Abstract
Transcranial motor evoked potentials (TcMEPs), which are muscle action potentials elicited by transcranial brain stimulation, have been the most popular method for the last decade to monitor the functional integrity of the motor system during surgery. It was originally difficult to record reliable and reproducible potentials under general anesthesia, especially when inhalation-based anesthetic agents that suppressed the firing of anterior horn neurons were used. Advances in anesthesia, including the introduction of intravenous anesthetic agents, and progress in stimulation techniques, including the use of pulse trains, improved the reliability and reproducibility of TcMEP responses. However, TcMEPs are much smaller in amplitude compared with compound muscle action potentials evoked by maximal peripheral nerve stimulation, and vary from one trial to another in clinical practice, suggesting that only a limited number of spinal motor neurons innervating the target muscle are excited in anesthetized patients. Therefore, reliable interpretation of the critical changes in TcMEPs remains difficult and controversial. Additionally, false negative cases have been occasionally encountered. Recently, several facilitative techniques using central or peripheral stimuli, preceding transcranial electrical stimulation, have been employed to achieve sufficient depolarization of motor neurons and augment TcMEP responses. These techniques might have potentials to improve the reliability of intraoperative motor pathway monitoring using TcMEPs.
Keywords: intraoperative neurophysiologic monitoring, transcranial motor evoked potential, principles, trends
Introduction
It is essential to monitor motor function during spinal surgery that may damage the spinal cord or the spinal nerve roots. Transcranial motor evoked potentials (TcMEPs), which are muscle action potentials elicited by transcranial brain stimulation, have been the most popular method for the last decade to monitor the functional integrity of the motor system during surgery. In 1980, Merton and Morton discovered that it was possible to stimulate the human brain through the intact scalp using a high-voltage single electrical stimulus, and that TcMEPs could be recorded from the limb muscles.1,2) Thereafter, this method was used on anesthetized patients in the operating theater to monitor motor function during neurosurgical operations on the spinal cord.3) However, it was difficult to record reliable and reproducible potentials under general anesthesia, especially when inhalation-based anesthetic agents that suppressed the firing of anterior horn neurons were used.4) Furthermore, a single electrical stimulus applied over the skull could not generate multiple descending volleys, which are required to generate TcMEPs, due to the suppression of motor cortical excitability under general anesthesia.5) Advances in anesthesia, including the introduction of intravenous anesthetic agents,6,7) and progress in stimulation techniques, including the use of pulse trains,8–10) improved the reliability and reproducibility of TcMEP responses.
Methodology
I. Anesthesia
Inhalational anesthetics such as isoflurane and nitrous oxide attenuate the amplitudes of TcMEPs, because of the suppression of the excitability of cortical and spinal motor neurons and interference with synapse transmission.4,11,12) Propofol causes less suppression of the excitability of motor neurons than inhalational agents.13–15) Consequently, total intravenous anesthesia using propofol and opioid is widely recommended as optimal.16) Other favorable intravenous agents include ketamine, etomidate, and benzodiazepines.16,17–20) Neuromuscular blockade is often omitted after intubation.19,21,22) Otherwise, muscle relaxant can be administered with a constant infusion according to the amplitude of muscle responses to peripheral nerve stimulation (“train of four” technique).22,23)
II. Stimulation
The electrode placement is on the skull based on the international 10–20 electroencephalograph (EEG) system.21) We prefer the method of Matsuda and Shimazu, with the electrode symmetrically on the skull 5 cm outside and 2 cm forward of Cz.23,24) Although EEG cup electrodes or needle electrodes may be used, cork screw-like needle electrodes are preferable because of their secure placement and low impedance.16,21) While the cathode becomes the stimulating electrode with increasing intensity of the current, anodal stimuli evoke potentials more efficiently than cathodal stimuli.16,21,25) Constant-current stimulators are better than constant-voltage stimulators because current delivered to the brain does not depend on the impedance of stimulating electrodes especially when impedance changes during surgery.5)
A short train of stimuli is preferable in anesthetized patients, while there remain controversies regarding the optimal parameters of the short train of stimuli including the number of pulses in the train, individual pulse duration, inter-pulse interval, and train repetition rate. We currently used a train of five biphasic stimuli with 0.5-ms in duration (two phases of 0.25 ms in each stimulus) and an inter-pulse interval of 2 ms.
III. Recording
TcMEPs can be recorded either with surface or needle electrodes. Needle electrodes inserted into the belly muscle yield greater amplitude due to their low impedance.5) Averaging of several trials is not always required because of high signal-to-noise ratios of TcMEPs.16) When choosing the muscles to record from for monitoring the functional integrity of corticospinal tract, small muscles in hands and feet should be included due to the rich corticospinal tract innervation.5)
Safety Issues
Safety concerns with intraoperative neurophysiologic monitoring using transcranial electrical stimulation include brain damage, scalp burns, seizure, bite injury, cardiac arrhythmias, and accidental injury resulting from patient movement.26) Continuous direct brain stimulation over a period of a few seconds with a frequency of 50–60 Hz was reported to easily induce seizures.27,28) In spite of rarity of seizure related to transcranial electrical stimulation, epilepsy should be included in the contraindication of transcranial electrical stimulation.5,26) The vigorous contractions and twitches of proximal muscle groups after transcranial electrical stimulation interfere with surgery and put patients at risk of spinal cord injury, and spinal nerve root, eye, tongue, and lip injuries.29) However, accidental injury resulting from patient movement can be avoided by brief surgical pauses (a few seconds) for monitoring of TcMEPs, coordinated between surgical and electrophysiological teams.30) Bite blocks are recommended to avoid tongue and lip lacerations.
Interpretations
In spite of the introduction of intravenous anesthetic agents and stimulation techniques using pulse trains,6–10) TcMEPs are much smaller in amplitude compared with compound muscle action potentials evoked by maximal peripheral nerve stimulation, and vary from one trial to another in clinical practice, suggesting that only a limited number of spinal motor neurons innervating the target muscle are excited by the currently used transcranial stimulation techniques in anesthetized patients.31) It is thought that TcMEPs reflect the activity of only 1.8–8.9% of the motor neuron pool innervating the target muscle during surgery,32–35) particularly as Taniguchi et al. demonstrated that the amplitude of TcMEPs corresponded to about 5% of the amplitude of compound muscle action potentials evoked by maximal peripheral nerve stimulation.36) The smaller amplitude of TcMEP may also be due to the desynchronization of the descending volley, which may occur and lead to a decrease in amplitude because of “phase cancellation” phenomena.37) We previously examined the proportion of recruited motor neurons by multipulse transcranial electrical stimulation after eliminating the desynchronization of the descending volley using Magistris’s technique,37) and demonstrated that only 20% of motor neurons innervating the target muscle are recruited during TcMEP monitoring under general anesthesia.38)
Regarding the fluctuation of TcMEPs, Kajiyama et al. reported that CMAPs vary from trial to trial even under partial neuromuscular blockade and under the strictly controlled low-dose propofol anesthesia.39) Similarly, it was reported that TcMEP responses degrade or fade over the duration of a surgery although the mechanism could not be explained.40) Therefore, reliable interpretation of the critical changes in TcMEPs remains difficult and controversial, including 50%,41,42) 70%,43) 80%,44,45) or 100%46,47) attenuation of amplitude. TcMEPs are demonstrated to be very sensitive to ischemic and compressive insults to the spinal cord although the disappearance of TcMEPs does not necessarily reflect a motor deficit.48,49) Additionally, in our clinical experience, we have occasionally encountered false negative cases in which patients have suffered from focal post-operative segmental motor weakness mostly due to single nerve root injury, despite no significant change in TcMEP activity during surgery.23) In such cases, TcMEPs may not have been reliable monitors of activity in motor units damaged intraoperatively, because of radicular overlap and different dominancy of each nerve root innervating the recorded muscle.50,51)
Recent Developments
To reduce the false negative cases, more spinal motor neurons innervating the target muscle should be recruited to augment TcMEP responses. Almost two decades passed without any significant developments in anesthetic technique except for the introduction of the short-acting muscle relaxant rocuronium. Sugammadex to reverse rocuronium was demonstrated to facilitate motor evoked potentials monitoring during spinal surgery.52)
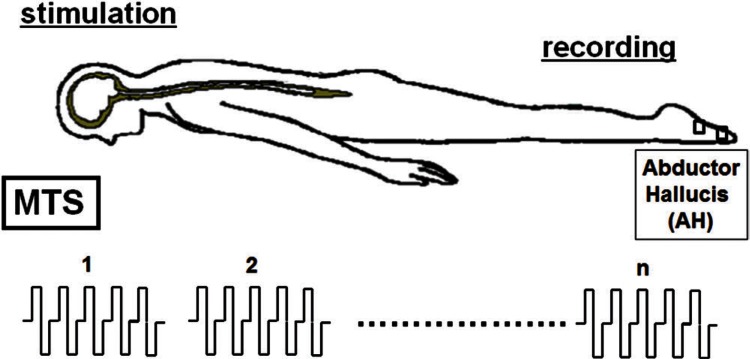
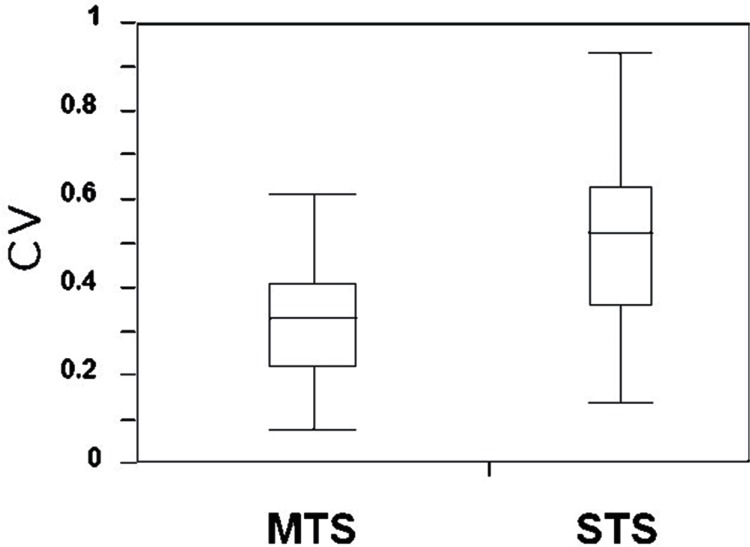
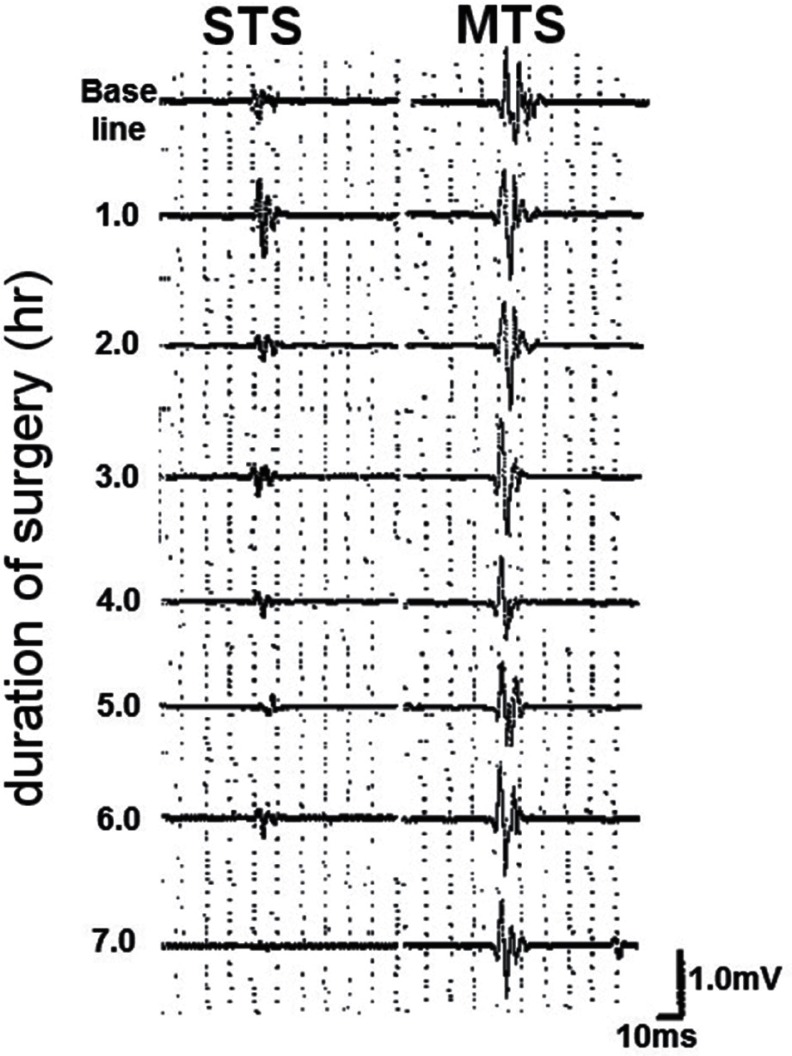
Recently, several facilitative techniques using central or peripheral stimuli (conditioning stimulation), preceding transcranial electrical stimulation, have been employed to achieve sufficient depolarization of motor neurons and augment TcMEP responses during surgery.29,53–57) According to Journée et al., conditioning stimulation can be classified into two categories: (1) heteronymous stimulation in which conditioning stimuli are applied at a different site from a test stimulus and (2) homonymous stimulation in which both conditioning and test stimuli are applied at the same site.57) One homonymous conditioning stimulation technique previously reported is recurrent pulse trains at low frequency (2–5 Hz), which was demonstrated to progressively facilitate TcMEP responses.21,58–60) Another homonymous conditioning is double-train stimulation developed by Journée et al.54) They demonstrated that double-train stimulation elicited a marked facilitation of TcMEPs when the inter-train interval (ITI) was short (10 ms ≤ ITI ≤ 40 ms) or long (ITI ≥ 0.1 s). Taking these previous homonymous conditioning techniques into account, we systematically investigated the optimal setting of multiple transcranial electrical pulse trains (Fig. 1), so-called multi-train stimulation (MTS) to enhance TcMEP responses, in which a pulse train was delivered repeatedly at repetitive rates of 2 Hz, 5 Hz, and 10 Hz (ITI; 0.5 s, 0.2 s, and 0.1 s, respectively).61) The amplitudes of TcMEPs increased with the number of train stimuli, and the strongest augmentation of TcMEPs was observed at a repetition rate of 5 Hz (ITI; 0.2 s). In addition, MTS significantly reduced the trial-to-trial variability of TcMEPs (Fig. 2), and enabled to obtain the stable responses throughout a surgery (Fig. 3), indicating that MTS could overcome “anesthetic fade.”
Fig. 1.
A schematic of the multi-train stimulation (MTS) technique, in which a pulse train is delivered repeatedly at constant repetitive rates (e.g., 2 Hz, 5 Hz, and 10 Hz). A pulse train consists of five biphasic stimuli with 0.5-ms in duration (two phases of 0.25 ms in each stimulus) and an inter-pulse interval of 2 ms. Transcranial motor evoked potentials are recorded from a pair of needle electrodes inserted in the muscle belly of the abductor hallucis (AH).
Fig. 2.
The within-patient variability of the amplitude of transcranial motor evoked potentials recorded from the abductor halluces muscle, which is assessed with the coefficient of variation (CV: standard deviation/mean). There is a statistically significant difference in CV between single-train stimulation (STS) and multi-train stimulation (MTS) (*p = 0.026, Mann-Whitney U test).
Fig. 3.
The trial-to-trial variability of transcranial motor evoked potentials recorded from the abductor halluces muscle. Multi-train stimulation (MTS) can reduce the trial-to-trial variability, and enables to obtain more stable responses throughout a surgery when compared to single-train stimulation (STS).
Conclusion
Intraoperative neurophysiologic monitoring has been indispensable with the recent advancement of surgical techniques for the treatment of more complicated spinal diseases which might damage neural tissues. Although the development of commercial equipment for intraoperative monitoring enabled reliable and reproducible evoked potentials, there have been some reports of false negative cases. Further advances in the TcMEP monitoring technique are required to enable it to be a good predictor of the patient’s motor function during spinal surgery.
References
- 1). Merton PA, Morton HB: Stimulation of the cerebral cortex in the intact human subject. Nature 285: 227, 1980. [DOI] [PubMed] [Google Scholar]
- 2). Merton PA, Morton HB: Electrical stimulation of human motor and visual cortex through the scalp. J Physiol 305: 9P– 10P, 1980. [Google Scholar]
- 3). Zentner J: Noninvasive motor evoked potential monitoring during neurosurgical operations on the spinal cord. Neurosurgery 24: 709– 712, 1989. [DOI] [PubMed] [Google Scholar]
- 4). Calancie B, Klose KJ, Baier S, Green BA: Isoflurane-induced attenuation of motor evoked potentials caused by electrical motor cortex stimulation during surgery. J Neurosurg 74: 897– 904, 1991. [DOI] [PubMed] [Google Scholar]
- 5). Deletis V, Sala F: Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: a review focus on the corticospinal tracts. Clin Neurophysiol 119: 248– 264, 2008. [DOI] [PubMed] [Google Scholar]
- 6). Jellinek D, Jewkes D, Symon L: Noninvasive intraoperative monitoring of motor evoked potentials under propofol anesthesia: effects of spinal surgery on the amplitude and latency of motor evoked potentials. Neurosurgery 29: 551– 557, 1991. [DOI] [PubMed] [Google Scholar]
- 7). Keller BP, Haghighi SS, Oro JJ, Eggers GW: The effects of propofol anesthesia on transcortical electric evoked potentials in the rat. Neurosurgery 30: 557– 560, 1992. [DOI] [PubMed] [Google Scholar]
- 8). Taylor BA, Fennelly ME, Taylor A, Farrell J: Temporal summation—the key to motor evoked potential spinal cord monitoring in humans. J Neurol Neurosurg Psychiatr 56: 104– 106, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Jones SJ, Harrison R, Koh KF, Mendoza N, Crockard HA: Motor evoked potential monitoring during spinal surgery: responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroencephalogr Clin Neurophysiol 100: 375– 383, 1996. [PubMed] [Google Scholar]
- 10). Pechstein U, Cedzich C, Nadstawek J, Schramm J: Transcranial high-frequency repetitive electrical stimulation for recording myogenic motor evoked potentials with the patient under general anesthesia. Neurosurgery 39: 335– 343; discussion 343–344, 1996. [DOI] [PubMed] [Google Scholar]
- 11). Zentner J, Ebner A: Nitrous oxide suppresses the electromyographic response evoked by electrical stimulation of the motor cortex. Neurosurgery 24: 60– 62, 1989. [DOI] [PubMed] [Google Scholar]
- 12). Zentner J, Kiss I, Ebner A: Influence of anesthetics—nitrous oxide in particular—on electromyographic response evoked by transcranial electrical stimulation of the cortex. Neurosurgery 24: 253– 256, 1989. [DOI] [PubMed] [Google Scholar]
- 13). Zentner J, Albrecht T, Heuser D: Influence of halothane, enflurane, and isoflurane on motor evoked potentials. Neurosurgery 31: 298– 305, 1992. [DOI] [PubMed] [Google Scholar]
- 14). Zhou HH, Zhu C: Comparison of isoflurane effects on motor evoked potential and F wave. Anesthesiology 93: 32– 38, 2000. [DOI] [PubMed] [Google Scholar]
- 15). Zhou HH, Jin TT, Qin B, Turndorf H: Suppression of spinal cord motoneuron excitability correlates with surgical immobility during isoflurane anesthesia. Anesthesiology 88: 955– 961, 1998. [DOI] [PubMed] [Google Scholar]
- 16). MacDonald DB, Skinner S, Shils J, Yingling C, American Society of Neurophysiological Monitoring : Intraoperative motor evoked potential monitoring—a position statement by the American Society of Neurophysiological Monitoring. Clin Neurophysiol 124: 2291– 2316, 2013. [DOI] [PubMed] [Google Scholar]
- 17). van Dongen EP, Schepens MA, Morshuis WJ, ter Beek HT, Aarts LP, de boer A, Boezeman EH: Thoracic and thoracoabdominal aortic aneurysm repair: use of evoked potential monitoring in 118 patients. J Vasc Surg 34: 1035– 1040, 2001. [DOI] [PubMed] [Google Scholar]
- 18). Jacobs MJ, Elenbaas TW, Schurink GW, Mess WH, Mochtar B: Assessment of spinal cord integrity during thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 74: S1864– S1866; discussion S1892–S1898, 2002. [DOI] [PubMed] [Google Scholar]
- 19). Weigang E, Hartert M, von Samson P, Sircar R, Pitzer K, Genstorfer J, Zentner J, Beyersdorf F: Thoracoabdominal aortic aneurysm repair: interplay of spinal cord protecting modalities. Eur J Vasc Endovasc Surg 30: 624– 631, 2005. [DOI] [PubMed] [Google Scholar]
- 20). Sutter M, Deletis V, Dvorak J, Eggspuehler A, Grob D, Macdonald D, Mueller A, Sala F, Tamaki T: Current opinions and recommendations on multimodal intraoperative monitoring during spine surgeries. Eur Spine J 16 Suppl 2: S232– S237, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Deletis V: Intraoperative neurophysiology and methodologies used to monitor the functional integrity of the motor system, in Deletis V, Shils JL. (eds): Neurophysiology in Neurosurgery. New York, Academic Press, 2002, pp 25–51 [Google Scholar]
- 22). Macdonald DB: Intraoperative motor evoked potential monitoring: overview and update. J Clin Monit Comput 20: 347– 377, 2006. [DOI] [PubMed] [Google Scholar]
- 23). Iwasaki H, Tamaki T, Yoshida M, Ando M, Yamada H, Tsutsui S, Takami M: Efficacy and limitations of current methods of intraoperative spinal cord monitoring. J Orthop Sci 8: 635– 642, 2003. [DOI] [PubMed] [Google Scholar]
- 24). Matsuda H, Shimazu A: Intraoperative spinal cord monitoring using electric responses to stimulation of caudal spinal cord or motor cortex, in Desmedt JE. (ed): Neuromonitoring in Surgery. Amsterdam, Elsevier, 1989, pp 175–190 [Google Scholar]
- 25). Rothwell JC, Thompson PD, Day BL, Dick JP, Kachi T, Cowan JM, Marsden CD: Motor cortex stimulation in intact man. 1. General characteristics of EMG responses in different muscles. Brain 110 (Pt 5): 1173– 1190, 1987. [DOI] [PubMed] [Google Scholar]
- 26). MacDonald DB: Safety of intraoperative transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol 19: 416– 429, 2002. [DOI] [PubMed] [Google Scholar]
- 27). Penfield W, Boldrey E: Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60: 339– 443, 1937. [Google Scholar]
- 28). Sartorius CJ, Wright G: Intraoperative brain mapping in a community setting—technical considerations. Surg Neurol 47: 380– 388, 1997. [DOI] [PubMed] [Google Scholar]
- 29). Kakimoto M, Kawaguchi M, Yamamoto Y, Inoue S, Horiuchi T, Nakase H, Sakaki T, Furuya H: Tetanic stimulation of the peripheral nerve before transcranial electrical stimulation can enlarge amplitudes of myogenic motor evoked potentials during general anesthesia with neuromuscular blockade. Anesthesiology 102: 733– 738, 2005. [DOI] [PubMed] [Google Scholar]
- 30). Hemmer LB, Zeeni C, Bebawy JF, Bendok BR, Cotton MA, Shah NB, Gupta DK, Koht A: The incidence of unacceptable movement with motor evoked potentials during craniotomy for aneurysm clipping. World Neurosurg 81: 99– 104, 2014. [DOI] [PubMed] [Google Scholar]
- 31). Woodforth IJ, Hicks RG, Crawford MR, Stephen JP, Burke DJ: Variability of motor-evoked potentials recorded during nitrous oxide anesthesia from the tibialis anterior muscle after transcranial electrical stimulation. Anesth Analg 82: 744– 749, 1996. [DOI] [PubMed] [Google Scholar]
- 32). Leppanen RE: From the electrodiagnostics lab: where transcranial stimulation, H-reflexes and F-responses monitor cord function intraoperatively. Spine J 4: 601– 603, 2004. [DOI] [PubMed] [Google Scholar]
- 33). Leppanen RE: Intraoperative applications of the H-reflex and F-response: a tutorial. J Clin Monit Comput 20: 267– 304, 2006. [DOI] [PubMed] [Google Scholar]
- 34). Sloan TB, Janik D, Jameson L: Multimodality monitoring of the central nervous system using motor-evoked potentials. Curr Opin Anaesthesiol 21: 560– 564, 2008. [DOI] [PubMed] [Google Scholar]
- 35). Malhotra NR, Shaffrey CI: Intraoperative electrophysiological monitoring in spine surgery. Spine 35: 2167– 2179, 2010. [DOI] [PubMed] [Google Scholar]
- 36). Taniguchi M, Cedzich C, Schramm J: Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery 32: 219– 226, 1993. [DOI] [PubMed] [Google Scholar]
- 37). Magistris MR, Rösler KM, Truffert A, Myers JP: Transcranial stimulation excites virtually all motor neurons supplying the target muscle. A demonstration and a method improving the study of motor evoked potentials. Brain 121 (Pt 3): 437– 450, 1998. [DOI] [PubMed] [Google Scholar]
- 38). Tsutsui S, Yamada H, Hashizume H, Minamide A, Nakagawa Y, Iwasaki H, Yoshida M: Quantification of the proportion of motor neurons recruited by transcranial electrical stimulation during intraoperative motor evoked potential monitoring. J Clin Monit Comput 27: 633– 637, 2013. [DOI] [PubMed] [Google Scholar]
- 39). Kajiyama S, Sanuki M, Kinoshita H: Effect of bolus propofol administration on muscle evoked potential during spine surgery. Masui Anesthesia 50: 867– 873, 2001. (Japanese) [PubMed] [Google Scholar]
- 40). Lyon R, Feiner J, Lieberman JA: Progressive suppression of motor evoked potentials during general anesthesia: the phenomenon of “anesthetic fade”. J Neurosurg Anesthesiol 17: 13– 19, 2005. [PubMed] [Google Scholar]
- 41). Meylaerts SA, Jacobs MJ, van Iterson V, De Haan P, Kalkman CJ: Comparison of transcranial motor evoked potentials and somatosensory evoked potentials during thoracoabdominal aortic aneurysm repair. Ann Surg 230: 742– 749, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Hilibrand AS, Schwartz DM, Sethuraman V, Vaccaro AR, Albert TJ: Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am 86-A: 1248–1253, 2004. [DOI] [PubMed] [Google Scholar]
- 43). Kobayashi S, Matsuyama Y, Shinomiya K, Kawabata S, Ando M, Kanchiku T, Saito T, Takahashi M, Ito Z, Muramoto A, Fujiwara Y, Kida K, Yamada K, Wada K, Yamamoto N, Satomi K, Tani T: A new alarm point of transcranial electrical stimulation motor evoked potentials for intraoperative spinal cord monitoring: a prospective multicenter study from the Spinal Cord Monitoring Working Group of the Japanese Society for Spine Surgery and Related Research. J Neurosurg Spine 20: 102– 107, 2014. [DOI] [PubMed] [Google Scholar]
- 44). Kombos T, Suess O, Ciklatekerlio O, Brock M: Monitoring of intraoperative motor evoked potentials to increase the safety of surgery in and around the motor cortex. J Neurosurg 95: 608– 614, 2001. [DOI] [PubMed] [Google Scholar]
- 45). Langeloo DD, Lelivelt A, Louis Journée H, Slappendel R, de Kleuver M: Transcranial electrical motor-evoked potential monitoring during surgery for spinal deformity: a study of 145 patients. Spine (Phila Pa 1976) 28: 1043– 1050, 2003. [DOI] [PubMed] [Google Scholar]
- 46). Kothbauer K, Deletis V, Epstein FJ: Intraoperative spinal cord monitoring for intramedullary surgery: an essential adjunct. Pediatr Neurosurg 26: 247– 254, 1997. [DOI] [PubMed] [Google Scholar]
- 47). Cioni B, Meglio M, Rossi GF: Intraoperative motor evoked potentials monitoring in spinal neurosurgery. Arch Ital Biol 137: 115– 126, 1999. [PubMed] [Google Scholar]
- 48). Nakatoh S, Kitagawa H, Kawaguchi Y, Nakamura H, Takano H: Change of muscle motor-evoked potentials after motor cortex stimulation caused by acute spinal cord injury in cats. J Spine Disord 14: 32– 38, 2001. [DOI] [PubMed] [Google Scholar]
- 49). Nakagawa Y, Tamaki T, Yamada H, Nishiura H: Discrepancy between decreases in the amplitude of compound muscle action potential and loss of motor function caused by ischemic and compressive insults to the spinal cord. J Orthop Sci 7: 102– 110, 2002. [DOI] [PubMed] [Google Scholar]
- 50). Tsutsui S, Tamaki T, Yamada H, Iwasaki H, Takami M: Relationships between the changes in compound muscle action potentials and selective injuries to the spinal cord and spinal nerve roots. Clin Neurophysiol 114: 1431– 1436, 2003. [DOI] [PubMed] [Google Scholar]
- 51). Macdonald DB, Stigsby B, Al Homoud I, Abalkhail T, Mokeem A: Utility of motor evoked potentials for intraoperative nerve root monitoring. J Clin Neurophysiol 29: 118– 125, 2012. [DOI] [PubMed] [Google Scholar]
- 52). Batistaki C, Papadopoulos K, Kalimeris KA, Soultanis K, Alevizou A, Pantazi M, Kostopanagiotou GG: Sugammadex to reverse rocuronium and facilitate intraoperative motor evoked potentials monitoring during spinal surgery. Anaesth Intensive Care 40: 1073– 1074, 2012. [PubMed] [Google Scholar]
- 53). Andersson G, Ohlin A: Spatial facilitation of motor evoked responses in monitoring during spinal surgery. Clin Neurophysiol 110: 720– 724, 1999. [DOI] [PubMed] [Google Scholar]
- 54). Journée HL, Polak HE, de Kleuver M, Langeloo DD, Postma AA: Improved neuromonitoring during spinal surgery using double-train transcranial electrical stimulation. Med Biol Eng Comput 42: 110– 113, 2004. [DOI] [PubMed] [Google Scholar]
- 55). Frei FJ, Ryhult SE, Duitmann E, Hasler CC, Luetschg J, Erb TO: Intraoperative monitoring of motor-evoked potentials in children undergoing spinal surgery. Spine (Phila Pa 1976) 32: 911– 917, 2007. [DOI] [PubMed] [Google Scholar]
- 56). Hayashi H, Kawaguchi M, Yamamoto Y, Inoue S, Koizumi M, Ueda Y, Takakura Y, Furuya H: Evaluation of reliability of post-tetanic motor-evoked potential monitoring during spinal surgery under general anesthesia. Spine (Phila Pa 1976) 33: E994– E1000, 2008. [DOI] [PubMed] [Google Scholar]
- 57). Journée HL, Polak HE, De Kleuver M: Conditioning stimulation techniques for enhancement of transcranially elicited evoked motor responses. Neurophysiol Clin 37: 423– 430, 2007. [DOI] [PubMed] [Google Scholar]
- 58). MacDonald DB, Al Zayed Z, Khoudeir I, Stigsby B: Monitoring scoliosis surgery with combined multiple pulse transcranial electric motor and cortical somatosensory-evoked potentials from the lower and upper extremities. Spine (Phila Pa 1976) 28: 194– 203, 2003. [DOI] [PubMed] [Google Scholar]
- 59). MacDonald DB, Al Zayed Z, Al Saddigi A: Four-limb muscle motor evoked potential and optimized somatosensory evoked potential monitoring with decussation assessment: results in 206 thoracolumbar spine surgeries. Eur Spine J 16 (Suppl 2): S171– S187, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Deletis V, Sala F: Corticospinal tract monitoring with D- and I-waves from the spinal cord and muscle MEPs from limb muscles, in Nuwer MR. (ed): Intraoperative Monitoring of Neural Function. Handbook of Clinical Neurophysiology , Vol. 8 Amsterdam, Elsevier, 2008, pp 235–251 [Google Scholar]
- 61). Tsutsui S, Iwasaki H, Yamada H, Hashizume H, Minamide A, Nakagawa Y, Nishi H, Yoshida M: Augmentation of motor evoked potentials using multi-train transcranial electrical stimulation in intraoperative neurophysiologic monitoring during spinal surgery. J Clin Monit Comput 29: 35– 39, 2015. [DOI] [PubMed] [Google Scholar]