). Altered As3mt expression and AS3MT polymorphism have been linked to changes in iAs metabolism and in susceptibility to iAs toxicity in laboratory models and in humans. As3mt-knockout mice have been used to study the association between iAs metabolism and adverse effects of iAs exposure. However, little is known about systemic changes in metabolism of these mice and how these changes lead to their increased susceptibility to iAs toxicity. Here, we compared plasma and urinary metabolomes of male and female wild-type (WT) and As3mt-KO (KO) C57BL6 mice and examined metabolomic shifts associated with iAs exposure in drinking water. Surprisingly, exposure to 1 ppm As elicited only small changes in the metabolite profiles of either WT or KO mice. In contrast, comparisons of KO mice with WT mice revealed significant differences in plasma and urinary metabolites associated with lipid (phosphatidylcholines, cytidine, acyl-carnitine), amino acid (hippuric acid, acetylglycine, urea), and carbohydrate (L-sorbose, galactonic acid, gluconic acid) metabolism. Notably, most of these differences were sex-specific. Sex-specific differences were also found between WT and KO mice in plasma triglyceride and lipoprotein cholesterol levels. Some of the differentially changed metabolites (phosphatidylcholines, carnosine, and sarcosine) are substrates or products of reactions catalyzed by other methyltransferases. These results suggest that As3mt KO alters major metabolic pathways in a sex-specific manner, independent of iAs treatment, and that As3mt may be involved in other cellular processes beyond iAs methylation.
Keywords: arsenic, metabolomics, As3mt knockout, mice, urine, plasma
Introduction
Chronic exposure to inorganic arsenic (iAs) via drinking water affects millions of people worldwide and is associated with an increased risk of cancer (NRC, 1999) as well as non-cancer diseases such as neurological (Tyler and Allen, 2014) and cardiometabolic disorders, including cardiovascular disease (Tsuji et al., 2014) and diabetes (Maull et al., 2012). In spite of the abundant evidence linking iAs exposure to a variety of adverse health effects, questions remain about the underlying biological mechanisms. Studies using powerful omics technologies, including metabolomics, may help to answer these questions.
Several recent studies have used metabolomics to characterize metabolic profiles associated with iAs exposure. A metabolomic study carried out in the general Chinese population identified several potential biomarkers of iAs exposure in urine (Zhang et al., 2014). Similarly, our recently published metabolomic analyses of urine and plasma from residents of Chihuahua, Mexico found 132 metabolites that were altered in iAs-exposed individuals (Martin et al., 2015). Metabolomic responses to iAs exposure have also been studied in laboratory animals, although most studies have focused on the effect of exposure to high levels of iAs (Garcia-Sevillano et al., 2014a,b; Wang et al., 2014). The only other study that examined effects of low iAs exposure was carried out in rats (Wang et al., 2015). However, rats are not considered a suitable animal model because avid binding of iAs metabolites to rat hemoglobin generates unique kinetics and pattern of iAs metabolism that differ from those in humans and other mammals (Lu et al., 2004). Overall, the effects of chronic iAs exposure on the metabolome of humans and of rodent species commonly used in laboratory studies of iAs toxicity, specifically mice, remain understudied.
Results of both population and laboratory studies suggest that severity of adverse effects of iAs exposure is determined, in part, by efficiency of iAs metabolism (Antonelli et al., 2014; Tseng et al., 2009). In humans and many other species, metabolism of iAs occurs by subsequent enzymatic methylation of iAs to form monomethyl-As (MAs) and dimethyl-As (DMAs), and in some species also trimethyl-As (TMAs) metabolites (Thomas et al., 2007). Arsenic (+3 oxidation state) methyltransferase (AS3MT) is the primary enzyme that catalyzes the methylation of iAs (Thomas et al., 2007). Experimental expression of rat As3mt in human urothelial (UROtsa) cells that do not express the AS3MT gene enables iAs methylation, and silencing of AS3MT in human hepatocellular carcinoma (HepG2) cells reduces the cell's capacity for iAs methylation (Drobna et al., 2005; Drobna et al., 2006). Furthermore, knockout of As3mt in mice results in a significant decrease in the rate of whole-body As clearance and in the accumulation of iAs in tissues (Drobna et al., 2009). For this reason, As3mt-catalyzed methylation of iAs is considered a detoxification process. However, we and others have shown that the methylated metabolites containing trivalent As (AsIII) contribute to the toxicities associated with iAs exposure (McCoy et al., 2015; Rehman et al., 2014; Styblo et al., 2000; Tseng, 2009; Watanabe et al., 2012). Since As3mt is the enzyme responsible for clearance of iAs from the body and for generation of trivalent methylated metabolites, As3mt-knockout (KO) cells or mice are useful models for studying the role of iAs metabolism in adverse effects of iAs exposure. These models can also be used to explore other potential functions of AS3MT. AS3MT orthologs are found in many living organisms, ranging from simple invertebrates to modern-day humans (Thomas et al., 2007). The conservation of AS3MT across species and during evolution suggests that, in addition to detoxifying iAs, this enzyme may act on other substrates (Thomas et al., 2007) and could play a role in metabolic pathways essential for survival (Uthus, 1992). While As3mt-KO mice are viable and do not show signs of dysfunction, a metabolomic analysis may reveal clues about the role of AS3MT beyond iAs methylation.
The present study examined plasma and urine metabolomes in As3mt-KO C57BL6 mice and C57BL6 wild-type (WT) mice exposed to iAs in drinking water and in unexposed control mice. The main goals were: (i) to characterize the metabolic response to iAs treatment, thus providing additional clues about the mechanisms underlying effects of iAs exposure, (ii) to compare the metabolic responses of WT mice that can efficiently methylate iAs with those of As3mt-KO mice that have severely impaired iAs methylation capacity, and (iii) to probe for altered pathways that may indicate novel roles of AS3MT.
Materials and Methods
Animals and treatment
All procedures involving mice were approved by the University of North Carolina (UNC) Institutional Animal Care and Use Committee. Male and female C57BL6 WT mice were obtained from Jackson Laboratories (Bar Harbor, ME) and let to acclimatize at the UNC Animal Facilities for one week. These WT mice were 17 weeks (±3 days) old at the beginning of the study. Male and female As3mt-KO mice on a C57BL6 background (Drobna, et al. 2009) were bred at the UNC Animal Facilities and were 17–20 weeks old at the beginning of the study. All mice were housed under controlled conditions (12-h light/dark cycle at 22+−1°C and 50±10% relative humidity) with ad libitum access to pelleted rodent chow (Prolab Isopro RMH 3000, LabDiet, St. Louis, MO). Mice drank either deionized water or deionized water containing 1 ppm As as sodium arsenite (AsNaO2, ≥99% pure; Sigma-Aldrich, St. Louis, MO, USA) ad libitum for four weeks (N=15–21 per experimental group). Water with iAsIII was freshly prepared every week to minimize oxidation of iAsIII to iAsV. Food and water consumption and body weight were monitored in all treatment groups every week and two weeks, respectively.
Urine and plasma collection
At the end of iAs-treatment, urine was collected from mice housed in metabolic cages (1 mouse per cage) for 24 hours without access to food to avoid food contamination in urine samples. Urine was aliquoted and stored at −80°C until analysis. Blood from non-fasted mice was collected prior to sacrifice via submandibular bleeding using heparinized Caraway micro-capillaries (Fisher Sc, Pittsburgh, PA). Plasma was isolated by centrifugation at 2000g for 10 minutes (at 4°C) and stored at −80°C until further analysis.
Analysis of iAs metabolites in urine
Hydride generation-atomic absorption spectrometry coupled with a cryotrap (HG-CT-AAS) was used to determine the concentration of As species, including total iAs (i.e., iAsIII + iAsV), total MAs (MAsIII + MAsV) and total DMAs (DMAsIII + DMAsV) (Hernandez-Zavala et al., 2008). As previously reported, limits of detection for this method are: 0.05 ng As/mL for MAs or DMAs and 0.1 ng As/mL for iAs (Martin et al., 2015). The concentration of total speciated As in urine was calculated as sum of iAs, MAs, and DMAs. For quality control, a certified standard reference material Arsenic Species in Frozen Human Urine (SRM 2669; National Institute of Standards and Technology) was analyzed with the mouse urine samples. The recoveries of As from this SRM ranged from 87% for DMAs to 91% for iAs,
Metabolomic analysis
Numbers of plasma and urine samples used for the metabolomic analysis ranged from 13 to 21 per experimental group (volumes collected from some mice were not sufficient for the analyses). Samples were prepared as previously described with some modifications (Ni et al., 2014; Qiu et al., 2014; Qiu et al., 2013). Briefly, the samples were extracted with cold organic solvents (for plasma - methanol:chloroform, 3:1; for urine - acetonitrile). The extract was split into two aliquots. One aliquot was used for untargeted metabolomic profiling with an Agilent 7890A gas chromatograph coupled to a Leco Pegasus time of flight mass spectrometer (GC-MS, Leco Corp., St Joseph, MI). Because previous studies in laboratory rodents found major effects of iAs exposure on metabolism of lipids and amino acids (Garcia-Sevillano et al., 2014a,b; Wang et al., 2014), we used the second aliquot for targeted metabolomic analysis of metabolites associated with amino acid and lipid metabolism by an Acquity ultra performance liquid chromatography coupled to a Xevo TQ-S mass spectrometer (LC-MS, Waters Corp., Milford, MA). The raw data files generated from LC-MS and GC-MS were processed, respectively, with TargetLynx Application Manager (Waters Corp., Milford, MA) and ChromaTOF software (Leco Corp., St Joseph, MI), in order to extract peak signal (normalized by dividing metabolite peak area by total chromatogram area), mass spectral data, and retention times for each metabolite. The detected metabolites from both analytical platforms were annotated and combined using an automated mass spectral data processing (AMSDP) software package (Ni et al., 2012).
Metabolomic data analysis
ANOVA and hierarchical clustering analysis were conducted using Partek Genomics SuiteTM Software (St. Louis, MO). Data were analyzed using a three-way multi-variate ANCOVA model including sex, genotype, and iAs treatment as covariates. To identify significantly changed metabolites between groups (i.e., differentially changed metabolites), significance was set with a False Discovery Rate (FDR) corrected q-value of q<0.05. Unsupervised two-way hierarchical clustering was performed on standardized data from LC-MS and GC-MS analysis with unknown metabolites excluded. Metabolites or animals were clustered using a Euclidean distance metric and average linkage. Results were displayed as a heatmap representing fold-difference from the overall mean of each metabolite. To maximize the probability of capturing relevant metabolites for pathway analysis, a less stringent cutoff value of p<0.05 was used to select metabolites for pathway analysis. Pathways with p<0.05 using the online tool, MetaboAnalyst (Xia, 2010), were determined to be significant. The KEGG Pathway database was used to create a pathway map specific to this investigation (Kanehisa, 2000).
Plasma lipid analysis
Plasma triglycerides and cholesterol associated with high density lipoproteins (HDL) fraction and with a combined low density/very low density lipoprotein (LDL/VLDL) fraction were analyzed using commercial enzymatic assays according to manufacturer's instructions (Wako Diagnostics, Mountainview, CA and Abcam, Cambridge, MA, respectively). Data was analyzed with a full factorial three-way ANOVA analysis considering sex, treatment, and genotype with post hoc Student's t tests assessing relevant comparisons using JMP 10.1 (SAS Institute Inc, Cary, NC).
Results
Phenotypic characteristics of WT and As3mt-KO mice
The average concentrations of total speciated As in the urine of WT and As3mt-KO mice treated with 1 ppm As in drinking water were about 10-fold higher than in the urine of the corresponding controls (466 ng/ml vs. 47 ng/ml in WT, 545 ng/ml vs. 57 ng/ml in KO mice) (Supplemental Figure 1). Knockout of As3mt had no statistically significant effects on total urinary As levels within the control groups. However, in iAs-treated animals, KO mice had significantly higher levels of total speciated As levels in urine as compared to iAs-treated WT mice. No significant differences were found between male and female mice in any groups. The majority of total speciated As in the urine of As3mt-KO mice was represented by iAs: on average 58% for controls and 91% for iAs-treated mice. In contrast, iAs accounted for only 8 and 10% of total speciated As in urine of WT controls and iAs-treated mice, respectively. DMAs was the major As metabolite, representing 40% of total urinary As in control KO mice and 89% in control WT mice. In iAs-treated animals, DMA constituted 9% of total urinary As in KO mice and 92% in WT mice. Notably, treatment with 1 ppm As had no significant effects on body weights of either WT or As3mt-KO mice (Suppl. Figure 2). There were no differences in the weights of female KO and WT mice, regardless of treatment. However, male KO mice treated with iAs were somewhat heavier at sacrifice than the iAs-treated, WT males. No significant differences were found in food consumption (Suppl. Figure 3A). Similarly, there were no differences in the amount of water consumed by female WT, female KO mice and male KO mice; however, male WT mice drank more water than mice in any other group (Suppl. Figure 3B). Treatment with iAs had no effect on the amount of water consumed by either WT or KO mice.
Plasma and urine metabolites identified by metabolomics analyses
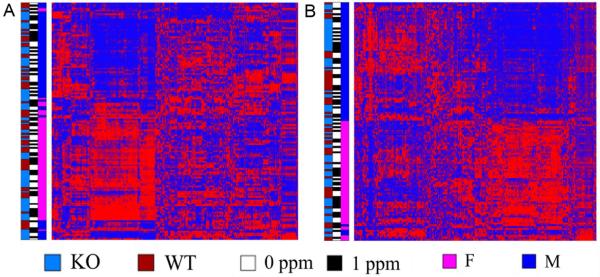
The targeted analysis using LC-MS identified 172 metabolites in plasma and 135 metabolites in urine. Global GC-MS profiling detected an additional 76 and 98 metabolites in plasma and urine, respectively, and 184 plasma and 320 urinary unidentified metabolites which were excluded from subsequent analyses. Thus, a total of 248 plasma and 233 urinary metabolites were identified (Suppl. Table 1) and used in the statistical analyses. Unsupervised two-way clustering analysis showed that animals with similar metabolite profiles clustered primarily by sex and, to a much lesser extent, by genotype or iAs treatment (Figure 1). Further comparisons characterized differences in the plasma and urine metabolomes between experimental groups differentiated by sex, genotype and treatment.
Figure 1.
Urinary and plasma metabolites identified by metabolomic analyses: Heat maps generated by unsupervised two-way clustering analysis show relative levels of metabolites (columns) in plasma (A) and urine (B) of control (0 ppm) and iAs-treated (1 ppm) male (M) and female (F) wild-type (WT) and As3mt-KO (KO) mice (rows). The metabolite levels are mean centered with high relative levels indicated in red and low relative levels indicated in blue.
Changes in plasma and urine metabolomes associated with iAs treatment
Direct comparisons of plasma and urinary metabolite concentrations of control mice and iAs-treated mice revealed only minor shifts associated with iAs treatment, and only in plasma of female As3mt-KO mice (Table 1). All three significantly changed urine metabolites (two acylcarnitine species and a sphingomyelin species) were associated with lipid metabolism. No metabolites were found significantly changed due to iAs treatment in other experimental groups. However, the effect of iAs treatment can be inferred indirectly by comparing the number of KO-associated metabolites in iAs-treated and control mice. For example, 42 metabolites were changed in plasma and urine of control female KO mice when compared with control female WT mice; however, only 15 metabolites were found significantly changed in comparison of KO and WT female mice treated with 1 ppm iAs (Table 2, Suppl. Figure 4). Similar differences in numbers and types of KO-associated metabolites can be seen in male control mice when compared to male iAs-treated mice (Table 2, Suppl. Figure 4). These observations imply that the effect of iAs-treatment on the plasma and urine metabolite profiles may be greater than that indicated by direct comparison.
Table 1.
Plasma and urinary metabolites found significantly changed (q<0.05) in comparisons assessing effect of iAs treatment in male and female wild-type (WT) and As3mt-knockout (KO) mice. Fold change indicates difference from the second group in the comparison.
| Urine | Plasma | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Comparison | Metabolite | q-value | Fold Change | Metabolite | q-value | Fold change |
| KO, female 1 ppm vs 0 ppm | C18:1 | 0.01 | −1.50 | none | ||
| C14 | 0.02 | −1.36 | ||||
| SM (OH) C14:1 | 0.01 | 1.79 | ||||
|
| ||||||
| KO, male 1 ppm vs 0 ppm | none | none | ||||
|
| ||||||
| WT, female 1 ppm vs 0 ppm | none | none | ||||
|
| ||||||
| WT, male 1 ppm vs 0 ppm | none | none | ||||
CX:Z, acyl-carnitine; SM(OH)CX:Z, sphingomyolipid (X indicates number of carbons of the fatty acid tail and Z indicates total number of double bonds).
Table 2.
Plasma and urinary metabolites found significantly changed (q<0.05) in comparisons of As3mt–KO (KO) and wild-type (WT) male and female mice. Fold change indicates difference from the second group in the comparison.
| Urine | Plasma | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Comparison | Metabolite | q-value | Fold Change | Metabolite | q-value | Fold change |
| Control, female KO vs WT (42)* | Urea | 0.03 | −5.97 | PC aa C38:3 | <0.01 | 1.34 |
| 2-Hydroxybutyric acid | <0.01 | −2.48 | PC aa C38:0 | <0.01 | 1.29 | |
| Suberic acid | 0.02 | −1.81 | PC aa C36:3 | 0.04 | 1.17 | |
| Adipic acid | 0.01 | −1.57 | PC aa C42:1 | 0.04 | 1.19 | |
| Pelargonic acid | 0.03 | −1.45 | PC ae C40:2 | 0.02 | 1.20 | |
| Glycolic acid | 0.03 | −1.39 | PC aa C36:1 | 0.02 | 1.20 | |
| 3-Methyl-2-oxovaleric acid | 0.04 | −1.22 | PC ae C42:2 | 0.04 | 1.21 | |
| Creatinine | 0.04 | −1.04 | PC ae C36:5 | 0.01 | 1.22 | |
| PC ae C36:5 | 0.04 | 1.21 | PC aa C42:2 | 0.02 | 1.23 | |
| PC aa C36:0 | 0.01 | 1.25 | PC aa C36:2 | 0.04 | 1.23 | |
| PC ae C36:3 | <0.01 | 1.26 | PC aa C40:1 | 0.02 | 1.24 | |
| PC ae C36:4 | <0.01 | 1.28 | PC aa C40:4 | 0.03 | 1.26 | |
| C18:1-OH | 0.04 | 1.38 | PC aa C40:6 | 0.02 | 1.27 | |
| C18 | 0.02 | 1.45 | PC aa C38:4 | 0.03 | 1.28 | |
| D-Threitol | 0.03 | 1.50 | Valine | 0.04 | 1.28 | |
| Ribonolactone | 0.04 | 1.52 | ||||
| Myoinositol | 0.02 | 1.60 | ||||
| C18:1 | <0.01 | 1.62 | ||||
| L-Arabitol | 0.01 | 1.63 | ||||
| 1,5-Anhydrosorbitol | 0.03 | 1.77 | ||||
| Adenosine | 0.03 | 1.78 | ||||
| Dopamine | 0.04 | 1.80 | ||||
| Cytidine | 0.04 | 2.21 | ||||
| Sarcosine | 0.04 | 2.21 | ||||
| Galactonic acid | <0.01 | 3.36 | ||||
| Gluconic acid | <0.01 | 3.36 | ||||
| L-Sorbose | 0.05 | 4.00 | ||||
|
| ||||||
| Control, male KO vs WT (17)* | Acetylglycine | <0.01 | −16.82 | Carnosine | 0.01 | −1.49 |
| Carnosine | <0.01 | −2.44 | PC aa C26:0 | <0.01 | −1.16 | |
| C14:2-OH | 0.02 | −2.31 | PC aa C32:1 | <0.01 | 1.47 | |
| Aspartate | <0.01 | −1.96 | Oxoglutaric acid | 0.04 | 1.59 | |
| C7-DC | 0.02 | −1.85 | ||||
| Uracil | 0.04 | −1.64 | ||||
| PC aa C36:0 | <0.01 | 1.29 | ||||
| PC aa C40:4 | <0.01 | 1.58 | ||||
| 2-Hydroxy-3-methylbutyric acid | <0.01 | 1.69 | ||||
| C6 (C4:1-DC) | 0.01 | 1.69 | ||||
| PC aa C40:5 | <0.01 | 1.82 | ||||
| Cytidine | <0.01 | 2.74 | ||||
| Hippuric acid | <0.01 | 8.51 | ||||
|
| ||||||
| iAs-treated, female KO vs WT (15)* | Citrulline | 0.01 | −3.58 | PC aa C26:0 | <0.01 | −1.19 |
| PC aa C26:0 | 0.02 | −2.50 | PC aa C38:3 | 0.01 | 1.29 | |
| Aminoadipic acid | 0.01 | −2.41 | PC aa C36:5 | 0.01 | 1.27 | |
| 2-Hydroxybutyric acid | <0.01 | −2.32 | PC aa C36:1 | <0.01 | 1.26 | |
| PC ae C30:2 | <0.01 | −2.30 | ||||
| Glutamate | 0.02 | −2.15 | ||||
| Ketoleucine | <0.01 | −1.64 | ||||
| PC ae C34:1 | <0.01 | −1.39 | ||||
| PC aa C38:6 | <0.01 | −1.33 | ||||
| PC ae C40:6 | <0.01 | −1.28 | ||||
| PC ae C36:4 | <0.01 | 1.24 | ||||
|
| ||||||
| iAs-treated, male KO vs WT (36)* | C14:2-OH | <0.01 | −2.91 | C18:1 | <0.01 | −1.99 |
| 4-Hydroxybenzoic acid | 0.03 | −2.81 | C18:2 | <0.01 | −1.97 | |
| Carnosine | <0.01 | −2.25 | C7-DC | 0.01 | −1.86 | |
| Gluconolactone | 0.01 | −2.01 | C18 | <0.01 | −1.86 | |
| C14:2 | 0.02 | −1.98 | Carnosine | <0.01 | −1.82 | |
| Uridine | 0.02 | −1.90 | C16 | <0.01 | −1.75 | |
| D-Glucuronic acid | 0.04 | −1.31 | Spermidine | <0.01 | −1.70 | |
| 2-Hydroxy-3-methylbutyric acid | <0.01 | 1.45 | C16:2-OH | <0.01 | −1.61 | |
| Adenine | 0.03 | 1.52 | C18:1-OH | 0.03 | −1.59 | |
| Succinic acid | 0.05 | 1.79 | Kynurenine | 0.02 | −1.51 | |
| O-Phosphoethanolamine | 0.01 | 1.86 | C14:2 | 0.01 | −1.50 | |
| Glycerol 3-phosphate | 0.01 | 1.94 | C16:1-OH | 0.03 | −1.45 | |
| PC aa C40:5 | <0.01 | 2.18 | t4-OH-Pro | <0.01 | −1.42 | |
| Cytidine | <0.01 | 2.49 | Phenylalanine | 0.03 | −1.37 | |
| Hippuric acid | 0.01 | 9.42 | Tryptophan | 0.04 | −1.33 | |
| PC ae C34:1 | 0.02 | −1.24 | ||||
| PC aa C26:0 | <0.01 | −1.14 | ||||
| PC aa C36:5 | 0.02 | 1.26 | ||||
| PC aa C40:4 | 0.01 | 1.34 | ||||
| PC aa C40:3 | 0.01 | 1.51 | ||||
| PC aa C32:1 | <0.01 | 1.59 | ||||
Numbers in parentheses indicate total number of changed metabolites in that comparison. CX; acylcarnitine where X indicates number of carbons of the fatty acid tail. SM; sphingomyolipid.
Phosphatidylcholine notation: PC; phosphatidylcholine, aa; two fatty acid ester linkages, ae; one ester and one ether linkage, CY:Z is a description of the fatty acid composition of two PC tails where Y indicates total number of carbons in both fatty acid tails and Z indicates total number of double bonds.
Changes in plasma and urine metabolomes associated with As3mt KO
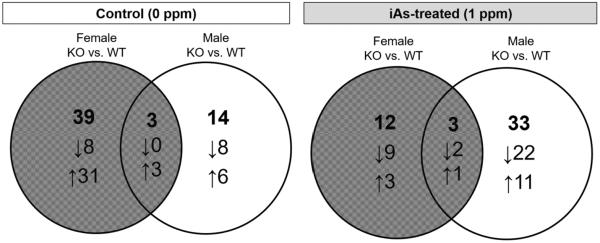
To characterize the effect of As3mt knockout on plasma and urine metabolomes, we identified all plasma and urinary metabolites that were shifted in control and iAs-treated male and female KO mice when compared to their respective wild-type counterparts (Table 2). From these comparisons, we selected the plasma and urinary metabolites that changed in both KO male and female mice, thus isolating metabolites associated with As3mt KO, regardless of sex. Control female KO mice had more than double the number of the KO-associated metabolites found in male KO mice (Figure 2). In the iAs-treated groups, female KO mice showed half as many KO-associated metabolites as compared to males (Figure 2). The KO-associated metabolites common to control male and female KO mice were two phosphatidylcholine (PC) species (PC aa C36:0, PC aa C40:4) and cytidine, all increased in KO mice. Three KO-associated metabolites, all PC species, were common to iAs-treated male and female KO mice (PC aa C26:0, PC aa C36:5, PC ae C34:1).
Figure 2.
Number of urinary and plasma metabolites significantly changed (q<0.05) in As3mt-knockout (KO) male and female mice in comparison with wild-type (WT) counterparts. Venn diagrams show overlapping and sex-specific plasma and urinary metabolites of control (left) and iAs-treated (right) mice. The bolded numbers represent the total number of changed metabolites in each section of the diagram; the arrows indicate metabolites that increased or decreased in response to knockout of As3mt.
KO-associated changes in urine metabolome
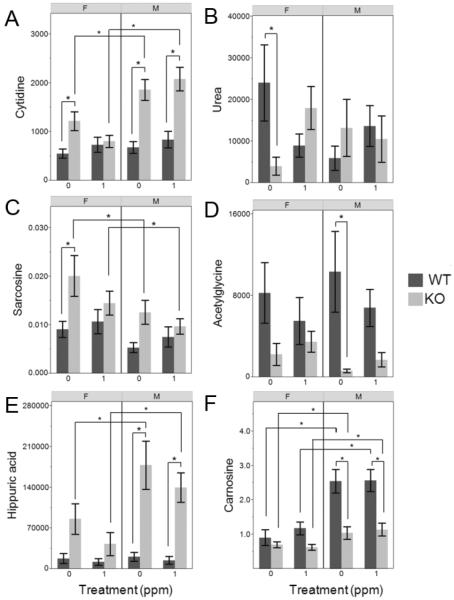
In comparisons of control animals, female KO mice had more differentially changed urinary metabolites than KO males when compared to respective WT mice. A total of 27 metabolites were changed in KO female controls but only seven metabolites had a fold change greater than 2: 2-hydroxybutyric acid, L-sorbose, gluconic acid, galactonic acid, cytidine, urea, and sarcosine (Table 2, Figure 3A–C). Thirteen metabolites were changed in control KO males, including five with fold changes greater than 2: cytidine, acetylglycine, hippuric acid, carnosine, and one acyl-carnitine species (C14:2-OH) (Table 2, Figure 3A, D–F). The highest fold changes were found for acetylglycine and hippuric acid, but acetylglycine decreased by 8-fold while hippuric acid increased by 16-fold. Similar, though not significant, changes in hippuric acid and acetylglycine were found in comparisons of female KO mice vs. WT mice. Cytidine was increased in urine of both male and female KO mice as compared to respective WT mice (Table 2, Figure 3A). In comparisons of iAs-treated mice, male KO mice had more significantly changed metabolites than KO females (15 vs. 11) when compared to the respective WT mice. Of the 11 changed metabolites in female KO mice, six metabolites had fold changes greater than 2: citrulline, aminoadipic acid, 2-hydroxybutyric acid, glutamate, and two PCs (PC aa C26:0 and PC ae C30:2) (Table 2). The urinary concentration of 2-hydroxybutyric acid remained significantly decreased in female KO mice after treatment with iAs (Table 2). In male KO mice, seven metabolites had fold changes equal to or greater than two: 4-hydroxybenzoic acid, carnosine, gluconolactone, cytidine, hippuric acid, and two acylcarnitine species (C14:2-OH and C14:2) (Table 2). Cytidine and hippuric acid were still significantly increased in iAs-treated male KO mice as compared to iAs-treated male WT mice (Figure 3A,E) but acetylglycine levels were no longer significantly different (Figure 3D). Hippuric acid and acetylglycine concentrations were lower in iAs-treated as compared to control mice, but these differences were not statistically significant (Figure 3E,D). The urinary levels of these two metabolites followed similar trends in female mice, but changes due to knockout of As3mt were not statistically significant.
Figure 3.
Relative concentrations of selected metabolites determined by GC-MS (A,B,D,E) or LC-MS (C, F) in urine of wild-type (WT) and As3mt-knockout (KO), male (M) and female (F) mice, control (0 ppm) or iAs-treated (1 ppm). Mean and SE are shown with N=13–21 per group. *Indicates statistically significant differences between experimental groups (q<0.05).
KO-associated changes in plasma metabolome
Fewer metabolites were found to be significantly changed due to KO of As3mt in plasma than in urine. Notably, most of the KO-associated metabolites in plasma were PC species. The greatest number of changed PCs was found in the comparison of control KO and WT females; all these PCs were increased in plasma of KO females, although the fold-change did not exceed 1.34 (Table 2). When male control KO mice were compared with control WT males, two PCs were changed (one increased and one decreased), along with carnosine (decreased) and oxoglutaric acid (increased). In comparisons of iAs-treated KO and WT mice, PCs were changed (generally increased) in plasma of both female and male KO mice (Table 2). All 4 changed metabolites in plasma of females were PCs (3 increased, 1 decreased). The iAs-treated male KO mice had more significantly changed PCs in the plasma than iAs-treated females (6 vs. 4), as well as more significantly changed metabolites overall (21 vs. 4), when compared to the respective WT mice. The KO-associated metabolite changes in iAs-treated male mice included decreased levels of carnosine, spermidine, kynurenine, and various acyl-carnitine species, though fold-changes did not exceed 2. Plasma acyl-carnitines were changed only in comparison of iAs-treated male KO and WT mice.
Metabolic pathways affected by iAs treatment and As3mt knockout
Because very few metabolites were found to be changed by iAs treatment using the q<0.05 filter (Table 1), a less stringent cutoff value of p<0.05 was used to identify significantly changed plasma and urinary metabolites for the analysis of affected pathways. With the less stringent cutoff, greater numbers of metabolites were identified as significantly changed due to iAs treatment (Suppl. Table 2) and due to As3mt knockout (Suppl. Table 3; Suppl. Figure 5). Using Metaboanalyst, many pathways were identified as enriched for these differentially changed metabolites; however, these pathways contained only one to three changed metabolites (hits) and often the same few metabolites were responsible for hits in multiple pathways (Supplemental Table 4). The pathways enriched as a result of iAs-treatment were genotype- and sex-specific. Pathways of amino acid metabolism were enriched only in iAs-treated male and female WT mice when compared to the respective controls (Supplemental Table 4). In KO mice, the pathways enriched by iAs treatment were associated mainly with metabolism of lipids (in males) and carbohydrates (in females). Pathways of amino acid metabolism were also enriched in comparisons of As3mt-KO and WT mice (Supplemental Table 4). Enrichment of pathways associated with lipid (including PC) metabolism was found in control and iAs-treated KO males, but not in KO female mice. Although most changes in individual PC metabolites were detected in the comparison of control female KO mice to control WT females (Table 2), no significantly enriched pathways of PC metabolism, or lipid metabolism in general, were found by the pathway analysis, likely because the program treated the numerous PC species as one metabolite. In iAs-treated mice, As3mt KO was also linked to the enrichment of pathways of carbohydrate metabolism, but the enriched carbohydrate pathways differed between males and females. Additional enriched pathways associated with As3mt KO in either male or female mice included the pathways of glutathione, propanoate, and panthothenate and CoA metabolism (Supplemental Table 4).
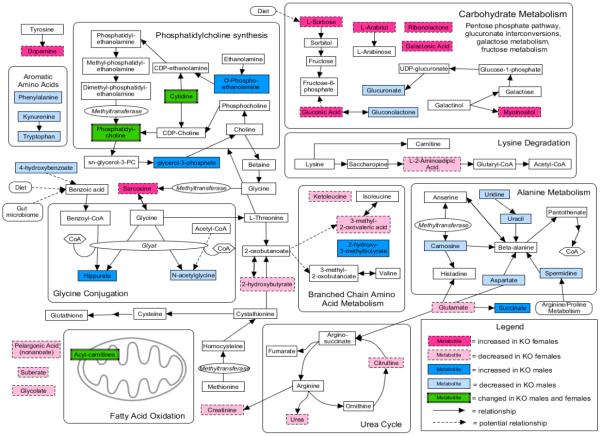
To generate a holistic picture of the pathways affected by As3mt KO without the restrictions inherent to the pathway analysis program, a pathway map of metabolites differentially changed (q<0.05) due to As3mt KO was constructed using KEGG's mus musculus-specific pathway map database (Figure 4). The resulting map includes the enriched pathways described in Table 2, but highlights the sex-specific differences, the direction of change, and depicts the metabolites that are reported to form connections between the affected pathways.
Figure 4.
Diagram of metabolic pathways involving metabolites significantly altered (q<0.05) in plasma or urine of control and/or iAs-treated male and female As3mt-knockout mice when compared to corresponding wild-type males and females. Arrows indicate a relationship based off KEGG pathways specific to mus musculus and may represent more than one enzymatic step.
Effects of iAs-treatment and As3mt KO on plasma lipid concentrations
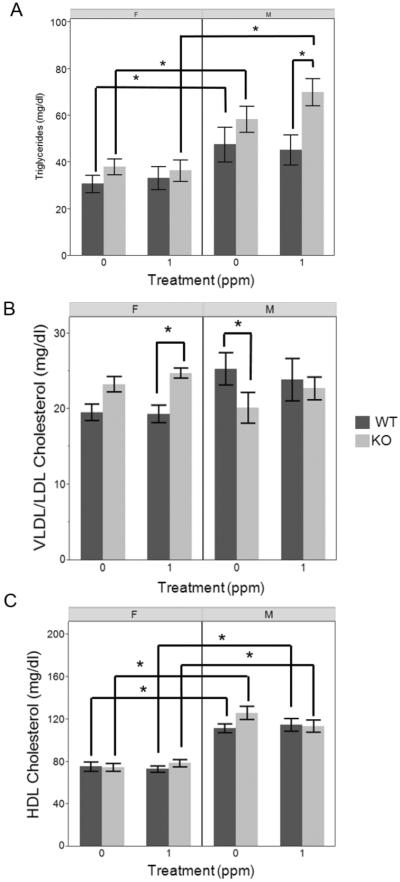
To further probe changes in plasma lipids, particularly the PC metabolites that were observed across all comparisons, we measured concentrations of plasma triglycerides and of lipoprotein cholesterol as indirect measures of plasma lipoprotein concentrations. Average triglyceride concentrations were generally higher in KO mice as compared to WT mice, but this difference was statistically significant only in iAs-treated males (Figure 5A). The plasma of KO male mice contained significantly more triglycerides than plasma of KO females, regardless of iAs treatment, and control WT male mice had higher levels of triglycerides than control WT females. Treatment with iAs had no significant effects of triglyceride levels in either WT or KO mice. Very few statistically significant differences were found in the VLDL/LDL-cholesterol levels (Figure 5B). WT females had lower VLDL/LDL cholesterol than KO females but this difference was only significant in iAs-treated animals. In contrast, WT males had higher VLDL/LDL-cholesterol levels than KO males, but only statistically significant in control males. Two-way ANOVA analysis of VLDL/LDL-cholesterol levels found the sex and genotype interaction to be statistically significant (p=0.005), thus confirming that the effect of genotype on VLDL/LDL cholesterol is different in males than in females. Treatment with iAs had no effect on VLDL/LDL- or HDL-cholesterol in any group. HDL-cholesterol levels were higher in plasma of male as compared to female mice, in both WT and KO animals (Figure 5C).
Figure 5.
Concentrations of lipids in plasma of wild-type (WT) and As3mt-knockout (KO), male (M) and female (F), control (0 ppm) and iAs-treated (1 ppm) mice: A, triglycerides (N=9–19); B, LDL/VLDL-cholesterol (N= 8-15); C, HDL-cholesterol (N= 8-15). Data shown as mean ± SE. * Indicates statistically significant differences between experimental groups (p<0.05)
Discussion
Effects of iAs treatment
We identified very few metabolites in KO and WT mice that were significantly changed due to iAs treatment using a direct ANOVA comparison. This result is consistent with data reported by Wang and associates (2015) who conducted a metabolomic study in male Sprague-Dawley rats exposed to 0.5, 2, or 10 ppm As in drinking water for 3 months. They found no or very few changes in the plasma metabolome at 0.5 and 2 ppm, but observed major changes in lipid, amino acid, and nucleotide metabolism at 10 ppm As. Thus, treatment with 1 ppm As for only 4 weeks may not be sufficient to result in metabolic disturbances in rats or mice. The lack of major metabolic shifts in mice exposed to 1 ppm As also raises questions about differences between rodents and humans in metabolism of iAs. Mice are thought to metabolize iAs more efficiently than humans (Vahter, 1994), and thus may be more resistant to iAs exposure. Our recent study in Chihuahua, Mexico showed that exposures to only ppb levels of As in drinking water (up to ~200 ppb) result in extensive metabolite shifts in human plasma and urine (Martin et al., 2015). This result may indicate higher susceptibility of humans to iAs toxicity and may justify application of higher doses of iAs in laboratory studies using mice to model iAs exposure in humans.
Effects due to As3mt KO: The role of sex
Unsupervised hierarchical clustering analysis showed that mice with similar metabolite profiles were clustered primarily by sex, and less by genotype or treatment (Figure 1). Significantly changed metabolites were different in males and females, further indicating that sex modifies the effect of the KO genotype on the metabolite profiles. Pathway analysis suggests that changes associated with knockout of As3mt were related to lipid, amino acid, and carbohydrate metabolism. Lipid metabolism was changed in both KO males and females but alterations in other pathways appeared to be largely sex specific.
Effects on lipid metabolism
Decreased levels of plasma PCs, increased triglycerides, increased acyl-carnitines and lyso-PCs have been previously reported in mice administered with 3 mg As/kg/day by gavage (Garcia-Sevillano et al., 2014a). Increased levels of acyl-carnitines and lyso-PCs were also found in plasma of rats exposed to 10 ppm As in drinking water (Wang et al., 2015). Changes in the same types of lipid metabolites were also found in the present study, but these changes were primarily associated with As3mt KO and to a much lesser extent with iAs treatment.
Knockout of As3mt was associated with extensive changes in PC species and a related metabolite, cytidine, across all comparisons, with the most prominent changes in the comparison of female KO vs. female WT mice (Table 2). Biosynthesis of PCs occurs via two pathways: the cytidine-diphosphate (CDP) pathway and the pathway catalyzed by phosphatidyethanolamine N-methyltransferase (PEMT). In the CDP-pathway, CDP-choline is combined with diacylglycerol to form PC. The PEMT pathway involves three subsequent methylations of phosphatidylethanolamine using S-adenosylmethionine (AdoMet) as a methyl group donor. The PEMT pathway occurs only in the liver and synthesizes ~30% of hepatic PCs (Sundler and Akesson, 1975). Notably, knockout of PEMT in mice has been shown to alter plasma lipoproteins levels in a sex-specific manner (Noga and Vance, 2003) and PEMT activity appears to be regulated by estrogen (Resseguie et al., 2007). Both CDP- and PEMT-pathways are necessary for normal production of plasma lipoproteins (Yao and Vance, 1988; Noga et al., 2002; Jacobs et al., 2004), specifically VLDL.
Results of the plasma triglyceride and lipoprotein cholesterol analyses performed in this study did not mirror the changes we observed in PC metabolites, although the higher levels of VLDL/LDL cholesterol in iAs-treated female KO vs. WT mice were consistent with the overall higher levels of plasma PCs (Table 2, Figure 5). Triglycerides in plasma of iAs-treated male KO mice were significantly higher than in plasma of WT counterparts; this difference may be associated with higher body weight of the KO mice (Suppl. Figure 2) which appeared obese at the end of this study. Nevertheless, we found differences due to sex in all lipid measures. Future studies should examine mechanisms by which knockout of As3mt affects pathways of PC synthesis and degradation, and lipid metabolism in general, in a sex-specific manner.
Acyl-carnitines were also among lipid metabolites changed in response to As3mt KO, particularly in male mice. Acyl-carnitines facilitate transfer of fatty acids into the mitochondria for fatty acid oxidation (Peng et al., 2013; Reuter and Evans, 2012). The changes in acyl-carnitines observed in KO mice in this study could reflect changes in rate of fatty acid oxidation, and consequently, in energy production.
Effects on amino acid and nitrogen metabolism
A number of metabolites related to amino acid metabolism were changed in As3mt-KO mice, particularly in male mice (Table 2). Two metabolites - hippuric acid and acetylglycine - had the greatest fold changes in the urine of male KO mice as compared to WT mice. However, while the hippuric acid level increased, the level of acetylglycine decreased in As3mt-KO mice (Figure 3D,E). The same trends, while not statistically significant, were found in female mice. Hippuric acid and acetylglycine are products of the conjugation of glycine with benzoyl-CoA and acetyl-CoA, respectively, which is catalyzed by glycine N-acyltransferase (Dempsey et al., 2014; Schachter and Taggart, 1954). Degradation of dietary polyphenols by gut microorganisms is thought to be a major source of benzoic acid and other glycine conjugation substrates (Knights and Miners, 2012; Lees et al., 2013). Elimination of the gut bacteria using antibiotics or germ-free mice was shown to eliminate hippuric acid excretion (Lees et al., 2013; Nicholls et al., 2003; Yap et al., 2008). As such, hippuric acid is considered a urinary microbial-mammalian cometabolite. Excretion of acetylglycine in urine was increased in mice after ingestion of alcohol, presumably due to increased formation of acetic acid (Manna et al., 2011). Colonic bacteria can ferment dietary fiber to form acetate, possibly explaining the association of acetylglycine with dietary fiber intake in humans (Cummings, 1983; Lustgarten et al., 2014). Thus, the changes in urinary levels of hippuric acid and acetylglycine found in this study could be associated with influences on the microbiome, possibly due to As3mt KO or due to different diets used at the UNC animal facility where the KO mice were bred and at the Jackson lab where the WT mice were purchased.
An almost 6-fold decrease in urea levels was found in urine of female KO, but not in urine of male KO mice, in comparisons with corresponding WT mice (Figure 3B). The production of urea in the urea cycle is the major mechanism for disposal of nitrogen during catabolism of amino acids. The decrease in urea production seen here in female KO mice may indicate a potential disruption of this mechanism in a sex-dependent manner.
Effects on energy/carbohydrate metabolism
Growing evidence suggests that iAs is an environmental diabetogen that impairs glucose homeostasis (Maull et al., 2012). Previous metabolomics studies have shown changes in carbohydrate metabolites in humans (Martin et al., 2015) and laboratory animals (Garcia-Sevillano et al., 2014 a,b; Wang et al., 2015) exposed to iAs. However, our metabolomics analysis found only minor effects of iAs treatment on pathways of carbohydrate metabolism, and only in KO female mice (Supplemental Table 4). Most of the changes in carbohydrate metabolites and metabolic pathways were associated with As3mt KO, and these changes were sex-specific (Table 2; Supplemental Table 4).
Effects on metabolites associated with methylation reactions
The inability of As3mt-KO mice to efficiently methylate iAs may have resulted in altered availability of AdoMet for other methylation reactions, thus influencing plasma or urinary levels of substrates and/or products of these reactions. The KO-associated increases in plasma PC levels may reflect higher activity of the PEMT pathway due to increased AdoMet availability. Currently, iAs is the only known substrate of As3mt but our metabolomic analyses in As3mt-KO mice could indicate other, so far unidentified, substrates. Here, we found that knockout of As3mt in mice affected levels of metabolites in two other methylation pathways: the metabolism of carnosine and sarcosine.
The concentration of carnosine was significantly decreased in the plasma and urine of both control and iAs-treated KO male animals as compared to corresponding WT males (Figure 3F). Carnosine is a dipeptide of beta-alanine and L-histidine and is found at high concentrations in skeletal muscle and the brain (Boldyrev et al., 2013). Methylation of carnosine by carnosine N-methyltransferase generates anserine, a storage form of carnosine in tissues (Boldyrev et al., 2013; McManus, 1962; Winnick and Winnick, 1959). A decrease in plasma carnosine in KO males could reflect an increased formation of anserine due to increased AdoMet availability. The sex differences in carnosine levels in plasma of WT mice found in this study (Figure 3F) are consistent with previous studies showing that males have more carnosine in skeletal muscle than females (Baguet et al., 2012; Penafiel et al., 2004). Sarcosine levels were significantly higher in urine of control KO females as compared to control WT females, and also in urine of control KO females as compared to KO males (Figure 3C). Sarcosine is formed by methylation of glycine, catalyzed by glycine N-methyltransferase (GNMT), and is also a byproduct of hydrolysis of dimethylglycine in the betaine homocysteine methyltransferase pathway (Luka et al., 2009). The formation of sarcosine by GNMT is thought to play a role in regulation of cellular methylation capacity (Cook et al., 1989; Horne et al., 1989; Luka et al., 2009; Mudd et al., 1980). Thus, an increase in sarcosine could indicate an increased availability of AdoMet in control KO mice that are not able to efficiently methylate iAs in the diet. The sex-dependence and the effects of iAs treatment on these changes, however, require further investigation.
Conclusions
This study was the first to examine metabolomic profiles associated with knockout of As3mt in mice treated with iAs and in control mice. Surprisingly, we found that treatment with 1 ppm As for four weeks elicited little or no change in the metabolite profile of both WT and As3mt-KO mice, males and females. This finding is consistent with results of previous metabolomics studies in rodents exposed to low levels of iAs, suggesting that higher exposure levels may be required to reproduce in mice the metabolomic changes observed in people exposed to iAs in the ppb range. In contrast, knockout of As3mt was associated with significant changes in both plasma and urine metabolomes and these changes were often sex-specific, implying that sex hormones may play an important role in regulation of the As3mt-catalyzed reactions or in systemic responses to the knockout of As3mt. Many of the altered metabolites were associated with lipid (specifically PC) and lipoprotein metabolism, carbohydrate metabolism, and amino acid metabolism. Some of the altered metabolites were substrates or products of AdoMet-dependent methylation reactions, suggesting that knockout of As3mt altered AdoMet utilization in these reactions or that As3mt may methylate other, As-unrelated substrates. It is unclear, why WT male mice drank more water than mice in any other group and whether this difference could explain some of the sex-or knockout-dependent differences in the urine or plasma metabolomes. However, this data suggest that studies comparing WT and As3mt-KO mice should monitor water consumption and account for possible differences in data evaluation. Future studies, using targeted, tissue-specific metabolomics or metabolite/enzyme-specific analyses are needed to explain the KO-associated changes in metabolite profiles observed in this study, as well as the sex-specific differences.
Supplementary Material
Acknowledgements
The authors thank Dr. David Thomas (US EPA) and Dr. Rosalind Coleman (Department of Nutrition, UNC Chapel Hill) for their helpful suggestions regarding interpretation of the metabolomics data.
Funding This work was supported by grants from the National Institute of Health (DK 034987, DK 056350, and 1R01ES022697), and in part by National Research Service Award from the National Institute of Environmental Health Sciences, NIH (T32 ES007126).
References
- Antonelli R, Shao K, Thomas DJ, Sams R, II, Cowden J. AS3MT, GSTO, and PNP polymorphisms: Impact on arsenic methylation and implications for disease susceptibility. Environ. Res. 2014;132:156–167. doi: 10.1016/j.envres.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Baguet A, Everaert I, Achten E, Thomis M, Derave W. The influence of sex, age and heritability on human skeletal muscle carnosine content. Amino Acids. 2012;43:13–20. doi: 10.1007/s00726-011-1197-3. [DOI] [PubMed] [Google Scholar]
- Boldyrev AA, Aldini G, Derave W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013;93:1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- Cook RJ, Horne DW, Wagner C. Effect of dietary methyl group deficiency on one-carbon metabolism in rats. J. Nutr. 1989;119:612–617. doi: 10.1093/jn/119.4.612. [DOI] [PubMed] [Google Scholar]
- Cummings JH. Fermentation in the human large intestine: evidence and implications for health. Lancet. 1983;1:1206–9. doi: 10.1016/s0140-6736(83)92478-9. [DOI] [PubMed] [Google Scholar]
- Dempsey DR, Bond JD, Carpenter A-M, Ospina SR, Merkler DJ. Expression, Purification, and Characterization of Mouse Glycine N-acyltransferase in Escherichia coli. Protein Expr. Purif. 2014;97:23–28. doi: 10.1016/j.pep.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobná Z, Waters SB, Devesa V, Harmon AW, Thomas DJ, Stýblo M. Metabolism and toxicity of arsenic in human urothelial cells expressing rat arsenic (+3 oxidation state)-methyltransferase. Toxicol. Appl. Pharmacol. 2005;207:147–159. doi: 10.1016/j.taap.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobna Z, Xing W, Thomas DJ, Stýblo M. shRNA silencing of AS3MT expression minimizes arsenic methylation capacity of HepG2 cells. Chem. Res. Tox. 2006;19:894–898. doi: 10.1021/tx060076u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobna Z, Naranmandura H, Kubachka KM, Edwards BC, Herbin-Davis K, Styblo M, Le XC, Creed JT, Maeda N, Hughes MF, et al. Disruption of the arsenic (+3 oxidation state) methyltransferase gene in the mouse alters the phenotype for methylation of arsenic and affects distribution and retention of orally administered arsenate. Chem. Res. Toxicol. 2009;22:1713–1720. doi: 10.1021/tx900179r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sevillano MA, Contreras-Acuña M, García-Barrera T, Navarro F, Gómez-Ariza JL. Metabolomic study in plasma, liver and kidney of mice exposed to inorganic arsenic based on mass spectrometry. Anal. Bioanal. Chem. 2014a;406:1455–1469. doi: 10.1007/s00216-013-7564-z. [DOI] [PubMed] [Google Scholar]
- García-Sevillano MÁ, García-Barrera T, Navarro-Roldán F, Montero-Lobato Z, Gómez-Ariza JL. A combination of metallomics and metabolomics studies to evaluate the effects of metal interactions in mammals. Application to Mus musculus mice under arsenic/cadmium exposure. J. Proteomics. 2014b;104:66–79. doi: 10.1016/j.jprot.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Horne DW, Cook RJ, Wagner C. Effect of dietary methyl group deficiency on folate metabolism in rats. J. Nutr. 1989;119:618–621. doi: 10.1093/jn/119.4.618. [DOI] [PubMed] [Google Scholar]
- Jacobs RL, Devlin C, Tabas I, Vance DE. Targeted deletion of hepatic CTP:phosphocholine cytidylyltransferase a in mice decreases plasma high density and very low density lipoproteins. J. Biol. Chem. 2004;279:47,402–47,410. doi: 10.1074/jbc.M404027200. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. Post-genome Informatics. Oxford University Press; Oxford, UK: 2000. [Google Scholar]
- Knights KM, Miners JO. Amino acid conjugation: A novel route of xenobiotic carboxylic acid metabolism in man. In: Lyubimov AV, editor. Encyclopedia of Drug Metabolism and Interactions. John Wiley and Sons; Somerset, USA: 2012. pp. 595–610. [Google Scholar]
- Lees HJ, Swann JR, Wilson ID, Nicholson JK, Holmes E. Hippurate: The Natural History of a Mammalian–Microbial Cometabolite. J. Proteome Res. 2013;12:1527–1546. doi: 10.1021/pr300900b. [DOI] [PubMed] [Google Scholar]
- Lu M, Wang H, Li X-F, Lu X, Cullen WR, Arnold LL, Cohen SM, Le XC. Evidence of Hemoglobin Binding to Arsenic as a Basis for the Accumulation of Arsenic in Rat Blood. Chem. Res. Toxicol. 2004;17:1733–1742. doi: 10.1021/tx049756s. [DOI] [PubMed] [Google Scholar]
- Luka Z, Mudd SH, Wagner C. Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J. Biol. Chem. 2009;284:22507–22511. doi: 10.1074/jbc.R109.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustgarten MS, Price LL, Chalé A, Fielding RA. Metabolites related to gut bacterial metabolism, peroxisome proliferator-activated receptor-alpha activation, and insulin sensitivity are associated with physical function in functionally-limited older adults. Aging Cell. 2014;13:918–925. doi: 10.1111/acel.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna SK, Patterson AD, Yang Q, Krausz KW, Idle JR, Fornace AJ, Gonzalez FJ. UPLC-MS-based urine metabolomics reveals indole-3-lactic acid and phenyllactic acid as conserved biomarkers for alcohol-induced liver disease in the Ppara-null mouse model. J. Proteome Res. 2011;10:4120–4133. doi: 10.1021/pr200310s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, González-Horta C, Rager J, Bailey KA, Sánchez-Ramírez B, Ballinas-Casarrubias L, Ishida MC, Gutiérrez-Torres DS, Hernández Cerón R, Viniegra Morales D, Baeza Terrazas FA, Saunders RJ, Drobná Z, Mendez MA, Buse JB, Loomis D, Jia W, García-Vargas GG, Del Razo LM, Stýblo M, Fry R. Metabolomic Characteristics of Arsenic-Associated Diabetes in a Prospective Cohort in Chihuahua, Mexico. Tox. Sci. 2015;144:339–346. doi: 10.1093/toxsci/kfu318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D. Evaluation of the Association between Arsenic and Diabetes: A National Toxicology Program Workshop Review. Environ Health Perspect. 2012;120:1658–1670. doi: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy CR, Stadelman BS, Brumaghim JL, Liu J-T, Bain LJ. Arsenic and Its Methylated Metabolites Inhibit the Differentiation of Neural Plate Border Specifier Cells. Chem. Res. Toxicol. 2015;28:1409–21. doi: 10.1021/acs.chemrestox.5b00036. [DOI] [PubMed] [Google Scholar]
- McManus IR. Enzymatic synthesis of anserine in skeletal muscle by N-methylation of carnosine. J. Biol. Chem. 1962;237:1207–1211. [Google Scholar]
- Mudd SH, Ebert MH, Scriver CR. Labile methyl group balances in the human: the role of sarcosine. Metab. Clin. Exp. 1980;29:707–720. doi: 10.1016/0026-0495(80)90192-4. [DOI] [PubMed] [Google Scholar]
- National Research Council . Arsenic in Drinking Water. National Academy Press; Washington, DC: 1999. Health Effects of Arsenic; pp. 83–149. [Google Scholar]
- Ni Y, Qiu Y, Jiang W, Suttlemyre K, Su M, Zhang W, Jia W, Du X. ADAP-GC 2.0: deconvolution of coeluting metabolites from GC/TOF-MS data for metabolomics studies. Anal. Chem. 2012;84:6619–6629. doi: 10.1021/ac300898h. [DOI] [PubMed] [Google Scholar]
- Ni Y, Xie G, Jia W. Metabonomics of human colorectal cancer: new approaches for early diagnosis and biomarker discovery. J. Proteome. Res. 2014;13:3857–3870. doi: 10.1021/pr500443c. [DOI] [PubMed] [Google Scholar]
- Nicholls AW, Mortishire-Smith RJ, Nicholson JK. NMR spectroscopic-based metabonomic studies of urinary metabolite variation in acclimatizing germ-free rats. Chem. Res. Toxicol. 2003;16:1395–404. doi: 10.1021/tx0340293. [DOI] [PubMed] [Google Scholar]
- Noga AA, Vance DE. A gender-specific role for phosphatidylethanolamine N-methyltransferase-derived phosphatidylcholine in the regulation of plasma high density and very low density lipoproteins in mice. J. Biol. Chem. 2003;278:21851–21859. doi: 10.1074/jbc.M301982200. [DOI] [PubMed] [Google Scholar]
- Noga A, Zhao Y, Vance DE. An unexpected requirement for phosphatidylethanolamine N-methyltransferase in the secretion of very low density lipoproteins. J. Biol. Chem. 2002;277:42,358–42,365. doi: 10.1074/jbc.M204542200. [DOI] [PubMed] [Google Scholar]
- Peñafiel R, Ruzafa C, Monserrat F, Cremades A. Gender-related differences in carnosine, anserine and lysine content of murine skeletal muscle. Amino Acids. 2003;26:53–58. doi: 10.1007/s00726-003-0034-8. [DOI] [PubMed] [Google Scholar]
- Peng M, Fang X, Huang Y, Cai Y, Liang C, Lin R, Liu L. Separation and identification of underivatized plasma acylcarnitine isomers using liquid chromatography-tandem mass spectrometry for the differential diagnosis of organic acidemias and fatty acid oxidation defects. J. Chromatogr. A. 2013;1319:97–106. doi: 10.1016/j.chroma.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Cai G, Zhou B, Li D, Zhao A, Xie G, Li H, Cai S, Xie D, Huang C, Ge W, Zhou Z, Xu XL, Jia W, Zheng S, Yen Y, Jia W. A distinct metabolic signature of human colorectal cancer with prognostic potential. Clin. Cancer. Res. 2014;20:2136–2146. doi: 10.1158/1078-0432.CCR-13-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Zhou B, Su M, Baxter S, Zheng X, Zhao X, Yen Y, Jia W. Mass spectrometry-based quantitative metabolomics revealed a distinct lipid profile in breast cancer patients. Int. J. Mol. Sci. 2013;14:8047–8061. doi: 10.3390/ijms14048047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman K, Fu YJ, Zhang YF, Wang QQ, Wu B, Wu Y, Zhou XY, Sun WH, Sun TF, Naranmandura H. Trivalent methylated arsenic metabolites induce apoptosis in human myeloid leukemic HL-60 cells through generation of reactive oxygen species. Metallomics. 2014;6:1502–1512. doi: 10.1039/c4mt00119b. [DOI] [PubMed] [Google Scholar]
- Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012;51:553–572. doi: 10.1007/BF03261931. [DOI] [PubMed] [Google Scholar]
- Resseguie M, Song J, Niculescu MD, da Costa K-A, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007;21:2622–2632. doi: 10.1096/fj.07-8227com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter D, Taggart JV. Glycine N-acylase: purification and properties. J Biol Chem. 1954;208(1):263–75. [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in human cells. Arch. Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Sundler R, Akesson B. Regulation of phospholipid biosynthesis in isolated rat hepatocytes. Effect of different substrates. J. Biol. Chem. 1975;250:3359–3367. [PubMed] [Google Scholar]
- Thomas DJ, Li J, Waters SB, Xing W, Adair BM, Drobna Z, Devesa V, Styblo M. Exp. Biol. Med. (Maywood) 2007;232:3–13. [PMC free article] [PubMed] [Google Scholar]
- Tseng C-H. A review on environmental factors regulating arsenic methylation in humans. Toxicol. Appl. Pharmacol. 2009;235:338–350. doi: 10.1016/j.taap.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Tsuji JS, Perez V, Garry MR, Alexander DD. Association of low-level arsenic exposure in drinking water with cardiovascular disease: A systematic review and risk assessment. Toxicology. 2014;323:78–94. doi: 10.1016/j.tox.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Allan AM. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr. Environ. Health Rep. 2014;1:132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthus EO. Evidence for arsenic essentiality. Environ Geochem Health. 1992;14:55–58. doi: 10.1007/BF01783629. [DOI] [PubMed] [Google Scholar]
- Vahter M. Species Differences in the Metabolism of Arsenic Compounds. Appl. Organomet. Chem. 1994;8:175–182. [Google Scholar]
- Wang X, Mu X, Zhang J, Huang Q, Alamdar A, Tian M, Liu L, Shen H. Serum metabolomics reveals that arsenic exposure disrupted lipid and amino acid metabolism in rats: a step forward in understanding chronic arsenic toxicity. Metallomics. 2015;7:544–552. doi: 10.1039/c5mt00002e. [DOI] [PubMed] [Google Scholar]
- Watanabe C, Inaoka T, Kadono T, Nagano M, Nakamura S, Ushijima K, et al. Males in rural Bangladeshi communities are more susceptible to chronic arsenic poisoning than females: analyses based on urinary arsenic. Environ Health Perspect. 2001;109:1265–1270. doi: 10.1289/ehp.011091265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnick T, Winnick RE. Pathways and the physiological site of anserine formation. Nature. 1959;183:1466–1468. doi: 10.1038/1831466a0. [DOI] [PubMed] [Google Scholar]
- Xia J, Wishart DS. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics. 2010;26:2342–2344. doi: 10.1093/bioinformatics/btq418. [DOI] [PubMed] [Google Scholar]
- Yao Z, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 1988;263:2998–3004. [PubMed] [Google Scholar]
- Yap IKS, Li JV, Saric J, Martin F-P, Davies H, Wang Y, Wilson ID, Nicholson JK, Utzinger J, Marchesi JR, Holmes E. Metabonomic and microbiological analysis of the dynamic effect of vancomycin-induced gut microbiota modification in the mouse. J. Proteome Res. 2008;7:3718–28. doi: 10.1021/pr700864x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shen H, Xu W, Xia Y, Barr DB, Mu X, Wang X, Liu L, Huang Q, Tian M. Urinary Metabolomics Revealed Arsenic Internal Dose-Related Metabolic Alterations: A Proof-of-Concept Study in a Chinese Male Cohort. Environ. Sci. Technol. 2014;48:12265–12274. doi: 10.1021/es503659w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.