Abstract
Objective
Neuroendocrine tumors (NETs) are a collection of complex tumors that arise from the diffuse endocrine system, primarily from the digestive tract. Carcinoid tumors most commonly originate from the small intestine. These tumors are either referred to as small intestinal neuroendocrine tumors or midgut carcinoids (MGCs). The purpose of this review article is to survey the diagnostic and therapeutic pathways for patients with MGC and provide an overview of the complex multidisciplinary care involved in improving their quality of life, treatment outcomes, and survival.
Methods
The current literature regarding the diagnosis and management of MGCs was reviewed.
Results
Dry flushing and secretory diarrhea are the hallmarks of the clinical syndrome of MGC. Managing MGC requires attention to the overall symptom complex, including the physical effects of the tumor and biomarker levels. The somatostatin analogs (SAs) octreotide and lanreotide are highly efficacious for symptomatic improvement. MGCs require resection to encompass the primary tumor and mesenteric lymph node metastases and should include cholecystectomy if the patient is likely to receive SA therapy. Debulking of liver metastasis by resection in combination with ablative therapies and other liver-directed modalities may help palliate symptoms and hormonal overproduction in carefully selected patients. Quality of life is an important measure of patients’ perception of the burden of their disease and impact of treatment modalities and may be a useful guide in deciding changes in therapy to alter apparent health status.
Conclusion
MGC is a challenging malignancy that requires the input of a multidisciplinary team to develop the best treatment plan. Consultation with expert centers that specialize in NETs may also be indicated for complex cases. With expert care, patients can be cured or live with the disease and enjoy good quality of life.
INTRODUCTION
Neuroendocrine tumors (NETs) are a collection of complex tumors that arise from the diffuse endocrine system, primarily from the digestive tract (1). The most common types of NETs are the carcinoid tumors that originate from the alimentary tract or the lung and pancreatic NETs. In general, NETs are considered to be slow-growing malignancies, but their biologic activity can vary widely. Carcinoid tumors most commonly originate from the small intestine (2). These tumors are either referred to as small intestinal NETs or midgut carcinoids (MGCs). The designation of midgut comes from the embryologic origins and vascular supply of the digestive tract: the foregut, midgut, and hindgut. The foregut includes tumors arising from the lungs, stomach, liver, biliary tract, pancreas, and first portion of the duodenum. The midgut includes the distal duodenum, the small intestines, the appendix, the right colon, and the middle of the transverse colon. The hindgut includes the distal transverse colon, the left colon, and the rectum.
Although generally considered a rare malignancy, the incidence and prevalence of NETs are rising. A review of the SEER database showed an increase in the incidence of the disease from 1973 (1.09 per 100,000) to 2004 (5.25 per 100,000), with an estimated prevalence of 103,312 cases in the United States (3). This increase in the number of cases is seen in other parts of the world as well (4,5). In addition, 71% of patients with MGC have metastatic disease at presentation (3).
MGC tumors are a particular challenge because patients suffer from both mechanical/oncologic complications and functional endocrine symptoms, typically flushing and diarrhea. These symptoms, known as the carcinoid syndrome, are common presenting complaints to the primary physician, endocrinologist, or the gastroenterologist. The purpose of this review article is to survey the diagnostic and therapeutic pathways for patients with MGC and provide an overview of the complex multidisciplinary care involved in improving their quality of life, treatment outcomes, and survival.
CLINICAL PRESENTATION
MGC tumors may present with signs and symptoms based on mechanical complications (pain, obstruction, bleeding) or due to the secreted bioactive factors (6). The carcinoid syndrome is a constellation of signs and symptoms associated with hypersecretion of vasoactive substances (e.g., serotonin, histamine, tachykinins, and prostaglandins) by the carcinoid tumor. The extent of these signs and symptoms is a function of the degree and type of substances that are secreted. Because the liver can inactivate these substances, hepatic metastases are typically present in MGCs presenting with carcinoid syndrome with bioactive substances released into the systemic circulation. The clinical manifestations of the carcinoid syndrome are generally seen in the skin, the digestive tract, and the heart, but other more widespread symptoms can include bronchospasm, myopathy, arthropathy, and edema (7).
Flushing
The hallmark presenting sign is flushing that primarily involves the face, neck, and upper chest. Flushes usually come on rapidly and may last up to 10 to 30 minutes, especially with more advanced disease. Flushing from MGC tends to be short-lived and occurs with metastases to the liver, whereas that from the foregut is protracted and occurs without metastases. Ovarian and pulmonary carcinoid tumors behave like foregut tumors. Patients may be unaware of the flush or describe warmth and redness in their face and neck. The flush specifically associated with carcinoid tends to be dry; a wet, diaphoretic flush suggests other diagnoses (postmenopausal state, panic attacks, medullary thyroid carcinoma, autonomic epilepsy, autonomic neuropathy, mastocytosis). Carcinoid flushing can be wet when there is superimposed anxiety. Both a reduction in blood pressure and presence of tachycardia may accompany flushing. Most flushes occur spontaneously, but they can be provoked by certain foods rich in serotonin (chocolate, some nuts, banana, avocado, red wine, blue cheese, and caffeine-containing drinks), alcohol, defecation, exercise, emotional events, palpation of the liver, and general anesthesia. Skin telangiectasia can occur after many months of flushing with fixed, violaceous vascular lesions due to prolonged vasodilatation on the face, especially the nose and upper lip. These may be associated with edema and cyanotic plethora.
Diarrhea and Abdominal Pain
Secretory diarrhea due to increased motility is present in over 80% of patients and is the most problematic manifestation of carcinoid syndrome. Stools are frequently watery, can be nocturnal, and may range from a few to more than 20 to 30 per day. Diarrhea can be associated with electrolyte loss, including hypokalemia, and may be accompanied by abdominal pain and cramps. Although the diarrhea may occur with the flushing, it is usually independent. Diarrhea persists with fasting and fails to disappear when feeding has been curtailed.
Abdominal pain and cramps are usually unrelated to flushing and may be a consequence of diarrhea, mesenteric fibrosis, or intestinal obstruction by the carcinoid tumor.
Carcinoid Heart Disease
Vasoactive substances lead to the development of carcinoid heart disease, which is characterized mainly by plaque-like, fibrous, endocardial thickening that principally involves the right side of the heart. The fibrous deposits cause retraction and fixation of the tricuspid and pulmonary valves. Tricuspid regurgitation is found in almost all cases, but tricuspid stenosis and both pulmonary regurgitation and stenosis may occur (8). The fibrous deposits may result in diminished right ventricular function as well. Left-sided heart disease is uncommon. The clinical manifestations of carcinoid heart disease include signs of right-sided heart failure with fatigue dyspnea, edema, ascites, and cardiac cachexia.
BLOOD AND URINE BIOMARKERS
Several circulating tumor markers have been evaluated for the diagnosis and follow-up management of NETs (Table 1). However, isolated elevation of marker levels is generally not sufficient for diagnosis without tissue confirmation. The most common markers for MGC are plasma chromogranin A and urinary 5-hydroindoleacetic acid (5-HIAA) excretion. New markers, such as pancreastatin, neurokinin A, and plasma 5-HIAA may improve the diagnostics and prognostication of MGC.
Table 1.
Specific Biochemical Markers for Each Neuroendocrine Tumor Type (18)
| Site | Tumor Type | Marker | Specificity |
|---|---|---|---|
| All | CgA and B PP, NSE, Neurokinin, Neurotensin HCGα and β |
High Intermediate Low |
|
| Thymus | Foregut carcinoid | ACTH | Intermediate |
| Bronchus | Foregut carcinoid, Small cell lung carcinoma |
ACTH, ADH, serotonin, 5-HIAA, histamine, GRP, GHRH, VIP, PTHrp |
Intermediate Low |
| Stomach | Foregut carcinoid, gastrinoma, ghrelinoma |
Histamine, gastrin ghrelin |
Intermediate Low |
| Pancreas | Gastrinoma, insulinoma, glucagonoma, somatostatinoma, PPoma, VIPoma. |
Gastrin, insulin, proinsulin, glucagon, somatostatin, pancreastatin, C-peptide, neurotensin, VIP, PTHrp, calcitonin |
High Low |
| Duodenum | Gastrinoma, somatostatinoma |
Gastrin, somatostatin | High |
| Ileum | Midgut carcinoid | Serotonin, 5-HIAA, pancreastatin neurokinin A, neuropeptide K, substance P |
High Intermediate |
| Colon and Rectum | Hindgut carcinoid | Peptide YY, somatostatin | Intermediate |
| Bone | Metastasis | Bone alkaline phosphatase, N-telopeptide, PTHrp |
High (blastic lesions) Modest (lytic lesions) Intermediate |
| Cardiac Involvement | Carcinoid | BNP | Intermediate |
Abbreviations: ACTH = adrenocorticotropic hormone; ADH = antidiuretic hormone; BNP = brain natriuretic peptide; Cg = chromogranin; GHRH = growth hormone–related hormone; GRP = gastrin-releasing peptide; HCG = human chorionic gonadotropin; 5-HIAA = 5-hydroxyindolacetic acid; NSE = neuron-specific enolase; PP = pancreatic polypeptide; PTHrp = parathyroid hormone–related protein; VIP = vasoactive intestinal peptide.
Chromogranin A (CgA)
CgA is a 49-kDa acidic polypeptide that is present in the secretory granules of neuroendocrine cells. Plasma CgA is elevated in 60 to 100% of patients with functioning or nonfunctioning NETs. The sensitivity and specificity of CgA for the detection of NETs range between 70 and 100% (9-11). The CgA level may correlate with tumor volume, but this should be interpreted carefully. Spuriously elevated levels of CgA have also been reported in patients using proton-pump inhibitors, in patients with renal or liver failure, malignant hypertension, and in those with chronic gastritis. Moreover, measurement of CgA is inconsistent between laboratories; therefore, sending serial samples to the same laboratory results in a more reliable value and constant normal range.
CgA can be used for prognosis and follow up. Jensen et al (12) found that a reduction in CgA levels greater than 80% after cytoreductive surgery for carcinoid tumors predicts symptom relief and disease control; it is associated with improved patient outcomes, even after incomplete cytoreduction. Falsely elevated CgA may make pancreastatin a more useful predictor of outcomes in some instances (see below).
5-HIAA (24-Hour Urine Collection)
Urinary 5-HIAA is a useful laboratory marker for carcinoid tumors. It is a metabolite of serotonin and is perhaps more useful than direct measurement of serum serotonin, which varies considerably during the day according to activity and stress level. The specificity of urinary 5-HIAA in carcinoid is 88% (8). However, certain foods and medications can increase urinary 5-HIAA levels and should be avoided during specimen collection (13). High serotonin concentrations occur with the ingestion of bananas, kiwis, pineapple, plantains, plums, and tomatoes. Moderate elevations are found with avocado, black olives, spinach, broccoli, cauliflower, eggplant, cantaloupe, dates, figs, nuts, grapefruit, and honeydew melon. Drugs that can increase 5-HIAA are: acetanalid, phenacetin, reserpine, glyceryl guiacolate (found in many cough syrups), and methocarbamol. Drugs that can decrease 5-HIAA levels include: chlorpromazine, heparin, impiramine, isoniazid, levodopa, monoamine oxidase inhibitors, methenamine, methyldopa, phenothiazines, promethazine, and tricyclic antidepressants. Therefore, a careful diet and medication history should be obtained in the assessment of abnormal 5-HIAA excretion.
The normal range for 5-HIAA excretion is 2 to 8 mg per 24 hours, and the quantitation of serotonin and all of its metabolites usually permits the detection of 84% of patients with MGC but fails to capture hindgut or foregut carcinoid tumors. No single measurement detects all cases of MGC, although the urine 5-HIAA appears to be the best screening procedure.
Neuron-Specific Enolase (NSE)
Another older blood marker, neuron-specific enolase (NSE), is a dimer of the glycolytic enzyme enolase. NSE is present in the cytoplasmic compartment of the cell, and its serum level is thought to be unrelated to the secretory activity of the tumor (9). Although it is less specific than CgA, NSE may be a useful marker for follow-up of patients with known diagnosis of NETs. NSE has been found in thyroid and prostatic carcinomas, neuroblastomas, small-cell lung carcinoma, NETs, and pheochromocytomas. Despite its high sensitivity (100%), its use is limited as a blood biochemical marker for NETs due to its very low specificity (32.9%) (9).
Pancreastatin
One of the posttranslational processing products of CgA, pancreastatin may be a negative prognostic indicator when its concentration in plasma is elevated before treatment in patients with NETs. A pancreastatin level >500 pmol/L is an independent marker of poor outcome. This marker is also known to correlate with the number of liver metastases, so it would have utility in the followup of NET patients. Furthermore, Stronge et al (14) found that an increase in pancreastatin levels following somatostatin analogue therapy is associated with poor survival. Other studies have suggested that pancreastatin should be measured prior to treatment and monitored during and after it to determine effectiveness and prognosis (15). Plasma levels of this marker above 5,000 pg/mL pretreatment were associated with increased periprocedure mortality in patients with NETs that underwent hepatic artery chemoembolization. These observations suggest that pancreastatin is potentially a very useful marker not only for diagnosis but also more importantly for monitoring treatment response and that it is unaffected by the use of protonpump inhibitors, which give false-positive results for CgA.
Neurokinin A (NKA)
NKA may have strong prognostic value. Turner et al (16) showed that in patients with MGC that have raised plasma NKA, a reduction of this biochemical marker after somatostatin analog therapy is associated with an 87% survival at 1 year, compared with 40% if NKA is increased. They also concluded that any alteration in NKA predicts improved or worsening survival (17,18).
Key Points Clinical Presentation, Blood and Urine Biomarkers
Dry flushing and secretory diarrhea are the hallmarks of the clinical syndrome of MGC.
The most reliable diagnostic marker for MGC is 5-HIAA, and predictors of adequate response to treatment, morbidity, and mortality include CgA, pancreastatin, and NKA, as well as NSE.
Managing MGC requires attention to the overall symptom complex, including the physical effects of the tumor and biomarker levels.
IMAGING
A major aspect in the evaluation of patients with suspected MGC is diagnostic imaging (19). The modalities typically used include both standard cross-sectional techniques as well as nuclear functional imaging with the following goals: making the diagnosis, determining the total tumor burden, determining the potential for surgical resection, establishing disease prognosis, and determining the potential for nonconventional therapies, especially for systemic or inoperable disease. Thorough workup before treatment intervention is preferable because features of the tumor and their metastases can be documented through imaging and compared for future clinical decision making.
Computed Tomography (CT)
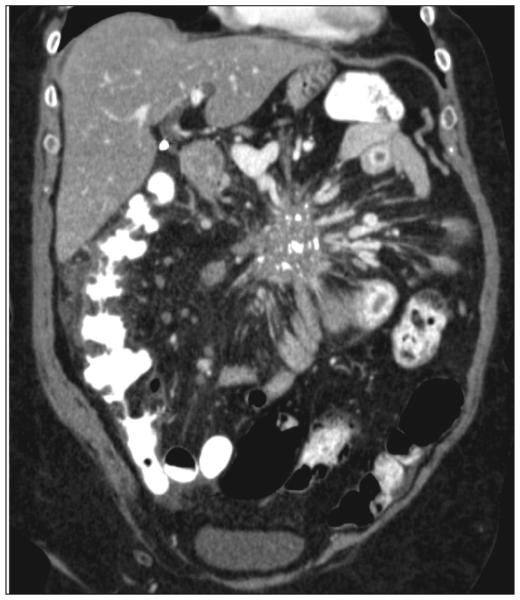
CT imaging has greatly improved the ability to diagnose MGC. With its wide availability and relatively standard administration, CT is the most common method of imaging carcinoids. However, high-level care for carcinoid patients requires understanding the strengths, limitations, and optimization of this imaging test to obtain the information necessary for complicated cases. The strengths of CT imaging of NETs are the wide field of view in the abdomen and pelvis, its accurate measurement of intra-abdominal lesions outside the liver, and the detail of the vascular anatomy (Fig. 1) (20). When planning surgery, it is particularly helpful for determining carcinomatosis and the vascular relationship of mesenteric metastases—both major causes of morbidity and mortality. It is also useful in detecting relatively large liver metastases.
Fig. 1.
Example of large calcified matted mesenteric metastasis from a midgut carcinoid causing venous and lymphatic congestion.
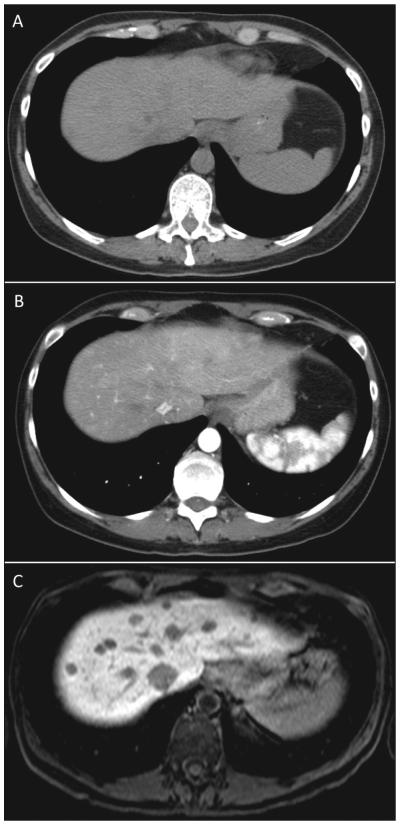
Despite the common use of CT to manage MGC, the specialist must be aware of several details to understand the quality of the information obtained from the test. Metastatic NETs are particularly sensitive to the timing of the administration of the contrast used. When evaluating the liver, MGC metastases are most visible on the arterial phase, difficult to visualize on the venous phase, and not visible on noncontrast phases (Fig. 2). More importantly, the resolution of the borders of the carcinoid metastasis can be difficult for the radiologist to discern, making determination of progression problematic. CT is also relatively insensitive in detecting small liver lesions. In addition, given the relatively long survival of patients with metastatic carcinoid, the repeated radiation exposure from frequent imaging can be a concern.
Fig. 2.
Comparison of the same patient with metastatic midgut carcinoid in the dome of the liver with different cross-sectional imaging techniques. (A) Computed tomography scan without contrast does not show lesions. (B) Computed tomography scan with arterial contrast showing some lesions. (C) Magnetic resonance imaging with Eovist showing multiple lesions more clearly.
Other modifications of CT, especially enterography, can be helpful in the evaluation of small intestine primary tumors. In the setting of emergent situations, such as small-bowel obstruction, CT remains the exam of choice.
Magnetic Resonance Imaging (MRI)
The wider availability of MRI and the development of new contrast agents have made MRI a powerful tool in the evaluation of NETs. Like CT, the information from the MRI must be used in the context of the strengths and weaknesses of the test. For MGC, MRI is most useful in the examination of liver metastases (Fig. 2) (21). Multiple sequences can improve the detection of very small lesions. NET metastases are uniquely vascular, which makes evaluation of water motion by MRI highly sensitive. T2-and diffusion-weighted imaging can detect small lesions not appreciated on CT. Moreover, with the introduction of hepatocyte-specific contrast agents (e.g., gadoxetic acid– or gadopentetic acid–based gadolinium), NETs can be seen with great detail and measured accurately. This information is particularly important for patients in follow-up assessment and also in patients potentially undergoing liver resection. Whereas the CT may only show disease in one area of the liver, bilobar disease detected on MRI may change the course of treatment. Outside of the liver, the MRI is not particularly strong in evaluating the small intestines or the mesentery because of movement artifacts.
Ultrasound and Endoscopy
Ultrasonography is an excellent tool in patients with NETs, especially as an adjunct for biopsy, but its role in MGC is more limited. However, ultrasound is a common method of diagnosing the disease as the patient is undergoing an evaluation for abdominal pain and will have an abdominal ultrasound for biliary examination. It is also highly effective in evaluating liver lesions intra-operatively. The most effective use of ultrasonography is echocardiography for carcinoid heart disease. In those patients with symptoms or elevated biologic markers, evaluation of the right-sided heart valves is critical prior to initiating a treatment plan. Right-sided heart failure can diminish the patient’s quality of life and adds significant morbidity to anyone being evaluated for surgery.
Endoscopy is a valuable diagnostic tool as well. Many of the tumors originate in the terminal ileum and can be seen on intubation of the small bowel with standard colonoscopy. However, small-bowel enteroscopy and pill-cam endoscopy may be necessary to see lesions beyond the reach of the standard endoscope.
Functional Imaging – Octreoscan
Single-photon emission computed tomography (SPECT) scans evaluate biologic properties utilizing radio-isotopes. For NETs, 111In-pentetreotide (Octreoscan) is the most widely used and available test in the United States (Fig. 3). It is based on the principle that most neuroendocrine tumors express somatostatin receptors (SSTRs), especially types 2 and 5. Pentetreotide is a somatostatin analogue similar to octreotide and is used with the SPECT-emitting isotope 111In. The tracer binds to tumors, and full-body imaging can be performed. In most centers, the SPECT imaging can be fused with CT to combine the functional information of the tumor with the cross-sectional imaging. The other strength of Octreoscan is that the whole body is usually imaged, so that distant metastases not normally in the field of view on CT or MRI can be detected (22).
Fig. 3.
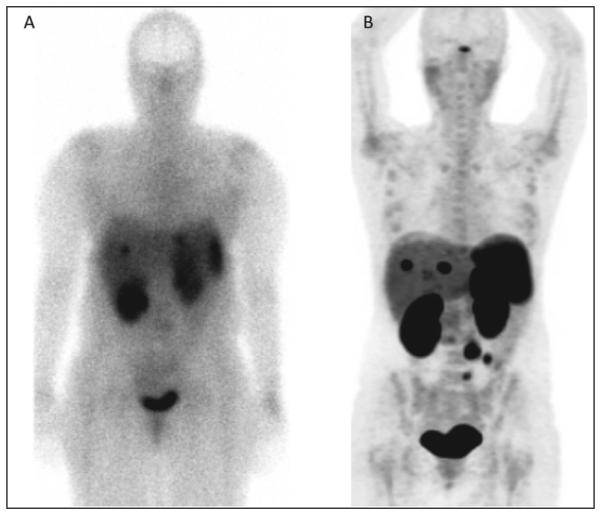
Comparison of patient with metastatic midgut carcinoid imaged with (A) Octreoscan and (B) 68Ga-DOTATATE PET.
Octreoscan is severely limited by 2 major issues: the requirement that the tumor express a somatostatin receptor (SSTR), and sensitivity. For MGC, up to 80% of tumors will express SSTR2, the receptor with the stronger binding affinity for the labelled ligand, and therefore be detectable. However, in some cases of carcinoid, if the primary tumor is already resected and a baseline Octreoscan was not obtained, it may be difficult to interpret the Octreoscan. Also, because of the nature of SPECT, it is relatively insensitive for lesions <1 cm. Also, physiologic activity in the kidney, spleen, liver, and bowel can obscure the tumors and reduce sensitivity. The test itself is somewhat cumbersome for the patient, requiring an initial intravenous injection of the tracer and scans 4 hours and 24 hours later.
Functional Imaging – Positron Emission Tomography (PET)
The next generation of functional imaging for NETs utilizes different imaging technology to evaluate these tumors. Positron emitters used in NETs include 68Ga and 11C. An emerging new technology is 68Ga-somatostatin analog imaging (Fig. 3). Although still binding to the SSTR, the PET technique offers greater sensitivity and resolution of images, especially for distant extra-abdominal metastases or difficult to locate abdominal lesions (23). The technique is also simpler, requiring the patient to only wait approximately 1 hour prior to a single imaging session. 68Ga-Somatostatin analog imaging is currently being evaluated in clinical trials in the United States and is unfortunately only available at a few specialty centers. 11C-Hydroxytryptophan is another agent that has been assessed in NETs and utilizes the amine precursor uptake machinery to detect tumors (24). However, it is only available in a few centers worldwide.
Key Points: Imaging
CT scan is limited in its evaluation of the liver and requires multiphase contrast evaluation.
MRI is an excellent examination of the liver.
Ultrasound is a highly effective method when used intra-operatively to evaluate liver lesions.
Octreoscan is a specific test for NETs but can give false-negative results.
68Ga-Somatostatin PET imaging is the emerging new
technology for functional imaging.
PATHOLOGY
Currently, there is no single standard system of nomenclature, grading, and staging of NETs, but the World Health Organization (WHO) system is the most adopted and can be applied without special immunostaining (25). NETs are divided broadly into well-differentiated and poorly differentiated categories. Poorly differentiated tumors are considered high-grade neuroendocrine carcinomas (including small- and large-cell neuroendocrine carcinomas) (Table 2) (26). Most systems recognize 3 grades: low, intermediate, and high, based on proliferative index (mitotic or Ki67 index) and the presence of necrosis. Pathology reports should include minimum required information, such as proliferative index, grade, and extent of involvement of bowel wall, other organs, and lymph nodes. Immunostaining of the proliferative marker Ki67 is the standard practice performed at most specialty/academic institutions and can support the evaluation of the tumor. Immunolabeling for general NET markers such as chromogranin A and synaptophysin are frequently performed to confirm the diagnosis.
Table 2.
Nomenclature for Midgut Carcinoid—Adapted from the NANETS Consensus Guidelines for the Diagnosis and Management of Neuroendocrine Tumors (26)
| Grade | Traditional | ENETs, WHO | Based on Moran |
|---|---|---|---|
| Low | Carcinoid tumor | Neuroendocrine tumor, grade 1 (G1) |
Neuroendocrine carcinoma, grade 1 |
| Intermediate | Carcinoid tumor | Neuroendocrine tumor, grade 2 (G2) |
Neuroendocrine carcinoma, grade 2 |
| High | Small-cell carcinoma | Neuroendocrine carcinoma, grade 3 (G3), small-cell carcinoma |
Neuroendocrine carcinoma, grade 3, small-cell carcinoma |
| Large-cell neuroendocrine carcinoma |
Neuroendocrine carcinoma, grade 3 (G3), large-cell neuroendocrine |
Neuroendocrine carcinoma, grade 3, large-cell neuroendocrine |
Abbreviations: ENETS = European Neuroendocrine Tumor Society; NANETS = North American Neuroendocrine Tumor Society; WHO = World Health Organization.
Key Points Pathology
Pathology reports should include minimum required information, such as proliferative index, grade, and tumor extent.
The WHO classification system is the most widely used and should be part of the pathologic evaluation of all specimens.
TREATMENT STRATEGIES
Because MGC patients frequently present with nonspecific symptoms, their diagnosis is often delayed by several years, and the primary tumor has already metastasized. The primary care physician, gastroenterologist, and endocrinologist may be the first to encounter such patients, who often complain of abdominal pain, diarrhea, and flushing. The goal of treatment is to control the carcinoid syndrome when present and to resect the primary tumor and its regional lymphatic drainage whenever possible.
SURGICAL TREATMENT
The aim of surgical treatment for MGCs should be the complete curative en block resection of the primary tumor and its mesenteric lymph node metastases/mass. Although a large majority of MGCs present initially with lymph node and distant metastases, surgery remains a worthwhile endeavor in these patients, and aims at palliation of the local and systemic tumor effects as well as extension of survival. MGCs present unique challenges for the surgeon: (1) the primary tumor is often small and can be difficult to identify (occult) on pre-operative imaging; (2) the mesenteric lymph node involvement can be bulky, difficult, or impossible to remove and can cause substantial morbidity; and (3) the hormonal effects of the tumor can be extreme and require specific therapy.
Resection of MGCs in the Absence of Liver Metastasis
Pre-operative imaging will often reveal mesenteric lymph node metastases without a clear localization of the primary tumor (Fig. 1). An occult primary should not deter attempts at resection, as the majority of these tumors are found intra-operatively after diligent and thorough inspection of the small bowel using open or laparoscopic techniques. At laparotomy, MGCs are characterized by small ileal, occasionally multiple, submucosal tumors, regularly associated with a larger mesenteric mass and intense fibrosis (27). Surgeons must be aware of the predilection of these tumors for spread along the bowel lymphatics, hence mimicking multiple small-bowel primaries. Loco-regional resection aims to remove the small-bowel primary and other small-bowel tumor deposits with an extensive mesenteric lymph node dissection, often requiring a right hemicolectomy, while respecting the blood supply to the remaining bowel and avoiding short bowel syndrome. The marked associated fibrosis tends to contract the mesentery, obstruct the small intestine, and sometimes the duodenum and ureters. The progressive fibrosis can involve the root of the mesentery and its vasculature, ultimately causing lymphatic obstruction, venous stasis, and intestinal ischemia.
Resection of MGCs in the Presence of Liver Metastasis
Although at times controversial, due to conflicting results, the loco-regional resection of the primary and mesenteric mass in patients presenting with distant metastatic MGC is currently advocated (28). This “prophylactic” surgical approach allows for a delay in the abdominal complications caused by the frequently unrelenting growth of the mesenteric mass. The median and 5-year overall survival after locoregional resection and selective systemic therapy of MGC were recently reported to be 8.4 years and 67%, respectively. Loco-regional resection of the primary tumor and its pathologic mesenteric lymph nodes may be an independent positive prognosticator of survival; poor survival is associated with more advanced mesenteric nodal metastases, distant abdominal node metastases, liver metastases, extra-abdominal metastases, and carcinomatosis (29). Debulking of liver metastasis by resection in combination with ablative therapies and other liver-directed modalities may help palliate symptoms and hormonal overproduction in carefully selected patients. In patients with asymptomatic primary tumors and liver metastases who may not benefit from primary tumor resection, addressing the liver metastasis to ascertain their response may be the best first approach (27,30-32).
When the carcinoid syndrome is present or suspected, the surgeon and the anesthesia team should be prepared for the possibility of a carcinoid crisis, with resulting hemodynamic instability, hyperthermia, shock, arrhythmia, flushing, or bronchial obstruction. Prior to a surgical procedure, patients should be evaluated for the presence of carcinoid valve disease, undergo valve repair, and be given prophylaxis with somatostatin analogues (SAs). Patients at high risk for carcinoid crisis, those with flushing and/or large bulky tumors, should be given peri-operative continuous intravenous octreotide (33). Lastly, because SAs are known to cause biliary stasis and the majority of patients with MGC will be treated with such agents at some point during their treatment course, cholecystectomy should be performed at the time of first laparotomy.
Resection of Appendix and Cecal Carcinoids
Carcinoids of the appendix are often found incidentally during appendectomy. The majority of appendiceal carcinoids are small (<1 cm), located at the tip of the appendix, and often cured with appendectomy. A right hemicolectomy with removal of the draining lymphatics is recommended when the primary tumor is ≥2 cm, incompletely resected, invades the base of the appendix or mesoappendix, displays lymphovascular invasion or lymph node metastases and unfavorable histology or grade (goblet cell, adenocarcinoid). Cecal carcinoids usually present as a bulky mass causing intestinal obstruction or hemorrhage. Complete resection of the tumor and its draining lymphatics is recommended (34).
Key Point Surgical Treatment
MGCs require resection to encompass the primary tumor and mesenteric lymph node metastases and should include cholecystectomy if the patient is likely to receive SA therapy.
MEDICAL TREATMENT
A mainstay of medical therapy for the carcinoid syndrome involves use of SAs. The rationale for use of SAs includes the high prevalence of SSTR types 1, 2, and 5 expression on NETs. The commonly used SAs, octreotide and lanreotide, bind to SSTR-2 and -5 and are useful in reducing the diarrhea and flushing in at least 80% of patients (35). In a review of 15 studies including 481 patients, the slow-release formulations octreotide LAR (long-acting release) and lanreotide autogel achieved symptomatic improvement in 74 and 68%, biochemical response in 51 and 39%, and tumor response in 70 and 64%, respectively (36). Overall, octreotide and lanreotide have similar efficacy in symptom control and reducing tumor markers and serotonin levels (37). The starting dose of lanreotide autogel is usually 90 to 120 mg subcutaneously monthly, whereas octreotide LAR is 30 to 120 mg intramuscularly monthly.
SAs control the growth of well-differentiated NETs as well. In a double blind, placebo controlled phase 3b study with octreotide LAR 30 mg monthly in 85 subjects, the median time to tumor progression was 6 months for placebo and 14 months for octreotide subjects (38). More recently, lanreotide autogel was evaluated as an antiproliferative agent in 30 subjects (including 12 with MGC) (39). In this open-label, phase 2 study involving lanreotide autogel at a dose of 120 mg every 4 weeks, the median progression-free survival (PFS) was 12.9 months, though there was no control group. These data suggest that SAs significantly extend the time to tumor progression.
In general, SAs are well tolerated, but patients may note nausea, abdominal discomfort, bloating, and/or steatorrhea, often during the first several weeks of therapy, after which the symptoms subside. Pancreatic malabsorption should be monitored and alleviated with pancreatic enzyme supplementation.
Although patients may be controlled with SAs for a number of years, refractory symptoms requiring dose escalation, increased dose frequency, or use of subcutaneous octreotide shots may occur in up to 40% of patients within 6 to 18 months (38,40). Slow progression over time of MGC tumors may contribute to reduction in SA efficacy within months to years (36).
Interferon-alpha (IFNα) may be useful in patients with refractory symptoms of carcinoid syndrome despite SA treatment. IFNα may reduce flushing and diarrhea in 40 to 50% of cases refractory to SAs, although objective tumor regression is less common (41). However, benefits are usually transient and there are limiting side effects, including fatigue, depression, and flu-like symptoms.
Everolimus, an oral inhibitor of the mammalian target of rapamycin (mTOR), has been studied as an adjuvant to SAs in patients with advanced NET, although this has not been approved by the Food and Drug Administration (FDA) for this indication. In the RADIANT-2 phase 3, double-blind placebo-controlled study in patients with advanced NETs and carcinoid syndrome, the combination of everolimus (mTOR inhibitor) plus octreotide LAR increased PFS from 11.3 months to 16.4 months versus everolimus alone (42). The role of mTOR inhibitors in the management of MGC and the carcinoid syndrome needs further investigation.
Pasireotide is a novel SA that has high affinity for SSTR-1, -2, -3, and -5 and displays a 30- to 40-fold higher affinity for SSTR-1 and SSTR-5 than octreotide or lanreotide. Pasireotide has been approved by the FDA for use in Cushing disease and has demonstrated efficacy in patients with acromegaly (43). Although generally well tolerated, pasireotide is associated with hyperglycemia, and close monitoring is therefore necessary (44). Kvols et al (45) administered pasireotide as depot monthly injections to 45 subjects with carcinoid syndrome who had lost octreotide responsiveness. In their study, pasireotide controlled diarrhea and flushing in 27% of patients. Tumors remained stable in 13 (57%) and progressed in 10. This study suggested a role for pasireotide in patients resistant to currently available SAs. In a more recent study, pasireotide administered as a monthly intramuscular injection to 42 subjects with primary or metastatic gastro-entero-pancreatic NET was well tolerated (46). Further study will help elucidate the role of pasireotide in patients with carcinoid tumors.
In addition to use of SAs, antidiarrheal agents such as loperamide and/or diphenoxylate/atropine and the opiates paregoric and tincture of opium may be useful. The serotonin-3-receptor antagonist ondansetron has been shown to be useful in reducing diarrhea in a small series of patients uncontrolled on a SA (47).
Key Points Medical Treatment
The SAs octreotide and lanreotide are highly efficacious for symptomatic improvement.
Tolerance to SAs is common within 6 to 12 months of therapy and requires dose escalation.
TREATMENT OF ADVANCED METASTATIC MGC
SAs remain the foundation of treatment for advanced MGC for both hormone and tumor control. Traditional chemotherapeutics have proven to be relatively ineffective against these slow-growing tumors; new methods are emerging that may improve quality of life (QOL) and survival. For those with advanced liver dominant disease, liver-directed therapy through interventional oncologic techniques is part of the treatment paradigm. Liver-directed therapy may include bland embolization, chemo-embolization, or radio-embolization. For metastatic carcinoid, 90-Yttrium based therapy is commonly used for treatment (48). It is effective at controlling symptoms but has not been shown to improve survival. In general, these patients undergo the procedure in 3 stages: (1) a mapping stage when the arterial anatomy of the liver is evaluated and potential shunting to the lung and foregut is determined; (2) treatment of disease dominant liver lobe; and (3) treatment of the remaining liver. In general, these procedures are performed as an outpatient and are well tolerated, with pain or ulceration as the major complications. Patients with poor hepatic synthetic function are not candidates.
A new emerging technology for treating systemic carcinoid is peptide receptor radiotherapy (PRRT) (49). Like nuclear functional imaging, this treatment modality uses the SSTR as a target of treatment. However, instead of carrying an imaging isotope, the SA is chelated to a betaemitting cytotoxic isotope, 90-Yttrium or 177-Lutetium. These therapies can have both rapid and delayed activity. Survival for this therapy has been reported to be as great as 44 months. 177-Lutetium-SA is currently being evaluated in a phase 3 clinical trial in the United States and is available at select centers around the world.
Key Points Treatment of Advanced Metastatic MGC
Liver-directed therapy may be in important modality to control symptoms.
PRRT is an emerging systemic therapy that is being tested in the United States.
QOL CONSIDERATIONS IN MGC PATIENTS
A major consideration in managing MGC is the impact of the disease on QOL. This includes the effects of the tumor per se, the clinical syndrome, impact of the hormones and cytokines produced, in addition to the impact of surgery, chemotherapy, and newer forms of intervention outlined above. Patients with NET, not surgically removed, with tumor recurrence after surgery and carcinoid syndrome symptoms, experience worse total QOL as well as impaired physical function, social activity, limitation of their physical role, depression, fatigue, and pain. A recent study showed that NET patients treated with PRRT had significant improvements in QOL scores, as measured by improvements in diarrhea, appetite, and insomnia. Patients with bone metastases or a decrease of 50% or more in CgA levels had improvement in the European Organization for Research and Treatment of Cancer QOL-C30 scores by at least 10 points. The Norfolk QOL-NET is a validated, comprehensive tool for evaluating QOL in patients specifically with NETs (50).
Key Points QOL
QOL is an important measure of patients’ perception of the burden of their disease and impact of treatment modalities and may be a useful guide in deciding changes in therapy to alter apparent health status.
Norfolk QOL-NET is particularly sensitive to health-related symptom change, physical functioning, emotional functioning, and respiratory and cardiovascular disease progression.
The QOL scores directly correlate with tumor burden and inversely with serotonin levels, accounting for the depression so common to patients with these tumors.
CONCLUSION
MGC is a challenging malignancy that requires the input of a multidisciplinary team to develop the best treatment plan. Even after treatment is initiated, good follow-up is necessary to monitor for disease persistence/recurrence and symptom progression. Although no standard follow-up protocol exists, checking biomarkers and scans every 3 to 12 months is generally encouraged, depending on the risk of recurrence or rate of disease progression (34). The time between monitoring tests can be extended based on clinical judgment. Consultation with expert centers that specialize in NETs may also be indicated for complex cases. With expert care, patients can be cured or live with the disease and enjoy good QOL.
Abbreviations
- CgA
chromogranin A
- CT
computed tomography
- 5-HIAA
5-hydroindoleacetic acid
- MGC
midgut carcinoid
- MRI
magnetic resonance imaging
- mTOR
mammalian target of rapamycin
- NET
neuroendocrine tumor
- NSE
neuron-specific enolase
- NKA
neurokinin A
- PET
positron emission tomography
- PRRT
peptide receptor radiotherapy
- QOL
quality of life
- SA
somatostatin analogue
- SPECT
single-photon emission computed tomography
- SSTR
somatostatin receptor
Footnotes
To purchase reprints of this article, please visit: www.aace.com/reprints.
The opinions represented in the AACE/ACE Disease State Clinical Review: Diagnosis and Management of Midgut Carcinoids are the expressed opinions of the Neuroendocrine and Pituitary Scientific Committee of the American Association of Clinical Endocrinologists. AACE/ACE Disease State Clinical Reviews are systematically developed documents written to assist health care professionals in medical decision making for specific clinical conditions, but are in no way a substitute for a medical professional’s independent judgment and should not be considered medical advice. Most of the content herein is based on literature reviews. In areas of uncertainty, professional judgment of the authors was applied.
This review article is a working document that reflects the state of the field at the time of publication. Because rapid changes in this area are expected, periodic revisions are inevitable. We encourage medical professionals to use this information in conjunction with, and not a replacement for, their best clinical judgment. The presented recommendations may not be appropriate in all situations. Any decision by practitioners to apply these guidelines must be made in light of local resources and individual patient circumstances.
DISCLOSURE
Dr. Eric Liu reports that he has received speaker bureau honoraria from Novartis. None of the other authors have any multiplicities of interest to disclose.
REFERENCES
- 1.Vinik AI, McLeod MK, Fig LM, Shapiro B, Lloyd RV, Cho K. Clinical features, diagnosis, and localization of carcinoid tumors and their management. Gastroenterol Clin North Am. 1989;18:865–896. [PubMed] [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 4.Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58–64. doi: 10.1007/s00535-014-0934-2. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18. doi: 10.1016/j.ecl.2010.12.005. vii. [DOI] [PubMed] [Google Scholar]
- 6.Vinik AI, Woltering EA, Warner RR, et al. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39:713–734. doi: 10.1097/MPA.0b013e3181ebaffd. [DOI] [PubMed] [Google Scholar]
- 7.Creutzfeldt W, Stockmann F. Carcinoids and carcinoid syndrome. Am J Med. 1987;82:4–16. doi: 10.1016/0002-9343(87)90422-0. [DOI] [PubMed] [Google Scholar]
- 8.Tormey WP, FitzGerald RJ. The clinical and laboratory correlates of an increased urinary 5-hydroxyindoleacetic acid. Postgrad Med J. 1995;71:542–545. doi: 10.1136/pgmj.71.839.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajetta E, Ferrari L, Martinetti A, et al. Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumors. Cancer. 1999;86:858–865. doi: 10.1002/(sici)1097-0142(19990901)86:5<858::aid-cncr23>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Bernini GP, Moretti A, Ferdeghini M, et al. A new human chromogranin ‘A’ immunoradiometric assay for the diagnosis of neuroendocrine tumours. Br J Cancer. 2001;84:636–642. doi: 10.1054/bjoc.2000.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goebel SU, Serrano J, Yu F, Gibril F, Venzon DJ, Jensen RT. Prospective study of the value of serum chromogranin A or serum gastrin levels in the assessment of the presence, extent, or growth of gastrinomas. Cancer. 1999;85:1470–1483. [PubMed] [Google Scholar]
- 12.Jensen EH, Kvols L, McLoughlin JM, et al. Biomarkers predict outcomes following cytoreductive surgery for hepatic metastases from functional carcinoid tumors. Ann Surg Oncol. 2007;14:780–785. doi: 10.1245/s10434-006-9148-z. [DOI] [PubMed] [Google Scholar]
- 13.Feldman JM, Lee EM. Serotonin content of foods: effect on urinary excretion of 5-hydroxyindoleacetic acid. Am J Clin Nutr. 1985;42:639–643. doi: 10.1093/ajcn/42.4.639. [DOI] [PubMed] [Google Scholar]
- 14.Stronge RL, Turner GB, Johnston BT, et al. A rapid rise in circulating pancreastatin in response to somatostatin analogue therapy is associated with poor survival in patients with neuroendocrine tumours. Ann Clin Biochem. 2008;45:560–566. doi: 10.1258/acb.2008.008033. [DOI] [PubMed] [Google Scholar]
- 15.O’Dorisio TM, Krutzik SR, Woltering EA, et al. Development of a highly sensitive and specific carboxyterminal human pancreastatin assay to monitor neuroendocrine tumor behavior. Pancreas. 2010;39:611–616. doi: 10.1097/MPA.0b013e3181c68d7a. [DOI] [PubMed] [Google Scholar]
- 16.Turner GB, Johnston BT, McCance DR, et al. Circulating markers of prognosis and response to treatment in patients with midgut carcinoid tumours. Gut. 2006;55:1586–1591. doi: 10.1136/gut.2006.092320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamikunian P, Ardill JE, O’Dorisio TM, et al. Validation of neurokinin a assays in the United States and Europe. Pancreas. 2011;40:1000–1005. doi: 10.1097/MPA.0b013e318232b6a2. [DOI] [PubMed] [Google Scholar]
- 18.Vinik AI, Silva MP, Woltering EA, Go VL, Warner R, Caplin M. Biochemical testing for neuroendocrine tumors. Pancreas. 2009;38:876–889. doi: 10.1097/MPA.0b013e3181bc0e77. [DOI] [PubMed] [Google Scholar]
- 19.Sundin A, Vullierme MP, Kaltsas G, Plöckinger U. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: radiological examinations. Neuroendocrinology. 2009;90:167–183. doi: 10.1159/000184855. [DOI] [PubMed] [Google Scholar]
- 20.Kumbasar B, Kamel IR, Tekes A, Eng J, Fishman EK, Wahl RL. Imaging of neuroendocrine tumors: accuracy of helical CT versus SRS. Abdom Imaging. 2004;29:696–702. doi: 10.1007/s00261-003-0162-3. [DOI] [PubMed] [Google Scholar]
- 21.Dromain C, de Baere T, Lumbroso J, et al. Detection of liver metastases from endocrine tumors: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J Clin Oncol. 2005;23:70–78. doi: 10.1200/JCO.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Chiti A, Fanti S, Savelli G, et al. Comparison of somatostatin receptor imaging, computed tomography and ultrasound in the clinical management of neuroendocrine gastroentero-pancreatic tumours. Eur J Nucl Med. 1998;25:1396–1403. doi: 10.1007/s002590050314. [DOI] [PubMed] [Google Scholar]
- 23.Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 24.Orlefors H, Sundin A, Garske U, et al. Whole-body (11)C-5-hydroxytryptophan positron emission tomography as a universal imaging technique for neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and computed tomography. J Clin Endocrinol Metab. 2005;90:3392–3400. doi: 10.1210/jc.2004-1938. [DOI] [PubMed] [Google Scholar]
- 25.Rindi G, Bosman FT, et al. WHO Classification of Tumours of the Digestive System. 4th. International Agency for Research on Cancer; Lyon: 2010. Nomenclature and classification of neuroendocrine neoplasms of the digestive system; p. 13. AR. [Google Scholar]
- 26.Boudreaux JP, Klimstra DS, Hassan MM, et al. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the jejunum, ileum, appendix, and cecum. Pancreas. 2010;39:753–766. doi: 10.1097/MPA.0b013e3181ebb2a5. [DOI] [PubMed] [Google Scholar]
- 27.Boudreaux JP, Putty B, Frey DJ, et al. Surgical treatment of advanced-stage carcinoid tumors: lessons learned. Ann Surg. 2005;241:839–845. doi: 10.1097/01.sla.0000164073.08093.5d. discussion 845-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Givi B, Pommier SJ, Thompson AK, Diggs BS, Pommier RF. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery. 2006;140:891–897. doi: 10.1016/j.surg.2006.07.033. discussion 897-898. [DOI] [PubMed] [Google Scholar]
- 29.Norlén O, Stålberg P, Öberg K, et al. Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J Surg. 2012;36:1419–1431. doi: 10.1007/s00268-011-1296-z. [DOI] [PubMed] [Google Scholar]
- 30.Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432–445. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 31.Chambers AJ, Pasieka JL, Dixon E, Rorstad O. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery. 2008;144:645–651. doi: 10.1016/j.surg.2008.06.008. discussion 651-653. [DOI] [PubMed] [Google Scholar]
- 32.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 33.Kvols LK, Martin JK, Marsh HM, Moertel CG. Rapid reversal of carcinoid crisis with a somatostatin analogue. N Engl J Med. 1985;313:1229–1230. doi: 10.1056/NEJM198511073131915. [DOI] [PubMed] [Google Scholar]
- 34.Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557–577. doi: 10.1097/MPA.0b013e31828e34a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kvols LK, Moertel CG, O’Connell MJ, Schutt AJ, Rubin J, Hahn RG. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986;315:663–666. doi: 10.1056/NEJM198609113151102. [DOI] [PubMed] [Google Scholar]
- 36.Modlin IM, Pavel M, Kidd M, Gustafsson BI. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Alimen Pharmacol Ther. 2010;31:169–188. doi: 10.1111/j.1365-2036.2009.04174.x. [DOI] [PubMed] [Google Scholar]
- 37.O’Toole D, Ducreux M, Bommelaer G, et al. Treatment of carcinoid syndrome: a prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer. 2000;88:770–776. doi: 10.1002/(sici)1097-0142(20000215)88:4<770::aid-cncr6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebocontrolled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Richard M, Massuti B, Pineda E, et al. Antiproliferative effects of lanreotide autogel in patients with progressive, well-differentiated neuroendocrine tumours: a Spanish, multicentre, open-label, single arm phase II study. BMC Cancer. 2013;13:427. doi: 10.1186/1471-2407-13-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofland LJ, Lamberts SW. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev. 2003;24:28–47. doi: 10.1210/er.2000-0001. [DOI] [PubMed] [Google Scholar]
- 41.Frank M, Klose KJ, Wied M, Ishaque N, Schade-Brittinger C, Arnold R. Combination therapy with octreotide and alpha-interferon: effect on tumor growth in metastatic endocrine gastroenteropancreatic tumors. Am J Gastroenterol. 1999;94:1381–1387. doi: 10.1111/j.1572-0241.1999.01090.x. [DOI] [PubMed] [Google Scholar]
- 42.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebocontrolled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 43.Colao A, Petersenn S, Newell-Price J, et al. A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med. 2012;366:914–924. doi: 10.1056/NEJMoa1105743. [DOI] [PubMed] [Google Scholar]
- 44.Petersenn S, Farrall AJ, De Block C, et al. Long-term efficacy and safety of subcutaneous pasireotide in acromegaly: results from an open-ended, multicenter, phase II extension study. Pituitary. 2014;17:132–140. doi: 10.1007/s11102-013-0478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kvols LK, Oberg KE, O’Dorisio TM, et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a phase II study. Endocr Relat Cancer. 2012;19:657–666. doi: 10.1530/ERC-11-0367. [DOI] [PubMed] [Google Scholar]
- 46.Wolin EM, Hu K, Hughes G, Bouillaud E, Giannone V, Resendiz KH. Safety, tolerability, pharmacokinetics, and pharmacodynamics of a long-acting release (LAR) formulation of pasireotide (SOM230) in patients with gastroenteropancreatic neuroendocrine tumors: results from a randomized, multicenter, open-label, phase I study. Cancer Chemother Pharmacol. 2013;72:387–395. doi: 10.1007/s00280-013-2202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiesewetter B, Raderer M. Ondansetron for diarrhea associated with neuroendocrine tumors. N Engl J Med. 2013;368:1947–1948. doi: 10.1056/NEJMc1301537. [DOI] [PubMed] [Google Scholar]
- 48.Kennedy AS, Dezarn WA, McNeillie P, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31:271–279. doi: 10.1097/COC.0b013e31815e4557. [DOI] [PubMed] [Google Scholar]
- 49.Kwekkeboom DJ, Krenning EP, Lebtahi R, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology. 2009;90:220–226. doi: 10.1159/000225951. [DOI] [PubMed] [Google Scholar]
- 50.Vinik E, Carlton CA, Silva MP, Vinik AI. Development of the Norfolk quality of life tool for assessing patients with neuroendocrine tumors. Pancreas. 2009;38:e87–e95. doi: 10.1097/MPA.0b013e31819b6441. [DOI] [PubMed] [Google Scholar]