Abstract
Instrumented spinal fixation is ordinarily required in patients who present with myelopathy or cauda equina syndrome secondary to vertebral collapse following osteoporotic thoracolumbar fracture. Posterior spinal fixation is a major surgical option, and partial vertebral osteotomy (PVO) through a posterior approach is occasionally reasonable for achievement of complete neural decompression and improvement of excessive local kyphosis. However, the indications and need for PVO remain unclear. The objectives of this retrospective study were to determine the efficacy and safety of posterior spinal fixation with or without PVO for osteoporotic thoracolumbar vertebral collapse and identify patients who require neural decompression and alignment correction by PVO. We retrospectively reviewed the clinical records of 20 patients (13 females, 7 males; mean age, 67.1 years) who underwent instrumented posterior fixation for osteoporotic thoracolumbar vertebral fracture. Clinical outcomes were assessed by the Japanese Orthopedic Association score and visual analog scale scores in the lumbar and leg areas. PVO was added with posterior spinal fixation in eight patients because neural decompression was incomplete after laminectomy as indicated by intraoperative echo imaging. Neurological and functional recovery significantly improved during follow-up. Clinical outcomes in patients who underwent PVO were similar to those in patients who did not undergo PVO. However, correction of the local kyphotic angle and improvement of spinal canal compromise after surgery was significant in patients who underwent PVO. The patients who required PVO had a less local kyphotic angle in the supine position and higher occupation rate of the fractured fragment in the spinal canal in the preoperative examination.
Keywords: osteoporosis, vertebral fracture, thoracolumbar spine, posterior fixation
Introduction
Thoracolumbar vertebral fracture associated with osteoporosis is becoming a serious social problem with the increasing elderly populations, especially in developed countries. Many osteoporotic vertebral fractures are controllable by conservative management for at least 6–8 weeks. If patients with a prolonged painful condition fail to respond to conservative treatment, minimally invasive surgery such as percutaneous kyphoplasty or vertebroplasty can be considered.1,2) Less invasive management should be indicated because these patients commonly have several associated problems including high age, various comorbidities, low bone quality, impaired cardiopulmonary function, and others. However, pseudarthrosis and subsequent vertebral collapse sometimes occur.3,4) Instrumented spinal fixation is ordinarily required in patients who present with progressive myelopathy or cauda equina syndrome secondary to vertebral collapse after osteoporotic thoracolumbar fracture.5–8) According to previous reports, the estimated incidence of delayed neurological deficits associated with vertebral collapse is 3% to 5% regardless of conservative treatment.9,10) Posterior instrumented spinal fixation is a major surgical option and may include additional augmentation with kyphoplasty or vertebroplasty. Partial vertebral osteotomy (PVO) and realignment combined with posterior fixation is reasonable for maximization of neural decompression and improvement in excessive local kyphosis in a portion of these patients.11) However, this technique is more invasive and is associated with a higher risk of perioperative neurovascular complications than other posterior surgical techniques. Moreover, resection of the skeletal structure in the middle and posterior spinal column to achieve vertebral osteotomy results in a greater loss of stability at the operated site compared with the preoperative status.
The indications and need for PVO have not been sufficiently determined in patients with delayed neurological deficits due to osteoporotic thoracolumbar vertebral fracture who have undergone instrumented posterior fixation. The objectives of this retrospective study were to determine the efficacy and safety of posterior spinal fixation for osteoporotic thoracolumbar fracture and elucidate the necessity of decompression and realignment by PVO.
Clinical Materials and Methods
We retrospectively reviewed the clinical records of 20 patients (13 females, 7 males) who underwent instrumented posterior fixation for osteoporotic thoracolumbar vertebral fracture from January 2009 to December 2013 at a single institute. The candidates were patients who presented with myelopathy or cauda equine syndrome caused by delayed vertebral collapse from the T12 to L2 level caused by osteoporotic vertebral fracture. Diagnosis of osteoporosis was defined as a T score of less than −1.5 points as evaluated by dual X-ray absorptiometry in the lumbar spine. Patients with multilevel vertebral collapses, spinal tumors, infectious disease, a history of back surgery, lumbar scoliosis of > 10°, or incomplete follow-up data were excluded. The mean age of the patients at surgery and the duration of illness were 67.1 (47–82) years and 7.8 (1–27) months, respectively. The minimum and mean follow-up periods were 12 months and 22.1 (12–49) months after surgery, respectively. The affected level of vertebral collapse was T12 in eight patients, L1 in nine, and L2 in three. Three patients had previous osteoporotic thoracolumbar fractures at other level of a collapsed vertebral fracture. Eleven patients were bedridden or restricted to a wheelchair because of a preoperative status of paraparesis. Six patients were ambulatory, but needed support to walk. Sphincter dysfunction was obvious in 18 patients.
Clinical outcomes were assessed using the Japanese Orthopedic Association (JOA) score and visual analogue scale (VAS) scores of 0 (no pain) to 10 (maximal pain) in the lumbar and leg areas. The recovery rates of the JOA and VAS scores, which indicated the degree of postoperative normalization, were calculated using the following formulas: (postoperative JOA score – preoperative JOS score) × 100 / (29 [full score] – preoperative JOS score) and (preoperative VAS – postoperative VAS) × 100 / preoperative VAS.
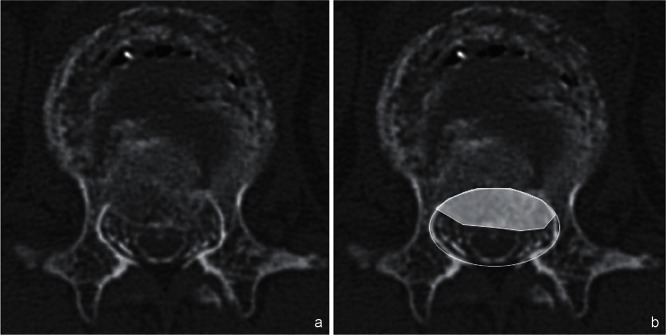
Radiological assessment included measurement of the sagittal Cobb angle [local kyphotic angle (LKA)] between the superior endplate of the vertebra above the fracture and the inferior endplate of the vertebra below the fracture on a preoperative standing X-ray (if the patient was unable to stand, the sitting position was used) and a preoperative lateral X-ray in the supine position (Fig. 1). The occupation rate of the protruded vertebral fragment into the spinal canal was calculated by computed tomography (CT) or CT myelography (Fig. 2). The patients were divided into a PVO and non-PVO group according to the surgical procedure performed (i.e., whether partial vertebral body resection was done or not, respectively). Clinical and radiological factors related to the necessity of PVO were also analyzed.
Fig. 1.
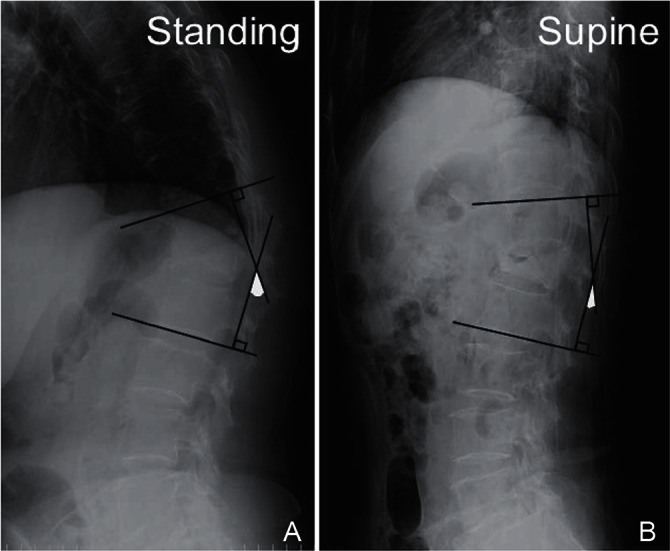
Evaluation of local kyphotic angle (LKA). Measurement of the sagittal Cobb angle between the superior endplate of the vertebra above the fracture and the inferior endplate of the vertebra below the fracture was defined as the LKA in the standing position (A) (if patient was unable to stand, the sitting position was used) and the supine position (B) on preoperative lateral X-rays.
Fig. 2.
The occupation rate was calculated as the percentage of the area of the protruded vertebral fragment into the spinal canal on axial computed tomography (CT) (a) or CT myelography (b).
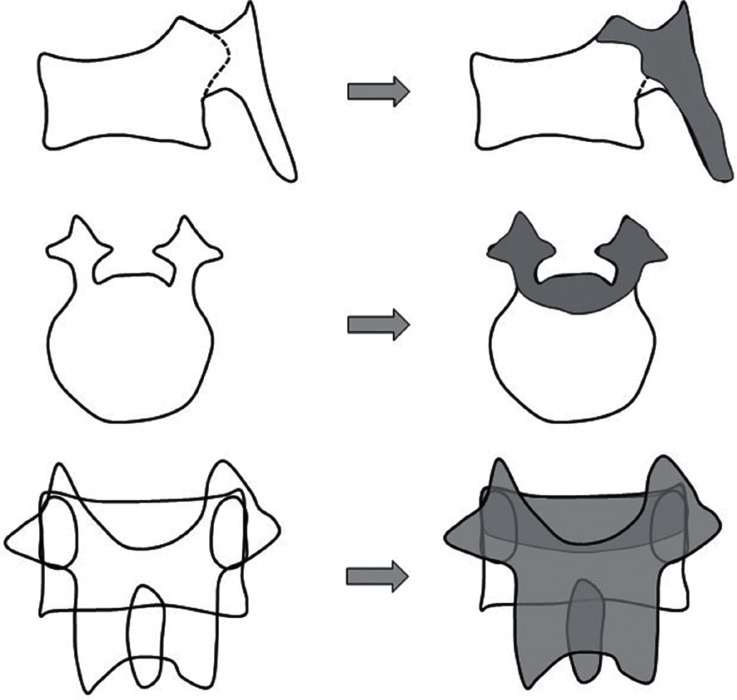
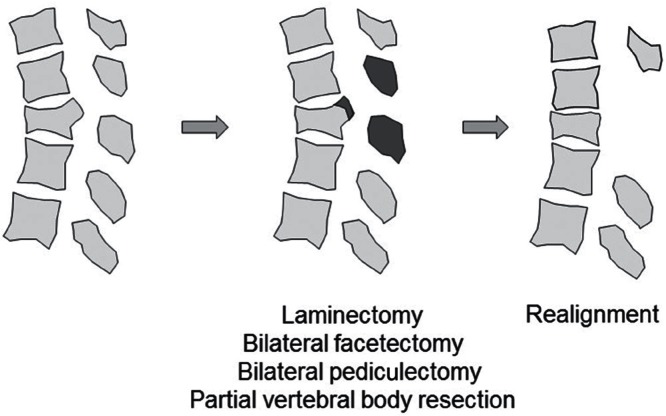
In all cases, the surgical procedure involved pedicle screw insertion at two or three levels above and below the fractured vertebral level initially. Laminectomy was also performed around the fractured vertebral level to observe spinal canal compromise and neural compression due to the protruded fragment of the collapsed vertebra from the ventral side by intraoperative echo imaging. PVO was added when remarkable indentation of the spinal cord or cauda equina was apparent upon intraoperative echo evaluation (Fig. 3). In such cases, partial vertebral body resection was carefully carried out to remove protruded osseous fragment avoiding excessive neural retraction through posterolateral space after bilateral facetectomy and pediculectomy (Fig. 4). Before final decompression maneuver, unilateral temporary rod was fixed to prevent neural injury. Achievement of decompression was evaluated by intraoperative echo imaging. Then, realignment procedure was gently done by adjusting rods precontoured to the acceptable alignment (Fig. 5). Finally, sublaminar taping was added at one or two above and below the residual laminae. All decompression maneuvers were performed under microscopy using a drill and a small osteotome. Vertebroplasty with hydroxyapatite blocks (Apaceram®; HOYA Technosurgical Corp., Shinjuku-ku, Tokyo) through transpedicular access was performed if an intravertebral cleft was obvious in patients who did not require PVO. In non-PVO cases, rod fixation and sublaminar taping were seated without realignment procedure after laminectomy.
Fig. 3.
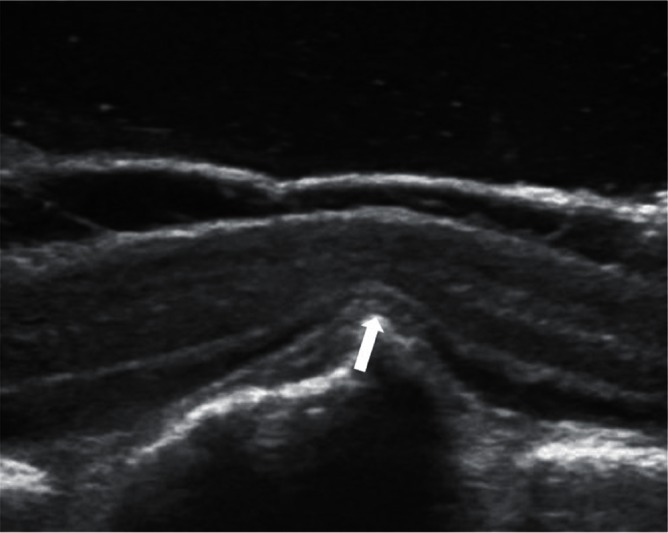
Spinal cord compression by a protruded vertebral fragment (arrow) into the spinal canal was observed upon intraoperative sagittal echo evaluation.
Fig. 4.
Schema of partial vertebral osteotomy. After laminectomy, bilateral facetectomy and pediculectomy were done. Then, partial vertebral body resection was carefully performed to remove protruded osseous fragment avoiding excessive neural retraction through posterolateral space using microscope.
Fig. 5.
Schema of osseous decompression and realignment. After osseous decompression, realignment was performed with the aid of posterior instrumentation.
All patients underwent operations with the posterior pedicle screw-rod instrumentation of the Mykles System (Century Medical Inc., Shinagawa, Tokyo) or CD HORIZON® SOLERATM Spinal System (Medtronic, Memphis, Tennessee, USA). Ultra-high-molecular-weight polyethylene tape (NESPLONTM Cable System; Alfresa Pharma Corp., Osaka city, Osaka) was used for sublaminar taping. Intraoperative neuromonitoring including SSEP and MEP was performed in 13 patients from February 2012. The postoperative management protocol was identical in both groups. The patients were allowed to walk 3 or 4 days after surgery and were required to wear a hard corset for 6 months after surgery.
Statistical analysis was performed using the Mann–Whitney test and Wilcoxon’s signed-rank test. A probability value of < 0.05 was considered statistically significant.
Results
Table 1 summarizes the patients’ demographic data. Neurological function and pain were significantly improved at the final follow-up in all patients as indicated by the JOA and VAS scores. The postoperative recovery rates according to the JOA score, lumbar VAS score, and leg VAS score were 43.9%, 58.4%, and 50.0%, respectively. Eight of 11 patients who were bedridden or restricted to a wheelchair preoperatively were able to walk with or without support. Five of six patients who needed a cane or walking frame became ambulatory without any support. The mean operation time and estimated blood loss were 401.7 (274–521) minutes and 245 (100–600) ml, respectively. The mean hospital stay was 47.6 (19–83) days. No perioperative major complications occurred in any patients; however, two patients required reoperation because of a vertebral compression fracture of the upper adjacent level or rod breakage.
Table 1.
Summary of clinical characteristics and operative information in all patients
| All cases (n = 20) | |
|---|---|
| Age (year) | 67.1 ± 10.5 |
| Sex | M 7, F 13 |
| Duration of illness (month) | 7.8 ± 7.3 |
| Level of fracture | |
| T12 | 8 |
| L1 | 9 |
| L2 | 3 |
| JOA score | preop. 6.9, postop. 16.6 |
| Recovery rate of JOA score | 43.9% |
| VAS in lumbar area | preop. 8.9, postop. 3.7 |
| Recovery rate of VAS in lumbar area | 58.4% |
| VAS in leg area | preop. 7.4, postop. 3.7 |
| Recovery rate of VAS in lumbar area | 50.0% |
| Operation time (minute) | 401.7 ± 65 |
| Blood loss (ml) | 245 ± 127 |
| Length of hospital stay (day) | 47.6 ± 19.6 |
Data are presented as mean ± standard deviation or mean, unless otherwise indicated. F: female, JOA: Japanese Orthopedic Association, M: male, VAS: visual analog scale.
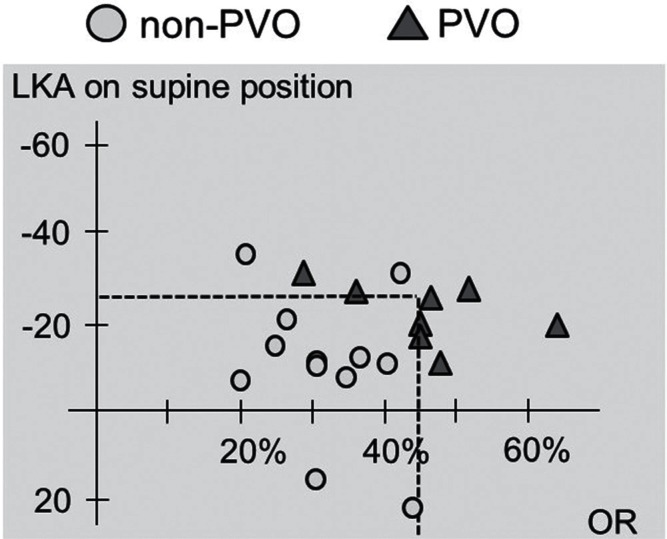
Posterior spinal fixation with or without vertebral augmentation using hydroxyapatite blocks was performed in 12 patients (non-PVO group). PVO with posterior spinal fixation was carried out in eight patients (PVO group) (Figs. 4–6). Table 2 summarizes the demographic data of the patients in the non-PVO and PVO groups. In both groups, the postoperative recovery rate of the JOA score and lumbar and leg VAS scores were significantly improved. There were no significant differences in the recovery rates between the two groups. The PVO group demonstrated significant reduction of the mean occupation rate and mean LKA correction postoperatively (P < 0.05). The operation time was significantly longer in the PVO than non-PVO group (P < 0.05). The patients’ age, sex, fracture level, preoperative JOA score, and LKA in the standing or sitting position were not correlated with the necessity of PVO. The occupation rate and LKA in the supine position were statistically associated with the necessity of PVO (P < 0.05) (Table 3). According to a plot of the relationship between the LKA in the supine position and the occupation rate, no patients with an occupation rate of < 45% and supine LKA of more than −25° underwent PVO (Fig. 7). Two cases who need reoperation were both in the non-PVO group. Rod breakage was observed in a case in whom titanium alloy rods was used.
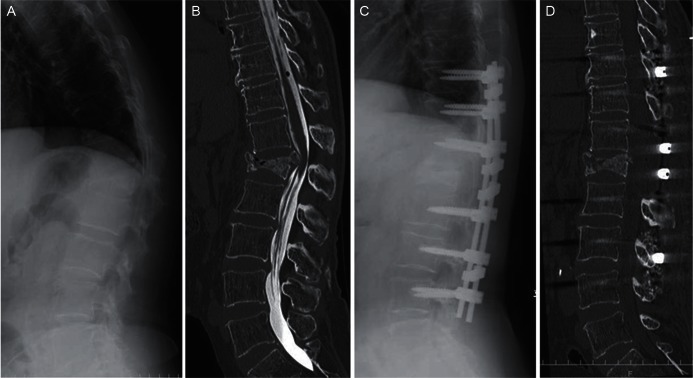
Fig. 6.
A, B: A preoperative lateral radiograph in the standing position (A) and a reconstruction sagittal computed tomography (CT) myelography image (B) of a patient with L1 vertebral collapse demonstrated severe local kyphosis and compression of the conus medullaris by the protruded fractured fragment. C, D: A postoperative lateral radiograph in the standing position (C) and reconstruction sagittal CT (D) revealed improvement in the spinal alignment and spinal canal compromise after posterior instrumented fixation with partial vertebral osteotomy.
Table 2.
Summary of clinical characteristics and operative information in the partial vertebral osteotomy (PVO) and non-PVO groups
| Non-PVO group (n = 12) | PVO group (n = 8) | |
|---|---|---|
| Age (year) | 67.7 ± 9.2 | 66.3 ± 12.5 |
| Sex | M 4, F 8 | M 3, F 5 |
| Duration of illness (month) | 4.4 ± 1.9 | 12.5 ± 9.4 |
| Level of fracture | ||
| T12 | 5 | 3 |
| L1 | 6 | 3 |
| L2 | 1 | 2 |
| Recovery rate of JOA score | 44.6% | 38.6% |
| Recovery rate of VAS in lumbar area | 61.9% | 54.2% |
| Recovery rate of VAS in lumbar area | 52.1% | 46.7% |
| Reduction of occupation area | 11.4% | 30.2%* |
| Correction of LKA (°) | 6.1 | 15.5* |
| Operation time (minute) | 377 ± 59 | 435 ± 60* |
| Blood loss (ml) | 228 ± 98 | 257 ± 148 |
| Length of hospital stay (day) | 49.0 ± 19.6 | 44.9 ± 19.9 |
Data are presented as mean ± standard deviation or mean unless otherwise indicated. F: female, JOA: Japanese Orthopedic Association, LKA: local kyphotic angle, M: male, PVO: partial vertebral osteotomy, VAS: visual analog scale,
P < 0.05.
Table 3.
Preoperative clinical and radiological factors related to partial vertebral osteotomy with posterior fixation
| Non-PVO group (n = 12) | PVO group (n = 8) | |
|---|---|---|
| Age (y) | 67.7 ± 9.2 | 66.3 ± 12.5 |
| Sex | M 4, F 8 | M 3, F 5 |
| Level of fracture | T12:5 L1:6 L2:1 | T12:3 L1:3 L2:2 |
| Preoperative JOA score | 5.9 ± 4.0 | 6.7 ± 4.7 |
| Occupation rate (%) | 30.1 ± 8.0 | 45.6 ± 10.7* |
| LKA on standing/sitting (°) | −25.2 ± 12.7 | −28.9 ± 8.9 |
| LKA on supine position (°) | −9.5 ± 12.1 | −22.3 ± 6.6* |
Data are presented as mean ± standard deviation unless otherwise indicated. F: female, JOA: Japanese Orthopedic Association, LKA: local kyphotic angle, M: male, PVO: partial vertebral osteotomy, VAS: visual analog scale,
P < 0.05.
Fig. 7.
According to the plot of the relationship between the local kyphotic angle (LKA) in the supine position and the occupation rate [OR], no patients with an occupation rate of < 45% and supine LKA of more than −25° underwent partial vertebral osteotomy (PVO).
Discussion
With the development of diagnostic modalities and medications that improve intolerable pain and fragile bone conditions, thoracolumbar vertebral fracture associated with osteoporosis is generally controllable by nonsurgical management. In spite of sufficient conservative therapy, however, some patients suffer from neurological symptoms due to deterioration of vertebral collapse and spinal instability.3) Less invasive surgery such as percutaneous vertebroplasty or kyphoplasty have a potential to improve neurological status, even in case with delayed neurological deficits. Nakamae et al. reported that 25 of 30 patients with delayed neurological deficits caused by osteoporotic vertebral compression fractures exhibited an improved neurological status at the final follow-up after percutaneous vertebroplasty.12) However, the principle concept of vertebroplasty or kyphoplasty is relief of intractable pain rather than spinal stability or neurological recovery. In these procedures, immediate neural decompression is not expected, and there is a risk of cement leakage through the damaged posterior vertebral wall. With reference to the limitations of vertebroplasty, Nakamae et al. also mentioned that vertebroplasty was not effective in patients who demonstrated high-grade spinal canal compromise and absence of remarkable dynamic instability.12) For these reasons, instrumented spinal fixation is commonly required in patients who present with progressive neurological symptoms caused by vertebral collapse after osteoporotic thoracolumbar fracture.13–15) Additionally, lesions at the thoracolumbar junction will be problematic because of a high rate of pseudarthrosis and high dynamic stress. However, the ideal surgical strategy has not been sufficiently investigated and remains controversial. A more sophisticated surgical strategy is also required in patients who need surgical treatment.
A retrospective comparative study of anterior decompression with plating versus posterior pedicle screw fixation with vertebroplasty for osteoporotic thoracolumbar collapse with neurological deficits demonstrated similar clinical results in both groups.8) In that study, intraoperative bleeding and the frequency of respiratory complications were higher in the anterior approach group. Instrumented surgery through a combined anterior and posterior approach is also presumed to be highly invasive and associated with a risk of respiratory or abdominal complications, although such a procedure is suggested to achieve the strongest stability and maintain favorable alignment accompanied with neural tissue decompression.7,16) The posterior approach with instrumentation is one potential surgical management option and has some benefits over the anterior or combined approach. The posterior approach is more familiar for treatment of thoracolumbar lesions, effectively avoids respiratory or abdominal complications, and can be modified by addition of a hook or sublaminar fixation technique, vertebral augmentation, vertebral osteotomy, percutaneous fixation, or other techniques. Posterior instrumentation may provide greater fixation strength than only anterior instrumentation because the vertebral body is more severely affected than the posterior spinal structure in patients with osteoporosis. PVO combined with posterior fixation, which shortens and realigns the fractured spine, is reasonable for neural decompression and improvement of excessive local kyphosis.11,17) However, this technique is also more invasive and is associated with a risk of perioperative neurovascular complications. Moreover, destructive procedures to resect the middle and posterior part of the vertebra will result in a loss of stability immediately after surgery and require more rigid spinal fixation. Adequate information is currently lacking about the indications and need for PVO when posterior fixation is selected.
Our clinical results illustrate that posterior instrumented fixation can significantly improve the activity of daily life and degree of pain in patients with myelopathy or cauda equina syndrome caused by osteoporotic vertebral collapse, regardless of whether PVO has been performed. Based on our intraoperative echo imaging findings, the factors related to the indications and need for PVO were excessive preoperative local kyphosis in the supine position and severe protrusion of the fractured fragment in the spinal canal. These findings indicate that reducibility of alignment by the lying position or absolute canal stenosis is indispensable for achievement of sufficient decompression of the spinal cord or cauda equina. The addition of PVO provided better decompression and correction of local spinal alignment in spite of the longer operation time. In this study, patients with an occupation rate of > 45% or supine LKA of less than −25° tended to need PVO, however, a cut-off point was not demonstrated because the sample size was very small. Based on our results, PVO should be performed in patients with a less LKA in the supine position or severity of spinal canal compromise in the preoperative examination. Suk et al. also reported that one-stage posterior closing wedge osteotomy demonstrated better surgical results with a significantly shorter mean operative time and less mean blood loss than combined anterior-posterior fixation.11) Even in their report, the mean blood loss volume associated with posterior closing wedge osteotomy was about 2,000 ml; therefore, we believe that the use of microscopy is helpful for reducing intraoperative blood loss regardless of the type of posterior surgery, while operation time will become longer.
The current study has several limitations. First, this study included a small sample size and short follow-up period. Second, this was a retrospective cohort study. Third, the indications for PVO were only defined by intraoperative echo findings. It is not clear that complete decompression and ideal realignment by PVO is reasonable rather than surgical risks of PVO. Therefore, further well-designed clinical studies are mandatory to evaluate the optimal indications for PVO in posterior instrumented surgery for delayed neurological deficits caused by osteoporotic thoracolumbar fracture.
Conclusion
The efficacy and safety of posterior spinal fixation for delayed neurological deficits caused by osteoporotic thoracolumbar fractures were investigated. The indications and need for decompression and realignment by PVO were also analyzed. The surgical results were acceptable in spite of invasive treatment. The factors related to the indications and need for PVO were less preoperative local kyphosis in the supine position and severe canal compromise caused by protrusion of the fractured fragment into the spinal canal.
References
- 1). Boonen S, Van Meirhaeghe J, Bastian L, Cummings SR, Ranstam J, Tillman JB, Eastell R, Talmadge K, Wardlaw D: Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J Bone Miner Res 26: 1627– 1637, 2011. [DOI] [PubMed] [Google Scholar]
- 2). Phillips FM: Minimally invasive treatments of osteoporotic vertebral compression fractures. Spine (Phila Pa 1976) 28(15 Suppl): S45–S53, 2003. [DOI] [PubMed] [Google Scholar]
- 3). Hoshino M, Nakamura H, Terai H, Tsujio T, Nabeta M, Namikawa T, Matsumura A, Suzuki A, Takayama K, Takaoka K: Factors affecting neurological deficits and intractable back pain in patients with insufficient bone union following osteoporotic vertebral fracture. Eur Spine J 18: 1279– 1286, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Tsujio T, Nakamura H, Terai H, Hoshino M, Namikawa T, Matsumura A, Kato M, Suzuki A, Takayama K, Fukushima W, Kondo K, Hirota Y, Takaoka K: Characteristic radiographic or magnetic resonance images of fresh osteoporotic vertebral fractures predicting potential risk for nonunion: a prospective multicenter study. Spine (Phila Pa 1976) 36: 1229– 1235, 2011. [DOI] [PubMed] [Google Scholar]
- 5). Kaneda K, Asano S, Hashimoto T, Satoh S, Fujiya M: The treatment of osteoporotic-posttraumatic vertebral collapse using the Kaneda device and a bioactive ceramic vertebral prosthesis. Spine (Phila Pa 1976) 17(8 Suppl): S295–S303, 1992. [DOI] [PubMed] [Google Scholar]
- 6). Kim DH, Vaccaro AR: Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J 6: 479– 487, 2006. [DOI] [PubMed] [Google Scholar]
- 7). Nakashima H, Imagama S, Yukawa Y, Kanemura T, Kamiya M, Deguchi M, Wakao N, Sato T, Matsuzaki K, Yoshida G, Matsuyama Y, Ishiguro N, Kato F: Comparative study of 2 surgical procedures for osteoporotic delayed vertebral collapse: anterior and posterior combined surgery versus posterior spinal fusion with vertebroplasty. Spine (Phila Pa 1976) 40: E120– E126, 2015. [DOI] [PubMed] [Google Scholar]
- 8). Sudo H, Ito M, Kaneda K, Abumi K, Kotani Y, Nagahama K, Minami A, Iwasaki N: Anterior decompression and strut graft versus posterior decompression and pedicle screw fixation with vertebroplasty for osteoporotic thoracolumbar vertebral collapse with neurologic deficits. Spine J 13: 1726– 1732, 2013. [DOI] [PubMed] [Google Scholar]
- 9). Ito M, Harada A, Nakano T, Kuratsu S, Deguchi M, Sueyoshi Y, Machida M, Yonezawa Y, Matsuyama Y, Wakao N: Retrospective multicenter study of surgical treatments for osteoporotic vertebral fractures. J Orthop Sci 15: 289– 293, 2010. [DOI] [PubMed] [Google Scholar]
- 10). Taneichi H, Kaneda K, Oguma T, Kokaji M: Risk factor analysis for osteoporotic vertebral collapse. Rinsyo Seikeigeka 37: 437– 442, 2002. [Google Scholar]
- 11). Suk SI, Kim JH, Lee SM, Chung ER, Lee JH: Anterior-posterior surgery versus posterior closing wedge osteotomy in posttraumatic kyphosis with neurologic compromised osteoporotic fracture. Spine (Phila Pa 1976) 28: 2170– 2175, 2003. [DOI] [PubMed] [Google Scholar]
- 12). Nakamae T, Fujimoto Y, Yamada K, Takata H, Shimbo T, Tsuchida Y: Percutaneous vertebroplasty for osteoporotic vertebral compression fracture with intravertebral cleft associated with delayed neurologic deficit. Eur Spine J 22: 1624– 1632, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Kanayama M, Ishida T, Hashimoto T, Shigenobu K, Togawa D, Oha F, Kaneda K: Role of major spine surgery using Kaneda anterior instrumentation for osteoporotic vertebral collapse. J Spinal Disord Tech 23: 53– 56, 2010. [DOI] [PubMed] [Google Scholar]
- 14). Matsuyama Y, Goto M, Yoshihara H, Tsuji T, Sakai Y, Nakamura H, Sato K, Kamiya M, Ishiguro N: Vertebral reconstruction with biodegradable calcium phosphate cement in the treatment of osteoporotic vertebral compression fracture using instrumentation. J Spinal Disord Tech 17: 291– 296, 2004. [DOI] [PubMed] [Google Scholar]
- 15). Uchida K, Nakajima H, Yayama T, Miyazaki T, Hirai T, Kobayashi S, Chen K, Guerrero AR, Baba H: Vertebroplasty-augmented short-segment posterior fixation of osteoporotic vertebral collapse with neurological deficit in the thoracolumbar spine: comparisons with posterior surgery without vertebroplasty and anterior surgery. J Neurosurg Spine 13: 612– 621, 2010. [DOI] [PubMed] [Google Scholar]
- 16). Kallemeier PM, Beaubien BP, Buttermann GR, Polga DJ, Wood KB: In vitro analysis of anterior and posterior fixation in an experimental unstable burst fracture model. J Spinal Disord Tech 21: 216– 224, 2008. [DOI] [PubMed] [Google Scholar]
- 17). Saita K, Hoshino Y, Kikkawa I, Nakamura H: Posterior spinal shortening for paraplegia after vertebral collapse caused by osteoporosis. Spine (Phila Pa 1976) 25: 2832–2835, 2000. [DOI] [PubMed] [Google Scholar]