Abstract
Background
Elevated left ventricular filling pressure (LVFP) is an important cause of exercise intolerance in patients with atrial fibrillation (AF). Exercise stress echocardiography could assess LVFP during exercise. The objective of this study was to investigate the relationship between exercise induced elevation of LVFP and exercise capacity in patients with AF.
Methods
This study included 145 consecutive patients (81 men and 64 women; mean age 65.5 ± 8.0 years) with persistent non-valvular AF and normal left ventricular systolic function (left ventricular ejection fraction ≥ 50%). All patients underwent a symptom-limited cardiopulmonary exercise test (CPET). Doppler echocardiography was performed both at rest and immediately after exercise. Five consecutive measurements of early diastolic mitral inflow velocity (E) and early diastolic mitral annular velocity (e') were taken and averaged. E/e' ratio was calculated. Elevated LVFP was defined as E/e' > 9, and patients with elevated LVFP at rest were excluded.
Results
Patients were classified into two groups according to LVFP estimated by E/e' ratio after exercise: 39 (26.9%) with elevated LVFP after exercise and 106 (73.1%) with normal LVFP. As compared with patients with normal LVFP, the ones with elevated LVFP after exercise had significantly lower peak oxygen uptake (VO2 peak) (21.7 ± 2.3 vs. 26.4 ± 3.8 mL/min per kilogram, P < 0.001), lower anaerobic threshold (19.9 ± 2.5 vs. 26.0 ± 4.0 mL/min per kilogram, P < 0.001), and shorter exercise time duration (6.2 ± 0.8 vs. 7.0 ± 1.3 min, P < 0.001). Multivariate analysis showed that age, gender and E/e' after exercise were significantly correlated with VO2 peak.
Conclusion
Elevated LVFP estimated by E/e' ratio after exercise is independently associated with reduced exercise capacity in AF patients.
Keywords: Atrial fibrillation; Diastolic dysfunction; Exercise capacity, Exercise stress echocardiography
1. Introduction
Atrial fibrillation (AF) is the most common rhythm disorder. It is a risk factor for increased mortality.[1] AF and left ventricular diastolic dysfunction often co-exist.[1]–[4] It was proven in previous studies, that diastolic dysfunction promotes the initiation[2] and recurrence of AF.[3],[4] On the other hand, patients with AF have a 3-fold higher risk of developing heart failure.[1] Thus, early recognition and appropriate therapy of diastolic dysfunction is worthwhile to prevent progression to diastolic heart failure and subsequent death in AF patients. Among the echocardiographic parameters of diastolic dysfunction, the ratio of early diastolic mitral inflow velocity (E) to early diastolic mitral annular velocity (e') has been reported to be a useful parameter for estimating left ventricular filling pressure (LVFP).[5] In the study by Li, et al.[6] E/e' > 9 has a sensitivity of 72.7% and a specificity of 70.4% for identification of elevated LVFP (> 15 mmHg) in AF patients.
AF is always associated with exercise intolerance.[7] Diastolic dysfunction could be an important cause of exercise induced dyspnea. Previous studies have shown that E/e' at rest correlates with exercise capacity in AF patients.[8],[9] However, in many cases it is only under conditions of cardiovascular stress that LVFP increases and exercise-limiting symptoms develop. Therefore, resting estimation of LVFP gives incomplete information. It has been reported that exercise stress echocardiography could assess LVFP during or after exercise, and is a non-invasive diagnostic test for early diastolic dysfunction.[5] However, whether E/e' after exercise is correlated with exercise capacity in AF patients was unknown.
So we hypothesized that exercise induced elevation of LVFP estimated by E/e' ratio is associated with reduced exercise capacity in AF patients.
2. Methods
2.1. Patient selection
This was a cross-sectional study. The study population consisted of 145 consecutive patients with persistent non-valvular AF and normal left ventricular systolic function [left ventricular ejection fraction (LVEF) ≥ 50%], who were screened from January 2012 to December 2014 at Peking University Third Hospital. Patients with elevated LVFP at rest were excluded (as defined below). Other exclusion criteria were: significant valvular heart disease or congenital heart disease; LVEF < 50%; NYHA class IV; coronary heart disease; ECG or echocardiographic evidence of exercise-induced ischemia; uncontrolled baseline heart rate (> 100 beats/min); moderate or severe respiratory disease, or functional disability. This study was approved by the ethics review boards of Peking University Health Science Center. Patients were explained about the study and written informed consent was taken.
2.2. Functional capacity assessment
All patients underwent a symptom-limited cardiopulmonary exercise test (CPET) with a treadmill using the Bruce protocol in accordance with the American Thoracic Society/American College of Chest Physicians (ATS/ ACCP).[10] Peak oxygen uptake (VO2 peak), anaerobic threshold (AT), carbon-dioxide production, and minute ventilation were measured during the test. VO2 peak and AT were computed as weighted terms (mL/min per kilogram). Patients were not asked to discontinue β-blockers, calcium channel blockers (CCBs) or digoxin before the test. A physician was present to encourage maximal exercise. The primary reason for discontinuing the exercise test included limiting dyspnea, chest pain, peripheral muscle fatigue, severe ST-segment depression, or severe ventricular arrhythmia.
2.3. Echocardiography
Comprehensive transthoracic echocardiography was performed using a Vivid S6 (GE) machine and a 3.5-MHz transducer. Standard M-mode, 2-dimensional, and color Doppler imaging were performed in parasternal and apical views with patients in the left lateral decubitus position before exercise. Doppler echocardiography was performed both at rest and immediately after exercise. E was measured using the pulsed wave Doppler method, by placing the sample volume at the level of the mitral valve leaflet tips. The e' was measured from the lateral corner of the mitral annulus in the apical 4-chamber view. Five consecutive measurements of E and e' were taken and averaged. E/e' ratio was calculated. Studies were performed by a single experienced operator. Images were recorded on videotape and interpreted by another experienced operator.
LVFP was estimated by E/e' ratio, and elevated LVFP was defined as E/e' > 9.[6] AF patients with normal LVFP (E/e' ≤ 9) at rest were classified into two groups according to LVFP after exercise: patients with elevated LVFP after exercise (E/e' > 9 after exercise) and the ones with normal LVFP (E/e' ≤ 9 after exercise).
2.4. Statistics
Continuous variables are expressed as mean ± SD, and compared by Student's unpaired t-tests between two groups. Percentage was calculated for categorical variables, and comparisons between groups were performed by Chi-Squared tests. Predictors of exercise capacity were identified by univariate analyses, and then all univariate predictors were entered in a stepwise manner into a multivariate linear regression model with the entry and retention set at a significance level of less than 0.10. Values for N-terminal pro-B-type natriuretic peptide (NT-proBNP) were normalized by logarithmic transformation (LnNT-proBNP). Spearman correlation was used to identify the bivariate correlations. Statistical significance was defined as P < 0.05. All analyses were performed with SPSS for Windows version 15.0 (SPSS, Chicago, IL).
3. Results
3.1. Clinical characteristics
A total of 145 AF patients (81 men and 64 women) were included in this study. The mean age was 65.5 ± 8.0 years. In all, 101 patients (69.7%) had a history of hypertension, 35 (24.1%) diabetes and 48 patients (33.1%) were current smokers.
Patients were classified into two groups according to LVFP estimated by E/e' after exercise: 39 (26.9%) with elevated LVFP after exercise and 106 (73.1%) with normal LVFP. There were no significant differences in age, gender, body mass index (BMI), concomitant illnesses, medications, heart rate, systolic blood pressure, or LnNT-proBNP between the two groups (all P > 0.05, Table 1).
Table 1. Clinical characteristics of patients.
| LVFP elevated after exercise (n = 39) | LVFP normal (n = 106) | P | |
| Age, yrs | 67.4 ± 6.3 | 64.9 ± 8.4 | 0.088 |
| Male | 18 (46.2%) | 63 (59.4%) | 0.153 |
| BMI, kg/m2 | 26.6 ± 3.2 | 25.6 ± 3.2 | 0.128 |
| Hypertension | 31 (79.5%) | 70 (66.0%) | 0.118 |
| Diabetes mellitus | 10 (25.6%) | 25 (23.6%) | 0.798 |
| Current smokers | 16 (41.0%) | 32 (30.2%) | 0.219 |
| Medications | |||
| β-blockers | 25 (64.1%) | 65 (61.3%) | 0.760 |
| CCBs | 14 (35.9%) | 28 (26.4%) | 0.264 |
| digoxin | 4 (10.3%) | 7 (6.6%) | 0.461 |
| ACE inhibitors/ARB | 13 (33.3%) | 32 (30.2%) | 0.717 |
| Antiplatelet agents | 20 (51.3%) | 53 (50.0%) | 0.891 |
| Anticoagulants | 12 (30.8%) | 22 (20.8%) | 0.207 |
| Stress response | |||
| Heart rate rest, beats/min | 80.8 ± 9.6 | 77.7 ± 9.3 | 0.208 |
| Heart rate peak, beats/min | 154.0 ± 37.8 | 150.9 ± 24.7 | 0.571 |
| Systolic BP rest, mmHg | 127.4 ± 22.3 | 136.2 ± 23.5 | 0.317 |
| Systolic BP peak, mmHg | 171.5 ± 18.1 | 172.2 ± 22.6 | 0.869 |
| LnNT-proBNP | 6.2 ± 1.1 | 5.8 ± 1.1 | 0.111 |
Data are represented as mean ± SD or n (%). ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; BMI: body mass index; BP: blood pressure; CCB: calcium channel blocker; LVFP: left ventricular filling pressure; NT-proBNP: N-terminal pro-B-type natriuretic peptide.
3.2. Echocardiographic parameters and exercise capacity
The average E/e' ratio of patients with elevated LVFP and normal LVFP were 10.2 ± 1.0 and 7.9 ± 0.7 separately. Table 2 summarizes the echocardiographic parameters and exercise capacity of the two groups. Echocardiographic parameters including left ventricular end diastolic diameter (LVEDD), left ventricular mass index (LVMI), LVEF and left atrial area (LAA) were not significantly different between the two groups (all P > 0.05). As compared with the patients with normal LVFP, the ones with elevated LVFP after exercise had significantly lower e' (P = 0.005) and higher E/e' at rest (P < 0.001), and higher E velocity (P = 0.001) and lower e' (P < 0.001) after exercise (P < 0.001). However, E velocity at rest was not significantly different between the two groups ( P = 0.920).
Table 2. Echocardiographic parameters and exercise capacity of patients.
| LVFP elevated after exercise (n = 39) | LVFP normal (n = 106) | P | |
| Echocardiography | |||
| LVEDD, mm | 42.3 ± 13.4 | 40.9 ± 15.7 | 0.519 |
| LVMI, g/m2 | 73.9 ± 11.7 | 71.3 ± 14.6 | 0.419 |
| LVEF, % | 69.3 ± 5.3 | 69.4 ± 5.1 | 0.909 |
| LAA, cm2 | 23.6 ± 4.3 | 22.5 ± 5.5 | 0.319 |
| E velocity rest, m/s | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.920 |
| e' rest, cm/s | 9.2 ± 1.7 | 10.4 ± 2.3 | 0.005 |
| E/e' rest | 7.9 ± 0.7 | 7.1 ± 0.9 | < 0.001 |
| E velocity stress, m/s | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.001 |
| e' stress, cm/s | 10.6 ± 1.6 | 12.3 ± 2.7 | < 0.001 |
| Exercise capacity | |||
| VO2 peak, mL/minper kilogram | 21.7 ± 2.3 | 26.4 ± 3.8 | < 0.001 |
| AT, mL/min per kilogram | 19.9 ± 2.5 | 26.0 ± 4.0 | < 0.001 |
| Exercise time duration, min | 6.2 ± 0.8 | 7.0 ± 1.3 | < 0.001 |
Data are represented as mean ± SD. AT: anaerobic threshold; E: early diastolic mitral inflow velocity; e': early diastolic mitral annular velocity; LAA: left atrial area; LVEDD: left ventricular end diastolic diameter; LVEF: Left ventricular ejection fraction; LVFP: left ventricular filling pressure; LVMI: left ventricular mass index; NT-proBNP: N-terminal pro-B-type natriuretic peptide; VO2 peak: peak oxygen uptake.
VO2 peak (21.7 ± 2.3 vs. 26.4 ± 3.8 mL/min per kilogram, P < 0.001), AT (19.9 ± 2.5 vs. 26.0 ± 4.0 mL/min per kilogram, P < 0.001) and exercise time duration (6.2 ± 0.8 vs. 7.0 ± 1.3 min, P < 0.001) were significantly lower in patients with elevated LVFP after exercise than the ones with normal LVFP.
3.3. Determinants of exercise capacity
Univariate regression analyses showed that age, gender, e' and E/e' at rest, e' and E/e' after exercise, and LnNT-proBNP were associated with VO2 peak. Multivariate analysis identified 3 significant variables that were predicative of VO2 peak: age (r = −0.351, P < 0.001), gender (26.4 ± 4.4 and 23.6 ± 3.0 mL/min per kilogram for male and female separately, P < 0.001) and E /e' after exercise (r = −0.632, P < 0.001) (Figure 1).
Figure 1. Linear regression of E/e' ratio with VO2 peak.
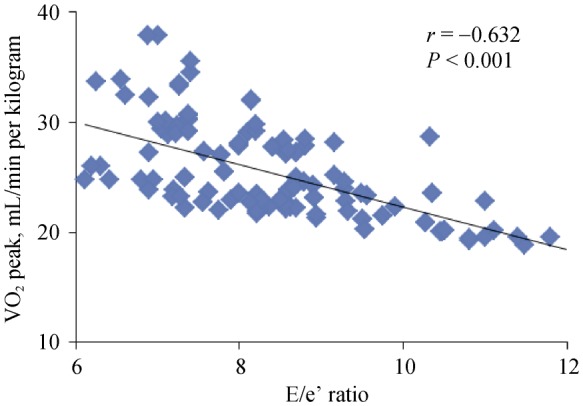
E: early diastolic mitral inflow velocity; e': early diastolic mitral annular velocity; VO2 peak: peak oxygen uptake.
Table 3. Clinical and echocardiographic variables as determinants of VO2 peak.
| Univariate |
Multivariate |
|||||
| β | t | P | β | t | P | |
| Age | −0.390 | −5.062 | < 0.001 | −0.274 | −3.559 | 0.001 |
| Gender (female) | −0.351 | −4.489 | < 0.001 | −0.239 | −3.500 | 0.001 |
| BMI | −0.017 | −0.220 | 0.826 | |||
| Hypertension | −0.063 | −0.824 | 0.411 | |||
| Diabetes mellitus | −0.049 | −0.634 | 0.527 | |||
| Current smokers | −0.036 | −0.470 | 0.639 | |||
| β-blockers | −0.109 | −1.434 | 0.153 | |||
| Heart rate rest | −0.019 | −0.226 | 0.822 | |||
| Heart rate peak | −0.046 | −0.537 | 0.592 | |||
| LVMI | −0.048 | −0.504 | 0.615 | |||
| LVEF | −0.099 | −1.147 | 0.253 | |||
| LAA | −0.013 | −0.148 | 0.883 | |||
| E velocity rest | 0.071 | 0.928 | 0.355 | |||
| e' rest | 0.360 | 5.038 | < 0.001 | |||
| E/e' rest | −0.402 | −5.725 | < 0.001 | |||
| E velocity stress | 0.025 | 0.321 | 0.748 | |||
| e' stress | 0.467 | 6.877 | < 0.001 | |||
| E/e' stress | −0.632 | −9.746 | < 0.001 | −0.530 | −7.614 | < 0.001 |
| LnNT-proBNP | −0.165 | −1.999 | 0.048 | |||
BMI: body mass index; E: early diastolic mitral inflow velocity; e': early diastolic mitral annular velocity; LAA: left atrial area; LVEF: Left ventricular ejection fraction; LVMI: left ventricular mass index; NT-proBNP: N-terminal pro-B-type natriuretic peptide; VO2 peak: peak oxygen uptake.
4. Discussion
In this study, our data showed that elevated LVFP after exercise was present in 26.9% of AF patients with normal LVFP at rest. Elevated LVFP after exercise was associated with reduced exercise capacity. Multivariate regression analysis showed age, gender and E/e' after exercise were independently associated with VO2 peak.
4.1. Left ventricular diastolic dysfunction in AF patients
AF impairs cardiac function by several mechanisms, such as the loss of atrioventricular synchrony and atrial contraction, the reduction of the diastolic filling and the induction of a tachycardia-induced cardiomyopathy.[11] The study by Kosiuk, et al.[12] showed that diastolic dysfunction was present in 37% of patients referred for AF catheter ablation. Park, et al.[13] reported that E/e' ratio was a useful independent prognostic parameter for predicting mortality in patients with AF whose left ventricular systolic function was preserved. In these studies, echocardiography was all done at rest. However, resting estimation of LVFP gives incomplete information. During exercise, to maintain adequate left ventricular filling and stroke volume, the filling pressures raise provoking symptoms to patients with diastolic dysfunction.[5],[14] Previous studies confirmed that in subjects with normal myocardial relaxation, E/e' remained unchanged during exercise because of a proportionally increase in both E and E' velocities. In patients with impaired myocardial relaxation, however, the increase in e' with exercise was lower than that of E velocity, so that the E/e' ratio increased.[5] Takagi, et al.[15] reported that elevated E/e' ratio after exercise was valuable in predicting new-onset of AF in non-ischemic elderly patients. This study found that elevated E/e' after exercise was present in 26.9% of AF patients with normal LVEP at rest, indicating a presence of early diastolic dysfunction in these patients.
4.2. Determinants of exercise capacity in AF patients
Exercise intolerance is one of the most important symptoms in patients with AF. The study by Kosiuk, et al.[12] showed that diastolic dysfunction was correlated with symptom severity in AF patients. Lee, et al.[8] reported a negative correlation between E/e' at rest and exercise capacity in AF patients. Because many patients may have normal LVFP in the resting state and cardiac symptoms may be precipitated only by exertion, it may be important to assess LVFP during exercise. Studies had shown LVFP during or after exercise was associated reduced exercise capacity in patients with heart failure[16] as well as non-obstructive hypertrophic cardiomyopathy.[17] However, the value of E/e' during exercise in AF patients were uncertain. As far as we know, this study was the first to show that AF patients with elevated E/e' after exercise had lower exercise capacity than patients with normal E/e'. So we concluded that early diastolic dysfunction detected by exercise stress echocardiography was associated with reduced exercise capacity.
In this study, both E/e' at rest and after exercise were correlated with VO2 peak in univariate analysis. However, multivariate regression analysis showed that E /e' after exercise, but not E/e' at rest, was an independent predictor of VO2 peak.
This study also showed age and gender were independently associated with VO2 peak in AF patents. It had been reported that elderly women were predominantly observed among patients with heart failure with preserved ejection fraction.[18] Previous study also showed that age and female gender were associated with heart failure in AF patients.[19]
In this study, heart rates neither at rest nor during exercise were correlated with VO2 peak. It has been proved that lenient heart rate control is as effective as strict rate control in terms of major clinical events.[20] However, previous studies had controversial results about the relationship between heart rate control and exercise capacity.[8],[21] In the study by Lee, et al.[8] heart rate at rest was an independent predictor of VO2 peak, while Cooper, et al.[21] found that heart rate was not significantly associated with 6-minute walking distance.
NT-proBNP, an established biomarker for heart failure, could predict exercise capacity in patients with heart failure.[22] In this study, patients with elevated E/e' after exercise did not have elevated LnNT-proBNP than patients with normal E/e'. Thus, NT-proBNP may be not as sensitive as E/e' in identifying early diastolic dysfunction in AF patients. In univariate regression analysis, LnNT-proBNP was associated with VO2 peak, however, LnNT-proBNP was not an independent predictor of VO2 peak in multivariate regression analysis.
4.3. Limitations
The accuracy and reproducibility of E/e' has been debated in AF patients because of the beat-to-beat variations. However, Li, et al.[6] demonstrating a good correlation between LVFP and E/E' in AF patients. In this study, five consecutive measurements of E and e' were taken and averaged in order to reduce the effect of variance from beat to beat.
4.4. Conclusions
Elevated LVFP estimated by E/e' ratio after exercise was independently associated with reduced exercise capacity. Exercise stress echocardiography could be a useful diagnostic test for early diastolic dysfunction in AF patients.
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China (81400177, CHEN SM) and Beijing Natural Science Foundation (7154249, CHEN SM). The authors have no financial disclosures.
References
- 1.Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long–term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 2.Tsang TS, Gersh BJ, Appleton CP, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–1644. doi: 10.1016/s0735-1097(02)02373-2. [DOI] [PubMed] [Google Scholar]
- 3.Melduni RM, Cullen MW. Role of left ventricular diastolic dysfunction in predicting atrial fibrillation recurrence after successful electrical cardioversion. J Atr Fibrillation. 2012;5:87–94. doi: 10.4022/jafib.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirai T, Cotseones G, Makki N, et al. Usefulness of left ventricular diastolic function to predict recurrence of atrial fibrillation in patients with preserved left ventricular systolic function. Am J Cardiol. 2014;114:65–69. doi: 10.1016/j.amjcard.2014.03.061. [DOI] [PubMed] [Google Scholar]
- 5.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Zhang J, Zhou C, et al. Will simultaneous measurement of E/e' index facilitate the non-invasive assessment of left ventricular filling pressure in patients with non-valvular atrial brillation? Eur J Echocardiogr. 2010;11:296–301. doi: 10.1093/ejechocard/jep218. [DOI] [PubMed] [Google Scholar]
- 7.Guglin M, Chen R. How much atrial fibrillation causes symptoms of heart failure? Int J Clin Pract. 2014;68:453–457. doi: 10.1111/ijcp.12262. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Jung JH, Choi SH, et al. Exercise intolerance in patients with atrial fibrillation: clinical and echocardiographic determinants of exercise capacity. J Am Soc Echocardiogr. 2005;18:1349–1354. doi: 10.1016/j.echo.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, He R, Li W, et al. Relationship between E/Em ratio and exercise capacity in patients with atrial fibrillation. Zhonghua Yi Xue Za Zhi. 2014;94:2745–2749. [Article in Chinese] [PubMed] [Google Scholar]
- 10.American Thoracic Society, American College of Chest Physicians ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 11.Thihalolipavan S, Morin DP. Atrial fibrillation and congestive heart failure? Heart Fail Clin. 2014;10:305–318. doi: 10.1016/j.hfc.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Kosiuk J, Van Belle Y, Bode K, et al. Left ventricular diastolic dysfunction in atrial fibrillation: predictors and relation with symptom severity. J Cardiovasc Electrophysiol. 2012;23:1073–1077. doi: 10.1111/j.1540-8167.2012.02368.x. [DOI] [PubMed] [Google Scholar]
- 13.Park SJ, Lee SC, Jang SY, et al. E/e' ratio is a strong prognostic predictor of mortality in patients with non-valvular atrial fibrillation with preserved left ventricular systolic function. Circ J. 2011;75:2350–2356. doi: 10.1253/circj.cj-11-0015. [DOI] [PubMed] [Google Scholar]
- 14.Borlaug BA, Nishimura RA, Sorajja P, et al. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takagi T, Takagi A, Yoshikawa J. Elevated left ventricular filling pressure estimated by E/E' ratio after exercise predicts development of new-onset atrial fibrillation independently of left atrial enlargement among elderly patients without obvious myocardial ischemia. J Cardiol. 2014;63:128–133. doi: 10.1016/j.jjcc.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Podolec P, Rubís P, Tomkiewicz-Pajak L, et al. Usefulness of the evaluation of left ventricular diastolic function changes during stress echocardiography in predicting exercise capacity in patients with ischemic heart failure. J Am Soc Echocardiogr. 2008;21:834–840. doi: 10.1016/j.echo.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Ma G, Xu M, Gao W, et al. Left ventricular filling pressure assessed by exercise TDI was correlated with early HFNEF in patients with non-obstructive hypertrophic cardiomyopathy. BMC Cardiovasc Disord. 2014;14:194. doi: 10.1186/1471-2261-14-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masoudi FA, Havranek EP, Smith G, et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 19.Imai K, Okura H, Tamada T, et al. Prediction of congestive heart failure in patients with non valvular atrial fibrillation. Intern Med. 2014;53:7–12. doi: 10.2169/internalmedicine.53.0962. [DOI] [PubMed] [Google Scholar]
- 20.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 21.Cooper HA, Bloomfield DA, Bush DE, et al. Relation between achieved heart rate and outcomes in patients with atrial fibrillation (from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management [AFFIRM] Study) Am J Cardiol. 2004;93:1247–1253. doi: 10.1016/j.amjcard.2004.01.069. [DOI] [PubMed] [Google Scholar]
- 22.Passino C, Poletti R, Bramanti F, et al. Neuro-hormonal activation predicts ventilatory response to exercise and functional capacity in patients with heart failure. Eur J Heart Fail. 2006;8:46–53. doi: 10.1016/j.ejheart.2005.05.007. [DOI] [PubMed] [Google Scholar]


