Abstract
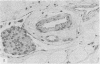
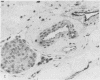
Immunocytochemical stains were used to find out whether they would increase the accuracy of detecting lymphatic and vascular invasion by primary breast cancer cells over conventional histological methods. Immune probes for type IV collagen were of value in confirming the conventional diagnosis of vascular invasion while stains for factor VIII assisted in differentiating small blood vessels from lymphatic channels. Antibodies to type IV collagen also increased the accuracy and rate of detection achieved by conventional histology.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Bettelheim R., Neville A. M. Lymphatic and vascular channel involvement within infiltrative breast carcinomas as a guide to prognosis at the time of primary surgical treatment. Lancet. 1981 Sep 19;2(8247):631–631. doi: 10.1016/s0140-6736(81)92760-4. [DOI] [PubMed] [Google Scholar]
- Dearnaley D. P., Ormerod M. G., Sloane J. P., Lumley H., Imrie S., Jones M., Coombes R. C., Neville A. M. Detection of isolated mammary carcinoma cells in marrow of patients with primary breast cancer. J R Soc Med. 1983 May;76(5):359–364. doi: 10.1177/014107688307600506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusterson B. A., Warburton M. J., Mitchell D., Ellison M., Neville A. M., Rudland P. S. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982 Nov;42(11):4763–4770. [PubMed] [Google Scholar]
- Jaffe E. A. Endothelial cells and the biology of factor VIII. N Engl J Med. 1977 Feb 17;296(7):377–383. doi: 10.1056/NEJM197702172960707. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Wicha M. S., Foidart J. M., Rennard S. I., Garbisa S., Kidwell W. R. Hormonal requirements for basement membrane collagen deposition by cultured rat mammary epithelium. Lab Invest. 1979 Dec;41(6):511–518. [PubMed] [Google Scholar]
- Mitchell D. P., Gusterson B. A. Simultaneous demonstration of keratin and mucin. J Histochem Cytochem. 1982 Jul;30(7):707–709. doi: 10.1177/30.7.6179986. [DOI] [PubMed] [Google Scholar]
- Mukai K., Rosai J., Burgdorf W. H. Localization of factor VIII-related antigen in vascular endothelial cells using an immunoperoxidase method. Am J Surg Pathol. 1980 Jun;4(3):273–276. doi: 10.1097/00000478-198006000-00008. [DOI] [PubMed] [Google Scholar]
- Nime F. A., Rosen P. P., Thaler H. T., Ashikari R., Urban J. A. Prognostic significance of tumor emboli in intramammary lymphatics in patients with mammary carcinoma. Am J Surg Pathol. 1977 Mar;1(1):25–30. doi: 10.1097/00000478-197701010-00003. [DOI] [PubMed] [Google Scholar]
- Rosen P. P., Saigo P. E., Braun D. W., Jr, Weathers E., DePalo A. Predictors of recurrence in stage I (T1N0M0) breast carcinoma. Ann Surg. 1981 Jan;193(1):15–25. doi: 10.1097/00000658-198101000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton M. J., Mitchell D., Ormerod E. J., Rudland P. Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating, and involuting rat mammary gland. J Histochem Cytochem. 1982 Jul;30(7):667–676. doi: 10.1177/30.7.6179984. [DOI] [PubMed] [Google Scholar]