Abstract
Recent studies reveal a conserved role for FoxO transcription factors in establishing neuronal structure and circuit function. In this issue, McLaughlin et al. (2016. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201601014) identify a novel Toll-like receptor–FoxO pathway that represses the mitotic kinesin Pavarotti/MKLP1 to promote dynamic microtubules required for axonal transport and activity-dependent remodeling of presynaptic terminals.
Nervous system function depends on circuits of synaptically connected neurons. To support information processing in neural circuits, developing neurons must establish a complex polarized structure, form functional connections with synaptic partners, and gain competence to modify their structure and connectivity in response to activity-dependent cues. Understanding the intrinsic and extrinsic mechanisms that underlie these processes is the subject of intensive study.
The microtubule cytoskeleton plays a key role in neurons during circuit formation. The stability of the microtubule network is regulated through a number of processes, including the cross-linking and bundling of microtubules by motor proteins such as kinesins. Similarly, kinesins can promote a dynamic microtubule network by driving the translocation of microtubules through a sliding mechanism (Kapitein and Hoogenraad, 2015). In developing neurons, microtubules are implicated in the polarized extension of neurites and transport of synaptic components that support circuit function. In contrast, little is known about the role of microtubules in established neural circuits. Studies in mouse hippocampal and cortical neurons have recently correlated activity-induced invasion of dendritic spines by dynamic microtubules and rapid growth of invaded spines (Hu et al., 2008; Jaworski et al., 2009). These studies highlight the likely importance of microtubules in neural circuit plasticity and the need for further study.
Broihier and colleagues became interested in the role of microtubules in neural circuits when they made the surprise earlier finding that FoxO limits microtubule stability at Drosophila melanogaster neuromuscular junctions (NMJs; Nechipurenko and Broihier, 2012). The FoxO family of transcription factors is well known for its role in cellular homeostasis and organismal aging downstream of insulin-like growth factors (Salih and Brunet, 2008). More recently, studies in worms and mice have implicated FoxO proteins in the establishment of neuronal polarity, neurite outgrowth, and memory consolidation (de la Torre-Ubieta et al., 2010; Christensen et al., 2011; Salih et al., 2012).
In the current issue, McLaughlin et al. have identified both upstream regulators of FoxO signaling in Drosophila motoneurons and a key downstream effector that determines the dynamic state of microtubules. Upon activation, FoxO translocates from the cytoplasm to the nucleus. To identify proteins upstream of FoxO, McLaughlin et al. (2016) screened flies with mutations in neuronal receptors for altered FoxO subcellular localization in motoneurons. Whereas loss of insulin, TGFβ, or Wnt receptors, which all regulate FoxO in other contexts, had no effect on FoxO subcellular localization, nuclear FoxO was significantly reduced in Toll-6 mutants.
Toll-6 is a member of the conserved family of Toll-like receptors (TLRs). TLRs were initially identified for their role in embryonic patterning and have long been studied in the context of innate immunity where they play a critical role (Morisalo and Anderson, 1995; Ferrandon et al., 2004). Conserved expression in post-mitotic neurons and glia suggested TLRs might have additional roles in nervous system development and function. This suggestion has been borne out by recent studies in Drosophila. Toll-6 and Toll-7 promote neuronal survival in the embryonic central nervous system downstream of neurotrophins (McIlroy et al., 2013). At the NMJ, a retrograde neurotrophin signal promotes synaptic growth through the Toll-8 receptor via a noncanonical c-Jun N-terminal kinase–dependent pathway (Ballard et al., 2014). In the adult olfactory system, noncanonical signaling through Toll-6 and Toll-7 directs dendritic and axonal targeting, respectively (Ward et al., 2015). Consistent with a conserved role for noncanonical TLR signaling in neurons, TLR8 signaling in mouse cultured cortical neurons inhibits neurite outgrowth and induces neuronal apoptosis independent of the canonical NF-κB pathway (Ma et al., 2006).
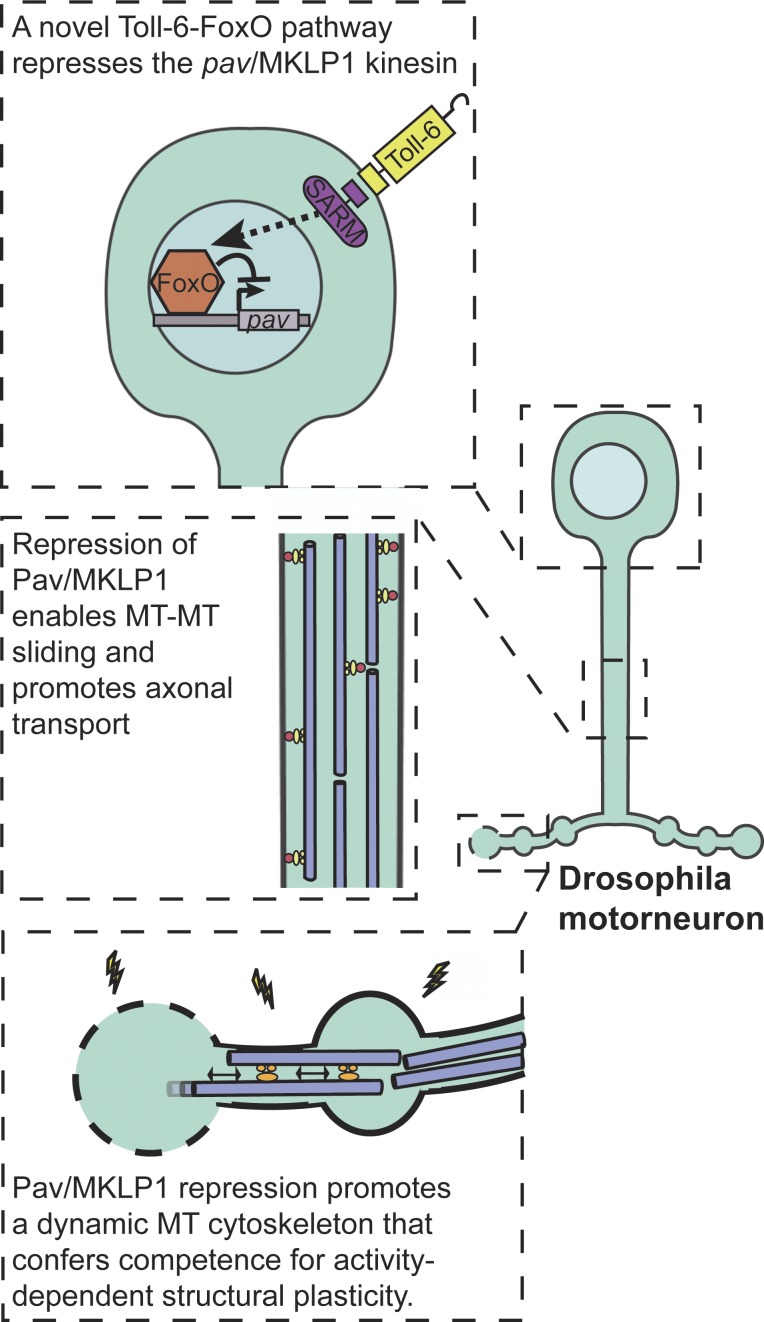
Moving downstream of Toll-6, McLaughlin et al. (2016) demonstrate that dSARM, a cytoplasmic adaptor protein that is proposed to bind TLRs through a conserved Toll-IL1 receptor domain and is currently under intense investigation for its role in injury-induced axon degeneration, promotes FoxO nuclear accumulation (Osterloh et al., 2012). The authors go on to link Toll-6 signaling to the modulation of AKT and c-Jun N-terminal kinase levels, consistent with their known roles in the phospho-regulation of FoxO localization. Together their findings support the existence of a novel signaling pathway in which Toll-6 signals through dSARM to modulate the activity of the FoxO transcription factor by promoting its nuclear localization (Fig. 1).
Figure 1.
Motoneuronal regulation of microtubule dynamics. A newly identified Toll-6–FoxO signaling pathway negatively regulates Pav-KLP (Pav) levels at the Drosophila NMJ. Repression of Pav, a negative regulator of microtubule sliding, is required for axonal trafficking of synaptic components and activity-dependent addition of new boutons. Modified from McLaughlin et al. (2016).
To investigate the functional significance of Toll-6 activation of FoxO in motoneurons, McLaughlin et al. (2016) first assessed synaptic growth at the well-characterized Drosophila NMJ. As previously demonstrated for FoxO, overexpression of either Toll-6 or dSARM in motoneurons spurred ectopic NMJ growth (Nechipurenko and Broihier, 2012). This overgrowth was dependent on the presence of FoxO, indicating that Toll-6 and dSARM act upstream of FoxO in a linear pathway that can promote the elaboration of NMJs. The authors also found that Toll-6 and foxO mutants exhibited a 20% decrease in the total length of synaptic arbors, confirming a common role in promoting the growth of NMJs.
As their earlier work had suggested that FoxO’s key in vivo function in motoneurons is to limit microtubule stability (Nechipurenko and Broihier, 2012), the authors next investigated whether this function is dependent on the Toll-6–dSARM pathway. Indeed, they found that microtubule stability, as measured by the presence of Futsch/MAP1-positive looped microtubules or acetylated α-tubulin in synaptic boutons, was increased at Toll-6 and dSARM mutant NMJs. Toll-6, foxO double mutants showed increases in microtubule stability similar to single mutants, consistent with the conclusion that they function in a linear pathway.
How does this new pathway regulate microtubule dynamics? To address this question, McLaughlin et al. (2016) sought to identify downstream targets of the Toll-6–FoxO pathway. To that end, they compiled a list of 50 known cytoskeletal regulators and assessed neuronal transcript levels in wild-type and Toll-6 mutant nerve cords. Four kinesins were consistently up-regulated in the absence of Toll-6. A genetic analysis demonstrated that knockdown of one of these kinesins, Pavarotti/MKLP1 (Pav-KLP), resulted in ectopic NMJ growth similar to that induced by overexpression of Toll-6, dSARM, and FoxO, as expected for an effector negatively regulated by the pathway.
Pav-KLP is a mitotic kinesin with an emerging role in post-mitotic neurons. In Drosophila, Pav-KLP was recently shown to limit neurite outgrowth through negative regulation of microtubule–microtubule sliding (del Castillo et al., 2015). An earlier study in mouse primary sympathetic neurons identified a role for Pav-KLP in establishing neuronal polarity (Sharp et al., 1997). Consistent with their transcript-level analysis, McLaughlin et al. (2016) found that Pav-KLP protein levels are increased twofold at the NMJs of Toll-6 and foxO mutants. If Pav-KLP is a key target of negative regulation by the endogenous Toll-6–FoxO pathway in motoneurons, then its overexpression would be expected to result in a significant increase in microtubule stability. The authors found that ectopic expression of Pav-KLP in motoneurons increases microtubule loops by ∼50%, phenocopying both Toll-6 and foxO loss-of-function mutants and motoneuron-specific knockdown by RNAi. This suggests that the loss of neuronal Toll-6–FoxO signaling leads to de-repression of Pav-KLP and diminished microtubule dynamics.
McLaughlin et al. (2016) next focused on determining key in vivo functions of this newly identified signaling pathway. A fundamental role of microtubules in motoneurons is the long-distance transport of synaptic components to presynaptic terminals, which are often very distant from the cell body. The authors found that Bruchpilot/CAST/ELKS, an integral component of presynaptic active zones, accumulates in the axons of animals lacking Toll-6, dSARM, or FoxO as well as those overexpressing Pav-KLP, indicating a defect in axonal transport to terminals. If misregulation of Pav-KLP underlies this defect, then a reduction in pav dosage is predicted to be protective. Consistent with this interpretation, the authors found that heterozygosity for a strong loss-of-function allele of pav suppressed the transport defect observed in Toll-6–FoxO pathway mutants. Finally, the authors directly test if the axonal transport defect could derive from pathway-dependent changes in microtubule stability by treating wild-type animals with the microtubule-stabilizing drug taxol. They found that nanomolar concentrations of taxol recapitulated the transport defects observed in the absence of Toll-6–FoxO signaling. Together, these results suggest that repressing Pav-KLP expression to maintain a dynamic microtubule cytoskeleton capable of supporting axonal trafficking is a critical function of the Toll-6–FoxO signaling pathway (Fig. 1).
McLaughlin et al. (2016) next investigated a role for Toll-6–FoxO–dependent microtubule regulation in activity-dependent neuronal remodeling. At the Drosophila NMJ, a well-established spaced high K+ depolarization paradigm induces the formation of nascent boutons within minutes (Ataman et al., 2008; Piccioli and Littleton, 2014). However, McLaughlin et al. (2016) found that this activity-dependent remodeling of the NMJ was completely lost in Toll-6–FoxO pathway mutants and sensitive to levels of Pav-KLP. The authors reasoned that if excessive stabilization of the microtubule network is prohibiting activity-dependent remodeling of NMJs in Toll-6–FoxO pathway mutants, the microtubule-destabilizing drug vinblastine might rescue structural plasticity. As predicted by their model, the authors found that pretreatment with vinblastine completely restored activity-dependent growth in Toll-6, dSARM, and foxO mutants as well as in motoneurons overexpressing Pav-KLP.
It is important to note that the morphological plasticity described here is rapid and independent of the nucleus, demonstrating that the Toll-6–FoxO pathway does not itself respond to the activity cue (Piccioli and Littleton, 2014). Rather, these results indicate that the pathway is critical for establishing the intrinsic competence of the neuron to restructure itself (Fig. 1). It is interesting to consider these findings in light of studies in mice demonstrating that FoxO6 is dispensable for learning, but required for memory consolidation, as this raises the intriguing possibility that FoxO-dependent regulation of intrinsic microtubule network dynamics might be a conserved mechanism for conferring neuronal competence to undergo activity-dependent plasticity (Salih et al., 2012).
The unexpected discovery that a Toll-6 signaling pathway regulates FoxO-dependent expression of a key microtubule regulator expands our understanding of FoxO and TLR signaling in neurons. It will be of interest to identify additional players in this new pathway. The Spätzle family of neurotrophin-like cysteine-knot proteins bind TLRs and represent good candidates for the ligand (McIlroy et al., 2013). At the Drosophila NMJ, Spätzle 3 is secreted from muscle where it binds presynaptic Toll-8 receptors to promote synaptic growth (Ballard et al., 2014). It will also be of interest to further investigate the mechanism through which ectopic Pav-KLP antagonizes axonal transport and synaptic remodeling. The recent finding that Pav-KLP blocks Kinesin-1–mediated microtubule sliding to limit neurite outgrowth in Drosophila motoneurons suggests that a similar mechanism may limit transport of synaptic components and activity-dependent remodeling (del Castillo et al., 2015). It was proposed that Pav-KLP controls the developmental down-regulation of microtubule sliding. This study by McLaughlin et al. (2016) suggests that precise regulation of microtubule sliding may be required to both limit neurite outgrowth late in development and establish competence to remodel in response to activity cues.
This leads to perhaps the most important outcome of the in vivo investigation of this novel pathway: the demonstration of the key role intrinsic microtubule state can play in neuronal plasticity. The implication that the maintenance of a dynamic microtubule network endows neurons with the morphological flexibility to undergo activity-dependent structural remodeling has broad significance for our basic understanding of neuronal plasticity and the clinical application of drugs that modulate microtubule stability. It will be of great interest to determine the extent to which the intrinsic state of the microtubule cytoskeleton determines plasticity in other neurons and organisms.
Acknowledgments
I thank Jill Wildonger for her insightful comments on the manuscript.
The O’Connor-Giles laboratory is funded by National Institutes of Health (R01NS078179 and R21NS088830) and the McKnight Foundation.
The authors declare no competing financial interests.
References
- Ataman B., Ashley J., Gorczyca M., Ramachandran P., Fouquet W., Sigrist S.J., and Budnik V.. 2008. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 57:705–718. 10.1016/j.neuron.2008.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard S.L., Miller D.L., and Ganetzky B.. 2014. Retrograde neurotrophin signaling through Tollo regulates synaptic growth in Drosophila. J. Cell Biol. 204:1157–1172. 10.1083/jcb.201308115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R., de la Torre-Ubieta L., Bonni A., and Colón-Ramos D.A.. 2011. A conserved PTEN/FOXO pathway regulates neuronal morphology during C. elegans development. Development. 138:5257–5267. 10.1242/dev.069062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre-Ubieta L., Gaudillière B., Yang Y., Ikeuchi Y., Yamada T., DiBacco S., Stegmüller J., Schüller U., Salih D.A., Rowitch D., et al. 2010. A FOXO–Pak1 transcriptional pathway controls neuronal polarity. Genes Dev. 24:799–813. 10.1101/gad.1880510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Castillo U., Lu W., Winding M., Lakonishok M., and Gelfand V.I.. 2015. Pavarotti/MKLP1 regulates microtubule sliding and neurite outgrowth in Drosophila neurons. Curr. Biol. 25:200–205. 10.1016/j.cub.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D., Imler J.L., and Hoffmann J.A.. 2004. Sensing infection in Drosophila: Toll and beyond. Semin. Immunol. 16:43–53. 10.1016/j.smim.2003.10.008 [DOI] [PubMed] [Google Scholar]
- Hu X., Viesselmann C., Nam S., Merriam E., and Dent E.W.. 2008. Activity-dependent dynamic microtubule invasion of dendritic spines. J. Neurosci. 28:13094–13105. 10.1523/JNEUROSCI.3074-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J., Kapitein L.C., Gouveia S.M., Dortland B.R., Wulf P.S., Grigoriev I., Camera P., Spangler S.A., Di Stefano P., Demmers J., et al. 2009. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 61:85–100. 10.1016/j.neuron.2008.11.013 [DOI] [PubMed] [Google Scholar]
- Kapitein L.C., and Hoogenraad C.C.. 2015. Building the neuronal microtubule cytoskeleton. Neuron. 87:492–506. 10.1016/j.neuron.2015.05.046 [DOI] [PubMed] [Google Scholar]
- Ma Y., Li J., Chiu I., Wang Y., Sloane J.A., Lü J., Kosaras B., Sidman R.L., Volpe J.J., and Vartanian T.. 2006. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J. Cell Biol. 175:209–215. 10.1083/jcb.200606016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy G., Foldi I., Aurikko J., Wentzell J.S., Lim M.A., Fenton J.C., Gay N.J., and Hidalgo A.. 2013. Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila melanogaster CNS. Nat. Neurosci. 16:1248–1256. 10.1038/nn.3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C.N., Nechipurenko I., Liu N., and Broihier H.T.. 2016. A Toll receptor–FoxO pathway represses Pavarotti/MKLP1 to promote microtubule dynamics in motoneurons. J. Cell Biol. 10.1083/jcb.201601014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisalo D., and Anderson K.V.. 1995. Signaling pathways that establish the dorsal-ventral pattern of the Drosophila embryo. Annu. Rev. Genet. 29:371–399. 10.1146/annurev.ge.29.120195.002103 [DOI] [PubMed] [Google Scholar]
- Nechipurenko I.V., and Broihier H.T.. 2012. FoxO limits microtubule stability and is itself negatively regulated by microtubule disruption. J. Cell Biol. 196:345–362. 10.1083/jcb.201105154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh J.M., Yang J., Rooney T.M., Fox A.N., Adalbert R., Powell E.H., Sheehan A.E., Avery M.A., Hackett R., Logan M.A., et al. 2012. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 337:481–484. 10.1126/science.1223899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccioli Z.D., and Littleton J.T.. 2014. Retrograde BMP signaling modulates rapid activity-dependent synaptic growth via presynaptic LIM kinase regulation of cofilin. J. Neurosci. 34:4371–4381. 10.1523/JNEUROSCI.4943-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih D.A., and Brunet A.. 2008. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 20:126–136. 10.1016/j.ceb.2008.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih D.A., Rashid A.J., Colas D., de la Torre-Ubieta L., Zhu R.P., Morgan A.A., Santo E.E., Ucar D., Devarajan K., Cole C.J., et al. 2012. FoxO6 regulates memory consolidation and synaptic function. Genes Dev. 26:2780–2801. 10.1101/gad.208926.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D.J., Yu W., Ferhat L., Kuriyama R., Rueger D.C., and Baas P.W.. 1997. Identification of a microtubule-associated motor protein essential for dendritic differentiation. J. Cell Biol. 138:833–843. 10.1083/jcb.138.4.833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A., Hong W., Favaloro V., and Luo L.. 2015. Toll receptors instruct axon and dendrite targeting and participate in synaptic partner matching in a Drosophila olfactory circuit. Neuron. 85:1013–1028. 10.1016/j.neuron.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]