Abstract
Background and Objective
Roxadustat is a hypoxia-inducible factor prolyl hydroxylase inhibitor in phase III development for the treatment of anaemia associated with chronic kidney disease. This study evaluated the effects of moderate hepatic impairment on roxadustat pharmacokinetics, pharmacodynamics and tolerability.
Methods
This was an open-label study in which eight subjects with moderate hepatic impairment (liver cirrhosis Child–Pugh score 7–9) and eight subjects with normal hepatic function (matched for body mass index, age and sex) received a single oral 100 mg roxadustat dose under fasted conditions. Blood samples were collected until 144 h post-dose in subjects with moderate hepatic impairment and until 96 h post-dose in subjects with normal hepatic function.
Results
In subjects with moderate hepatic impairment, area under the concentration–time curve (AUC) from the time of drug administration to infinity (AUC∞) and observed maximum concentration (Cmax) were 23 % higher [geometric least-squares mean ratio (GMR) 123 %; 90 % CI 86.1–175] and 16 % lower (GMR 83.6 %; 90 % CI 67.5–104), respectively, than in subjects with normal hepatic function. Mean terminal half-life (t½) appeared to be longer (17.7 vs. 12.8 h) in subjects with moderate hepatic impairment, however intersubject variability on apparent total systemic clearance after single oral dosing (CL/F), apparent volume of distribution at equilibrium after oral administration (Vz/F) and t½ was approximately twofold higher. Erythropoietin (EPO) baseline-corrected AUC from administration to the last measurable EPO concentration (AUCE,last) and maximum effect (Emax) were 31 % (GMR 68.95 %; 90 % CI 29.29–162.29) and 48 % (GMR 52.29 %; 90 % CI 28.95–94.46) lower, respectively, than in subjects with normal hepatic function. The single oral roxadustat dose was generally well tolerated.
Conclusions
This study demonstrated the effect of moderate hepatic impairment on the pharmacokinetics and pharmacodynamics of roxadustat relative to subjects with normal hepatic function. These differences are not expected to be of clinical significance.
Key Points
| Subjects with moderate hepatic impairment exhibit changes in roxadustat pharmacokinetics and pharmacodynamics relative to subjects with normal hepatic function. |
| These differences are not expected to be clinically significant or to warrant a different dosing strategy for subjects with moderate hepatic impairment. |
| The single 100 mg oral dose of roxadustat was generally well tolerated in subjects with moderate hepatic impairment and subjects with normal hepatic function. |
Introduction
Chronic kidney disease (CKD) is a long-term progressive disease leading to reduced kidney function, progression to kidney failure and end-stage renal disease (ESRD) [1, 2]. ESRD ultimately leads to the requirement for dialysis and/or kidney transplantation [3]. Worldwide, CKD has become a major public health concern, with its incidence increasing continuously in recent decades; this is likely to be related to an increase in risk factors such as obesity, diabetes and hypertension [1]. A systematic review of 26 population-based studies found that the median prevalence of CKD was 7.2 % in those aged >30 years and, in those aged >64 years, prevalence ranged from 23.4 to 35.8 % [4].
Anaemia is a common complication in patients with CKD, and its incidence increases as the disease progresses [5]. The pathogenesis of anaemia is multifactorial, but decreased erythropoietin (EPO) production and the impaired ability of the body to absorb and use iron are considered to be important aetiological factors [5]. Anaemia is thought to contribute to excess morbidity and mortality in patients with CKD and ESRD [5], and the severity of anaemia has been shown to correlate with the risk of hospitalisation and number of comorbid conditions [6].
Current treatment options for CKD include injectable erythropoiesis-stimulating agents (ESAs), iron supplements and blood transfusions. Current treatments are associated with side effects such as iron toxicity [7], anaphylactic reactions [8], cardiovascular risk [9], thromboembolic complications [10] and the risk for allosensitisation (due to blood transfusions), which is associated with the increased rejection of organ transplants [11].
Roxadustat (FG-4592) is a novel, orally active, small-molecule hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitor in clinical development for the treatment of anaemia in patients with CKD [12]. HIF is a protein transcription factor that responds to low oxygen in the cellular environment by inducing erythropoiesis [12, 13]. It induces erythropoiesis at lower EPO levels compared with ESAs.
Roxadustat is predominantly eliminated by phase I oxidation and phase II conjugation (glucuronidation and glucosidation). Furthermore, roxadustat is highly bound to proteins in human plasma, predominantly albumin.
The primary objective of this study was to determine the effect of moderate hepatic impairment on the pharmacokinetics of roxadustat following administration of a single oral roxadustat dose of 100 mg. Secondary objectives of the study included pharmacodynamic assessments and evaluation of roxadustat tolerability.
Methods
Study Design
This was a single-centre (University Hospital St Ivan Rilski, Sofia, Bulgaria), single-dose, open-label study investigating the pharmacokinetics, pharmacodynamics and tolerability of roxadustat in subjects with moderate hepatic impairment and control subjects with normal hepatic function. Pairs of subjects with moderate hepatic impairment and normal hepatic function were well matched for sex, age (±5 years) and body mass index (BMI) (±15 %). After being admitted to the clinic the previous day, and undergoing an overnight fast of at least 10 h, subjects received a single oral dose of 100 mg roxadustat. Standard meals were allowed after 4 h post-dose. Subjects with normal hepatic function were housed in the clinical unit for a period of 5 days, while those with moderate hepatic impairment were in the clinical unit for a period of 7 days (discharge on day 7). The 100 mg dose of roxadustat was selected because it is within the therapeutic dose range and allows for a potential increase in systemic exposure to roxadustat in subjects with moderate hepatic impairment. Overall, a single dose of 100 mg was considered to be clinically relevant, safe to administer and able to provide an accurate representation of the pharmacokinetics of roxadustat in this population.
Blood samples, for both the determination of plasma EPO levels and roxadustat pharmacokinetic analysis, and urine samples, for roxadustat (pharmacokinetic) analysis, were collected up to 96 h post-dose in subjects with normal hepatic function and up to 144 h post-dose in subjects with moderately impaired hepatic function to account for a potentially longer terminal half-life (t½) of roxadustat in subjects with moderate hepatic impairment.
The study was conducted in accordance with the protocol, Good Clinical Practice and International Conference on Harmonisation (ICH) guidelines, applicable regulations and guidelines governing clinical study conduct and the ethical principles of the Declaration of Helsinki [14, 15]. All subjects provided written informed consent and the trial protocol had undergone approval via independent Ethics Committees.
Subjects
Adult men and women (aged 18–80 years, BMI 18.5–34.0 kg/m2, weight ≥50 kg at screening) were included in the study. Participants with moderate hepatic impairment were assessed according to the Child–Pugh classification (liver cirrhosis Child–Pugh B [score 7–9 points]). Moderate hepatic impairment was defined by the presence of two or more of the following criteria: decreased serum albumin (≤28 g/l), increased serum bilirubin (≥51.312 µmol/l or 3 mg/dl), prolonged prothrombin time (≤6 s prolonged to the upper limit of normal), ascites and encephalopathy.
Exclusion criteria included known or suspected allergy to the study drugs, known history or diagnosis of any other relevant medical condition, and use of any prescribed or over-the-counter medication (including vitamins, oral contraceptives, natural and herbal remedies) within the 2 weeks prior to study drug administration. Subjects with moderately impaired hepatic function were excluded if the dose regimen of their medically required medication had changed in the 4 weeks before screening. Restrictions during the study included food and drink that could interact with circulatory, gastrointestinal, liver or renal function (e.g. alcohol, caffeine or xanthine-containing products). If a prohibited medication was needed during the study, the subject had to be withdrawn unless the study investigator was convinced that it would not affect study outcomes in any way. For subjects with moderate hepatic impairment, the underlying cause of hepatic impairment could not include the presence of a hepatocellular carcinoma, acute liver disease caused by an infection or drug toxicity, biliary liver cirrhosis, biliary obstruction, or any other cause of hepatic impairment not related to parenchymal disorder and/or disease of the liver.
Bioanalytical Methodology
Concentrations of roxadustat in human Na-Heparin plasma were measured with a liquid chromatography tandem mass spectrometry method validated according to the European Medicines Agency bioanalytical method validation guidance (21 July 2011) and using a stable isotope label ([13C2, D3]-roxadustat) as the internal standard. In brief, roxadustat and the internal standard were extracted from 100 µl human plasma by solid-phase extraction, and afterwards separated by reversed-phase liquid chromatography on a C18 column using a gradient of water/acetonitrile, with formic acid as the mobile phase. Detection was carried out by tandem mass spectrometry on a 4000 QTrap mass spectrometer using positive Turbo ion spray ionisation. The method was linear in the range 1–1000 ng/ml, with a lower limit of quantification of 1 ng/ml. The method was selective and did not show any evidence of carryover. The inter-run accuracy (%RE) varied between −4.6 and 10.0, whereas the inter-run precision (%CV) ranged between 0.7 and 4.2. The unbound fraction of roxadustat was determined by equilibrium dialysis.
Concentrations of roxadustat in human urine were measured as above from 100 µl human urine. The %RE varied between −7.9 and 0.3, the %CV ranged between 1.6 and 9.3, and the lower limit of quantification for urine was 1 ng/ml.
EPO concentration in human lithium heparin plasma was measured using the EPO assay kit LKEPN1 with a Siemens Immulite 1000 immunoanalyzer. The method, based on a chemiluminescent immunoturbidimetric assay, uses 100 µl of plasma and is suitable for the determination of EPO over the range 1–750 mU/ml. Intra-assay precision in terms of %CV was ≤6.7, and inter-assay was ≤10.4. Accuracy was ±15.9 (intra-assay) and ±15.3 (inter-assay) in terms of %RE. EPO was shown to be stable up to three freeze-thaw cycles at nominally −20 and −80 °C.
Pharmacokinetic Assessments
Blood samples for the assessment of roxadustat in plasma were collected pre-dose and 30 min, 1, 2, 3, 4, 6, 8, 12, 16, 24, 36, 48, 72, and 96 h post-dose in subjects with normal hepatic function, and pre-dose and 30 min, 1, 2, 3, 4, 6, 8, 12, 16, 24, 36, 48, 72, 96, 120, and 144 h post-dose in subjects with moderate hepatic impairment. Where possible, the following pharmacokinetic parameters were calculated for each subject using non-compartmental analysis: area under the concentration–time curve (AUC) from the time of drug administration to infinity (AUC∞), AUC from the time of administration to the last measurable concentration (AUClast), observed maximum concentration (Cmax), apparent total systemic clearance after single oral dosing (CL/F), time to reach Cmax (tmax), t½, and apparent volume of distribution at equilibrium after oral administration (Vz/F). Plasma pharmacokinetic and pharmacodynamic parameters were calculated using Phoenix® WinNonlin® 6.3 (Certara, L.P., St Louis, MO, USA). Urine pharmacokinetic parameters were calculated using SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Blood samples for protein binding analysis of roxadustat were collected at 3, 12 and 24 h post-dose. The geometric mean for the fraction unbound (fu) was used to calculate the unbound pharmacokinetic parameters for roxadustat. Unbound parameters of roxadustat were calculated by multiplying (en and women were initially scremax, AUClast, or AUC∞) or dividing (CL/F, Vz/F, or renal clearance [CLR]) the individual parameter by its fu in plasma.
Urine samples for the assessment of roxadustat concentration were collected up to 96 h post-dose in subjects with normal hepatic function, and up to 144 h post-dose in subjects with moderate hepatic impairment. The following elimination pharmacokinetic parameters were calculated: CLR, cumulative amount of drug excreted (Ae) from the time of administration extrapolated to infinity (Ae∞), percentage Ae∞ (Ae∞%), Ae up to the time of the last measurable concentration (Aelast), and percentage Aelast (Aelast%). CLRwas calculated as the amount excreted in urine divided by the AUC in plasma (Aelast/AUClast). It was judged to be dose independent, therefore Ae∞ was calculated as CLR multiplied by AUC∞.
Pharmacodynamic Assessments
Blood samples for the evaluation of EPO were collected up to 96 h post-dose in subjects with normal hepatic function, and up to 144 h post-dose for the group with moderate hepatic impairment. Pharmacodynamic parameters based on EPO measurements in plasma included the maximum effect (Emax) and the AUC from administration to the last measurable EPO concentration (AUCE,last), absolute and corrected for EPO values at baseline.
Tolerability Assessments
Tolerability assessments included treatment-emergent adverse events (TEAEs; frequency, seriousness, relation to study drug and severity [National Cancer Institute Common Terminology Criteria for Adverse Events (NCI–CTCAE) v4.03]), clinical laboratory variables [biochemistry, including liver function tests (LFTs), haematology and urinalysis], vital signs [diastolic blood pressure (DBP), systolic blood pressure (SBP) and pulse] and electrocardiogram (ECG).
Statistical Analysis
No formal sample size calculation was performed. The study had a matched-pairs design whereby eight subjects with moderate hepatic impairment were matched per protocol to eight subjects with normal hepatic function. The trial was not designed to be a head-to-head comparison of subjects with moderate hepatic impairment and normal hepatic function. Therefore, eight independent pairs (16 subjects in total) were considered sufficient to explore the pharmacokinetic properties of roxadustat in subjects with moderate hepatic impairment. The overall sample size was in accordance with guidance for industry provided by the US FDA and the European Committee for Medicinal Products for Human Use [14, 15].
To assess the effect of moderate hepatic impairment on the pharmacokinetics of roxadustat in plasma, an analysis of covariance (ANCOVA) was performed on each natural log-transformed pharmacokinetic parameter, with hepatic function status (normal or moderately impaired), sex, age and BMI as fixed effects. The primary pharmacokinetic parameters were AUC∞ and Cmax. Least squares (LS) means for the factor hepatic function status, the estimated LS means difference (moderate hepatic impairment−normal hepatic function) and its 90 % confidence interval (CI) on the log scale were constructed. For each primary pharmacokinetic parameter (as well as unbound AUC and Cmax), an estimate of the adjusted geometric least-squares mean ratio (GMR) for the comparison of interest (moderate hepatic impairment/normal hepatic function) was calculated by exponentiating the difference between LS means. Similarly, the 90 % CI of the estimated LS means difference was back-transformed to obtain the results on the original scale. A similar analysis was performed on the pharmacodynamic parameters.
Results
Subject Disposition
A total of twenty men and women were initially screened; of these, sixteen were enrolled into the study: eight subjects with moderate hepatic impairment and eight subjects with normal hepatic impairment, matched for BMI, age and sex with normal hepatic function (Table 1). All subjects were Caucasian. All enrolled subjects completed the study, were dosed per protocol, and were included in the analyses. Subjects were individually matched. The hepatic impairment group had a moderate distribution of Child–Pugh scores in the 7–9 range: Child–Pugh 7, n = 2; Child–Pugh 8, n = 2; Child–Pugh 9, n = 4. The aetiology of liver disease was chronic hepatitis B-related liver cirrhosis (n = 4) and alcohol-related liver cirrhosis (n = 4). All subjects with moderate hepatic impairment received concomitant medication for cirrhosis throughout the study, as well as medication for prostatic hyperplasia (one subject), ascites (one subject), chronic gastritis (one subject) or gastritis (one subject) and thrombocytopenia (one subject). No subject with normal hepatic function received concomitant medication.
Table 1.
Baseline demographics and other characteristics of the study subjects
| Characteristics | Subjects with moderate hepatic impairment [N = 8] | Subjects with normal hepatic impairment [N = 8] |
|---|---|---|
| Sex, n (%) | ||
| Male | 5 (62.5) | 5 (62.5) |
| Female | 3 (37.5) | 3 (37.5) |
| Age, years | 57.0 (11.1) | 54.5 (10.2) |
| Weight, kg | 80.8 (16.4) | 83.6 (10.2) |
| BMI, kg/m2 | 27.8 (3.9) | 28.1 (2.0) |
| Albumin, g/dl | 35.5 (5.7) | 43.1 (2.0) |
| Bilirubin, µmol/l | 30.2 (15.4) | 9.7 (4.5) |
| Subjects with grade 2–3 prothrombin time, n a | 1 | – |
| Subjects with grade 2–3 encephalopathy, n b | 7 | 0 |
| Subjects with grade 2–3 ascites, n c | 6 | 0 |
Data are expressed as mean (SD) unless otherwise specified
BMI body mass index, SD standard deviation
aOne patient had grade 2, and 7 patients had grade 1
bEncephalopathy: grade 0–normal consciousness, personality, neurological examination, ECG; grade 1–restless, irritable, tremor, impaired handwriting, five cycles/s waves; grade 2–lethargic, time-disorientated, asterixis, ataxia, slow triphasic waves. Seven patients had grade 2 encephalopathy and 1 patient had grade 1
cFive patients had grade 2 ascites, and 1 patient had grade 3
Pharmacokinetics
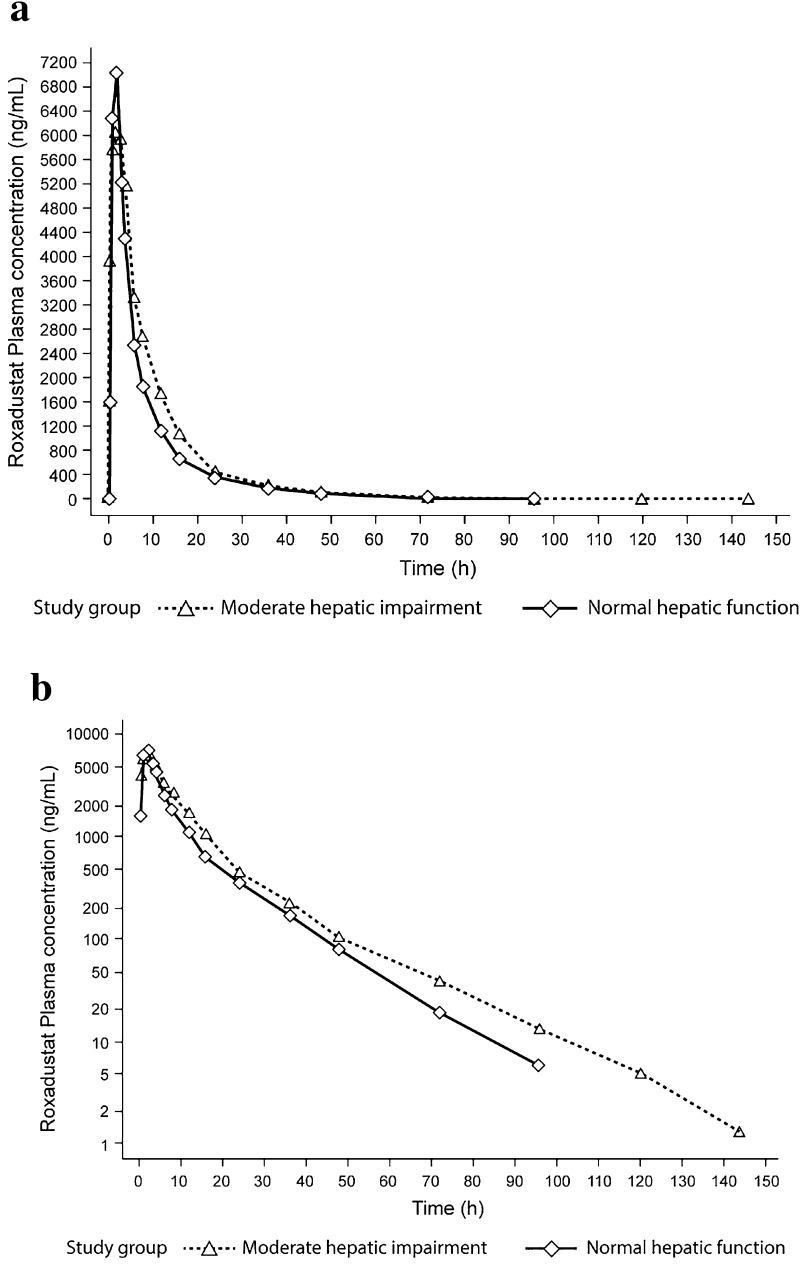
Plasma pharmacokinetic parameters for roxadustat are summarised in Table 2 and Fig. 1. Based on the comparison of roxadustat administered as a 100 mg dose in subjects with moderate hepatic impairment versus subjects with normal hepatic function, AUC∞ was 23 % higher (GMR 122.8 %; 90 % CI 86.1–175.1), whereas Cmax was 16 % lower (GMR 83.6 %; 90 % CI 67.5–103.5). The fraction of roxadustat unbound (fu) (Table 2) was higher in those with moderate hepatic impairment than in those with normal hepatic function (1.1 vs. 0.8 %). AUC∞,u and Cmax,u of roxadustat were 70 % (GMR 170.4 %; 90 % CI 119.4–243.2) and 16 % higher (GMR 116.0 %; 90 % CI 93.1–144.6), respectively, in the moderate hepatic impairment group compared with controls. Mean half-life appeared to be longer for subjects with moderate hepatic impairment than for subjects with normal hepatic function (17.7 vs. 12.8 h); however, the intersubject variability (expressed as CV) in CL/F, Vz/F and, consequently, t½ was approximately twofold higher (Table 2). The percentage of unchanged roxadustat excreted in urine, and the CLR, were higher (2.4 vs. 1.6 % and 0.05 vs. 0.03 l/h, respectively) in the hepatic impairment group than in the control group, whereas the CLR of unbound roxadustat appeared to be unaffected (Table 2). Table 3 shows the GMRs and 90 % CIs for AUC∞, Cmax, AUC∞,u and Cmax,u for those with moderate hepatic impairment and normal hepatic function.
Table 2.
Summary of the plasma roxadustat pharmacokinetic parameters
| Parameter | Subjects with moderate hepatic impairment [N = 8] | Subjects with normal hepatic impairment [N = 8] |
|---|---|---|
| Pharmacokinetic parameters of total (bound and unbound) roxadustat concentration | ||
| AUC∞, ng·h/ml | 63,693 (30,947) | 49,807 (15,111) |
| C max, ng/ml | 6975 (1514) | 8498 (2203) |
| t max, ha | 2.0 (0.5–3.0) | 1.5 (1.0–2.0) |
| t ½, ha | 14.7 (10.0–30.1) | 12.6 (10.2–16.8) |
| Aelast, mg | 2.4 (1.8) | 1.6 (0.6) |
| CLR, l/h | 0.05 (0.04) | 0.03 (0.01) |
| Pharmacokinetic parameters of unbound roxadustat concentration | ||
| AUC∞,u, ng·h/ml | 708.2 (314.8) | 396.9 (94.8) |
| AUClast,u, ng·h/ml | 2.4 (1.8) | 396.0 (94.9) |
| C max,u, ng/ml | 78.4 (17.0) | 67.7 (13.4) |
| CLR,u, l/h | 4.1 (3.0) | 4.0 (1.6) |
| f u, % | 1.1 (0.16) | 0.81 (0.07) |
Data are expressed as mean (SD) unless otherwise specified
Ae last cumulative amount of drug excreted from the time of administration to the last measurable concentration, AUC last area under the concentration–time curve from the time of administration to the last measurable concentration, AUC ∞ area under the concentration–time curve from the time of drug administration to infinity, C max maximum concentration, CL R renal clearance, SD standard deviation, t ½ terminal half-life, F u fraction of unbound drug, t max time to maximum concentration, u unbound
aMedian (range)
Fig. 1.
Mean plasma roxadustat concentrations in subjects with normal and moderately impaired hepatic function. a Concentration versus time; b log-transformed concentration versus time
Table 3.
Statistical assessment of roxadustat exposure parameters after single-dose roxadustat administered to subjects with moderate hepatic impairment, compared with administration to subjects with normal hepatic function
| Parameter | Moderate hepatic impairmenta [N = 8] | Normal hepatic functiona [N = 8] | GLSM ratio (%)b | 90 % CI |
|---|---|---|---|---|
| AUC∞, ng·h/ml | 60,108 | 48,967 | 122.75 | 86.1–175.1 |
| C max, ng/ml | 6928 | 8291 | 83.57 | 67.5–103.5 |
| AUC∞,u, ng·h/ml | 668 | 392 | 170 | 119.4–243.2 |
| C max,u, ng/ml | 77.0 | 66.4 | 116 | 93.1–144.6 |
AUC ∞ area under the concentration–time curve from the time of drug administration to infinity, CI confidence interval, C max maximum concentration, GLSM geometric least-squares means, u unbound
aData are expressed as GLSM
bRatio defined as (GLSM moderate hepatic impairment)/(GLSM normal hepatic function)
Mean values of CLR unbound (CLR,u) were 4.2 and 4.0 l/h for subjects with moderate hepatic impairment and normal hepatic function, respectively. The CV in Ae and CLR was higher in subjects with moderate hepatic impairment, with values ranging from 72.8 to 84.6 %, compared with subjects with normal hepatic function, with values ranging from 39.4 to 46.5 %.
Pharmacodynamics
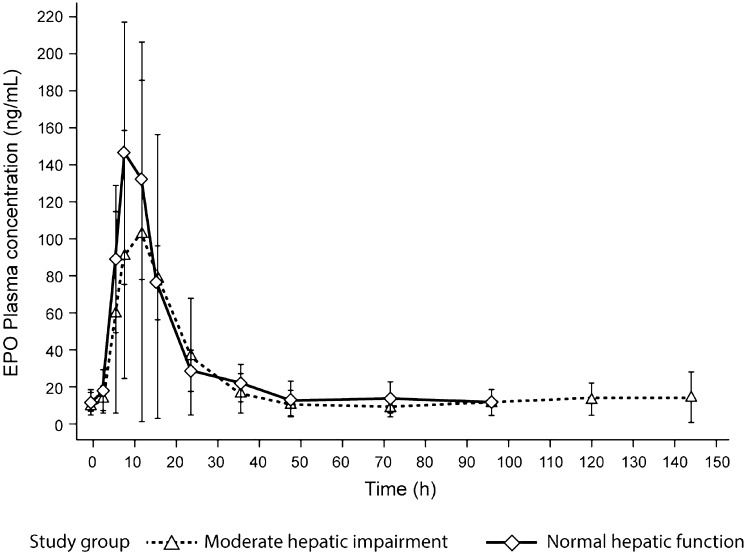
Mean plasma EPO concentrations over time are shown in Fig. 2. For subjects with moderate hepatic impairment, EPO AUCE,last levels were similar (GMR 100.4 %; 90 % CI 66.8–151.0), whereas Emax was 44 % lower (GMR 56.4 %; 90 % CI 33.3–95.7) compared with subjects with normal hepatic function. The EPO baseline-corrected AUCE,last and Emax were 31 % (GMR 69.0 %; 90 % CI 29.3–162.3) and 48 % lower (GMR 52.3 %; 90 % CI 29.0–94.5), respectively. Intersubject variation (expressed as CV%) in the EPO exposure parameters (measured and baseline-corrected) was approximately two- to threefold higher in subjects with moderate hepatic impairment (see Table 4).
Fig. 2.
Mean (SD) plasma EPO concentrations in subjects with normal and moderately impaired hepatic function over time. SD standard deviation, EPO erythropoietin
Table 4.
Summary of plasma erythropoietin pharmacodynamic parameters
| Parameter | Subjects with moderate hepatic impairment [N = 8] | Subjects with normal hepatic impairment [N = 8] |
|---|---|---|
| E max, mU/ml | 113.9 (104.2) | 154.7 (65.38) |
| Baseline-corrected E max, mU/ml | 102.8 (101.2) | 141.2 (65.78) |
| t max, ha | 10.0 (8.0–12.2) | 8.0 (8.00–16.0) |
| AUCE,last, mU·h/ml | 3231 (1973) | 3009 (823.1) |
| Baseline-corrected AUCE,last, mU·h/ml | 1635 (1591) | 1716 (502.9) |
Data are expressed as mean (SD) unless otherwise specified
AUC E,last area under the concentration–time curve from administration to the last measurable erythropoietin concentration, E max maximum effect, SD standard deviation, t max time to maximum concentration
aMedian (range)
Tolerability
A single dose of roxadustat was generally well tolerated. No deaths or serious adverse events were reported. In total, two TEAEs were reported in two different subjects, with moderate hepatic impairment: one event of neutropenia and one event of headache; both were graded as mild. No TEAEs were reported for subjects with normal hepatic function, and no events led to study discontinuation.
A single case of worsening neutropenia was the only TEAE considered by the investigator to be possibly related to study drug. The individual who developed neutropenia was a female subject with moderate hepatic impairment. The subject’s leucocyte count was 3.26 × 109/l at baseline, decreasing to a low of 1.67 × 109/l on day 3 (i.e. 2 days after administration of a single dose of 100 mg roxadustat), and was 2.45 × 109/l at the end of study visit (ESV). The associated neutrophil count was 2300 × 106/l at baseline, decreasing to a low of 1110 × 106/l on day 2 (i.e. 1 day after administration of roxadustat), and was 1800 × 106/l at the ESV.
No subject with moderate hepatic impairment showed twofold or more increase in LFTs from screening. No subject with normal hepatic function showed either elevated LFTs at screening or LFT elevations during the study. Changes reflecting normal diurnal variation were observed for mean SBP and DBP and mean pulse, and there were no apparent clinically significant study drug-related trends. No relevant changes in clinical laboratory analyses or ECG parameters were noted.
Discussion
The purpose of this phase I clinical study was to evaluate the effects of moderate hepatic impairment (Child–Pugh score 7–9 [Class B]) on the pharmacokinetics, pharmacodynamics and tolerability of a single 100 mg dose of roxadustat. Subjects with moderate hepatic impairment were evaluated alongside subjects with normal hepatic function, and matched for sex, age and BMI. Exposure to roxadustat (AUC∞) was 23 % higher in those with moderate hepatic impairment, while Cmax was 16 % lower compared with subjects with normal hepatic function. Roxadustat was absorbed rapidly in both groups, with a median tmax of 1.5–2 h, although it appeared to be eliminated more slowly in subjects with moderate hepatic impairment than in subjects with normal hepatic function (17.72 vs. 12.79 h, respectively). In subjects with moderate hepatic impairment, the average percentage unbound was higher (1.1 %) compared with subjects with normal hepatic function (0.81 %), and is not considered to be of clinical relevance.
In subjects with moderate hepatic impairment, after baseline correction, EPO AUCE,last and Emax, respectively, were 31 and 48 % lower than in subjects with normal hepatic function. EPO levels returned to baseline levels within 48 h. The effects on roxadustat pharmacokinetics and pharmacodynamics seen in subjects with moderate hepatic impairment, compared with matched subjects with normal hepatic function, are not considered to warrant a different dosing strategy for subjects with moderate hepatic impairment.
A single oral 100 mg dose of roxadustat was well tolerated by subjects with moderate hepatic impairment and those with normal hepatic function, with only two TEAEs reported (neutropenia and headache), both of which were graded as mild. Neutropenia was considered by the investigator to be possibly related to the study drug. In the case of neutropenia, leucocyte and neutrophil counts subsequently returned to normal levels. No trends were seen among the other subjects with respect to declining neutrophil count during the study drug treatment.
In subjects with moderate hepatic impairment, an approximately twofold higher intersubject variability on the pharmacokinetic parameters of roxadustat was observed compared with subjects with normal hepatic function.
The potential limitations of this study were the wide age range and the sample size, as a larger sample size might have reduced the variability observed. In addition, this study did not include patients with severe hepatic impairment, which limits the results to those with only moderate impairment. However, as the eight subjects with moderate hepatic impairment were on an individual level matched for age, BMI, and sex, to eight subjects with normal hepatic function, a reliable estimate of the effect of hepatic impairment on the pharmacokinetics of roxadustat could still be determined. These results show the effect of moderate hepatic impairment on the pharmacokinetics and pharmacodynamics of roxadustat.
Conclusions
This study demonstrated the effect of moderate hepatic impairment on the pharmacokinetics and pharmacodynamics of roxadustat relative to subjects with normal hepatic function. The differences observed are not expected to be of clinical significance.
Compliance with Ethical Standards
Funding
This study was co-funded by FibroGen, AstraZeneca and Astellas Pharma. Medical writing and editorial assistance was provided by Carole Manners and Kay Chapman of Darwin Healthcare Communications, and was co-funded by FibroGen, AstraZeneca and Astellas Pharma.
Conflict of interest
Dorien Groenendaal-van de Meent, Martin den Adel, Sanne Rijnders, Axel Krebs-Brown, and Marloes Schaddelee are employees of Astellas Pharma. Jan Noukens (Kinesis Pharma) participated in the interpretation of the data and writing of the report, co-funded by FibroGen, AstraZeneca and Astellas Pharma. Lyudmila Mateva and Assen Alexiev have no conflicts of interest to report.
Ethical approval
The study was conducted in accordance with the protocol, Good Clinical Practice and International Conference on Harmonisation (ICH) guidelines, applicable regulations and guidelines governing clinical study conduct and the ethical principles of the Declaration of Helsinki. The clinical study protocol had undergone approval via an independent Ethics Committee.
Informed consent
All subjects provided written informed consent.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.KDIGO. Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:1–150. [DOI] [PubMed]
- 3.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Zhang QL, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–1634. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebben JP, Gilbertson DT, Foley RN, Collins AJ. Hemoglobin level variability: associations with comorbidity, intercurrent events, and hospitalizations. Clin J Am Soc Nephrol. 2006;1:1205–1210. doi: 10.2215/CJN.01110306. [DOI] [PubMed] [Google Scholar]
- 7.Slotki I. Intravenous iron supplementation in the anaemia of renal and cardiac failure: a double-edged sword? Nephrol Dial Transplant. 2005;20(Suppl 7):vii16–23. [DOI] [PubMed]
- 8.Macdougall IC, Casadevall N, Locatelli F, Combe C, London GM, Di PS, et al. Incidence of erythropoietin antibody-mediated pure red cell aplasia: the Prospective Immunogenicity Surveillance Registry (PRIMS) Nephrol Dial Transplant. 2015;30:451–460. doi: 10.1093/ndt/gfu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krapf R, Hulter HN. Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA) Clin J Am Soc Nephrol. 2009;4:470–480. doi: 10.2215/CJN.05040908. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X, Perazella MA. Nonhematologic complications of erythropoietin therapy. Semin Dial. 2006;19:279–284. doi: 10.1111/j.1525-139X.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 11.Scornik JC, Bromberg JS, Norman DJ, Bhanderi M, Gitlin M, Petersen J. An update on the impact of pre-transplant transfusions and allosensitization on time to renal transplant and on allograft survival. BMC Nephrol. 2013;14:217. doi: 10.1186/1471-2369-14-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Besarab A, Provenzano R, Fishbane S, Sun CH, Belo S, Neff TB, et al. FG-4592 Oral hypoxia-Inducible factor prolyl hydroxylase inhibitor corrects anemia in nondialysis CKD patients without IV iron. J Am Soc Nephrol. 2011;22:196A. [Google Scholar]
- 13.Jelkmann W. Physiology and pharmacology of erythropoietin. Transfus Med Hemother. 2013;40:302–309. doi: 10.1159/000356193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. 2003. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072123.pdf. Accessed 13 June 2016.
- 15.Committee for Medicinal Products for Human Use. Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with impaired hepatic function. EMEA 2005. CPMP/EWP/2339/02. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003122.pdf. Accessed 13 June 2016.