Abstract
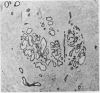
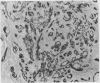
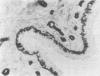
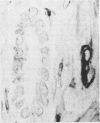
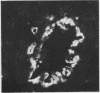
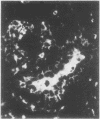
The distributions of defined basement membrane proteins in nine pure tubular carcinomas, 10 cases of sclerosing adenosis, and 15 ductal adenocarcinomas were compared. Sections of formalin fixed, paraffin embedded specimens were pretreated with pepsin and then immunostained for laminin, type IV collagen, and basement membrane proteoglycan, components specific for basement membranes. In sclerosing adenosis the tubules were surrounded by a continuous intact basement membrane composed of laminin, type IV collagen, and basement membrane proteoglycan, while the epithelium in the tubular carcinomas was negative for these proteins. The tumours were also analysed for the distribution of the apocrine epithelial antigen (AEA). In contrast to the benign lesions the tubular carcinomas expressed the AEA in a distinct non-polar fashion throughout the cell surface. In normal ducts and in adenosis the AEA was confined exclusively to the luminal surface. These studies suggest that there is a disturbance of cell polarity in tubular carcinomas. It is concluded that a combined analysis of basement membrane proteins and luminal surface antigens is a reliable and convenient way to differentiate between tubular carcinoma and sclerosing adenosis of the breast.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrechtsen R., Nielsen M., Wewer U., Engvall E., Ruoslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981 Dec;41(12 Pt 1):5076–5081. [PubMed] [Google Scholar]
- Asch B. B., Kamat B. R., Burstein N. A. Interactions of normal, dysplastic, and malignant mammary epithelial cells with fibronectin in vivo and in vitro. Cancer Res. 1981 Jun;41(6):2115–2125. [PubMed] [Google Scholar]
- Brozman M. Immunohistochemical analysis of formaldehyde- and trypsin- or pepsin-treated material. Acta Histochem. 1978;63(2):251–260. doi: 10.1016/S0065-1281(78)80032-4. [DOI] [PubMed] [Google Scholar]
- Carstens P. H., Huvos A. G., Foote F. W., Jr, Ashikari R. Tubular carcinoma of the breast: a clinicopathologic study of 35 cases. Am J Clin Pathol. 1972 Sep;58(3):231–238. doi: 10.1093/ajcp/58.3.231. [DOI] [PubMed] [Google Scholar]
- Deos P. H., Norris H. J. Well-differentiated (tubular) carcinoma of the breast. A clinicopathologic study of 145 pure and mixed cases. Am J Clin Pathol. 1982 Jul;78(1):1–7. doi: 10.1093/ajcp/78.1.1. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Alitalo K., Vaheri A., Timpl R., Saxén L. Induction of a basement membrane glycoprotein in embryonic kidney: possible role of laminin in morphogenesis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):485–489. doi: 10.1073/pnas.77.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P., Miettinen M., Rapola J., Foidart J. M. Demonstration of laminin, a basement membrane glycoprotein, in routinely processed formalin-fixed human tissues. Histochemistry. 1982;75(3):301–307. doi: 10.1007/BF00496733. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Enami J., Pitelka D. R., Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson R. A., Carstens P. H. Ultrastructure of tubular carcinoma of the breast. Cancer. 1972 Apr;29(4):987–995. doi: 10.1002/1097-0142(197204)29:4<987::aid-cncr2820290446>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Fenoglio C., Lattes R. Sclerosing papillary proliferations in the female breast. A benign lesion often mistaken for carcinoma. Cancer. 1974 Mar;33(3):691–700. doi: 10.1002/1097-0142(197403)33:3<691::aid-cncr2820330313>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Foidart J. M., Bere E. W., Jr, Yaar M., Rennard S. I., Gullino M., Martin G. R., Katz S. I. Distribution and immunoelectron microscopic localization of laminin, a noncollagenous basement membrane glycoprotein. Lab Invest. 1980 Mar;42(3):336–342. [PubMed] [Google Scholar]
- Harris M., Ahmed A. The ultrastructure of tubular carcinoma of the breast. J Pathol. 1977 Oct;123(2):79–83. doi: 10.1002/path.1711230203. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Robey P. G., Barrach H. J., Wilczek J., Rennard S. I., Martin G. R. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4494–4498. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagios M. D. Multicentricity of breast carcinoma demonstrated by routine correlated serial subgross and radiographic examination. Cancer. 1977 Oct;40(4):1726–1734. doi: 10.1002/1097-0142(197710)40:4<1726::aid-cncr2820400449>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Lagios M. D., Rose M. R., Margolin F. R. Tubular carcinoma of the breast: association with multicentricity, bilaterality, and family history of mammary carcinoma. Am J Clin Pathol. 1980 Jan;73(1):25–30. doi: 10.1093/ajcp/73.1.25. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Vembu D., Kleinman H. K., Martin G. R., Boone C. Collagen required for proliferation of cultured connective tissue cells but not their transformed counterparts. Nature. 1978 Apr 13;272(5654):622–624. doi: 10.1038/272622a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Wicha M. S., Foidart J. M., Rennard S. I., Garbisa S., Kidwell W. R. Hormonal requirements for basement membrane collagen deposition by cultured rat mammary epithelium. Lab Invest. 1979 Dec;41(6):511–518. [PubMed] [Google Scholar]
- Nemoto T., Vana J., Bedwani R. N., Baker H. W., McGregor F. H., Murphy G. P. Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer. 1980 Jun 15;45(12):2917–2924. doi: 10.1002/1097-0142(19800615)45:12<2917::aid-cncr2820451203>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Shannon J. M., Pitelka D. R. The influence of cell shape on the induction of functional differentiation in mouse mammary cells in vitro. In Vitro. 1981 Nov;17(11):1016–1028. doi: 10.1007/BF02618428. [DOI] [PubMed] [Google Scholar]
- Siegal G. P., Barsky S. H., Terranova V. P., Liotta L. A. Stages of neoplastic transformation of human breast tissue as monitored by dissolution of basement membrane components. An immunoperoxidase study. Invasion Metastasis. 1981;1(1):54–70. [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Tobon H., Salazar H. Tubular carcinoma of the breast. Clinical, histological, and ultrastructural observations. Arch Pathol Lab Med. 1977 Jun;101(6):310–316. [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- Warburton M. J., Mitchell D., Ormerod E. J., Rudland P. Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating, and involuting rat mammary gland. J Histochem Cytochem. 1982 Jul;30(7):667–676. doi: 10.1177/30.7.6179984. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Garbisa S., Kidwell W. R. Basement membrane collagen requirements for attachment and growth of mammary epithelium. Exp Cell Res. 1979 Nov;124(1):181–190. doi: 10.1016/0014-4827(79)90268-4. [DOI] [PubMed] [Google Scholar]