Abstract
Instrumented lumbar fusion can provide immediate stability and assist in satisfactory arthrodesis in patients who have pain or instability of the lumbar spine. Lumbar adjunctive fusion with decompression is often a good procedure for surgical management of degenerative spondylolisthesis (DS). Among various lumbar fusion techniques, lumbar interbody fusion (LIF) has an advantage in that it maintains favorable lumbar alignment and provides successful fusion with the added effect of indirect decompression. This technique has been widely used and represents an advancement in spinal instrumentation, although the rationale and optimal type of LIF for DS remains controversial. We evaluated the current status and role of LIF in DS treatment, mainly as a means to augment instrumentation. We addressed the basic concept of LIF, its indications, and various types including minimally invasive techniques. It also has acceptable biomechanical features, and offers reconstruction with ideal lumbar alignment. Postsurgical adverse events related to each LIF technique are also addressed.
Keywords: lumbar spine, degenerative spondylolisthesis, instrumentation, spinal alignment, lumbar interbody fusion
Introduction
Fusion procedures for degenerative diseases of the lumbar spine, in which mechanical stress and age-related morphological change can easily induce instability and deformity, comprise one of the mandatory techniques in lumbar spinal surgery to maintain sufficient stability and good spinal alignment. The use of lumbar fusion with instrumentation has become widespread because it can produce immediate stability of the index lumbar spine, thereby achieving a shorter hospital stay and earlier recovery. These favorable outcomes depend on advances in spinal implants and the development of safe, less-invasive surgical methods. This new technology for instrumented spinal surgery makes it possible to apply spinal reconstruction in complicated cases in which there is a severe spinal deformity or a fragile spine due to osteoporosis.
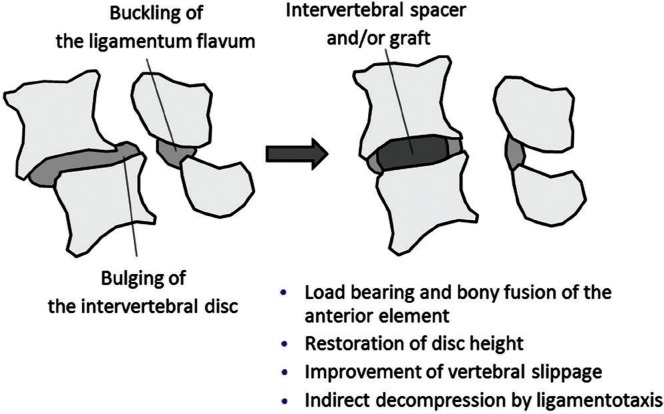
Among various lumbar fusion techniques, interbody arthrodesis supported by intervertebral and transpedicular instrumentation offers several advantages. It provides immediate structural support, restores disc height, and corrects spinal misalignment (Fig. 1). Maintaining sufficient stability and ideal load bearing of the anterior spinal element can result in successful clinical results and a high fusion rate.1) Therefore, lumbar interbody fusion (LIF) has become an important option for surgical management of lumbar degenerative disease with spinal instability and spondylolisthesis, although the rationale and appropriate technique for spinal fusion remain controversial.
Fig. 1.
Schema of before and after lumbar interbody fusion (LIF). Merits of LIF include load bearing and bony fusion of the anterior element, restoration of disc height, improvement of vertebral slippage, and indirect decompression by the ligamentotaxis effect.
We address the current status of instrumented LIF for degenerative spondylolisthesis, mainly augmented with instrumentation, with regard to not only the basic concept and traditional methods, but also advanced techniques, including minimally invasive surgery (MIS), biomechanical features, and reconstruction ending in ideal lumbar alignment. Surgical indications and adverse events associated with LIF techniques are also described.
Indications for Lumbar Fusion for Degenerative Spondylolisthesis
Lumbar spondylolisthesis has several etiologies, including spinal degeneration. It may also be caused by dysplastic (congenital), isthmic (presence of a defect in the pars interarticularis), traumatic, iatrogenic, and pathologic factors. Among these categories, degenerative spondylolisthesis (DS) is the most frequent. DS is characterized by slippage of one vertebral body on another due to intervertebral degenerative change without disruption of the neural arch. DS predominantly affects older women and occurs frequently at the L4–L5 vertebral level. It is estimated to affect 5–7% of the general population.2,3) Symptoms are related to neural compression, segmental instability, and spinal imbalance, which commonly presents with back pain, radicular pain, and neurogenic claudication. If conservative treatment fails, surgical management is usually indicated, similar to lumbar canal stenosis. Some well-designed studies have indicated that surgical management provides a more favorable clinical outcome than conservative therapy in symptomatic patients with stenosis associated with DS.4–6)
When planning a surgical strategy for DS, important factors for decision making include the degree of facet resection needed for decompression, grade of vertebral spondylolisthesis, dynamic segmental instability, severity of low back pain, spinal alignment, and spinopelvic balance. Surgical invasiveness and risks, as well as patient characteristics, influence the decisions. Based on these factors, lumbar fusion with decompression is recommended in symptomatic patients who have substantial intervertebral translation or instability, considerable segmental pain, and spinal misalignment. Lumbar fusion is also indicated in cases of over-resection of the facet joint that was performed to achieve complete neural decompression at the surgical site. In certain situations, decompression alone is an effective surgical option for patients with DS. Several factors—low-grade slippage, absence of instability, reduced range of motion, older age—were previously cited as negative factors with regard to recommending fusion surgery.7–9) Decompression alone, however, has a potential risk of iatrogenic destabilization of the spine and spondylolisthesis progression, leading to restenosis, local deformity, and persistent pain at the same segment.
Lumbar fusion with decompression has been widely accepted and is performed for stenosis associated with DS.10,11) Guidelines were established regarding the performance of fusion procedures for degenerative disease of the lumbar spine and were updated in 2014 on behalf of the American Association of Neurological Surgeons/Congress of Neurological Surgeons (AANC/CNS) Joint Section on Disorders of the Spine and Peripheral Nerves. According to these guidelines, surgical decompression and fusion are recommended as effective treatment for symptomatic stenosis associated with DS in patients who desire surgical treatment, although the standard fusion technique has not been established. The surgical strategy should be individualized for each patient to maximize fusion potential while minimizing the risk of complications. The strategy should include determining the patient’s anatomy as well as his or her desires and concerns and the surgeon’s experience.12) The role of LIF is to improve the success rate in patients undergoing lumbar fusion. However, the improved fusion rates with the addition of interbody fusion is not directly related to improved clinical outcomes.13)
Despite the fact that an absolute indication for this surgery is still unclear, decisions about performing lumbar fusion for DS should be undertaken by considering not only the patient’s condition but also the social circumstances, medical insurance system, economic effects, and the surgeon’s preference and experience. Also, a new classification system is needed to help establish the indications for lumbar fusion. Kepler et al.14) thus proposed a clinical and radiographic degenerative spondylolisthesis (CARDS) classification. Their system evaluates patients based on the presence of disc collapse, spinal instability, focal kyphosis, and symptoms, which should indicate whether lumbar fusion is needed.
Variations of LIF
There are several surgical approaches to LIF, depending on the access route to the lumbar intervertebral space.15) These operative approaches are traditionally divided into two categories according to the anterior or posterior approach being used. The anterior approach means anterior LIF (ALIF) traditionally, however, lateral LIF (LLIF) has been developed as a modified, minimally invasive technique as alternatives of ALIF recently. LLIF is recognized as less invasive because it uses a retroperitoneal transpsoas or parapsoas approach. The posterior approach includes posterior LIF (PLIF) and transforaminal LIF (TLIF). Each approach has some advantages and disadvantages, depending on the techniques and application of instrumentation (Table 1). The choice of the interbody fusion technique is left to the discretion of the surgeons.
Table 1.
Characteristics of lumbar interbody fusion techniques
| Characteristics | Posterior approach | Anterior approach | Lateral approach | |||||
|---|---|---|---|---|---|---|---|---|
| PLIF | MI PLIF | TLIF | MI TLIF | ALIF | MI ALIF | XLIF | OLIF | |
| Advantages | ||||||||
| Direct neural decompression | ○ | ○ | ○ | ○ | × | × | × | × |
| Area of fusion site | ○ | ○ | ▵ | ▵ | ◎ | ◎ | ◎ | ◎ |
| Disc height restoration | ○ | ○ | ○ | ○ | ◎ | ◎ | ◎ | ◎ |
| Alignment correction | ○ | ○ | ▵ | ▵ | ◎ | ◎ | ◎ | ◎ |
| Disadvantages | ||||||||
| Invasiveness | ○ | ▵ | ○ | ▵ | ◎ | ▵ | ▵ | ▵ |
| Risk of neural tissue traction | ○ | ○ | ▵ | ▵ | × | × | ▵ | × |
| Risk of vascular injury | ▵ | ▵ | ▵ | ▵ | ○ | ○ | ○ | ○ |
| Back muscle damage | ○ | ▵ | ○ | ▵ | × | × | × | × |
◎: more compatible, ○: compatible, ▵: less compatible, ×: not compatible. ALIF: anterior LIF, LIF: lumbar interbody fusion, MI: minimally invasive, OLIF: oblique LIF, PLIF: posterior LIF, TLIF: transforaminal LIF, XLIF: extreme lateral LIF.
I. Posterior approach
The posterior approach (Fig. 2) is the most common and familiar technique for spinal surgeons. Decompression and fusion procedures can be carried out through the same approach even though there may be concomitant back muscle damage.
Fig. 2.
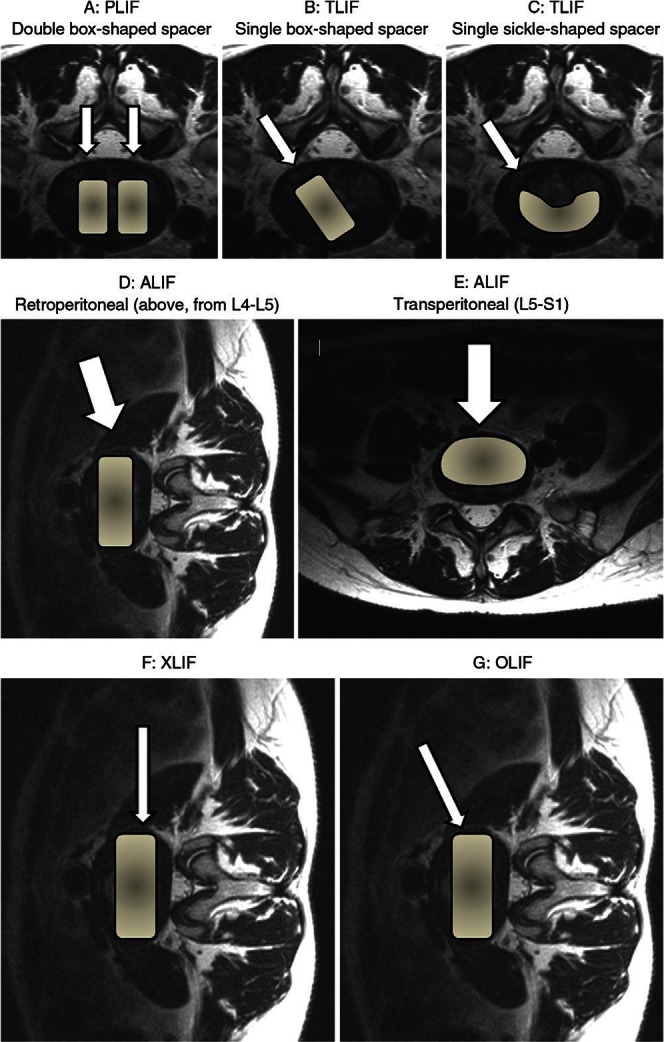
Access routes and implantation of interbody spacers through posterior approach. A: Posterior lumbar interbody fusion (PLIF) with double box-shaped spacers (arrows). B: Transforaminal lumbar interbody fusion (TLIF) with a single box-shaped spacer (arrow). C: TLIF with a single sickle-shaped spacer (arrow). Approach and implantation of interbody spacers (arrows) for anterior lumbar interbody fusion (ALIF). D: Ordinarily, the retroperitoneal anterolateral approach is done at a lumbar level above L4–L5. E: At the L5–S1 level, the transperitoneal anterior approach is selected. Access route and implantation of interbody spacers through the lateral approach for lateral lumbar interbody fusion (LLIF). LLIF is performed via a retroperitoneal approach using a less-invasive original retractor. F: Extreme lateral interbody fusion (XLIF) is a lateral transpsoas approach. G: Oblique lumbar interbody fusion (OLIF) accesses the disc between the aorta and the psoas muscle.
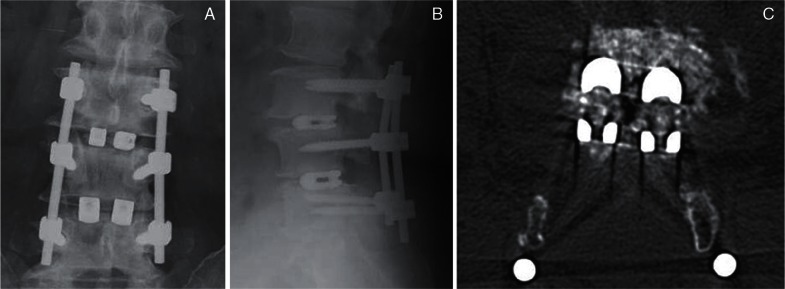
PLIF (Figs. 2A, 3) is the traditional LIF technique. Here, access to the intervertebral space is accomplished by retracting bilateral intraspinal portions along with their neural structures. Although bilateral total facetectomy can reduce the risk of excessive neural retraction, the potential for postoperative spinal instability must be considered.
Fig. 3.
Postoperative radiographs (A, B) and computed tomography (C) after posterior lumbar interbody fusion with double box-shaped spacers (titanium cage).
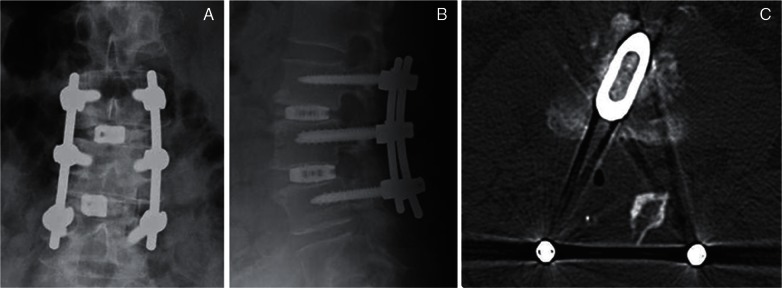
The advantage of TLIF (Figs. 2B, C, 4) over PLIF is avoidance of intraoperative over-retraction of the thecal sac or lumbar roots at the unilateral foraminal portion. Minimal neural retraction is particularly important during reoperation or in the presence of an upper level lesion in the lumbar spine to avoid neurological or dural injury. There appears to be less exposed area of the endplate, however, with TLIF than with other LIF techniques.
Fig. 4.
Postoperative radiographs (A, B) and computed tomography (C) after transforaminal lumbar interbody fusion with a single box-shaped spacer (titanium cage).
Medical evidence derived from MIS TLIF/PLIF is of low to very low quality compared to with that derived from open TLIF/PLIF. This comparison suggests that the surgical and clinical outcomes are almost equal with regard to intraoperative surgical complications but there are fewer perioperative medical complications with MIS TLIF/PLIF.16)
II. Anterior approach
The anterior approach for LIF provides the best opportunity for fusion because of the improved loading of the graft and the large surface area available for fusion compared with that provided using the posterior techniques. Indirect decompression due to the ligamentotaxis effect can also be expected for foraminal and intraspinal stenosis. Muscle damage in the back is reduced if there is no posterior instrumentation or if it is installed in a minimally invasive manner. Unique complications are possible with the anterior approach, such as ureteral injury, intestinal injury, major vessel injury, and disturbed sexual function.
ALIF (Fig. 2D, E) is a traditional anterior approach through the retroperitoneal or transperitoneal space. The patient’s surgical positioning depends on the level of the lumbar spine, taking into consideration the vascular anatomy, pelvic structure, and surgeon’s preference. The maximum graft and implant can be inserted into the intervertebral space directly. Posterior instrumentation is commonly augmented to increase its biomechanical strength.
III. Lateral approach
LLIF is recognized as less invasive through a 90° off-midline retroperitoneal approach using an original retractor. During the past decade, LLIF has increasingly been used as an alternative to conventional anterior approaches. LLIF is anatomically justified at all levels of the lumbar spine from L1–2 to L4–5 (Fig. 2F, G).17)
Extreme lateral lumbar interbody fusion (XLIF) (Fig. 2F) is an MIS lateral transpsoas approach to the lumbar spine. The advantages of XLIF include less blood loss, shorter operative times, shorter hospital stay, and less postoperative pain than is experienced with traditional open surgery. However, it has the potential to be associated with postoperative paresthesia, thigh pain, and motor weakness caused by injury to the ilioinguinal, iliohypogastric, lateral femoral cutaneous, and genitofemoral nerves and to the psoas major muscle in the retroperitoneal space.18) To avoid injury of the lumbar plexus, this technique is supported by the use of advanced neuromonitoring modalities.
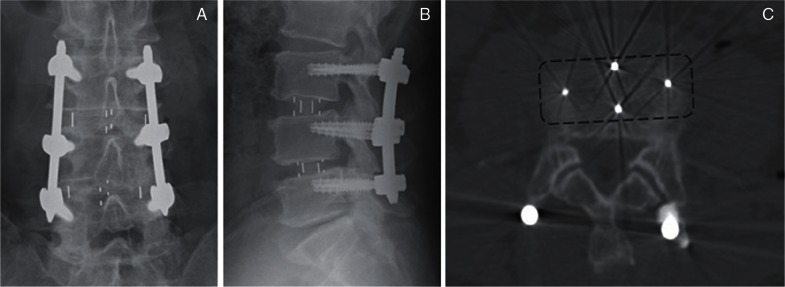
Oblique lumbar interbody fusion (OLIF) (Figs. 2G, 5) is also a lateral approach, but it obliquely accesses the disc between the aorta and the psoas muscle to avoid neural injury.
Fig. 5.
Postoperative radiographs (A, B) and computed tomography (C) after oblique lumbar interbody fusion with a polyetheretherketone (PEEK) spacer (dashed line rectangle).
There is evidence of moderate strength to support the appearance of reproducible and reasonable complications, side effects, and outcome profiles following LLIF that may be technique-dependent. There is low to moderate strength evidence that LLIF is cost-effective.17)
Current Status of LIF
Among the lumbar fusion techniques, interbody arthrodesis supported by instrumentation provides sufficient stability and ideal load bearing of anterior spinal elements to maintain a favorable disc contour.1) Medical evidence suggests that addition of an interbody fusion technique enhances higher fusion rates in patients with DS, as noted in the updated guidelines.
Kepler et al. reported that 96% of patients in the American Board of Orthopedic Surgery database who underwent surgical treatment for DS in 2011 had fusion surgery.19) They also reported that the percentage of patients treated with LIF increased significantly throughout the study period, from 13.6% (1999–2001) to 32% (2009–2011), although up to 53% of surgeons would recommend decompression alone in certain patients; most commonly, those who are older patients without significant low back pain or spinal instability. In another analysis, the US Nationwide Inpatient Sample database of estimated national trends revealed that LIF through a posterior approach represented the most frequently used procedure for surgical treatment of DS. The proportion of patients undergoing this procedure was estimated at about 75% in 2010.20) According to the results of a questionnaire survey about surgical treatment for DS from all members of the Lumbar Spine Research Society and AOSpine members in the world, both the presence of instability on flexion/extension radiographs and low back pain made spinal fusion statistically more likely to be selected than isolated decompression. The surgeon’s preference to perform LIF was primary based on the conclusion that the patient needed direct decompression of the foramen by complete facetectomy. Other reasons for selecting LIF were the need to correct coronal or sagittal balance, improve fusion rate, and use an MIS approach. Conversely, surgeons reported that the most important factors for avoiding LIF were the presence of a collapsed disc with advanced spondylotic changes, lack of motion on flexion/extension radiographs, minimal or no low back pain, osteoporosis, age > 65 years, and normal lordosis.11) In another study, LIF was considered appropriate in patients with high-grade slippage.21)
There is no high-level evidence to support the statement that there are better clinical outcomes with LIF than with other lumbar fusion techniques. LIF, however, has great potential to enhance a solid arthrodesis, which has been often confirmed as a superior rationale to support the increased cost and assumed complications of LIF. Furthermore, the latest minimally invasive approaches associated with LIF techniques may reduce the morbidity and produce earlier clinical recovery than is seen when using traditional surgical techniques.
Contribution of LIF to Gaining Ideal Lumbar Alignment
Lumbar spinal alignment is influenced not only by local spondylolisthesis or instability, but also by vertebral deformity, disc collapse, or wedging. According to enlightened, modern concepts relating to spinal deformity surgery, it is important to maintain favorable alignment in the lumbar spine, global spinal balance, and spinopelvic harmony. Keeping good alignment after fusion can be useful for preventing persistent low back pain, adjacent-segment disease, and disability with regard to the activities of daily life.22–24) Therefore, the presence or absence of spinal deformity before surgery has become an indispensable factor when deciding on a surgical strategy for DS. Notably, realignment of the lower lumbar spine is important, considering that misalignment is responsible for approximately 60% of lumbar lordosis cases.25) Several reports have reported that LIF has superior ability to restore disc height and lordotic alignment compared with posterior or posterolateral fusion.23,24,26,27)
Each LIF technique has some merits and demerits with regard to achieving lumbar realignment. The advantages of LIF through the anterior approach are that it provides a maximum area of the endplate interface, permitting a larger intervertebral spacer or graft that can maximize correction of disc height and lordosis at the surgical site. Lordotic alignment is easier to accomplish using an interbody spacer with a tilting angle of the lordosis. In contrast, LIF through the posterior approach provides a limited area to access a smaller intervertebral space and shape than that provided by LLIF. However, concomitant posterior decompression by bilateral facetectomy can contribute the shortening effect of a posterior osseous element to create an acceptable lordosis. When TLIF is performed in patients for symptomatic DS with a large scoliotic angle or coronal imbalance of the lumbar spine, unilateral use of a box-shaped implant reportedly had insufficient effect for coronal realignment.28) Additionally, with the LIF procedures, the biomechanical and biological properties are influenced not only by variations in the technique, the type and position of interbody implants, or the supplemented posterior instrumentation but also by the grade of the deformity, bone quality, and status of the end plate, especially in older patients.29,30)
Adverse Events after LIF
Numerous perioperative complications associated with LIF have been reported, including postoperative hematoma, surgical-site infection, cerebrospinal fluid leakage, visceral injury, vascular injury, instrumentation-related problems, and neurological deficits. These complications have appeared with all the delineated LIF procedures. However, accessing the intervertebral space and then inserting devices into it also has a potential risk of complications. Although early clinical results after LIF have been good, postsurgical problems do occur, such as pseudarthrodesis, adjacent-segment disease, instrument breakage or migration, and correction loss of spinal alignment over the long time. The incidence of each complication is influenced by many factors, including patient conditions, the LIF technique, the type and location of the implant, and the follow-up period. From an analysis of the Nationwide Inpatient Sample database, patients who underwent LIF had a higher risk of death than patients with posterolateral fusion (PLF). Also, patients with any type of LIF were more likely to develop complication than those with PLF.20)
With regard to LIF through the posterior approach, the greatest advantage of TLIF compared with PLIF is avoidance of intraoperative over-retraction of the dural sac or lumbar roots. Some reports indicated that use of TLIF could reduce the incidences of incidental durotomy and postoperative neuralgia and neurological deficits.31,32)
ALIF is a well-known procedure and an ideal surgical treatment in selected patients. The traditional open access needed for ALIF, however, is associated with unique complications, including vascular and intestinal problems and sexual disorders. Vascular complications have been reported in 0.5–6.7% of traditional ALIF procedures for degenerative lumbar disease.33) Retrograde ejaculation due to damage to the superior hypogastoric plexus during exposure of the anterior lumbosacral spine is estimated to occur using the transperitoneal approach rather than the retroperitoneal approach.34)
More recently, MIS has been developed for both the anterior and posterior approaches. These minimally invasive techniques can reduce approach-related complications, length of hospital stay, and prolonged time needed to recover before returning to work. Minimally invasive LLIF is a modest technique that uses a special tool and retractor. In a systematic review of the literature, LLIF had higher rates of both temporary and permanent neurological symptoms, although the intraoperative and wound complication rates were lower than with MIS-TLIF.35) Even with minimally invasive LLIF, catastrophic complications related to intestinal, vascular, and urological organ injuries are possible. Thus, systematic support by general, vascular, and urological specialists is desirable. Spinal surgeons who perform MIS procedures should also recognize and perform traditional open surgery.
Long-standing problems after surgery for DS, adjacent-segment disease (ASD), and same-segment disease (SSD) are common reasons for reoperation. Few comparison studies of LIF versus other surgical management are currently available to demonstrate which type of surgery would decrease the reoperation rate related to ASD or SSD. Sato et al. reported that the incidence of reoperation in patients surgically treated for DS was reported to be 23.2% during a mean follow-up of 5.9 years.36) A significantly higher incidence of reoperation was observed in patients treated with laminectomy alone compared with those treated with PLIF (33.8% vs. 14.4%). Body mass index and residual disc height were identified as independent risk factors for SSD, whereas male sex and facet degeneration were identified as independent risk factors for ASD.
Conclusion
LIF is commonly accepted as a surgical option in patients with symptomatic DS, although the absolute necessity of LIF remains controversial. High-quality arthrodesis and realignment at the surgical site achieved by LIF provides superior clinical outcomes in selected patients. However, the risk of adverse surgical events and its economic efficiency must be considered when this technique is selected. The role of LIF should be better defined based on future well-designed studies that include benefits of the MIS techniques.
References
- 1). McAfee PC, DeVine JG, Chaput CD, Prybis BG, Fedder IL, Cunningham BW, Farrell DJ, Hess SJ, Vigna FE: The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine (Phila Pa 1976) 30(6 Suppl): S60–S65, 2005. [DOI] [PubMed] [Google Scholar]
- 2). Frymoyer JW: Back pain and sciatica. N Engl J Med 318: 291– 300, 1988. [DOI] [PubMed] [Google Scholar]
- 3). Jacobsen S, Sonne-Holm S, Rovsing H, Monrad H, Gebuhr P: Degenerative lumbar spondylolisthesis: an epidemiological perspective: the Copenhagen Osteoarthritis Study. Spine (Phila Pa 1976) 32: 120– 125, 2007. [DOI] [PubMed] [Google Scholar]
- 4). Pearson AM, Lurie JD, Blood EA, Frymoyer JW, Braeutigam H, An H, Girardi FP, Weinstein JN: Spine patient outcomes research trial: radiographic predictors of clinical outcomes after operative or nonoperative treatment of degenerative spondylolisthesis. Spine (Phila Pa 1976) 33: 2759– 2766, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, Blood EA, Birkmeyer NJ, Hilibrand AS, Herkowitz H, Cammisa FP, Albert TJ, Emery SE, Lenke LG, Abdu WA, Longley M, Errico TJ, Hu SS: Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med 356: 2257– 2270, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Weinstein JN, Lurie JD, Tosteson TD, Zhao W, Blood EA, Tosteson AN, Birkmeyer N, Herkowitz H, Longley M, Lenke L, Emery S, Hu SS: Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. Four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 91: 1295– 1304, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Epstein NE: Decompression in the surgical management of degenerative spondylolisthesis: advantages of a conservative approach in 290 patients. J Spinal Disord 11: 116– 122; discussion 123, 1998. [DOI] [PubMed] [Google Scholar]
- 8). Herron LD, Trippi AC: L4-5 degenerative spondylolisthesis. The results of treatment by decompressive laminectomy without fusion. Spine (Phila Pa 1976) 14: 534– 538, 1989. [PubMed] [Google Scholar]
- 9). Kristof RA, Aliashkevich AF, Schuster M, Meyer B, Urbach H, Schramm J: Degenerative lumbar spondylolisthesis-induced radicular compression: nonfusion-related decompression in selected patients without hypermobility on flexion-extension radiographs. J Neurosurg 97 (3 Suppl): 281– 286, 2002. [DOI] [PubMed] [Google Scholar]
- 10). Herkowitz HN, Kurz LT: Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am 73: 802– 808, 1991. [PubMed] [Google Scholar]
- 11). Schroeder GD, Kepler CK, Kurd MF, Vaccaro AR, Hsu WK, Patel AA, Savage JW: Rationale for the surgical treatment of lumbar degenerative spondylolisthesis. Spine (Phila Pa 1976) 40: E1161– E1166, 2015. [DOI] [PubMed] [Google Scholar]
- 12). Resnick DK, Watters WC, Sharan A, Mummaneni PV, Dailey AT, Wang JC, Choudhri TF, Eck J, Ghogawala Z, Groff MW, Dhall SS, Kaiser MG: Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: lumbar fusion for stenosis with spondylolisthesis. J Neurosurg Spine 21: 54– 61, 2014. [DOI] [PubMed] [Google Scholar]
- 13). Mummaneni PV, Dhall SS, Eck JC, Groff MW, Ghogawala Z, Watters WC, Dailey AT, Resnick DK, Choudhri TF, Sharan A, Wang JC, Kaiser MG: Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 11: interbody techniques for lumbar fusion. J Neurosurg Spine 21: 67– 74, 2014. [DOI] [PubMed] [Google Scholar]
- 14). Kepler CK, Hilibrand AS, Sayadipour A, Koerner JD, Rihn JA, Radcliff KE, Vaccaro AR, Albert TJ, Anderson DG: Clinical and radiographic degenerative spondylolisthesis (CARDS) classification. Spine J 15: 1804– 1811, 2015. [DOI] [PubMed] [Google Scholar]
- 15). Rodgers WB, Gerber EJ, Patterson J: Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976) 36: 26– 32, 2011. [DOI] [PubMed] [Google Scholar]
- 16). Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR: Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res 472: 1727– 1737, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Lehmen JA, Gerber EJ: MIS lateral spine surgery: a systematic literature review of complications, outcomes, and economics. Eur Spine J 24 (Suppl 3): 287– 313, 2015. [DOI] [PubMed] [Google Scholar]
- 18). Lykissas MG, Aichmair A, Hughes AP, Sama AA, Lebl DR, Taher F, Du JY, Cammisa FP, Girardi FP: Nerve injury after lateral lumbar interbody fusion: a review of 919 treated levels with identification of risk factors. Spine J 14: 749– 758, 2014. [DOI] [PubMed] [Google Scholar]
- 19). Kepler CK, Vaccaro AR, Hilibrand AS, Anderson DG, Rihn JA, Albert TJ, Radcliff KE: National trends in the use of fusion techniques to treat degenerative spondylolisthesis. Spine (Phila Pa 1976) 39: 1584– 1589, 2014. [DOI] [PubMed] [Google Scholar]
- 20). Norton RP, Bianco K, Klifto C, Errico TJ, Bendo JA: Degenerative spondylolisthesis: an analysis of the nationwide inpatient sample database. Spine (Phila Pa 1976) 40: 1219– 1227, 2015. [DOI] [PubMed] [Google Scholar]
- 21). Cheng L, Nie L, Zhang L: Posterior lumbar interbody fusion versus posterolateral fusion in spondylolisthesis: a prospective controlled study in the Han nationality. Int Orthop 33: 1043– 1047, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Hara M, Nishimura Y, Nakajima Y, Umebayashi D, Takemoto M, Yamamoto Y, Haimoto S: Transforaminal lumbar interbody fusion for lumbar degenerative disorders: mini-open TLIF and corrective TLIF. Neurol Med Chir (Tokyo) 55: 547– 556, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Kim KH, Lee SH, Shim CS, Lee DY, Park HS, Pan WJ, Lee HY: Adjacent segment disease after interbody fusion and pedicle screw fixations for isolated L4–L5 spondylolisthesis: a minimum five-year follow-up. Spine (Phila Pa 1976) 35: 625– 634, 2010. [DOI] [PubMed] [Google Scholar]
- 24). Kim MK, Lee SH, Kim ES, Eoh W, Chung SS, Lee CS: The impact of sagittal balance on clinical results after posterior interbody fusion for patients with degenerative spondylolisthesis: a pilot study. BMC Musculoskelet Disord 12: 69, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Scheer JK, Auffinger B, Wong RH, Lam SK, Lawton CD, Nixon AT, Dahdaleh NS, Smith ZA, Fessler RG: Minimally invasive transforaminal lumbar interbody fusion (TLIF) for spondylolisthesis in 282 patients: in situ arthrodesis versus reduction. World Neurosurg 84: 108– 113, 2015. [DOI] [PubMed] [Google Scholar]
- 26). Kong LD, Zhang YZ, Wang F, Kong FL, Ding WY, Shen Y: Radiographic restoration of sagittal spinopelvic alignment after posterior lumbar interbody fusion in degenerative spondylolisthesis. J Spinal Disord Tech 2014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27). Zhou ZJ, Zhao FD, Fang XQ, Zhao X, Fan SW: Meta-analysis of instrumented posterior interbody fusion versus instrumented posterolateral fusion in the lumbar spine. J Neurosurg Spine 15: 295– 310, 2011. [DOI] [PubMed] [Google Scholar]
- 28). Takahashi T, Hanakita J, Watanabe M, Kawaoka T, Takebe N, Kitahara T: Lumbar alignment and clinical outcome after single level asymmetrical transforaminal lumbar interbody fusion for degenerative spondylolisthesis with local coronal imbalance. Neurol Med Chir (Tokyo) 54: 691– 697, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Hueng DY, Chung TT, Chuang WH, Hsu CP, Chou KN, Lin SC: Biomechanical effects of cage positions and facet fixation on initial stability of the anterior lumbar interbody fusion motion segment. Spine (Phila Pa 1976) 39: E770– E776, 2014. [DOI] [PubMed] [Google Scholar]
- 30). Lowe TG, Hashim S, Wilson LA, O’Brien MF, Smith DA, Diekmann MJ, Trommeter J: A biomechanical study of regional endplate strength and cage morphology as it relates to structural interbody support. Spine (Phila Pa 1976) 29: 2389– 2394, 2004. [DOI] [PubMed] [Google Scholar]
- 31). Humphreys SC, Hodges SD, Patwardhan AG, Eck JC, Murphy RB, Covington LA: Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine 26: 567– 571, 2001. [DOI] [PubMed] [Google Scholar]
- 32). Zhang Q, Yuan Z, Zhou M, Liu H, Xu Y, Ren Y: A comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion: a literature review and meta-analysis. BMC Musculoskelet Disord 15: 367, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Sasso RC, Best NM, Mummaneni PV, Reilly TM, Hussain SM: Analysis of operative complications in a series of 471 anterior lumbar interbody fusion procedures. Spine (Phila Pa 1976) 30: 670– 674, 2005. [DOI] [PubMed] [Google Scholar]
- 34). Sasso RC, Kenneth Burkus J, LeHuec JC: Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine (Phila Pa 1976) 28: 1023– 1026, 2003. [DOI] [PubMed] [Google Scholar]
- 35). Joseph JR, Smith BW, La Marca F, Park P: Comparison of complication rates of minimally invasive transforaminal lumbar interbody fusion and lateral lumbar interbody fusion: a systematic review of the literature. Neurosurg Focus 39: E4, 2015. [DOI] [PubMed] [Google Scholar]
- 36). Sato S, Yagi M, Machida M, Yasuda A, Konomi T, Miyake A, Fujiyoshi K, Kaneko S, Takemitsu M, Machida M, Yato Y, Asazuma T: Reoperation rate and risk factors of elective spinal surgery for degenerative spondylolisthesis: minimum 5-year follow-up. Spine J 15: 1536– 1544, 2015. [DOI] [PubMed] [Google Scholar]