Abstract
The major problems of revision surgery for recurrent lumbar disc herniation (LDH) include limited visualization due to adhesion of scar tissue, restricted handling of neural structures in insufficient visual field, and consequent higher risk of a dura tear and nerve root injury. Therefore, clear differentiation of neural structures from scar tissue and adhesiolysis performed while preserving stability of the remnant facet joint would lower the risk of complications and unnecessary fusion surgery. Biportal endoscopic spine surgery has several merits including sufficient magnification with panoramic view under very high illumination and free handling of instruments normally impossible in open spine surgery. It is supposed to be a highly recommendable alternative technique that is safer and less destructive than the other surgical options for recurrent LDH.
Keywords: Lumbosacral region, Intervertebral disc displacement, Minimally invasive surgical procedure, Endoscopic
Recurrent lumbar disc herniation (LDH) that develops at least 6 months after previous open discectomy shows a relatively frequent incidence of 7%–10%.1,2) Surgical options for recurrent LDH could be determined based on the type of previous procedure. If the previous one was percutaneous endoscopic lumbar discectomy (PELD) through a far lateral approach, revision PELD could be performed using a posterior approach despite the high risk of dura tear associated with the use of one portal during the procedure,3) which requires steep learning curve and special instruments for epidural scarring.4) In patients with primary open lumbar microdiscectomy (OLM), the secondary OLM showed much higher complication rates than wider decompression with fusion surgery in terms of postoperative back pain, neurologic deficit, and dura tear. Therefore, wider decompression could be justified for less neural damage despite decreased stability and fusion surgery in some cases.5) However, this approach is not recommendable for relatively young patients.
Biportal endoscopic spine surgery (BESS) is a newly introduced method in the field of minimally invasive spine surgery. It has fewer limitations and is safer in terms of approaches than the conventional PELD due to free handling of instruments by another hand and less irritation of the roots. During the procedure, handling of an endoscope is so dynamic under very higher magnification and brightness that delicate manipulation of the soft tissue and neural tissue is possible.6,7) Owing to these merits of BESS, we tried to perform revision surgery for recurrent LDH and described the procedure in detail for recurred LDH to avoid unnecessary wider decompression and consequent need for instrumentation with fusion surgery.
TECHNIQUE
Step 1. Patient Preparation and Disc Staining
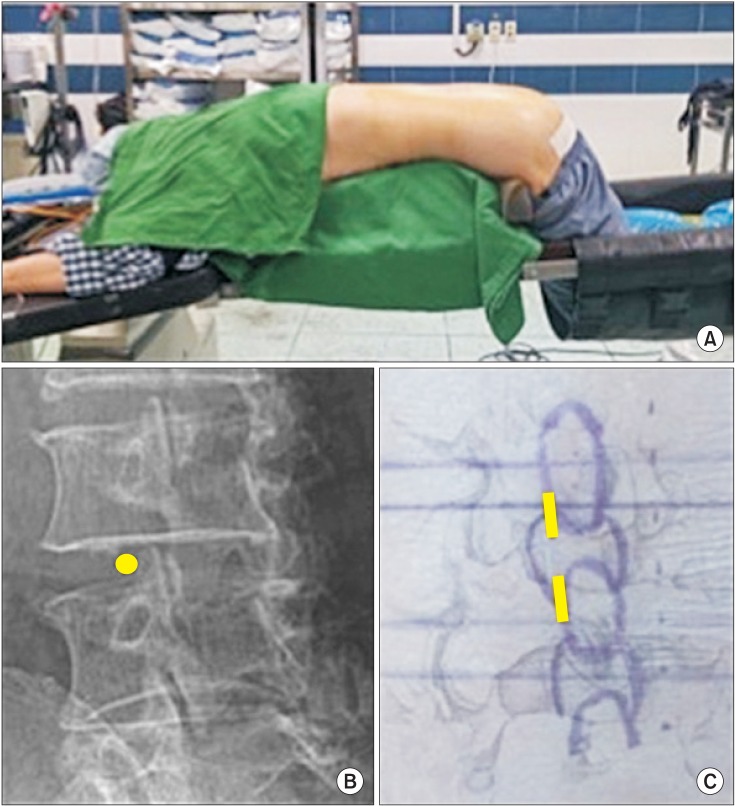
The patient was prepared in the prone position over the radiolucent chest frame in a flexed position. The head and the upper back should be placed slightly lower than the lower back and buttocks for sufficient circulation of the head when the systolic blood pressure should be maintained at 90–100 mmHg to reduce bleeding from the surgical field. The hip was flexed naturally about 70° and the knee needed to be protected with soft pillows. The surgeon examined the pressure under the patient's knees by inserting the hand under the patient's knees and made the hip and knee comfortable. If too tight, the patient would feel and complain anterior pain of the hip and knee within a few postoperative days. Fluoroscopic confirmation of a certain level should be made before incision. Using a 22-gauge spinal needle, dyeing of the disc was performed with indigo carmine just lateral side of the superior articular process at the disc level under the guidance of a 30° rotated fluoroscopy (Fig. 1) to locate the torn site of the annulus or ruptured disc material under the scar tissue.
Fig. 1. Preparation of the procedure. (A) The position of the patient with the back flat, the head down and the hip flexed. (B) The target point of spinal needle insertion for dyeing of the disc. (C) Biportal inlets were located over the margin of the interlaminar space rather than the level of disc space.
Step 2. Lamina Margin Exposure and Release from Scar Tissue
Two standard entry points on the skin were made at the upper and lower end margins of the inter-lamina space just beside the spinous process. The closer to the medial side of it, the better and wider the view on the inner side of the spinal canal. The mid-position was already covered by granulation tissue after the previous surgery and normal anatomic barrier of ligamentum flavum was absent. Surrounding nominated structures were already imbedded by scar tissue so that there was no suspected referencing landmark just as in the primary surgery. Bone touching on the lamina by a blunt dilator for making working space and water flow was the important step. The scar tissue was scratched off on the dorsal surface and medial edge of the lamina until a shaver and a radiofrequency device differentiated it from the naked bony margin of the lamina. Scar release should be done from the medial side of the facet, distal lamina and then proximal lamina so that the dura under the heavy scar could be retracted medially with less tension from the surrounding scar tissue.
Step 3. Adhesiolysis to the Base of the Pedicle
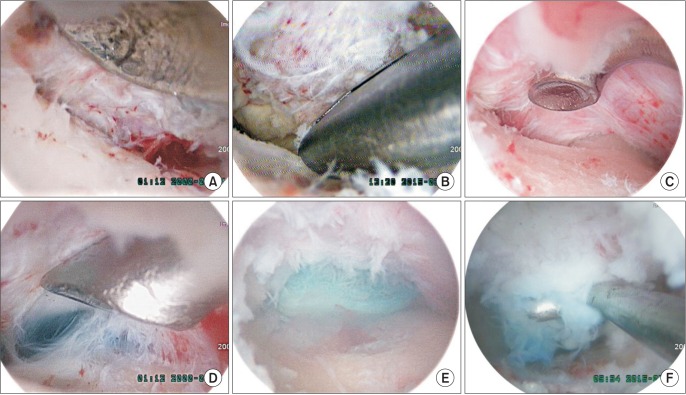
An attempt was made to trace the bony margin of the pedicle to the base where adhesiolysis was performed in a piecemeal fashion with a freer and small-headed (2 mm) angled curette until the dye-tinged annulus or ruptured disc was exposed. An additional root retractor was not necessary due to the magnified view if the distal and proximal release from the lamina was done. There was little bleeding from the scar tissue so that the general field was far clearer than the primary surgery. Minor bleeding could be controlled easily with a small tipped radiofrequency device (Fig. 2).
Fig. 2. Revision biportal endoscopic spine surgery. (A) With the bony margin of the facet exposed, adhesiolysis of scar tissue was safely performed from the bony tissue of the lamina under excellent magnification and bright illumination with curetting to differentiate it from soft tissue scar. (B) Decompression somewhat wider with a Kerrison punch to make a room for inspecting the disc space. (C) Probing and searching the disc space along the bony margin of the pedicle to the base. (D) Stained basal area indicated the ruptured disc level. (E) The ruptured disc fragment stained by indigo carmine was exposed at the ruptured site. (F) The ruptured fragment was removed by a hook.
Step 4. Probing of the Ruptured Disc and Confirmation of the Dura Pulsation
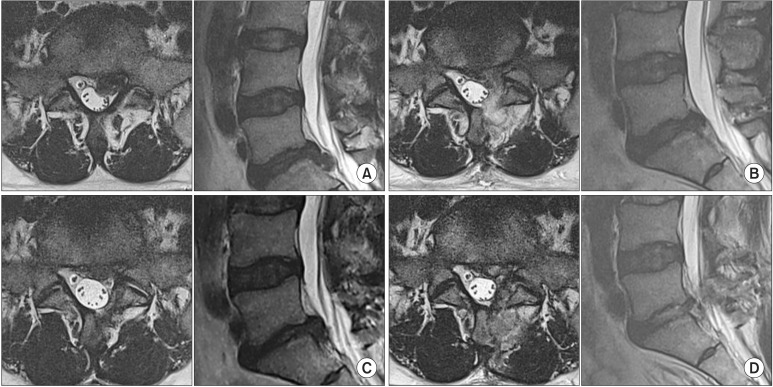
Due to the heavy scar, the mainly ruptured fragment might be missed at the initial probing. So repeated probing under the dura and just beneath the torn site of the annulus was necessary. Forceful irrigation of the inner side through the torn annulus and by a syringe with a long elastic needle should be followed to remove remnant fragments. If the causative fragment was successfully removed, pulsation of the dura and root could be observed. If there was no pulsation after removing of some fragments, the release state of the basal adhesion of the dura and root at the distal side should be confirmed again. Postoperative magnetic resonance imaging was performed on the first postoperative day (Fig. 3).
Fig. 3. A 43-year-old male patient with recurring lumbar disc herniation (LDH) at L5–S1. (A) Magnetic resonance imaging (MRI) scans of the ruptured disc in the first event. (B) Postoperative axial MRI scans after open microdiscectomy. (C) Axial and sagittal views of the recurring LDH at 7 months after the first operation. (D) Postoperative MRI scans after the revision surgery: biportal endoscopic spine surgery with decompression of the affected disc and preservation of the facet.
DISCUSSION
In this study, the BESS was performed under about 28 to 35 times magnification and very bright illumination of 2,700 to 6,700 lux. These conditions were sufficient to enable differentiation of adhesive scar tissue surrounding neural structures from bony structures. In addition, the view was not fixed by a microscope and panoramic view could be obtained with free moving of an endoscope and dynamic handing of instruments without any disturbance by soft tissue retractors. This newly introduced technique is not yet prevalent and has not been recommended especially for revision surgery.
Until accustomed to the magnified endoscopic view and new anatomy, the surgeon is advised to dye the disc to make a reference point on the over-clouded scar tissue. Sometimes, the dye overflowed from the torn annulus to the target area. Differentiation of the scar tissue should be initiated from the bony margin of the lamina. A 2 mm-angled curette can be helpful to scrape the bony surface. The bony surface of the lamina should be exposed from the proximal to the distal of the facet for the dura to be totally released from the scar tension. This would help the dura and the root to avoid excessive tension during insertion of various instruments into the small gap. The scar tissue of soft tissue was clearly differentiated from the bony tissue under the endoscopic view so that the abutting margin between them was easily diverged by curetting. In order to protect the neural tissue, deeper intrusion along the bony surface from the facet to the base of the pedicle should be performed; however, care should be taken not to go deeper inside while dividing the center of the scar tissue. By follow the bony tissue to the base of the pedicle, the disc level can be easily differentiated. Disc fragments can be several pieces. The remnant disc fragments under the dura and torn disc space could be eliminated by forceful saline irrigation. After removal of the ruptured fragments, the traversing root should be decompressed by adhesiolysis from the basal scar tissue and distal laminectomy until pulsation of the dura and root could be felt under endoscopic visualization. Even without the use of an additional root retractor, the magnified view could provide sufficient working space. For achievement of proper visualization, inlets should be located closely medial to the spinous process. The BESS also has a weak point: due to the use of water, minor bleeding could completely block the view of the surgical site. Therefore, intraoperative bleeding should never be neglected. Radiofrequency devices with a big head could generate higher energy that could cause the muscle to twitch backward excessively before sufficient coagulation. However, the small-tipped radiofrequency device could coagulate only the bleeding foci or epidural vessels one by one with lower energy. If the surgical field becomes blurred, fluent water flow and minor bleeding should be checked first.
The potential benefits of BESS include preservation of the facet joint, obtainment of the working space through trimming of the lamina, and adhesiolysis of the dura without restriction. The range of view in the floating technique of BESS is much wider than that of the conventional PELD with a docking technique into the base of the lesion. This technical aspect can make quiet different results and overcome the limitation of the conventional PELD. An adhesive surgical scar could make it harder to observe the base of the pedicle. Repeated and aggressive retraction of the dura and traversing root during probing under the dura could result in battered root syndrome or a dura tear in OLM. However, BESS with sufficient magnification for inspection of the base facilitates probing and discectomy even through a very little space without a root retraction. Insertion of a certain instrument can widen the view to examine the base of the pedicle and annulus. The 1.5 mm-diametered, upbite, pituitary dually plays as a retractor by turning its back on the dura and a grasper with medial rotation and insertion under the dura; therefore, the small space is sufficient for the instrument to probe and remove the disc fragments.
Obtainment of a clear surgical field under water by control of epidural bleeding and bone bleeding is essential for successful performance of BESS. If the surgical view were turbid due to bleeding, the procedure could not proceed any more. Care should be taken not to raise the saline bag higher or compress it to make the turbid and blurred field washed out to see the structures more clearly. The outflow is narrower when an instrument is inserted and the pushed saline could go higher to the epidural space. Then, the patient could feel and complain discomfort and pain in the neck due to the increased intracranial pressure. If lots of small bleeding from the fibrous scar tissue and trimmed cancellous bone surface could not be controlled in the early stage of learning curve, it is advised to ask an anesthesiologist to maintain systolic pressure below 100 mmHg just as in shoulder arthroscopy. The systolic pressure of 100–120 mmHg of the arm in the prone position could mean much higher pressure in the back.
BESS in revision for recurrent LDH could permit wider microscopic decompression of a traversing root through successful removal of causative disc fragment. Delicate and gentle handling of the neural structures in the surgery could also decrease the risk of over-manipulation by forceful retraction for visualization through tight scar tissue in a very narrow surgical field in revision surgery. Therefore, BESS could provide an enough working space and a sufficiently magnified and brightly illuminated view. We believe BESS could be a viable alternative for recurrent LDH to the conventional OLM or fusion surgery.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Kim MS, Park KW, Hwang C, et al. Recurrence rate of lumbar disc herniation after open discectomy in active young men. Spine (Phila Pa 1976) 2009;34(1):24–29. doi: 10.1097/BRS.0b013e31818f9116. [DOI] [PubMed] [Google Scholar]
- 2.Oh JT, Park KS, Jung SS, et al. Surgical results and risk factors for recurrence of lumbar disc herniation. Korean J Spine. 2012;9(3):170–175. doi: 10.14245/kjs.2012.9.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou T, Zhou Q, Dai F, et al. Repeated microendoscopic discectomy for recurrent lumbar disk herniation. Clinics (Sao Paulo) 2015;70(2):120–125. doi: 10.6061/clinics/2015(02)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin KH, Chang HG, Rhee NK, Lim KS. Revisional percutaneous full endoscopic disc surgery for recurrent herniation of previous open lumbar discectomy. Asian Spine J. 2011;5(1):1–9. doi: 10.4184/asj.2011.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Shazly AA, El Wardany MA, Morsi AM. Recurrent lumbar disc herniation: a prospective comparative study of three surgical management procedures. Asian J Neurosurg. 2013;8(3):139–146. doi: 10.4103/1793-5482.121685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi CM, Chung JT, Lee SJ, Choi DJ. How I do it? Biportal endoscopic spinal surgery (BESS) for treatment of lumbar spinal stenosis. Acta Neurochir (Wien) 2016;158(3):459–463. doi: 10.1007/s00701-015-2670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soliman HM. Irrigation endoscopic discectomy: a novel percutaneous approach for lumbar disc prolapse. Eur Spine J. 2013;22(5):1037–1044. doi: 10.1007/s00586-013-2701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]