Abstract
Despite substantial opportunity and commercial interest in developing drugs that modulate the complement system in a broad range of non-orphan indications, several obstacles remain to be overcome. Among these issues is the biophysical nature of complement proteins, whose circulating levels are typically very high and whose turnover rates are relatively rapid, especially in the setting of chronic inflammatory conditions. This situation necessitates the use of very high levels of therapeutic compounds in order to achieve both multi-pathway and multiple effector mechanism inhibition. In addition, one must avoid infectious complications or the systemic impairment of the other important physiological functions of complement. Herein we focus on the development of a novel therapeutic strategy based on injured tissue-specific targeting of complement inhibitors using the antigen-combining domains of a small subset of natural IgM antibodies, which as endogenous antibodies specifically recognize sites of local damage across a broad range of tissues and locally activate complement C3, resulting in C3 fragment covalent fixation. Because the use of such recombinant tissue-targeting inhibitors precludes the utility of measuring systemic levels of complement biomarkers or function, since a goal of this targeting strategy is to leave those processes intact and unimpeded, we also briefly describe a new method designed to quantitatively measure using imaging modalities the inhibition of generation of fixed C3 fragments at sites of inflammation/injury. In addition to the ability to determine whether complement activation is locally constrained with the use of inhibitors, there is also a broader application of this imaging approach to inflammatory and autoimmune diseases characterized by local complement activation.
Keywords: Therapeutics, Targeting, Natural Antibodies, Cell Injury, Neoepitopes, Imaging
1. Overview – Dysregulation of Local Complement Activation is of Paramount Importance in Human Diseases
The complement system consists of soluble activation pathway proteins as well as membrane-bound receptors and both soluble and membrane-bound regulatory proteins [1]. Importantly, the outcomes of complement system activation depend on the specific context in which the process occurs, and the number, localization and levels of the effector molecules generated. There are also many beneficial effects of complement activation that must be accommodated and allowed to function normally in any attempt to modulate the system. Beneficial effects include recognition and clearance of foreign and non-self-antigens and pathogens [2], enhancement of humoral [3] and cellular [4] immune responses, non-inflammatory clearance of apoptotic bodies containing self-antigens [5], transport of immune complexes for disposal in the reticuloendothelial system [6], tissue regeneration following injury [7, 8], appropriate pruning of neurons during development [9], and shaping the composition of the endogenous natural antibody (NatAb) repertoire [10]. There is also an increasing appreciation that intracellular complement activation may play an important role in modulating T cell responses [11].
A key concept that members of this author group have focused on for nearly two decades is that, despite the near universal therapeutic focus on systemic inhibition of the complement system, the effects of pathogenic dysregulation of complement are almost exclusively manifest locally by tissue-specific injury and impaired function. That is especially evident in diseases such as atypical hemolytic uremic system (aHUS) and age-related macular degeneration (AMD), where germ line mutations of complement factors with potential systemic effects result in very localized tissue injury [12, 13]. Additionally, even in diseases such as systemic lupus erythematosus (SLE) in which complement is characterized as “systemically activated”, the actual damage in individual patients is most often focused on a subset of tissues such as the kidney [14]. Thus, in a disease context when one desires to block complement activation locally, for example to protect the retina, brain or kidney, it is only logical to design a strategy in which one would maintain elsewhere the positive protective functions that are listed above, and focus inhibition to the site where it is needed.
Another important consideration with regard to therapeutic development in the complement system is that it is activated by three interacting recognition mechanisms through the classical, alternative and lectin pathways. Essential issues with regard to the choice of inhibitors for each pathway relative to the disease of interest are addressed elsewhere [15, 16]. Importantly, though, each pathway converges on the centrally important C3 protein when multi-component C3 convertases are formed and C3 is cleaved. In this activation process, a thioester bond in C3 allows the covalent attachment through ester or amide linkages to other molecules of the C3 protein in the C3b form [17]. The activation of C3 is associated with the soluble anaphylatoxin C3a release and is followed by C5 cleavage and activation, the coincident release of C5a [18] and formation of the pore-like membrane attack complex (MAC) [19]. Because the generated C3b forms additional C3 convertase, the fixation of C3b to tissues creates a favorable microenvironment for further complement activation. Subsequent cleavage of C3b generates the iC3b/C3dg/C3d forms and binding of the specific molecules to their cognate C3 fragment receptors [20]. This process effectively “marks” the C3 fragment-bound targets as immunologically different. It is the deposition of tissue-localized C3 fragments and their subsequent processing to fragments with altered neoepitope presentation that underlies the imaging of local C3 activation described below in this review.
2. Rationale, History and Previous Strategies for Development of Local Tissue Targeted Complement Inhibitors
The vast majority of historical and even contemporary approaches to complement inhibition focus on systemic blocking activity of the compound. That could be considered the case even in the context of “local” delivery in an organ such as the eye, where the injurious complement activation is occurring at the posterior pole (RPE, Bruch's membrane, choriocapillaris) and not in the vitreous at the site of injection of an inhibitor such as anti-Factor D monoclonal antibody [21]. The basic rationale behind the development of site-targeted complement inhibitors, however, is the supposition that localized delivery will not only increase inhibitor bioavailability and durability at sites of disease, but will minimize disruption of the normal physiological and protective mechanisms of complement. In addition, although advantages of targeted inhibition are most relevant for the treatment of chronic diseases, avoidance of short-term disruption of host defense mechanisms is desirable even during acute conditions, especially so if the patient is immunocompromised. The choice of which linked inhibitor to use (Crry, DAF, FH, CR1, CD59) is also important and is dependent upon several factors, including the need in the particular indication being studied to block at the point of terminal MAC formation (CD59), or the desire to impair C3 and C5 convertase function in order to obtain either alternative pathway/amplification inhibition modulation alone (FH) or inhibitory effects on all three pathways (Crry, CR1, DAF).
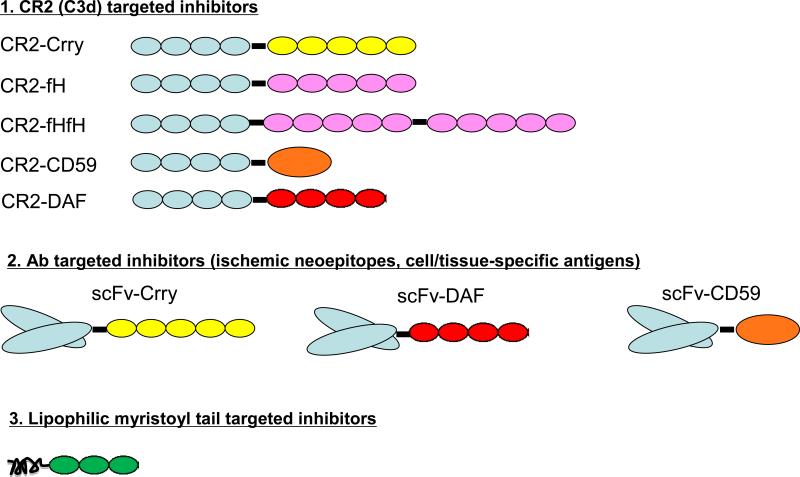
The first publications focusing on targeted complement inhibition to sites of injury appeared in 1999 and described two basic approaches. In one approach, cell specific targeting of CD59 was achieved by linking the inhibitor to an antibody or antibody fragment (Figure 1). The fusion proteins specifically targeted cells expressing cognate antigen and provided targeted, but not untargeted cells, with effective protection from complement-mediated lysis and injury [22]. In another approach, it was shown that the decoration of soluble CR1 (sCR1), an inhibitor of the C3 and C5 convertases derived from all three pathways, with sialyl Lewisx (sLex) moieties significantly enhanced the protective effect of the inhibitor in a rat model of selectin-dependent lung injury [23] and in a mouse model of ischemic stroke [24]. The sLex carbohydrate moiety binds to both P and E selectin, adhesion molecules that are upregulated on activated endothelial cells. In the lung injury model, the enhanced protective effect of sCR1 sLex correlated with increased binding of sCR1 sLex to the lung vasculature when compared to binding of sCR1. Thus, decoration with the sLex moiety represents a strategy to increase the efficacy of complement inhibitors by targeting them to selectin-expressing activated endothelial surfaces. Importantly, however, the efficacy of sCR1 sLex was still dependent on the ability to maintain systemic complement inhibition at the same time.
Figure 1.
Illustration of the three major tissue/cell specific targeting strategies utilized where specific localization of the drug in vivo has been shown experimentally to be necessary and sufficient for its beneficial effect. The first embraces the use of the C3d-binding domain of CR2 and includes the different types of murine inhibitors created and tested. The second illustrates the use of ScFv directed to either ischemia-induced or cell/tissue specific antigens, along with four linked regulatory domains that have been used. The third illustrates the use of a lipophilic myristoyl tail to “paint” membranes with which it comes in contact.
A short form of sCR1, designated APT070, has also been targeted to cell membranes, albeit nonspecifically, by incorporation of a membrane targeting myristoylated peptide to the 3 N-terminal SCRs of CR1. APT70 was shown to be 100-fold more active than its parent protein in in vitro complement inhibition assays, although the untargeted CR1(SCR1-3) was itself much less active than sCR1 [25]. APT070 has demonstrated targeting-specific benefit in a rat model of antigen-induced arthritis [26], intestinal ischemia-reperfusion injury [27] and a model of Miller Fisher syndrome [28]. An application that may be especially well-suited to this strategy of membrane localization and is currently under study in a clinical trial is graft protection after cold storage, as perfusion of donor rat kidneys with APT070 reduced tubular injury and increased graft survival after cold ischemia and transplantation [29].
Another approach to the targeting of complement inhibitors has been developed using single chain antibody (scFv) fragments as delivery vehicles. Human DAF and mouse Crry were shown to target mouse RBC's when linked to a scFv specific for mouse glycophorin A and protect mouse RBCs from complement lysis [30]. Rat Crry and CD59 have also been linked to a scFv specific for an antigen expressed on rat tubular epithelium and shown to be protective in a model of experimental nephrotic syndrome [31]. In this approach, the scFv-inhibitor constructs demonstrated very short circulatory half-lives (around 30 minutes) and thus effectively protected against renal injury without systemically inhibiting complement activation.
A different strategy has been applied to target complement inhibition that involves the use of a pro-drug. In this approach, DAF and CD59 were attached to an antibody Fc region by means of a linker incorporating a cleavage site for matrix metalloproteases and/or aggrecanases [32]. The fusion proteins demonstrated limited functional activity (thus limiting their ability to systemically inhibit complement), but an active form of the inhibitor was released upon exposure to enzymes from cytokine-stimulated chondrocytes and synovial fluid that are present in high concentrations at sites of inflammation. Once released, though, there is no mechanism to retain the inhibitor specifically at that site. In addition, newer modular therapeutics are being developed linking inhibitors to putative tissue- or cell-targeting domains; however, none to our knowledge have yet been demonstrated to exhibit in vivo specific tissue-targeting or durable binding in a comparable manner as the inhibitors discussed above, and thus are not included in this review.
3. Complement Receptor 2 (CR2)-Mediated Targeting of Complement Inhibitors
CR2 in its normal role is a multi-functional receptor expressed on B cells, follicular dendritic cells and a subset of peripheral and thymic T cells (reviewed in [33]). CR2 has four distinct types of ligands, including complement C3 fragments iC3b/C3dg and C3d [34], the gp350/220 viral coat protein of the Epstein-Barr virus [35], the immunoregulatory protein CD23 [36] and interferon-alpha [37] .
CR2-complement inhibitor fusion proteins, specifically CR2-DAF and CR2-CD59, were initially constructed using the human proteins [38]. In these studies, it was shown in vitro that both CR2-DAF and CR2-CD59 bound to C3-opsonized CHO cells and erythrocytes. The in vivo validation of the use of CR2-targeted complement inhibitors in a rodent model required the generation of the mouse CR2-Crry construct, with Crry serving as a murine orthologue of human sCR1. CR2-Crry was first characterized in a mouse model of intestinal ischemia reperfusion injury where its characteristics were compared to the systemically inhibitory counterpart Crry-Ig [39]. In these studies, CR2-Crry was shown to be 20-fold more effective and localize to tissue sites of complement activation, providing protection from both local intestinal and remote lung complement deposition and injury. Importantly, the minimum effective dose of Crry-Ig also markedly increased susceptibility to infection in the cecal ligation sepsis model of polymicrobial infection, whereas even multiple injections of CR2-Crry had no effect on survival [39]. It was also shown that the circulatory half-life of CR2-Crry was only 8.7 hours and the therapeutic dose of CR2-Crry exhibited minimal effect on serum complement activity. The question of tissue retention at a targeted site was addressed in the MRL/lpr spontaneous model of lupus. Following a single 0.25 mg intravenous injection of CR2-Crry, biodistribution studies demonstrated localization to the kidneys with a tissue half-life of ~24 hours [40]. Subsequently, CR2-Crry and a related molecule that inhibits the alternative pathway and amplification loop, designated CR2-FH, have shown significant benefit in many other experimental murine models (reviewed in [41]). With regard to durability of binding, in a murine model of choroidal neovascularization, CR2-FH targeted to the lesions in the eye could be identified 24 hours after injection of a therapeutic dose [42], whereas in ischemic stroke in mouse, CR2-FH targeted to the ischemic brain with a tissue half-life of greater than 48 hours [43]. Because targeted inhibitors are retained in local targeted tissue for a long period of time, a short circulatory half-life is a positive attribute since it will minimize systemic complement inhibition. A human version of CR2-FH has been created [44], tested to be efficacious in mouse models (e.g., [45]), and found to be safe and non-immunogenic during single ascending dose studies in humans.
4. Natural Antibody-Mediated Tissue Injury
The concept of DAMPS (damage associated molecular patterns), or self-antigens that induce acute sterile inflammation, is well established [46]. The general principle is that trauma or stress leads to non-physiologic exposure of nuclear or cytoplasmic antigens in a process that triggers an innate immune response similar to that mediated by microbial infection. Although intracellular cytoplasmic pathways are commonly studied sensors of DAMPS, extracellular recognition by innate proteins also provides an important recognition mechanism [10, 47]. Recently, the unexpected finding that a subset of natural antibodies (NatAbs) represents a novel class of recognition proteins that resemble the innate danger-sensing receptors has been made. These particular NatAbs have evolved to bind highly conserved self-ligands exposed during cell injury and stress responses that accompany autoimmune disease, ischemia-reperfusion (IR), and many other forms of tissue damage [10].
NatAbs are primarily produced by a subset of B lymphocytes termed B-1. In contrast to conventional B-2 B cells, B-1 cells are known to be derived early during development from a distinct self-renewing progenitor [48]. B-1 cells exhibit a limited repertoire as they do not undergo somatic hyper-mutation and have restricted junctional diversity. Accordingly, they are considered as innate lymphocytes, and their antigen-binding repertoire is thought to be shaped through evolution [49].
A unique feature of a subset of self-tissue-directed recognition by NatAbs that have been described and studied by this author group is the focus of reactivity to a single definable epitope, rather than the polyreactivity that is characteristic of pathogen-directed NatAbs. The structures recognized by this unique NatAb subset comprise neoepitopes that are typically exposed on cells following injury during early and late stages of apoptosis and/or necrosis [10], and includes modified annexin-4 (ANX4) and modified phospholipids. This recognition process is essential to the development of experimental complement-dependent intestinal IR injury following ligation of the mesenteric artery [50] and murine laser-induced choroidal neovascularization [51].
One particular pro-inflammatory NatAb has been described through the characterization of a highly informative pathogenic IgM monoclonal antibody (mAb) [50] and is among those being utilized in the development of tissue-targeted complement inhibitors. The mAb, designated B4, recognizes annexin-4 (ANX4) [50]. ANX4 belongs to a family of Ca2+ and phospholipid-binding proteins (reviewed in [52]); annexins including ANX4 have been shown to be associated with the external cell membrane, especially of cells undergoing injury [53].
Recent work by the authors of this review have identified many ANX4-expressing target cells and tissues in both ischemic and non–ischemic disease states against which recognition by anti-ANX4 NatAbs is key to initiating injury (Table 1). In general, IgM NatAbs promote tissue injury through the engagement of complement-dependent pro-inflammatory mechanisms through the classical and lectin pathways [54] (Figure 1) and the alternative pathway amplification loop [55]. Therapeutic modulation of these NatAb-dependent pro-inflammatory processes in vivo has been validated in IR injury and the laser-induced mouse choroidal neovascularization model by using a blocking protein that mimics the tissue neoepitope on ANX4 [50, 51] (Rohrer, unpublished observations). Using high doses of purified single IgM mAb in RAG−/− mice, the anti-ANX4 mAb B4 led to IR injury in the otherwise protected RAG−/− mice [50, 56]. Finally, this same pathogenic NatAb system also appears to be present in humans, based on the: 1) Presence of human serum NatAbs that recognize recombinant ANX4 [50]; 2) Cross-reactivity of mAb B4 with neoepitopes elaborated on oxidatively stressed human endothelial cells [57] and human retinal pigmented epithelial cells [51] (Rohrer, unpublished observations); and 3) Expression of the neoepitope recognized by the B4 NatAb in injured human tissues (unpublished observations).
Table 1.
| Model | Experimental Outcome | Publication |
|---|---|---|
| Intestinal IR Injury | Induction of injury in RAG−/− mice/Protection in WT mice with ANX4 | J. Immunol. 182:5363-5373, 2009 |
| Stroke | Induction of injury in RAG−/− mice/Protection in WT mice with ANX4 | J. Immunol. 188:1460-1408, 2012 |
| Cardiac Allotransplantation | Recognition of murine cardiac vessel neoantigen and induction of injury/Protection with B4-Crry | Circulation 131:1171-1180, 2015 |
| Choroidal Neovascularization | Recognition of B4 neoantigen on retinal pigmented epithelial cells from humans and mice/amplification of injury and neovascularization in RAG−/− mice | Unpublished observations; Rohrer, Couglin and Joseph, 2013 |
| Adriamycin-Induced Glomerulosclerosis | Enhancement of injury in mice by natural antibodies/ targeting of mAb B4 and Nat Abs to injured glomerulus | J. Immunol. 185:4393-4400, 2010 |
5. Natural Antibody Mediated Targeting of Complement Inhibitors
Following from the success of utilizing the recognition of fixed C3 using the CR2 targeting moiety along with the unexpected realization that there was an essentially “universal” means by which injured tissue is recognized by a small number of pathogenic NatAbs, a new type of tissue-targeted complement inhibitor was envisioned and proof-of-concept studies recently completed (Figure 2). The idea put forward was that one could build a dual therapeutic approach using bi-functional constructs that can both inhibit the binding of some of the endogenous pathogenic IgM NatAbs and also inhibit subsequent complement activation. To accomplish this task, the new constructs consist of single chain antibodies (scFv) that recognize the injury specific neoepitopes expressed in injured tissues, linked to a complement inhibitor. There are a number of potential advantages with this novel NatAb scFv targeting strategy. First, the approach is based on and beneficially affects the proximal event in complement activation, which is recognition by pathogenic IgM of neoepitopes in the injured tissue. Second, unlike CR2, the targeting vehicle itself contributes to therapeutic activity by blocking the binding of complement activating endogenous pathogenic IgM NatAbs, which in turn reduces the activation of the classical and lectin pathways. Third, unlike CR2-mediated targeting, the scFv strategy will likely be more specific for sites of injury since CR2 also binds other ligands as well as sites of spontaneous complement activation in kidney tubules and lymphoid follicles. Fourth, both functional arms of this kind of construct can be modified in terms of target tissue/site specificity as well as the complement pathway(s)/product(s) inhibited. And finally, neoepitope targeting will likely be less immunosuppressive since CR2 can in principle also bind pathogens marked for destruction by C3 opsonization, and thus inhibit their clearance.
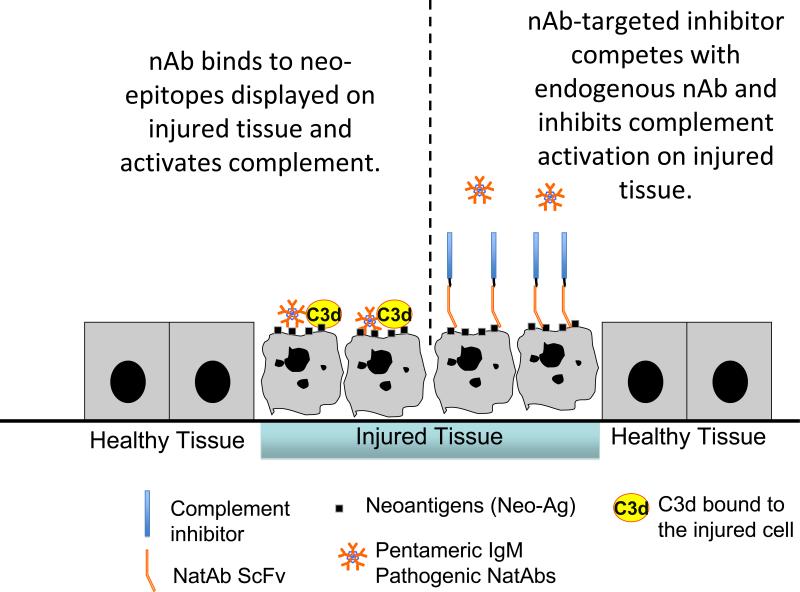
Figure 2.
Schematic view of the process of NatAb-mediated targeting of a therapeutic complement inhibitor and the pharmacodynamics effects thereof, which are read out by C3d-dependent imaging. Natural antibody and NatAb scFv targeted complement inhibitors do not bind to healthy tissue. 1. Natural antibody binds to neoepitopes expressed on injured tissues, and C3d is fixed to the tissue surface. 2. Treatment with a NatAb scFv targeted complement inhibitor prevents natural antibody from binding and controls C3d deposition. 3. An anti-C3d probe binds to the C3d and can be non-invasively detected. By clinically assessing the capability of the complement inhibitor to the ability to detect both initial C3d fixation and the subsequent downstream effects of the localization of the therapeutic to that site, one can perform both patient segmentation and initial in vivo proof-of-concept studies in patients and experimental animals.
This new tissue-targeted therapeutic concept has recently been evaluated by first transplanting hearts from wild-type donor mice into antibody-deficient mice reconstituted with specific pathogenic IgM NatAbs. One neoepitope identified as being expressed post-transplant was ANX4, and this molecule was recognized by mAb B4 [58]. Subsequently, an anti-ANX4 scFv and the same scFv linked to Crry (B4-Crry) was constructed. In an allograft heart transplant model, in which recipients contain a full antibody repertoire, the B4-Crry construct blocked graft pathogenic IgM binding and complement C3 activation, and significantly reduced inflammation and tissue injury. Although the B4scFv construct alone provided some protection, indicating a functional contribution of the targeting moiety, B4-Crry provided optimal and more durable protection. In addition, as expected B4-Crry did not affect immunity to infection, as unlike C3 deficient mice, B4-Crry treated mice did not demonstrate accelerated death in a cecal ligation-sepsis model. Additional studies and disease models are currently being evaluated with B4-Crry as well as other chimeric inhibitors containing injury-specific neoepitope recognition.
6. Imaging of Local Complement Activation: Overview, Rationale and Emerging Opportunities
The goal of tissue-targeted complement inhibitors is to block pathologic complement activation in diseased tissues without inhibiting systemic complement activity. Consequently, the standard assays of complement function using patient serum will not accurately reflect the pharmacokinetics/pharmacodynamics of these drugs. For example, the total complement activity (CH50) or alternative pathway activity (AH50) assays are unlikely to be fully suppressed by these drugs, even when complement activation is completely inhibited within tissues. Tissue biopsies are frequently stained for deposited C3 fragments and can be used to verify complement inhibition within the target tissue. Biopsies are invasive procedures, however, and cannot always be safely performed. Alternative methods of detecting complement activation within tissues will thus improve the ability to monitor complement inhibition in patients treated with targeted complement inhibitors.
One approach to detecting tissue C3 fragment deposits without a biopsy is to develop radiologic imaging probes that bind to C3 deposits (Figure 3). A technical barrier to the development of such a probe is that it must recognize the tissue bound fragments (C3b/iC3b/C3d) but not C3 and C3b in plasma. As outlined above, CR2 binds iC3b, C3dg, and C3d. Similar to the CR2-targeted therapeutic agents, a recombinant protein that contains the C3d binding region of CR2 has been used to deliver imaging contrast agents to C3 fragment deposits. The recombinant CR2 protein was conjugated to the surface of superparamagnetic iron oxide nanoparticles. After injection into live mice the nanoparticles were detected using T2-weighted magnetic resonance imaging (MRI). In a murine model of lupus nephritis, MRI of the kidneys after injection with the CR2-targeted nanoparticles successfully distinguished diseased kidneys from healthy [59]. Furthermore, the abundance of C3 deposition in the kidneys increases as the disease severity worsens, and the degree of renal enhancement with the CR2-targeted nanoparticles also increased as more C3 fragments were seen in the kidneys [60]. A recombinant CR2 construct has also been radiolabeled with technetium-99, and was shown to bind C3d-opsonized erythrocytes in vitro [61].
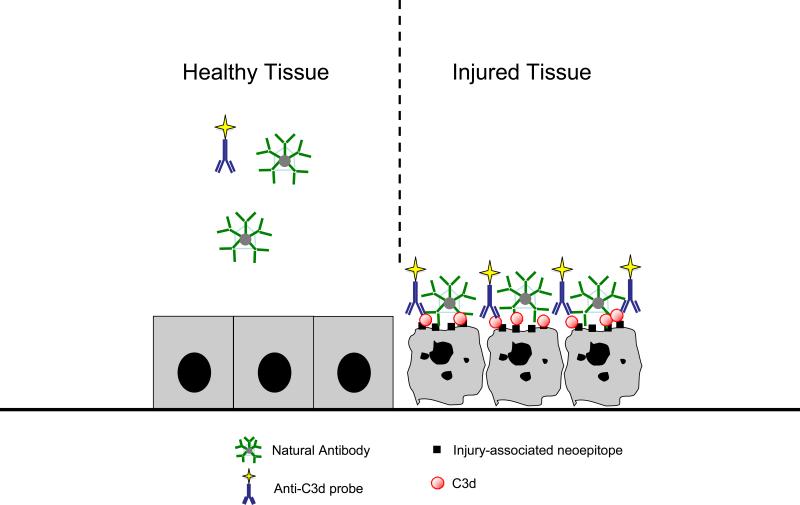
Figure 3.
Illustration of a strategy utilized to identify in vivo complement C3d fixation following the activation of complement by NatAbs to injured tissues. Various methods to direct and detect through external scanning methods imaging reagents that recognize C3d are described in the text.
CR2 binds to C3d with a relatively low affinity. One of the recombinant CR2 constructs mentioned above, for example, binds to C3d with a KD of ~500 nM [61]. In order to detect C3 deposits with greater sensitivity, we generated monoclonal antibodies to C3d that bind the iC3b/C3d fragments with high affinity (KD < 1 nM) but do not bind to intact C3 or C3b [62]. When injected systemically, these antibodies target sites of complement activation. In a murine model of laser induced choroidal neovascularization, one of these antibodies (mAb 3d29) accumulated in the ocular lesion and was detected in live mice using optical imaging [62]. Ongoing experiments are testing whether a radiolabeled form of mAb 3d29 can be used to non-invasively monitor complement activation within tissues by positron-emission tomography (PET) and single photon emission computed tomography (SPECT). Preliminary data from these experiments indicates that this approach can successfully detect and localize C3 fragments in tissues with high sensitivity.
7. Summary
Based on a wide variety of approaches, it appears that substantial therapeutic advantage can be gained by the targeting of complement inhibitors to specific sites. Initial targeting was accomplished by several means, using sLex binding characteristics to selectins (sCR1 sLex), physical membrane insertion (APT070), linkage to ScFv that direct inhibitors to specific proteins (ScFv-DAF), and the CR2-mediated approach. Much was learned during the early periods of development in both the design of inhibitors as well as the optimal means to target appropriate sites. The most recent generation of Nat-Ab-directed inhibitors incorporates insights from several key features of the tissue injury process, including the role of “universal” NatAb recognition of injured cells and tissues, creation of molecules with short circulating half-lives but prolonged and durable tissue localization, the use of different complement inhibitors that provide inhibition of the pathway(s) and activation fragment(s) desired for a particular indication. These features, in addition to the decreased risk of infection as compared to systemically active inhibitors, should promote the successful development of this most recent generation of injured tissue-targeted complement inhibitors.
Targeted complement inhibitors pose several unique challenges. Based on their mechanism of action, retention of the drug in tissues and tissue pharmacodynamics are more important than the level of free drug in plasma or suppression of the CH50. The deposition of C3 on tissue surfaces provides an excellent readout of drug efficacy, but currently there are no reliable biomarkers of localized complement activation except for biopsy. Imaging methods for detecting C3 deposits in diseased tissues will be invaluable for quantitatively measuring fixation of C3 fragments to tissue sites. This will allow the assessment of the “coverage” of the target by the targeted inhibitors when circulating biomarkers are neither informative nor relevant. It is also easy to envision an important role for these imaging methods in for guiding treatment with other immunomodulatory and chemotherapeutic drugs. C3 deposition the kidneys is an important prognostic finding in lupus nephritis [63] for example, and a method of performing serial measurements of C3 deposition in the kidneys of lupus patients could be an important tool for assessing disease activity and the response to any immunomodulatory treatment.
The currently available drugs for targeting the complement cascade act systemically, yet complement activation is a local phenomenon. Although many of the proteins that activate or regulate the complement system circulate in plasma, it is activation on tissue surfaces that causes complement-mediated disease. A new generation of drugs is in development that will block pathologic activation of the complement cascade without neutralizing the entire system. Molecular imaging techniques to detect complement activation within tissues will also be a significant advance beyond the currently available laboratory tests. The complement system has a wide range of physiologic functions, and this approach will allow clinicians to treat complement-mediated diseases without completely neutralizing the homeostatic and protective functions of the complement system.
Acknowledgements
This work discussed herein was supported by a National Institutes of Health (NIH) grants R01 AR051749 (VMH), R01 DK076690 (JMT), R01 EY019320 (BR), R01 HL091944 (CA), and P20 GM109040, in addition to a grant from the KIDNEEDS Foundation (JMT), a grant from the Lupus Research Institute (JMT), the Department of Veterans Affairs Merit Awards 1I01RX001141 (ST), 1BX001218 (ST) and I01 RX000444 (BR), and an unrestricted grant to MUSC from Research to Prevent Blindness (BR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
VMH, JT, BR and ST hold licensed patents for complement inhibitors and have received royalties from Alexion Therapeutics.
References
- 1.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 3.Carroll MC. The role of complement in B cell activation and tolerance. Adv. Immunol. 2000;74:61–88. doi: 10.1016/s0065-2776(08)60908-6. [DOI] [PubMed] [Google Scholar]
- 4.Kaya Z, Afanasyeva M, Wang Y, Dohmen KM, Schlichting J, Tretter T, Fairweather D, Holers VM, Rose N. Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nature Immunology. 2001;2:739–745. doi: 10.1038/90686. [DOI] [PubMed] [Google Scholar]
- 5.Botto M, Walport MJ. C1q, autoimmunity and apoptosis. Immunobiology. 2002;205:395–406. doi: 10.1078/0171-2985-00141. [DOI] [PubMed] [Google Scholar]
- 6.Davies KA, Schifferli JA, Walport MJ. Complement deficiency and immune complex disease. Springer Sem. Immunopath. 1994;15:397–416. doi: 10.1007/BF01837367. [DOI] [PubMed] [Google Scholar]
- 7.Strey CW, Markiewski M, Matellos D, Tudoran R, Spruce LA, Greenbaum LE, Lambris JD. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J. Exp. Med. 2003;198:913–923. doi: 10.1084/jem.20030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He S, Atkinson C, Qiao F, Cianflone K, Chen C, Tomlinson S. A complement-dependent balance between hepatic ischemia/reperfusion injury and liver regeneration in mice. J. Clin. Invest. 2009;119:2304–2316. doi: 10.1172/JCI38289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Ann. Rev. Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 10.Holers VM, Carroll MC. Holers, Innate Autoimmunity. Adv. Immunol. 2005;86:137–157. doi: 10.1016/S0065-2776(04)86004-8. [DOI] [PubMed] [Google Scholar]
- 11.Kolev MLF, G., Kemper C. Complement - tapping into new sites and effector systems. Nat. Rev. Immunol. 2014;14:811–820. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- 12.Goicoechea de Jorge E, Caesar JJE, Malik TH, Patel M, Colledge M, Johnson S, Hakobyan S, Morgan BP, Harris CL, Pickering MC, Lea SM. Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc. Natl. Acad. Sci. 2013;110:4685–4690. doi: 10.1073/pnas.1219260110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tortajada A, Yebenes H, Abarrategui-Garrido C, Anter J, Garcia-Fernandez JM, Martinez-Barricarte R, Alba-Dominguez M, Malik TH, Bedoya R, Perez RC, Trascasa ML, Pickering MC, Harris CL, Sanchez-Corral P, Llorca O, de Cordoba SR. C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J. Clin. Invest. 2013;123:2434–2446. doi: 10.1172/JCI68280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsokos GC. Systemic Lupus Erythematosus. NEJM. 2012;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 15.Morgan BP, Harris CL. Complement, a target for therapy in inflammatory and degenerative diseases. Nature Rev. Drug Discovery. 2014;14:857–877. doi: 10.1038/nrd4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricklin D, Lambris JD. Progress and trends in complement therapeutics. Adv. Exp. Med. Biol. 2013;735:1–22. doi: 10.1007/978-1-4614-4118-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambris JD, Sahu A. Lambri, Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol. Rev. 2001;180:35–48. doi: 10.1034/j.1600-065x.2001.1800103.x. [DOI] [PubMed] [Google Scholar]
- 18.Wetsel RA. Structure, function and cellular expression of complement anaphylatoxin receptors. Cur. Opin. Immunol. 1995;7:48–53. doi: 10.1016/0952-7915(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 19.Morgan BP. Regulation of the complement membrane attack pathway. Crit. Rev. Immunol. 1999;19:173–198. [PubMed] [Google Scholar]
- 20.Carroll MC. The role of complement and complement receptors in the induction and regulation of immunity. Ann. Rev. Immunol. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- 21.Van Lookerin Campagne MS, E.C., Yaspan BL. Age-related macular degeneration: Complement in action. Immunobiology. 2015 doi: 10.1016/j.imbio.2015.11.007. doi:10.1016/j.imbio.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H-F, Yu J, Bajwa E, Morrison SL, Tomlinson S. Targeting of functional antibody-CD59 fusion proteins to a cell surface. J. Clin. Invest. 1999;103:55–61. doi: 10.1172/JCI4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulligan MS, Warner RL, Rittershaus CW, Thomas LJ, Ryan US, Foreman KE, Crouch LD, Till GO, Ward PA. Endothelial targeting and enhanced antiinflammatory effects of complement inhibitors possessing Sialyl Lewisx moieties. J. Immunol. 1999;162:4952–4959. [PubMed] [Google Scholar]
- 24.Huang J, Kim LJ, Mealey R, Marsh HC, Jr., Zhang Y, Tenner AJ, Connelly ES, Jr., Pinsky DJ. Neuronal protection in stroke by an sLex-glycosylated complement inhibitory protein. Science. 1999;285:595–599. doi: 10.1126/science.285.5427.595. [DOI] [PubMed] [Google Scholar]
- 25.Smith RA. Targeting anticomplement agents. Biochemical Society Transactions. 2002;30:1037–1041. doi: 10.1042/bst0301037. [DOI] [PubMed] [Google Scholar]
- 26.Linton SM, Williams AS, Dodd I, Smith RA, Williams BD, Morgan BP. Therapeutic efficacy of a novel membrane-targeted complement regulator in antigen-induced arthritis in the rat. Arth. Rheum. 2012;43:2590–2597. doi: 10.1002/1529-0131(200011)43:11<2590::AID-ANR29>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Souza DG, Esser D, Bradford R, Vieira AT, Teixeira MM. APT070 (Mirococept), a membrane-localized complement inhibitor, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Brit. J. Pharm. 2005;145:1027–1034. doi: 10.1038/sj.bjp.0706286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halstead SK, Humphreys PD, Goodfellow JA, Wagner ER, Smith ER, Willison HJ. Complement inhibition abrogates nerve terminal injury in Miller Fisher syndrome. Ann. Neurol. 2005;58:203–210. doi: 10.1002/ana.20546. [DOI] [PubMed] [Google Scholar]
- 29.Patel H, Smith RAG, Sacks SH, Zhou W. Therapeutic strategy with a membrane-localizing complement regulator to increase the number of usable donor organs after prolonged cold storage. J. Am. Soc. Nephrol. 2006;17:1102–1111. doi: 10.1681/ASN.2005101116. [DOI] [PubMed] [Google Scholar]
- 30.Spitzer D, Unsinger J, Mao D, Wu X, Molina H, Atkinson JP. In vivo correction of complement regulatory protein deficiency with an inhibtior of targeting the red blood cell membrane. J. Immunol. 2005;175:7763–7770. doi: 10.4049/jimmunol.175.11.7763. [DOI] [PubMed] [Google Scholar]
- 31.He C, Imai M, Song H, Quigg RJ, Tomlinson S. Complement inhibitors targeted to the proximal tubule prevent injury in experimental nephrotic syndrom and demonstrate a key role for C5b-9. J. Immunol. 2005;174:5750–5757. doi: 10.4049/jimmunol.174.9.5750. [DOI] [PubMed] [Google Scholar]
- 32.Harris CL, Fraser DA, Morgan BP. Tailoring anti-complement therapeutics. Biochem. Soc. Trans. 2002;30:1019–1026. doi: 10.1042/bst0301019. [DOI] [PubMed] [Google Scholar]
- 33.Carroll MC. CD21/CD35 in B cell activation. Semin. Immunol. 1998;10(4):279–286. doi: 10.1006/smim.1998.0120. [DOI] [PubMed] [Google Scholar]
- 34.Iida K, Mornaghi R, Nussenzweig V. Complement receptor (CR1) deficiency in erythrocytes from patients with systemic lupus erythematosus. J. Exp. Med. 1982;155:1427–1438. doi: 10.1084/jem.155.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weis JJ, Toothaker LE, Smith JA, Weis JH, Fearon DT. Structure of the human B lymphocyte receptor for C3d and the Epstein-Barr virus and relatedness to other members of the family of C3/C4 binding proteins. J. Exp. Med. 1988;167:1047–1066. doi: 10.1084/jem.167.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aubry JP, Pochon S, Graber P, Jansen KU, Bonnefoy JY. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992;358:505–507. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- 37.Asokan R, Hua J, Young KA, Gould HJ, Hannan JP, Kraus DM, Szakonyi G, Grundy GJ, Chen XS, Crow MK, Holers VM. Characterization of human complement receptor type 2 (CR2/CD21) as a receptor for interferon-alpha: a potential role in systemic lupus erythematosus. J. Immunol. 2006;177:383–394. doi: 10.4049/jimmunol.177.1.383. [DOI] [PubMed] [Google Scholar]
- 38.Song H, He C, Knaak C, Guthridge JM, Holers VM, Tomlinson S. Complement receptor 2-mediated targeting of complement inhibitors to sites of complement activation. J. Clin. Invest. 2003;111:1875–1885. doi: 10.1172/JCI17348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atkinson C, Song H, Lu B, Qiao F, Burns TA, Holers VM, Tsokos GC, Tomlinson S. Targeted complement inhibition by C3d recognition ameliorates tissue injury without apparent increase in susceptibility to infection. J. Clin. Invest. 2005;115:2444–2453. doi: 10.1172/JCI25208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atkinson C, Qiao H, Song H, Gilkeson GS, Tomlinson S. Low-dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/lpr mice. J. Immunol. 2008;180:1231–1238. doi: 10.4049/jimmunol.180.2.1231. [DOI] [PubMed] [Google Scholar]
- 41.Holers VM, Rohrer B, Tomlinson S. CR2-mediated targeting of complement inhibitors: bench-to-bedside using a novel strategy for site-specific complement modulation. Adv. Exp. Med. Biol. 2013;735:137–154. doi: 10.1007/978-1-4614-4118-2_9. [DOI] [PubMed] [Google Scholar]
- 42.Rohrer B, Long Q, Coughlin B, Wilson RB, Huang Y, Qiao F, Tang PH, Kunchithapautham K, Gilkeson GS, Tomlinson S. A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Invest. Opthal. Vis. Sci. 2009;50:3056–3064. doi: 10.1167/iovs.08-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alawieh A, Elvington A, Zhu H, Yu J, Kindy MS, Atkinson C, Tomlinson S. Modulation of post-stroke degenerative and regenerative processes and subacute protection by site-targeted inhibition of the alternative pathway of complement. 2015;12 doi: 10.1186/s12974-015-0464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fridkis-Hareli M, Storek M, Maszaroff I, Risitano AM, Lundberg AS, Horvath CJ, Holers VM. Design and development of TT30, a novel C3d-targeted C3/C5 convertase inhibitor for treatment of human complement alternative pathway-mediated diseases. Blood. 2011;118:4705–4713. doi: 10.1182/blood-2011-06-359646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rohrer B, Coughlin B, Holers VM. Systemic human CR2-targeted complement alternative pathway inhibitor treatment ameliorates mouse laser-induced choroidal neovascularization. Journal of Ocular Pharmacology and Therapeutics. 2012;28:402–409. doi: 10.1089/jop.2011.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 47.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp 3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1166. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardy RR, Hayakawa K. B cell development pathways. Ann. Rev. Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 49.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nature Rev. Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 50.Kulik L, Fleming SD, Moratz C, Reuter JW, Novikov A, Chen K, Andrews KA, Markaryan A, Quigg RJ, Silverman GJ, Tsokos GJ, Holers VM. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J. Immunol. 2009;182:5363–5373. doi: 10.4049/jimmunol.0803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joseph K, Kulik L, Couglin B, Coughlin B, Kunchithapautham K, Bandyopadhyay M, Thiel S, Thielens NM, Holers VM, Rohrer B. Oxidative stress sensitizes retinal pigmented epithelial (RPE) cells to complement-mediated injury in a natural antibody-, lectin pathway-, and phospholipid epitope-dependent manner. J. Biol. Chem. 2013;288:12753–12765. doi: 10.1074/jbc.M112.421891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rescher U, Gerke V. Annexins - unique membrane binding proteins with diverse functions. J. Cell Sci. 2004;117:2631–2639. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- 53.Mayran N, Traverso V, Maroux S, Massey-Harroche D. Cellular and subcellular localization of annexins I, IV, and V in lung epithelia. Am. J. Physiol. 1996;270:L863–L871. doi: 10.1152/ajplung.1996.270.5.L863. [DOI] [PubMed] [Google Scholar]
- 54.Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q. J. Immunol. 2005;174:6373–6380. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- 55.Stahl GL, Xu Y, Hao L, Miller M, Buras JA, Fung M, Zhao H. Role for the alternative complement pathway in ischemia/reperfusion injury. Am. J. Pathol. 2003;162:449–455. doi: 10.1016/S0002-9440(10)63839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang M, Austen WG, Jr., Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr., Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Pro. Natl. Acad. Sci. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elvington A, Atkinson C, Kulik L, Zhu H, Yu J, Kindy MS, Holers VM, Tomlinson S. Pathogenic natural antibodies propagate cerebral injury following ischemic stroke in mice. J. Immunol. 2012;188:1460–1468. doi: 10.4049/jimmunol.1102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atkinson C, Qiao F, Yang X, Zhu P, Reaves N, Kulik L, Goddard M, Holers VM, Tomlinson S. Targeting pathogenic postischemic self-recognition by natural IgM to protect against posttransplantation cardia reperfusion injury. Circulation. 2015;131:1171–1180. doi: 10.1161/CIRCULATIONAHA.114.010482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serkova N, Renner B, Larsen B, Stoldt C, Halebroock KM, Bradshow-Pierce E, Holers VM, Thurman JM. Renal Inflammation: Targeted iron oxide nanoparticles for moecular MR imging in mce. Radiology. 2012;210:517–526. doi: 10.1148/radiol.09091134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sargsyan SA, Thurman JM. Molecular imaging of autoimmune Diseases and Inflammation. Molecular Imaging. 2012;11:251–264. [PubMed] [Google Scholar]
- 61.Badar A, DeFreitas S, McDonnell JM, Yahya N, Thakor D, Razavi R, Smith R, Sacks S, Mullen GED. Recombinant complement receptor 2 radiolabeled with [99mTc(CO)3]+ : a potential new radiopharmaceutical for imaging activated complement. PloS one. 2010;6(4):e18275. doi: 10.1371/journal.pone.0018275. 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thurman JM, Kulik L, Orth H, Wong M, Renner B, Sargsyan SA, Mitchell LM, Hourcade DE, Hannan JP, Kovacs JM, Coughlin B, Woodell AS, Pickering MC, Rohrer B, Holers VM. Detection of complement activation using monoclonal antibodies against C3d. J. Clin. Invest. 2013;123:2218–2230. doi: 10.1172/JCI65861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hill JS, Delahousse M, Nochy D, Tomkiewics E, Remy P, Mignon F, Mery JP. A new morphologic index for the evaluation of renal biopsies in lupus nephritis. 2000;58:1160–1173. doi: 10.1046/j.1523-1755.2000.00272.x. [DOI] [PubMed] [Google Scholar]