Abstract
A major feature of the human cortex is its huge morphological variability. Although a comprehensive literature about the sulco-gyral pattern of the central region is available from post-mortem data, a reliable and reproducible characterization from in vivo data is still lacking. The aim of this study is to test the reliability of morphological criteria of the central region sulci used in post-mortem data, when applied to in vivo magnetic resonance imaging (MRI) data. Thirty right-handed healthy individuals were included in the study. Automated segmentation and three dimensional (3D) surface-based rendering were obtained from clinical 3D T1-weighted MRI. Two senior radiologists labeled the three sulci composing the central region (precentral [PreCS], central [CS] and postcentral [PostCS]) and analyzed their morphological variations using 47 standard criteria derived from Ono’s atlas based on post-mortem data. For each criterion, inter-rater concordance and comparison with the occurrence frequency provided in Ono’s atlas were estimated. Overall, the sulcal pattern criteria derived from MRI data were highly reproducible between the raters with a high mean inter-rater concordance in the three sulci (CS: κ = 0.92 in left hemisphere/κ = 0.91 in right hemisphere; PreCS: κ = 0.91/κ = 0.93; PostCS: κ = 0.84/0.79). Only a very limited number of sulcal criteria significantly differed between the in vivo and the post-mortem data (CS: 2 criteria in the left hemisphere/3 criteria in the right hemisphere; PreCS: 3 in the left and right hemispheres; PostCS: 3 in the left hemisphere and 5 in the right hemisphere). Our study provides a comprehensive description of qualitative sulcal patterns in the central region from in vivo clinical MRI with high agreement with previous post-mortem data. Such identification of reliable sulcal patterns of the central region visible with standard clinical MRI data paves the way for the detection of subtle variations of the central sulcation associated with variations of normal or pathological functioning.
Keywords: central sulcus, pre-central sulcus, post-central sulcus, magnetic resonance imaging, cortex, sulcal patterns
Introduction
Sulci and gyri provide a natural topographic partition of the cortical anatomy. A huge inter- and intra-individual variability in the morphology of the cortical gyri and sulci—including size, shape and spatial pattern—has been previously reported (Ono et al., 1990; Rademacher et al., 1993; Rajkowska and Goldman-Rakic, 1995; Bartley et al., 1997; Blanton et al., 2001). Such variability raises methodological issues for functional brain mapping (Hunton et al., 1996) as well as for lesion localization in neurosurgical planning (Signorelli et al., 2001; Quiñones-Hinojosa et al., 2003). Difficulties in disentangling normal from abnormal morphological variations affect the detection of subtle dysmorphology associated with neurological or psychiatric conditions, e.g., neurodevelopmental impairments, schizophrenia (Yücel et al., 2002; Nakamura et al., 2007a; Plaze et al., 2011, 2015; Cachia et al., 2015) or malformation of cortical development (Barkovich et al., 2012).
The central region, located between the precentral sulcus (PreCS) and the postcentral sulcus (PostCS) and centered on the central sulcus (CS), provides major anatomo-functional landmarks. For instance, the CS hand knob is a stable morphological landmark (Yousry et al., 1997) and limits the primary motor area in the precentral gyrus and the primary somatosensory area in the postcentral gyrus. Descriptions of the central region and its variations have been based on visual inspection of post-mortem brains (Broca, 1888; Campbell, 1905; Cunningham, 1905). More recently, the sulcal anatomy from 25 post-mortem brains provided standardized morphological criteria to define and identify each sulcus of the human brain (Ono et al., 1990). This atlas provided by Ono introduced 47 criteria to characterize the central region sulci (PreCS, CS and PostCS). It is unclear, however, whether these criteria from post-mortem data can be transposed to in vivo data obtained from clinical magnetic resonance imaging (MRI). To this end, we evaluated the inter-rater concordance of each Ono criterion estimated from three dimensional (3D) reconstructions of brain surface (Mangin et al., 2004) obtained from anatomical MRI from 30 control subjects and compared their occurrence frequency with that from post-mortem data in Ono’s seminal study (Ono et al., 1990). Only the standard Ono atlas was used in this study as it is the only atlas that provides quantitative description (i.e., distribution percentage) of the morphological sulcal pattern variations.
Materials and Methods
Subjects
Thirty healthy, right-handed individuals (14 men, 16 women; median age = 30 years, range = 22–50 years) were participants in the study. Imaging was performed with the informed written consent of the participants and the approval of the ethics committee Ile-de-France III.
MRI Acquisition
In all the subjects, images were acquired on a 1.5 T Signa MR scanner (Signa 1.5T, General Electrics Healthcare, Milwaukee, WI, USA) using an inversion recovery 3D T1-weighted fast-spoiled gradient recalled acquisition (repetition time/echo time/flip angle: 10/2 ms/15°, 1.2 mm slice thickness, no gap, in-plane resolution: 0.93 × 0.93 mm, acquisition time: 6 min 14 s).
Image Analysis
In order to generate 3D images of the sulci, the raw MRI data were subjected to automatized segmentation (Mangin et al., 2004) and 3D surface-based rendering using BrainVisa software1 using standard parameters. Briefly, an automated pre-processing step skull-stripped T1-weighted MRI, segmented the brain tissues (cerebrospinal fluid, gray matter, and white matter), separated the two hemispheres and reconstructed the 3D surfaces corresponding to the gray–white matter and gray matter–cerebrospinal fluid interface. The cortical folds were then automatically segmented throughout the cortex from the skeleton of the gray matter–cerebrospinal fluid mask, with the cortical folds corresponding to the crevasse bottoms of the “landscape”, the altitude of which is defined by the intensity on MRI. This definition provides a stable and robust sulcal surface definition that is not affected by variations of the gray–white matter contrast. The cortical folds were then converted to a graph-based representation of the cortex containing information relating to shape (area, depth and length) and spatial organization (position and orientation). No spatial normalization was applied to the MRI data to overcome potential bias due to the sulcus shape deformations induced by the warping process.
Sulcal Characterization
The three sulci of the central region (preCS, CS and postCS) were manually labeled and analyzed independently by two senior radiologists (CM and M-NL) based on the presence or absence of the 47 morphological criteria used in Ono’s atlas (Ono et al., 1990). Only anatomical landmark derived from standard clinical MR images were used to identify the sulci. Table 1 provides a description of the 16 criteria defining the CS, Table 2 the description of the 18 criteria defining the PreCS and Table 3 the description of the 13 criteria defining the PostCS.
Table 1.
Description of the 16 criteria defining the pattern of the CS.
| Region/pattern | Criteria | Description |
|---|---|---|
| Whole sulcus | Continuous or discontinuous | Interruption (discontinuity) is caused by a transitional convolution |
| Inferior end | Extension into the SF | Connection between CS and SF |
| Anterior direction | Compared to the general axis of the central sulcus | |
| Posterior direction | ||
| Straight shape | General shape of the inferior end of the sulcus | |
| “Y” shape | ||
| “T” shape | ||
| Superior end | Extension into the medial surface | |
| Straight shape | General shape of the superior end of the sulcus | |
| “Y” shape | ||
| “T” shape | ||
| Side branches | Over precentral gyrus | Small sulci having the same depth as CS, cutting anteriorly |
| Over postcentral gyrus | Small sulci having the same depth as CS, cutting posteriorly | |
| Connections | With precentral sulcus | Connections result from the union of two sulci that run in the same direction and at the same level |
| With postcentral sulcus | ||
| With small free sulcus in precentral gyrus |
SF, Sylvian fissure; CS, central sulcus.
Table 2.
Description of the 18 criteria defining the pattern of the preCS.
| Region/Pattern | Criteria | Description |
|---|---|---|
| Whole sulcus | Number of segments | Interruption between segments are caused by a transitional convolution |
| Marginal PreCS | Present or absent | Horizontally oriented sulcus, over the lateral surface and above the superior side of the preCS |
| Medial PC sulcus | Present or absent | Notches the superior margin of the hemisphere above the superior preCS. |
| Of note, two medial precentral sulci can coexist in the same hemisphere (on each side of the preCS axis) | ||
| Superior segment | Arcuate termination with Y-shaped end | According to the sulcus curvature (arcuate or T-shaped) and the end shape (straight or Y-shaped) |
| T-shaped side anastomosis with Y-shaped end | ||
| Inferior segment | Arcuate form | According to the general shape of the sulcus |
| Ramified form | ||
| Bayonet form | ||
| Y-shaped end | ||
| Inferior end | Extension into SF | |
| Straight shape | ||
| Y-shape | ||
| Connections | With central sulcus | Connections result from the union of two sulci that run in the same direction and at the same level |
| With superior frontal sulcus | ||
| With intermediate frontal sulcus | ||
| With inferior frontal sulcus |
SF, Sylvian fissure; preCS, precentral sulcus.
Table 3.
Description of the 13 criteria defining the pattern of the postCS.
| Region/Pattern | Criteria | Description |
|---|---|---|
| Whole sulcus | Number of segments | Interruption between segments are caused by a transitional convolution |
| Inferior end | Extension into SF | Connection between postCS and SF |
| Double parallel pattern | With intraparietal sulcus | Orientation of the postCS parallel to the intraparietal sulcus |
| With posterior subcentral sulcus | Orientation of the postCS parallel to the posterior subcentral sulcus | |
| Superior end | Y-shape | According to the general shape of the sulcus |
| Straight | ||
| Extension to the medial surface | ||
| Side branches | Over postcentral gyrus | Small sulci having the same depth as postCS, cutting anteriorly or posteriorly |
| Over superior parietal lobule | ||
| Over inferior parietal lobule | ||
| Connections | With central sulcus | Connections result from the union of two sulci that run in the same direction and at the same level |
| With intraparietal sulcus | ||
| With superior temporal sulcus |
SF, Sylvian fissure; postCS, postcentral sulcus.
Statistical Analysis
For each of the 47 Ono’s criteria in the left and right hemispheres, we estimated: (1) the inter-rater concordance (kappa index); and (2) the difference with the data obtained by Ono from post-mortem data (Chi square tests).
Results
Inter-Rater Concordance for In Vivo Data
The mean inter-rater concordance (kappa index) was calculated for all sulcal pattern criteria.
For the CS, mean inter-rater concordance was excellent in both left (κ = 0.92, range: 0.77–1.0) and right (κ = 0.91, range: 0.77–1.0) hemisphere (Table 4). The concordance was good only for the number of sulcal branches over the precentral gyrus (κ = 0.77).
Table 4.
Mean occurrence frequency and inter-rater concordance of each criterion of the CS in the left and right hemispheres.
| Criteria | Variations | Left hemisphere | Right hemisphere | ||
|---|---|---|---|---|---|
| Mean (%) | κ | Mean (%) | κ | ||
| Interruptions | Continuous | 97 | 1 | 97 | 1 |
| Inferior end | Extension into the SF | 13 | 0.96 | 17 | 0.92 |
| Anterior direction | 40 | 0.86 | 53 | 0.79 | |
| Posterior direction | 60 | 0.86 | 47 | 0.79 | |
| Straight shape | 100 | 0.82 | 90 | 0.94 | |
| “Y” shape | 0 | 1 | 10 | 0.95 | |
| “T” shape | 0 | 0.91 | 0 | 1 | |
| Superior end | Extension into the medial surface | 57 | 0.80 | 50 | 0.80 |
| Straight shape | 100 | 1 | 93 | 0.94 | |
| “Y” shape | 0 | 1 | 3 | 0.95 | |
| “T” shape | 0 | 1 | 3 | 0.95 | |
| Side branches | Over precentral gyrus | ||||
| 0 | 67 | 67 | |||
| 1 | 27 | 20 | |||
| 2 | 3 | 0.77 | 10 | 0.77 | |
| 3 | 3 | 3 | |||
| 4 | 0 | 0 | |||
| Over postcentral gyrus | |||||
| 0 | 85 | 83 | |||
| 1 | 13 | 17 | |||
| 2 | 2 | 0.88 | 0 | 0.84 | |
| 3 | 0 | 0 | |||
| 4 | 0 | 0 | |||
| Connections | With precentral sulcus | 27 | 0.92 | 10 | 0.95 |
| With postcentral sulcus | 0 | 1 | 0 | 1 | |
| With small free sulcus in preC gyrus | 0 | 0.95 | 7 | 0.95 | |
Sulcal criteria with high inter-rater concordance (κ > 0.8) are shown in bold font. Measures with a significant difference between in vivo and ex vivo data values (p-value < 0.05) are underlined. preC, precentral.
For the PreCS, mean inter-rater concordance was also excellent in both left (κ = 0.91, range: 0.76–1.0) and right (κ = 0.93, range: 0.61–1.0) hemisphere (Table 5). It was good only for the left medial and superior segment variation and the right inferior end variation (κ < 0.8).
Table 5.
Mean occurrence frequency and inter-rater concordance of each sulcal criterion of the preCS in the left and right hemispheres.
| Criteria | Variations | Left hemisphere | Right hemisphere | ||
|---|---|---|---|---|---|
| Mean (%) | κ | Mean (%) | κ | ||
| Number of segments | 2 | 60 | 0.95 | 70 | 1 |
| 3 | 37 | 0.94 | 30 | 1 | |
| 4 | 3 | 1 | 0 | 1 | |
| Marginal PreCS | 30 | 0.89 | 37 | 0.90 | |
| Medial PreCS | 0 | 13 | 0.83 | 23 | 0.90 |
| 1 | 60 | 0.77 | 53 | 0.86 | |
| 2 | 27 | 0.89 | 23 | 0.98 | |
| Superior segment | Arcuate termination | 43 | 0.82 | 33 | 0.95 |
| With Y-shaped end | 10 | 0.93 | 10 | 1 | |
| T-shaped side anastomosis | 33 | 0.77 | 47 | 0.91 | |
| With Y-shaped end | 13 | 0.76 | 10 | 0.93 | |
| Inferior segment | Arcuate form | 33 | 0.86 | 17 | 0.94 |
| Ramified form | 33 | 0.90 | 40 | 0.95 | |
| Bayonet form | 10 | 0.88 | 27 | 1 | |
| Y-shaped end | 23 | 0.94 | 17 | 1 | |
| Inferior end | Extension into SF | 60 | 0.90 | 50 | 1 |
| Straight shape | 97 | 1 | 90 | 0.61 | |
| Y-shape | 3 | 1 | 10 | 0.61 | |
| Connections | With CS | 27 | 0.91 | 10 | 1 |
| With superior FS | 80 | 1 | 73 | 0.91 | |
| With intermediate FS | 23 | 1 | 27 | 0.91 | |
| With inferior FS | 77 | 1 | 57 | 1 | |
Sulcal criteria with high inter-rater concordance (κ > 0.8) are shown in bold font. Measures that significantly differ between in vivo and ex vivo data values (p-value < 0.05) are underlined. PreCS, precentral sulcus; CS, central sulcus, SF, sylvian fissure; FS, frontal sulcus.
Finally, for the PostCS, the mean inter-rater concordance was globally excellent with κ = 0.84 (range: 0.59–1.0) for the left hemisphere and 0.79 (range: 0.60–1.0) for the right hemisphere (Table 6), even though the concordant was good only for several criteria (left number of segments, superior end variation, side branches over postcentral gyrus and right number of segments, inferior and superior end variation, and number of side branches over postcentral gyrus and inferior parietal lobule).
Table 6.
Mean occurrence frequency and inter-rater concordance of each criterion of the postCS in the left and right hemispheres.
| Criteria | Variations | Left hemisphere | Right hemisphere | ||
|---|---|---|---|---|---|
| Mean (%) | κ | Mean (%) | κ | ||
| Number of segments | Continuous | 60 | 0.92 | 73 | 0.73 |
| Two | 30 | 0.84 | 20 | 0.79 | |
| Three | 10 | 0.78 | 7 | 0.65 | |
| Inferior end | Extension into SF | 40 | 0.92 | 37 | 0.71 |
| Double parallel pattern | With intraparietal sulcus | 27 | 0.59 | 33 | 0.92 |
| With posterior subcentral sulcus | 3 | 1 | 7 | 1 | |
| Superior end | Y-shape | 43 | 0.79 | 27 | 0.64 |
| Straight | 57 | 0.79 | 73 | 0.64 | |
| Extension to the medial surface | 27 | 0.65 | 30 | 0.73 | |
| Side branches | Over postcentral gyrus | ||||
| 0 | 17 | 0.72 | 33 | 0.74 | |
| 1 | 47 | 50 | |||
| 2 | 20 | 10 | |||
| 3 | 13 | 7 | |||
| 4 | 3 | 0 | |||
| 5 | 0 | 0 | |||
| Over superior parietal lobule | |||||
| 0 | 17 | 18 | |||
| 1 | 12 | 0.93 | 12 | 0.86 | |
| 2 | 3 | 0 | |||
| Over inferior parietal lobule | |||||
| 0 | 77 | 87 | |||
| 1 | 23 | 0.90 | 13 | 0.60 | |
| 2 | 0 | 0 | |||
| Connections | With CS | 0 | 1 | 0 | 1 |
| With intraparietal sulcus | 80 | 0.88 | 77 | 0.83 | |
| With superior temporal sulcus | 0 | 1 | 17 | 1 | |
Sulcal criteria with high inter-rater concordance (κ > 0.8) are shown in bold font. Measures with a significant difference between in vivo and ex vivo data values (p-value < 0.05) are underlined. PostC, postcentral; CS, central sulcus; SF, Sylvian fissure.
Comparison of In Vivo and Post-Mortem Data
Overall, 80% of the criteria in the in vivo analysis did not differ from the analysis based on Ono’s data regarding their frequency of occurrence. Only a limited number of sulcal criteria significantly differed (p < 0.05) between the in vivo and the post-mortem data: there were 3 (18%) (resp. 2 [12%]) in the left (resp. right) CS (Table 4), 3 (13%) (resp. 3 [13%]) in the left (resp. right) PreCS (Table 5) and 3 (20%) (resp. 5 [33%]) in the left (resp. right) PostCS (Table 6).
The criteria that differed between Ono’s post-mortem data and in vivo MRI data were:
-
–
for the CS: inferior end straight shape, inferior end T-shape and the number of connections with small free sulcus in the left precentral gyrus;
-
–
for the preCS: extension to the left Sylvian fissure (SF), number of connections with left CS, right superior segment anastomosis, inferior end arcuate form and connections with right frontal sulcus (FS);
-
–
for the post CS: left and right superior end variations, right inferior end variation and right double parallel pattern.
Details of features that showed excellent inter-rater concordance (κ > 0.80; Landis and Koch, 1977) and that did not differ from Ono’s post-mortem values are represented in Figure 1 for the CS, Figure 2 for the Pre-CS and Figure 3 for the Post-CS.
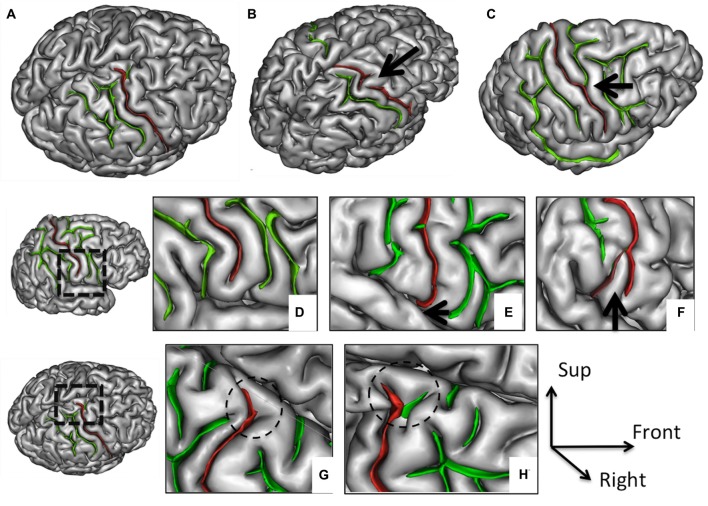
Figure 1.
Morphological features of the central sulcus (CS) with an excellent inter-rater concordance (κ > 0.80) and with values (frequency occurrence) that did not differ from Ono’s post-mortem values. The CS (in red) is represented on a three dimensional (3D) mesh-based reconstruction of the cortex surface. Continuous (A) or interrupted (B, arrow) CS. Connection with the precentral sulcus (PreCS; C, arrow). Inferior end without (D) or with extension to the sylvian fissure (SF; E, arrow). Inferior end “Y” shape (F). Superior end: “T” shape (G) or “Y” shape (H).
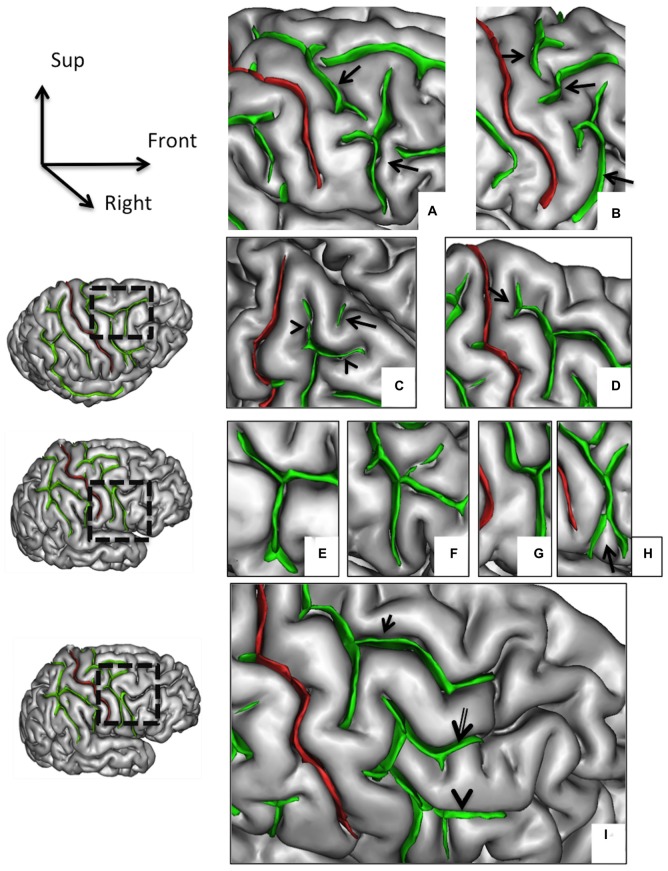
Figure 2.
Morphological features of the precentral sulcus (PreCS) with an excellent inter-rater concordance (κ > 0.80) and with values (frequency occurrence) that do not differ from Ono’s post-mortem values. The PreCS (in green) represented on a 3D mesh-based reconstruction of the cortex surface. PreCS with two (A) or three (B) segments (arrows). PreCS superior end patterns (C) with marginal PreCS (arrow heads) and medial PreCS (arrow). PreCS superior segment shape with arcuate termination with Y-shaped end (D, arrow). Pre-CS inferior segment patterns with arcuate form (E), ramified form (F), bayonet form (G) and Y-shaped end (H, arrow). PreCS connections (I) with superior frontal sulcus (arrow), intermediate frontal sulcus (double arrow) or inferior frontal sulcus (arrow head).
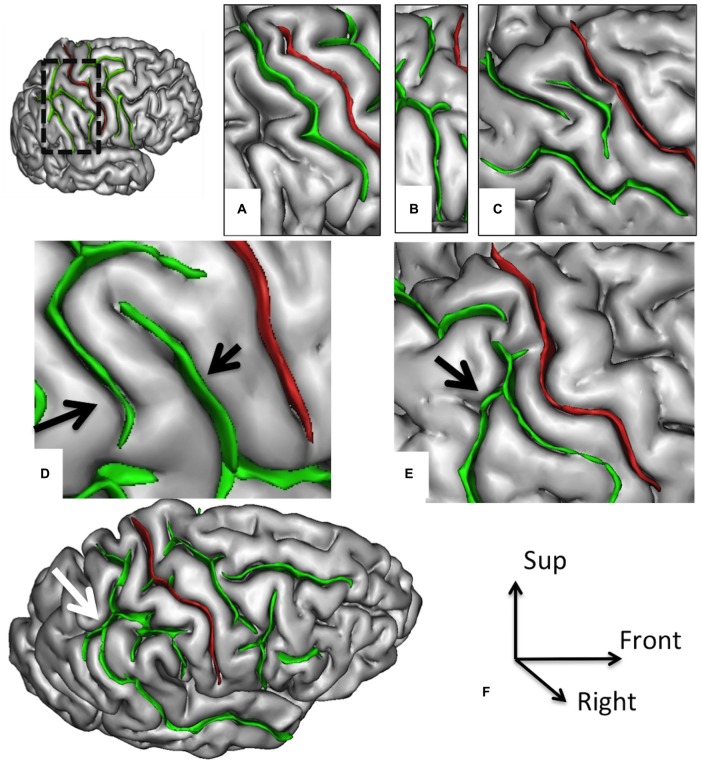
Figure 3.
Morphological features of the postcentral sulcus (PostCS) with an excellent inter-rater concordance (κ > 0.80) and with values (frequency occurrence) that do not differ from Ono’s post-mortem values. The PostCS (in green) represented on a 3D mesh-based reconstruction of the cortex surface. PostCS with one (A), two (B) or three (C) segments. PostCS double parallel pattern (D, arrows). PostCS connections with intraparietal sulcus (E, arrow) or with superior temporal sulcus (F, arrow).
Discussion
The present MRI study on the sulcal variability in the central region confirms the sulcal pattern distributions reported in Ono’s atlas and its applicability to MRI studies. It provides two main findings: (1) most of the 47 sulcal criteria characterizing the sulcal morphology of the central region, initially developed by Ono for post-mortem data (Ono et al., 1990), can be used for in vivo MRI data; (2) analysis of in vivo (MRI) and ex vivo (post-mortem) data provide similar variations of the normal sulcal anatomy of the CS, preCS and postCS. The most noticeable difference between the analysis based on 3D MR images and the analysis based on post-mortem data concerns the number of side branches and sulcal connections, with more indentations in the post-mortem description than in the MRI analysis. Such differences likely result from a lower spatial resolution in MRI data compared to ex vivo data, and from a post-processing regularization step (spatial pruning in the sulcal skeleton of small folds) used to limit the effect of a minor segmentation error (Mangin et al., 1995).
Relevance for Clinical Studies
By providing reference data on normal variations of MRI-derived sulcal features of the central area, this work has important clinical applications as it allows clinicians detecting unusual sulcal patterns. This may be useful, for instance, in patients referred for intractable epilepsy and suspected of having focal cortical dysplasia (FCD) in the central region with a normal MRI based on conventional analysis. Up to 50% of such dysplasia affect the central region (Mellerio et al., 2012). Sulcal abnormalities have been reported in FCD, such as abnormally deep sulci (Besson et al., 2008). Such features are difficult to be detected by the human eye and are likely underestimated due to the absence of standard criteria to disentangle normal from abnormal sulcal patterns. Based on the visual analysis of the 3D surface rendering MR images, one study describes a specific sulcal pattern associated with the presence of an FCD in the central region (Mellerio et al., 2015) even in patients with “normal” MRI. The present study could provide reference data for such an individual sulcal-based analysis. For instance, in a patient referred for intractable epilepsy and suspected of having cortical dysplasia in the central region, the presence of an unusual value for one or several morphological criteria could help to detect an underlying lesion, even in the case of negative MRI.
In addition to neurological conditions, the analysis of the central region sulcation may also be of interest in psychiatric disorders, notably in schizophrenia. Indeed, motor impairments have been reported in schizophrenia (Strube et al., 2014), but it is still not clear to what extent they can be attributed to the anatomy of the central region. Hence, neurological soft signs—i.e., observable defects in motor coordination, motor integration and sensory integration—have been reported at all stages of schizophrenia, including in first episode psychosis patients and in antipsychotic naïve patients (Bombin et al., 2005). A recent study of schizophrenia patients detected an association between the presence of neurological soft signs and the sulcal surface area in several cortical regions but not in the central region (Gay et al., 2013). It will be interesting to test whether some sulcal descriptors of the central region identified in the present study, which are more reliable than a simple measure of surface area, can pinpoint abnormal sulcal patterns of CS, preCS or postCS associated with neurological soft signs.
Investigating the Long Term Effect of Sulcal Region Ontogenesis
The sulcal pattern results from early processes during fetal life that shape the cortex anatomy from a smooth lissencephalic structure to a highly convoluted surface (Mangin et al., 2010). Several genetic and environmental factors (Dehay et al., 1996; Molko et al., 2003; Rakic, 2004; Barkovich et al., 2012) contribute to the neurodevelopmental processes that influence the shape of the folded cerebral cortex, including structural connectivity through axonal tension forces (Van Essen, 1997; Hilgetag and Barbas, 2006). These mechanical constraints lead to a compact layout that optimizes the transmission of neuronal signals between brain regions (Klyachko and Stevens, 2003) and thus brain network functioning. This association between cortical folding and network functioning may explain why the sulcal pattern is an early marker of later functional development (Dubois et al., 2008).
The qualitative features of the sulcal pattern, like the 47 criteria of the central region investigated in the current study, are markers of early brain development (Welker et al., 1988). Indeed, as opposed to quantitative measures of the cortex anatomy—such as the Gyrification Index (Armstrong et al., 1995; White et al., 2010; Zilles et al., 2013) or the thickness, surface, and volume of the cortex (Gogtay et al., 2004; Giedd et al., 2009) that vary from childhood through early adulthood—the sulcal pattern is a stable feature of the brain anatomy not affected by brain maturation occurring after birth (Sun et al., 2012; Cachia et al., 2016). The analysis of the qualitative features of the sulcal pattern therefore raises new possibilities to investigate the long term effect of fetal life on symptom variability in neurological (Kim et al., 2008; Régis et al., 2011; Roca et al., 2015) and psychiatric (Yücel et al., 2002; Nakamura et al., 2007b; Cachia et al., 2008; Plaze et al., 2015) disorders and also on normal variability in cognitive efficiency in healthy subjects (Cachia et al., 2014; Borst et al., 2016).
The exact link between the folding pattern and functional competence is a complex issue (Welker et al., 1988; Zilles et al., 2013). However, strong correspondences between cortical folding features and functional activations have been found not only in primary areas but also in higher level areas, including the visual areas (Watson et al., 1993; Dumoulin et al., 2000), the paracentral sulcus (Grosbras et al., 1999), the PreCS (Sun et al., 2015), the frontal operculum (Amiez et al., 2016), the midcingulate cortex (Amiez et al., 2013), the dorsal premotor region (Amiez et al., 2006) and the fusiform gyrus (Weiner et al., 2014). The main difficulty limiting the investigation of such sulcal morphology/function correspondence is the variability of the folding pattern (Ono et al., 1990; Régis et al., 2005; Petrides et al., 2012). A critical issue is therefore the possible identification of reliable sulcal patterns from MRI data.
Limitations
The results of this study are best understood in the context of some methodological issues. Firstly, only right-handed subjects were selected in this study in order to optimize the sample homogeneity and limit the reported handedness-related bias on the sulcal anatomy, notably in the central region (Amunts et al., 2000; Klöppel et al., 2010; Sun et al., 2012). Further studies, investigating sulcal patterns in left-handed as well as mixed-handed subjects are needed. Secondly, the so-called “plis de passage”–small gyri deeply buried in the main sulci (Gratiolet, 1854; Broca, 1888; Cunningham, 1892) associated with U-shaped white matter fibers—are important landmarks to characterize the sulcal patterns (Régis et al., 2005) but were not used in this study because their detection from structural MRI is difficult (Cachia et al., 2003). Future multimodal analyses, combining 3D reconstruction of the cortical surface from structural MRI with white-matter bundle tractography from diffusion MRI, should help to clarify the interactions between the sulcal patterns and the underlying white matter connectivity (Van Essen, 1997; Hilgetag and Barbas, 2006). In addition, it would be interesting to investigate the use of microstructural information (e.g., cortical myelination Glasser et al., 2014; Lutti et al., 2014) along with morphological features of the cortical surface for sulcal identification. Furthermore, comparison of in vivo and ex vivo data is a critical issue for translating the findings obtained from cadaver to living human brain. Hence, recent brain imaging approaches for “in vivo Brodmann mapping” allowing direct correlations between microstructure and function in living human brains (Geyer et al., 2011) would represent a major step forward for the brain mapping of human brain.
In conclusion, our study provides a description of sulcal patterns in the central region from in vivo clinical MRI which is in high agreement with previous ex vivo data. Such identification of reliable sulcal patterns of the central region visible with standard clinical MRI data paves the way for the detection of subtle variations of the central sulcation associated with variations of normal or pathological functioning.
Author Contributions
CM, CO and AC contributed to the conception of the work. CM, CO, M-NL, LL, J-FM participated in the acquisition of the data. CM, AC, CO, PR, SC participated in the analysis and interpretation of the data. CM, CO and AC drafted the work. M-NL, PR, SC, LL, J-FM revised the work critically for important intellectual content. All authors approved the final version of the article and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
- Amiez C., Kostopoulos P., Champod A.-S., Petrides M. (2006). Local morphology predicts functional organization of the dorsal premotor region in the human brain. J. Neurosci. 26, 2724–2731. 10.1523/jneurosci.4739-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C., Neveu R., Warrot D., Petrides M., Knoblauch K., Procyk E. (2013). The location of feedback-related activity in the midcingulate cortex is predicted by local morphology. J. Neurosci. 33, 2217–2228. 10.1523/jneurosci.2779-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C., Wutte M. G., Faillenot I., Petrides M., Burle B., Procyk E. (2016). Single subject analyses reveal consistent recruitment of frontal operculum in performance monitoring. Neuroimage 133, 266–278. 10.1016/j.neuroimage.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Amunts K., Jäncke L., Mohlberg H., Steinmetz H., Zilles K. (2000). Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia 38, 304–312. 10.1016/s0028-3932(99)00075-5 [DOI] [PubMed] [Google Scholar]
- Armstrong E., Schleicher A., Omran H., Curtis M., Zilles K. (1995). The ontogeny of human gyrification. Cereb. Cortex 5, 56–63. 10.1093/cercor/5.1.56 [DOI] [PubMed] [Google Scholar]
- Barkovich A. J., Guerrini R., Kuzniecky R. I., Jackson G. D., Dobyns W. B. (2012). A developmental and genetic classification for malformations of cortical development: update 2012. Brain 135, 1348–1369. 10.1093/brain/aws019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley A. J., Jones D. W., Weinberger D. R. (1997). Genetic variability of human brain size and cortical gyral patterns. Brain 120, 257–269. 10.1093/brain/120.2.257 [DOI] [PubMed] [Google Scholar]
- Besson P., Andermann F., Dubeau F., Bernasconi A. (2008). Small focal cortical dysplasia lesions are located at the bottom of a deep sulcus. Brain 131, 3246–3255. 10.1093/brain/awn224 [DOI] [PubMed] [Google Scholar]
- Blanton R. E., Levitt J. G., Thompson P. M., Narr K. L., Capetillo-Cunliffe L., Nobel A., et al. (2001). Mapping cortical asymmetry and complexity patterns in normal children. Psychiatry Res. 107, 29–43. 10.1016/s0925-4927(01)00091-9 [DOI] [PubMed] [Google Scholar]
- Bombin I., Arango C., Buchanan R. W. (2005). Significance and meaning of neurological signs in schizophrenia: two decades later. Schizophr. Bull. 31, 962–977. 10.1093/schbul/sbi028 [DOI] [PubMed] [Google Scholar]
- Borst G., Cachia A., Tissier C., Ahr E., Simon G., Houdé O. (2016). Early cerebral constraints on reading skills in school-age children: an MRI Study. Mind Brain Educ. 10, 47–54. 10.1111/mbe.12098 [DOI] [Google Scholar]
- Broca P. (1888). Mémoires d’Anthropologie. Paris: C. Reinwald. [Google Scholar]
- Cachia A., Amad A., Brunelin J., Krebs M.-O., Plaze M., Thomas P., et al. (2015). Deviations in cortex sulcation associated with visual hallucinations in schizophrenia. Mol. Psychiatry 20, 1101–1107. 10.1038/mp.2014.140 [DOI] [PubMed] [Google Scholar]
- Cachia A., Borst G., Tissier C., Fisher C., Plaze M., Gay O., et al. (2016). Longitudinal stability of the folding pattern of the anterior cingulate cortex during development. Dev. Cogn. Neurosci. 19, 122–127. 10.1016/j.dcn.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachia A., Borst G., Vidal J., Fischer C., Pineau A., Mangin J.-F., et al. (2014). The shape of the ACC contributes to cognitive control efficiency in preschoolers. J. Cogn. Neurosci. 26, 96–106. 10.1162/jocn_a_00459 [DOI] [PubMed] [Google Scholar]
- Cachia A., Mangin J. F., Rivière D., Kherif F., Boddaert N., Andrade A., et al. (2003). A primal sketch of the cortex mean curvature: a morphogenesis based approach to study the variability of the folding patterns. IEEE Trans. Med. Imaging 22, 754–765. 10.1109/tmi.2003.814781 [DOI] [PubMed] [Google Scholar]
- Cachia A., Paillère-Martinot M.-L., Galinowski A., Januel D., de Beaurepaire R., Bellivier F., et al. (2008). Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. Neuroimage 39, 927–935. 10.1016/j.neuroimage.2007.08.049 [DOI] [PubMed] [Google Scholar]
- Campbell A. W. (1905). Histological Studies on the Localisation of Cerebral Function. Cambridge: University Press. [Google Scholar]
- Cunningham D. J. (1892). Contribution to the Surface Anatomy of the Cerebral Hemispheres. Dublin: Academy House. [Google Scholar]
- Cunningham D. J. (1905). Text-Book of Anatomy. New York, NY: W. Wood and company. [Google Scholar]
- Dehay C., Giroud P., Berland M., Killackey H., Kennedy H. (1996). Contribution of thalamic input to the specification of cytoarchitectonic cortical fields in the primate: effects of bilateral enucleation in the fetal monkey on the boundaries, dimensions and gyrification of striate and extrastriate cortex. J. Comp. Neurol. 367, 70–89. [DOI] [PubMed] [Google Scholar]
- Dubois J., Benders M., Borradori-Tolsa C., Cachia A., Lazeyras F., Leuchter R. H.-V., et al. (2008). Primary cortical folding in the human newborn: an early marker of later functional development. Brain 131, 2028–2041. 10.1093/brain/awn137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin S. O., Bittar R. G., Kabani N. J., Baker C. L., Le Goualher G., Pike G. B., et al. (2000). A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb. Cortex 10, 454–463. 10.1093/cercor/10.5.454 [DOI] [PubMed] [Google Scholar]
- Gay O., Plaze M., Oppenheim C., Mouchet-Mages S., Gaillard R., Olié J.-P., et al. (2013). Cortex morphology in first-episode psychosis patients with neurological soft signs. Schizophr. Bull. 39, 820–829. 10.1093/schbul/sbs083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S., Weiss M., Reimann K., Lohmann G., Turner R. (2011). Microstructural parcellation of the human cerebral cortex–from Brodmann’s post-mortem map to in vivo mapping with high-field magnetic resonance imaging. Front. Hum. Neurosci. 5:19. 10.3389/fnhum.2011.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J. N., Lalonde F. M., Celano M. J., White S. L., Wallace G. L., Lee N. R., et al. (2009). Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry 48, 465–470. 10.1097/CHI.0b013e31819f2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M. F., Goyal M. S., Preuss T. M., Raichle M. E., Van Essen D. C. (2014). Trends and properties of human cerebral cortex: correlations with cortical myelin content. Neuroimage 93, 165–175. 10.1016/j.neuroimage.2013.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J. N., Lusk L., Hayashi K. M., Greenstein D., Vaituzis A. C., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U S A 101, 8174–8179. 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratiolet L. P. (1854). Mémoire sur les plis cérébraux de l’homme et des primatès: Mit einem Atlas (4 pp. XIV pl.) in fol. 33i. Paris: A. Bertrand. [Google Scholar]
- Grosbras M.-H., Lobel E., Van de Moortele P.-F., LeBihan D., Berthoz A. (1999). An anatomical landmark for the supplementary eye fields in human revealed with functional magnetic resonance imaging. Cereb. Cortex 9, 705–711. 10.1093/cercor/9.7.705 [DOI] [PubMed] [Google Scholar]
- Hilgetag C. C., Barbas H. (2006). Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comput. Biol. 2:e22. 10.1371/journal.pcbi.0020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunton D. L., Miezin F. M., Buckner R. L., van Mier H. I., Raichle M. E., Petersen S. E. (1996). An assessment of functional-anatomical variability in neuroimaging studies. Hum. Brain Mapp. 4, 122–139. [DOI] [PubMed] [Google Scholar]
- Kim H., Bernasconi N., Bernhardt B., Colliot O., Bernasconi A. (2008). Basal temporal sulcal morphology in healthy controls and patients with temporal lobe epilepsy. Neurology 70, 2159–2165. 10.1212/01.wnl.0000313150.62832.79 [DOI] [PubMed] [Google Scholar]
- Klöppel S., Mangin J.-F., Vongerichten A., Frackowiak R. S. J., Siebner H. R. (2010). Nurture versus nature: long-term impact of forced right-handedness on structure of pericentral cortex and basal ganglia. J. Neurosci. 30, 3271–3275. 10.1523/JNEUROSCI.4394-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyachko V. A., Stevens C. F. (2003). Connectivity optimization and the positioning of cortical areas. Proc. Natl. Acad. Sci. U S A 100, 7937–7941. 10.1073/pnas.0932745100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. (1977). The measurement of observer agreement for categorical data. Biometrics 33, 159–174. 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- Lutti A., Dick F., Sereno M. I., Weiskopf N. (2014). Using high-resolution quantitative mapping of R1 as an index of cortical myelination. Neuroimage 93, 176–188. 10.1016/j.neuroimage.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Mangin J.-F., Frouin V., Bloch I., Régis J., López-Krahe J. (1995). From 3D magnetic resonance images to structural representations of the cortex topography using topology preserving deformations. J. Math. Imaging Vis. 5, 297–318. 10.1007/bf01250286 [DOI] [Google Scholar]
- Mangin J.-F., Jouvent E., Cachia A. (2010). In-vivo measurement of cortical morphology: means and meanings. Curr. Opin. Neurol. 23, 359–367. 10.1097/WCO.0b013e32833a0afc [DOI] [PubMed] [Google Scholar]
- Mangin J.-F., Rivière D., Cachia A., Duchesnay E., Cointepas Y., Papadopoulos-Orfanos D., et al. (2004). A framework to study the cortical folding patterns. Neuroimage 23, S129–S138. 10.1016/j.neuroimage.2004.07.019 [DOI] [PubMed] [Google Scholar]
- Mellerio C., Labeyrie M.-A., Chassoux F., Daumas-Duport C., Landre E., Turak B., et al. (2012). Optimizing MR imaging detection of type 2 focal cortical dysplasia: best criteria for clinical practice. Am. J. Neuroradiol. 33, 1932–1938. 10.3174/ajnr.A3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellerio C., Roca P., Chassoux F., Danière F., Cachia A., Lion S., et al. (2015). The power button sign: a newly described central sulcal pattern on surface rendering mr images of type 2 focal cortical dysplasia. Radiology 274, 500–507. 10.1148/radiol.14140773 [DOI] [PubMed] [Google Scholar]
- Molko N., Cachia A., Rivière D., Mangin J. F., Bruandet M., Le Bihan D., et al. (2003). Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron 40, 847–858. 10.1016/s0896-6273(03)00670-6 [DOI] [PubMed] [Google Scholar]
- Nakamura M., Nestor P. G., McCarley R. W., Levitt J. J., Hsu L., Kawashima T., et al. (2007a). Altered orbitofrontal sulcogyral pattern in schizophrenia. Brain 130, 693–707. 10.1093/brain/awm007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Salisbury D. F., Hirayasu Y., Bouix S., Pohl K. M., Yoshida T., et al. (2007b). Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol. Psychiatry 62, 773–783. 10.1016/j.biopsych.2007.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Kubik S., Abernathey C. D. (1990). Atlas of the Cerebral Sulci. Stuttgart: Thieme. [Google Scholar]
- Petrides M., Tomaiuolo F., Yeterian E. H., Pandya D. N. (2012). The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex 48, 46–57. 10.1016/j.cortex.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Plaze M., Mangin J.-F., Paillère-Martinot M.-L., Artiges E., Olié J.-P., Krebs M.-O., et al. (2015). “Who is talking to me?”–Self-other attribution of auditory hallucinations and sulcation of the right temporoparietal junction. Schizophr. Res. 169, 95–100. 10.1016/j.schres.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Plaze M., Paillère-Martinot M.-L., Penttilä J., Januel D., de Beaurepaire R., Bellivier F., et al. (2011). “Where do auditory hallucinations come from?”—a brain morphometry study of schizophrenia patients with inner or outer space hallucinations. Schizophr. Bull. 37, 212–221. 10.1093/schbul/sbp081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones-Hinojosa A., Ojemann S. G., Sanai N., Dillon W. P., Berger M. S. (2003). Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. J. Neurosurg. 99, 311–318. 10.3171/jns.2003.99.2.0311 [DOI] [PubMed] [Google Scholar]
- Rademacher J., Caviness V. S., Jr., Steinmetz H., Galaburda A. M. (1993). Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping and neurobiology. Cereb. Cortex 3, 313–329. 10.1093/cercor/3.4.313 [DOI] [PubMed] [Google Scholar]
- Rajkowska G., Goldman-Rakic P. S. (1995). Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the talairach coordinate system. Cereb. Cortex 5, 323–337. 10.1093/cercor/5.4.323 [DOI] [PubMed] [Google Scholar]
- Rakic P. (2004). Neuroscience. Genetic control of cortical convolutions. Science 303, 1983–1984. 10.1126/science.1096414 [DOI] [PubMed] [Google Scholar]
- Régis J., Mangin J.-F., Ochiai T., Frouin V., Riviére D., Cachia A., et al. (2005). “Sulcal root” generic model: a hypothesis to overcome the variability of the human cortex folding patterns. Neurol. Med. Chir. (Tokyo) 45, 1–17. 10.2176/nmc.45.1 [DOI] [PubMed] [Google Scholar]
- Régis J., Tamura M., Park M. C., McGonigal A., Rivière D., Coulon O., et al. (2011). Subclinical abnormal gyration pattern, a potential anatomic marker of epileptogenic zone in patients with magnetic resonance imaging-negative frontal lobe epilepsy. Neurosurgery 69, 80–93. 10.1227/NEU.0b013e318212bb1a [DOI] [PubMed] [Google Scholar]
- Roca P., Mellerio C., Chassoux F., Rivière D., Cachia A., Charron S., et al. (2015). Sulcus-based MR analysis of focal cortical dysplasia located in the central region. PLoS One 10:e0122252. 10.1371/journal.pone.0122252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli F., Guyotat J., Isnard J., Schneider F., Mohammedi R., Bret P. (2001). The value of cortical stimulation applied to the surgery of malignant gliomas in language areas. Neurol. Sci. 22, 3–10. 10.1007/s100720100016 [DOI] [PubMed] [Google Scholar]
- Strube W., Wobrock T., Bunse T., Palm U., Padberg F., Malchow B., et al. (2014). Impairments in motor-cortical inhibitory networks across recent-onset and chronic schizophrenia: a cross-sectional TMS Study. Behav. Brain Res. 264, 17–25. 10.1016/j.bbr.2014.01.041 [DOI] [PubMed] [Google Scholar]
- Sun Z. Y., Klöppel S., Rivière D., Perrot M., Frackowiak R., Siebner H., et al. (2012). The effect of handedness on the shape of the central sulcus. Neuroimage 60, 332–339. 10.1016/j.neuroimage.2011.12.050 [DOI] [PubMed] [Google Scholar]
- Sun Z. Y., Pinel P., Rivière D., Moreno A., Dehaene S., Mangin J.-F. (2015). Linking morphological and functional variability in hand movement and silent reading. Brain Struct. Funct. 1–11. 10.1007/s00429-015-1106-8 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Van Essen D. C. (1997). A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 385, 313–318. 10.1038/385313a0 [DOI] [PubMed] [Google Scholar]
- Watson J. D., Myers R., Frackowiak R. S., Hajnal J. V., Woods R. P., Mazziotta J. C., et al. (1993). Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb. Cortex 3, 79–94. 10.1093/cercor/3.2.79 [DOI] [PubMed] [Google Scholar]
- Weiner K. S., Golarai G., Caspers J., Chuapoco M. R., Mohlberg H., Zilles K., et al. (2014). The mid-fusiform sulcus: a landmark identifying both cytoarchitectonic and functional divisions of human ventral temporal cortex. Neuroimage 84, 453–465. 10.1016/j.neuroimage.2013.08.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker E., Hoogland P. V., Van der Loos H. (1988). Organization of feedback and feedforward projections of the barrel cortex: a PHA-L study in the mouse. Exp. Brain Res. 73, 411–435. 10.1007/bf00248234 [DOI] [PubMed] [Google Scholar]
- White T., Su S., Schmidt M., Kao C.-Y., Sapiro G. (2010). The development of gyrification in childhood and adolescence. Brain Cogn. 72, 36–45. 10.1016/j.bandc.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry T. A., Schmid U. D., Alkadhi H., Schmidt D., Peraud A., Buettner A., et al. (1997). Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120, 141–157. 10.1093/brain/120.1.141 [DOI] [PubMed] [Google Scholar]
- Yücel M., Stuart G. W., Maruff P., Wood S. J., Savage G. R., Smith D. J., et al. (2002). Paracingulate morphologic differences in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biol. Psychiatry 52, 15–23. 10.1016/s0006-3223(02)01312-4 [DOI] [PubMed] [Google Scholar]
- Zilles K., Palomero-Gallagher N., Amunts K. (2013). Development of cortical folding during evolution and ontogeny. Trends Neurosci. 36, 275–284. 10.1016/j.tins.2013.01.006 [DOI] [PubMed] [Google Scholar]