Abstract
Hermansky-Pudlak Syndrome (HPS) is an autosomal recessive disorder that is associated with oculocutaneous albinism, bleeding diathesis, granulomatous colitis, and highly penetrant pulmonary fibrosis in some subtypes, including HPS-1, HPS-2, and HPS-4. HPS pulmonary fibrosis exhibits many of the clinical, radiologic, and histologic features found in Idiopathic Pulmonary Fibrosis, but occurs at a younger age. All patients with oculocutaneous albinism and easy bruising or bleeding should be screened for HPS, though the degree of albinism is variable and can be subtle. All adult patients with HPS should be screened for pulmonary involvement with chest computerized tomography. Lung biopsy is not required for diagnosis in HPS and is contraindicated because of bleeding complications. Despite knowledge of the underlying genetic defects, there are currently no definitive therapeutic or preventative approaches for HPS pulmonary fibrosis other than lung transplantation. The Hermansky–Pudlak Syndrome Network, Inc. (http://www.hpsnetwork.org) is a support organization available for patients with HPS. Delineating molecular mechanisms responsible for fibrotic susceptibility in HPS holds promise for development of targeted therapies.
Keywords: Hermansky-Pudlak Syndrome, pulmonary fibrosis, interstitial lung disease, albinism, biogenesis of lysosome related organelle complex, adaptor protein 3
Introduction
Hermansky-Pudlak Syndrome (HPS) is an autosomal recessive disorder associated with highly penetrant pulmonary fibrosis in young adults with subtypes HPS-1, HPS-2, and HPS-4. Other clinical features of HPS include oculocutaneous albinism and bleeding diathesis, enabling identification of at-risk individuals prior to the onset of lung disease. In this review, we summarize current knowledge about the clinical features and natural history of HPS, provide current recommendations for diagnosis and management of HPS, and highlight recent progress in elucidating disease pathogenesis and ongoing work in this field.
Epidemiology
Hermansky-Pudlak Syndrome (HPS) is a very rare autosomal recessive disease with 10 known subtypes1. HPS has been described in many regions of the world and in different ethnic backgrounds, including Western Europe, Indian subcontinent, Japan, China, Middle-East, and non-Puerto-Rican Hispanics2-7. To date, over 1200 individuals with HPS have registered with The HPS Network, Inc., a volunteer self help, not for profit support group for people and families dealing with HPS that was founded in 1992 (personal communication, Donna Appell, RN). The true frequency of the disease and of the different subtypes is not known. In northwest Puerto Rico, 1 in 21 persons is a carrier of the founder mutation (16bp duplication in exon 15 of HPS18) and 1 in 1800 persons suffers from HPS-1 9. Due to another founder mutation (3,904bp deletion in HPS3), one in 4000 people in central Puerto Rico is affected with HPS-3 10,11. Multiple other HPS1 mutations have been identified and HPS-1 is also the most common subtype of HPS in non-Puerto Ricans (25%)1. A handful of case reports describe subjects affected by the other HPS subtypes especially HPS-3 in individuals with Ashkenazi Jewish background12.
Pathogenesis
The 10 reported genetically distinct subtypes of HPS (denoted HPS-1-10) share the common features of oculocutaneous albinism (OCA) and a platelet storage pool deficiency2,13,14,15. HPS gene products are ubiquitously expressed and assemble into hetero-oligomeric complexes called BLOCs (biogenesis of lysosome-related organelle complexes), which are critical in trafficking to lysosome-like organelles, such as melanosomes and platelet dense granules16. The HPS1 gene product is a component of BLOC3, and HPS1 mutations cause highly penetrant pulmonary fibrosis. In contrast, the HPS3 gene product is part of BLOC2, a different trafficking complex; pulmonary fibrosis has not been observed in patients with HPS-3. The best characterized of the HPS gene products is HPS2, which in mice and humans is the β3A subunit of the Adapter Protein-3 (AP-3) complex (named independently of the BLOCs)17. AP-3 is a ubiquitously expressed hetero-oligomer which functions in organelle biogenesis and early endosomal protein trafficking. In HPS-2, mutations in the β3A subunit of AP-3 result in instability and ubiquitin-mediated degradation of the entire AP-3 complex.
There is a growing body of work focusing on the cell biology of HPS. HPS patients have macrophage-mediated inflammation preceding the onset of pulmonary fibrosis. Studies show that BAL fluid from HPS patients contains increased numbers of BAL macrophages and that these macrophages are constitutively activated, elaborating excess cytokines and chemokines18. One study suggests abnormalities in fibroblasts, with increased expression of galectin-3, a β-galactosidase-binding lectin with profibrotic effects19. Further, HPS patients with pulmonary fibrosis (HPS-PF) have been found to have increased numbers of circulating CXCR4-positive fibrocytes compared to subjects with HPS without lung disease or healthy control subjects20.
Limited availability of human lung tissue has prompted substantial HPS research in murine models. In mice, as in humans, there is considerable locus heterogeneity for HPS-like syndromes. Most of the HPS mouse models are naturally-occurring and maintained as congenic mutants on the C57BL/6J inbred strain. HPS mice reliably model important features of the human disease, including hypopigmentation, deficiency of platelet dense granules, and genotype-specific constitutive alveolar macrophage activation and susceptibility to pro-fibrotic stimuli. While spontaneous fibrosis does not occur in naturally-occurring HPS mice, HPS-1 and HPS-2 models have an exaggerated fibrotic response to fibrotic stimuli, including silica21 and bleomycin22-24. Spontaneous lung fibrosis has been reported in a HPS-1/2 double-mutant model25.
Macrophage mediated inflammation is also present in HPS mouse models26,27. However, murine bone marrow transplant studies have demonstrated that macrophage abnormalities and fibrotic susceptibility are due to epithelial dysfunction, not intrinsic macrophage defects22. Additional studies implicate alveolar epithelial cell apoptosis,25 IL-13Rα2 localization and signaling24, and autophagy28 as potential mechanisms. The mechanisms underlying how HPS trafficking defects result in alveolar epithelial cell dysfunction and resultant pulmonary fibrosis remain incompletely understood.
Diagnosis
Clinical Features
To satisfy criteria for diagnosis, all patients with HPS –regardless of subtype-must have 1) tyrosinase positive oculocutaneous albinism (OCA), and 2) a bleeding disorder due to platelet dysfunction which ranges from mild to severe (3). OCA is characterized by hypopigmentation of the hair and skin. Retinal hypopigmentation is characterized by reduced iris and retinal pigment associated with a severe decline in visual acuity and horizontal nystagmus13,29. Tyrosinase positive OCA implies that eumelanin or brown/black pigment is absent from hair, eyes and skin while pheomelanin or yellow/orange pigment is present and builds up with age30. Importantly, the degree of albinism is variable and can be subtle in HPS patients, potentially masked by use of hair-coloring products.
In the absence of overt oculocutaneous albinism, HPS could be confused with idiopathic pulmonary fibrosis (IPF), nonspecific interstitial pneumonia (NSIP), or pulmonary fibrosis due to a variety of other causes. The differential diagnosis of HPS also includes Chediak–Higashi syndrome (CHS), a recessive disorder which shares the features of mild albinism and bleeding. CHS is also associated with innate immunodeficiency, often with recurrent infections and an accelerated lymphoproliferative phase.
Diagnostic testing
Platelet electron microscopy
The bleeding disorder in HPS stems from the absence of dense bodies (δ granules) in platelets despite the presence of normal numbers of platelets. . Platelet storage granules (α and δ granules) release their content (ATP, ADP, serotonin) to attract other platelets after the initiation of the platelet aggregation cascade. Historically, the bleeding time was used to test for platelet aggregation defects, but this test has been shown to be unreliable and is not recommended. 29. The most accurate diagnostic test remains the study of freshly isolated plasma with electron microscopy to establish the complete (or near-complete) absence of δ granules31. Platelet transmission electron microscopic (PTEM) study is currently available only in selected labs (www.mayomedicallaboratories.com).
Genetic testing
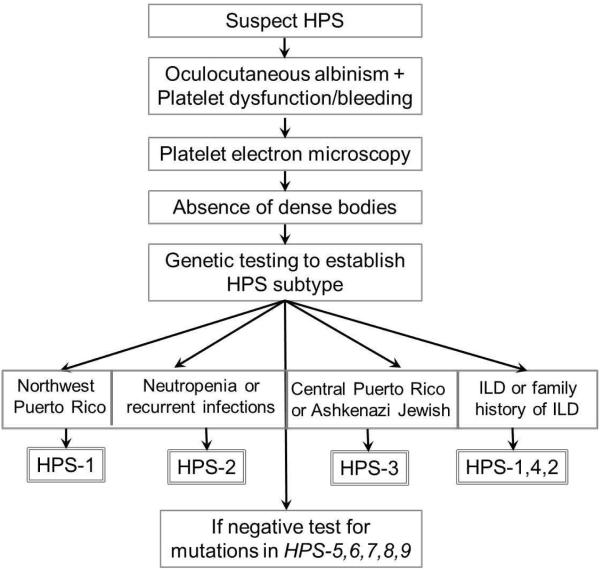
Genetic testing is recommended to determine the specific disease subtype in individuals with HPS because there are important phenotypic differences between subtypes that have critical implications for follow-up and prognosis. As suggested by Gahl et al.1, if available, one strategy could be to test a multi-gene panel containing the now 10 genes identified to cause HPS.. Alternatively, a testing strategy focusing on the ethnic background or the specific phenotypic presentation of patients with OCA and a bleeding disorder would be appropriate (Figure 1).
Figure 1.
Approach to diagnostic testing for HPS.
- HPS1 should be investigated in subjects from northwest Puerto Rico and those who are severely affected and present with interstitial lung disease (ILD).
- HPS3 testing is particularly indicated in subjects from Central Puerto Rico or with Ashkenazi Jewish ancestry
- HPS2 should be tested in subjects with neutropenia or infections
- HPS4 should be tested in severely affected subjects and those with ILD.
- Other HPS subtypes can be pursued in patients with a milder phenotype or in whom testing for HPS1-4 is negative.
Diagnosis of interstitial lung disease
High resolution computed tomography of the chest (HRCT)
Patients with HPS-1 universally develop pulmonary fibrosis. The frequency of HPS-PF in HPS-4 and HPS-2 is not known. Patients generally develop ILD in the third decade of life32, however some reports indicate the presence of symptomatic lung disease in late adolescence29. The diagnosis of ILD is established with a HRCT scan. Characteristic HRCT findings are increased reticular opacities, thickened interlobular septa, and ground- glass infiltrates in addition to fibrotic changes including traction bronchiectasis and honeycombing (Figure 2)33. These imaging findings evolve over time. With advanced disease there is universal development of reticular changes and traction bronchiectasis, with ~60% of patients developing honeycombing and ground-glass infiltrates33. The severity of changes on HRCT has been shown to correlate with decline in lung function and mortality33. Intriguingly, when patients were grouped by the severity of their disease by PFTs (no disease, mild, moderate and severe changes) and HRCT findings, the average age was similar among all groups, highlighting the heterogeneity of lung function decline in HPSPF, even among individuals with the same HPS mutations32. The timing of the first screening HRCT should likely be in late adolescence based on reports of earliest onset of ILD29.
Figure 2.
Example Chest CT imaging findings in HPS
Importantly, lung biopsy is not recommended for ILD diagnosis in individuals with HPS, as the risk of bleeding is considerable and the high pretest likelihood that fibrotic changes are due to HPS obviate the need for a surgical lung biopsy in this setting. However, examination of the lung tissue from HPSPF patients when available has revealed changes that are similar to usual interstitial pneumonia (UIP) pattern characteristic of idiopathic pulmonary fibrosis (IPF). Additional characteristic changes are foamy swelling of alveolar macrophages and epithelial cells7,34-36.
Natural history and prognosis
Data regarding the rate of lung function decline and prognosis in HPS are derived from studies conducted at the National Institutes of Health37,38. The natural history of pulmonary fibrosis in HPS has been reported as variable but universally progressive, with mortality commonly occurring in the 4th-5th decades of life. However, the age of death has ranged from 26-74 years. Indeed, Gahl et al. have reported a biphasic distribution of lung function and rate of decline37. A first peak was seen in young adults (20-25 years old) with a steady decline in forced vital capacity (FVC) % predicted from 88 to 63% by the age of 36-40 years. For those patients with normal FVC (mean 89% predicted) at 45 years of age, there seems to be a subsequent steady decline to 67% predicted by the age of 56-60 years37. When outcomes were analyzed independent of age for subjects starting with mild to moderate disease (FVC>50% predicted), average annual decline in FVC and DLCO were −2.8±5.0 (% predicted) and −2.0±4.5 (% predicted), respectively38. However, additional data indicate that some subjects may experience a much more rapid decline37,38
Similarly, a recent study examining the role of circulating fibrocytes as biomarkers of disease progression in HPSPF enrolled 40 subjects with HPS-1, 37 of whom had ILD and an average FVC of 71% predicted (60-80 interquartile range). Over the average study follow up period of more than 600 days, average lung function was stable. However, individual patients suffered lung function decline20. In this study, a threshold level of CXCR4 positive circulating fibrocytes was associated with increased risk or death during a median follow-up duration of 6.1 years20. However, fibrocyte levels were not predictive of lung function decline (FVC or DLCO) in this cohort. In summary, beyond an accelerated decline in lung function (500ml/year) that has been reported to be associated with death from HPSPF37, there are no clinically available biomarkers to predict disease progression in HPS.
Clinical management
Management of pulmonary disease
Patients with HPS-1,-4 and -2 subtypes at risk for pulmonary fibrosis should have a thorough lung examination, an early HRCT scan and serial PFTs to determine their own rate of lung function decline.
Pirfenidone in HPSPF
Although there are no known specific therapies for HPSPF, patients with HPSPF were the first to enroll in clinical trials with Pirfenidone in the United States37,38. There have been two placebo-controlled trials of Pirfenidone in HPSPF conducted at the National Institutes of Health (NIH) Clinical Center. The first trial enrolled 23 subjects with HPSPF with mildly to severely decreased lung function (FVC 40-75% predicted), with 1:1 randomization to placebo or Pirfenidone (800mg three times a day). Analysis by the repeated measures model showed that Pirfenidone treated patients lost lung function as assessed by FVC at a rate that was 5% of predicted (approximately 400mL) per year slower than placebo-treated patients, with post-hoc analysis showing greater benefit to patients with an initial FVC of at least 50% of predicted. After 44 months, based on assessment of efficacy, the trial was stopped on the recommendation of the Data Safety and Monitoring Board, though FDA approval for pirfenidone was not granted based on the trial data.
Therefore, based on the results of the first Pirfenidone trial, a second controlled trial in which patients with mild to moderate disease were randomized to Pifenidone vs. placebo in a 2:1 ratio . An interim analysis, performed 12 months after 30 of the targeted 39 patients were enrolled, showed that subjects in the group treated with Pirfenidone (801mg three times a day) had a rate of decline in FVC that was 0.7% predicted per month less than that in the placebo group. However, since FVC in the placebo group declined at a much slower rate (2.5% per year) than expected, the trial was stopped for futility, because demonstrating a difference between groups had become a statistical impossibility37. In these studies, Pirfenidone was safe and well-tolerated in patients with HPSPF.
Lung transplantation
HPS patients with pulmonary fibrosis –i.e. HPS-1, -4 and HPS-2- should be referred for lung transplant evaluation early on in the disease process. Successful lung transplant has been performed in HPS-1 despite the risks of bleeding39. Careful evaluation for risk of bleeding and other potential complications while on the wait list and after transplant are of critical importance and early referral to a lung transplant center would mitigate some of the potential barriers to transplant.
Additional recommendations for pulmonary management
Similar to other patients with pulmonary disease, patients with HPSPF should receive yearly influenza immunizations. Pneumococcal Polysaccharide Vaccinations (PPSV23) should be offered to all HPS patients with ILD or at risk for ILD, and Pneumococcal conjugate vaccine (PCV13) to those older than the age of 65. Patients should also be referred to pulmonary rehabilitation programs as needed and in preparation for lung transplant evaluation and listing.
Management of extrapulmonary complications
Skin and eye protection
Patients with albinism are at increased risk for skin cancers such as squamous and basal cell carcinomas as well as melanoma. Protections from the sun from a young age as well as yearly screening examinations by a dermatologist are indicated.
Bleeding complications
Patients with HPS are counseled to wear medical alert bracelets. Medications such as aspirin, ibuprofen, and warfarin are generally avoided due to bleeding complications. Platelet transfusions may be required in the setting of trauma, severe bleeding episodes, or surgical procedures, with recommendation for use of a single donor when possible to reduce cumulative antibody sensitization over time. Desmopressin (DDAVP) may also be utilized to prevent bleeding complications.
Inflammatory bowel disease
Involvement of the gastro-intestinal tract by a granulomatous colitis has been described in patients with HPS-1, 4 and 640-43. The disease resembles Crohn’s colitis clinically and pathologically, and most often involves the colon to a greater extent than other regions of the gastrointestinal tract. In a study of 122 subjects with HPS, 8% were found to have colitis, and among those who presented with gastrointestinal symptoms, 33% were diagnosed with colitis42. Treatment of HPS related colitis mirrors that of the treatment of Crohn’s disease with anti-inflammatory drugs, immunosuppressants, and Infliximab41,42. Because of the universal presence of platelet dysfunction, the role of aminosalicylates is controversial. Surgery should be a last resort, for intractable cases29.
Future Directions
Natural history studies and investigations into the molecular basis of HPS are ongoing at the NIH Clinical Center (NCT00001456). A longitudinal study of HPS Pulmonary Fibrosis (NCT02368340) is being conducted by the Rare Lung Diseases Consortium which is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, with funding through collaboration between NCATS and NHLBI. Additional efforts are needed to further characterize the molecular and cellular pathogenesis of HPS pulmonary fibrosis, to define the overlap between HPS pathogenesis and IPF, to identify biomarkers, and to develop targeted therapies for this devastating disease.
Summary
Hermansky-Pudlak Syndrome is an autosomal recessive disorder that is associated with oculocutaneous albinism, bleeding diatheses, granulomatous colitis, and highly penetrant pulmonary fibrosis in some subtypes, including HPS-1, HPS-2, and HPS-4. HPS pulmonary fibrosis exhibits many of the clinical, radiologic, and histologic features found in Idiopathic Pulmonary Fibrosis, but occurs at a younger age. Improved understanding of the pathogenesis of HPS holds great promise for development of molecularly targeted therapies for this rare disease and may have implications for other fibrotic lung disorders.
KEY POINTS.
Hermansky-Pudlak Syndrome (HPS) is an autosomal recessive disorder that is associated with oculocutaneous albinism, bleeding diatheses, granulomatous colitis, and highly penetrant pulmonary fibrosis in some subtypes, including HPS-1, HPS-2, and HPS-4.
HPS pulmonary fibrosis exhibits many of the clinical, radiologic, and histologic features found in Idiopathic Pulmonary Fibrosis (IPF), but occurs at a younger age.
The extent of albinism in HPS is variable, and may not be readily appreciated in patients with presumed IPF unless carefully assessed.
HPS gene products are ubiquitously expressed and assemble into hetero-oligomeric complexes called BLOCs (biogenesis of lysosome-related organelle complexes), which are critical in trafficking to lysosome-like organelles, such as melanosomes, platelet dense granules, and lamellar bodies.
Although HPS is a very rare disease reported worldwide, it is common in Puerto Rico where 1 in 1800 individuals are affected due to a founder mutation.
SYNOPSIS.
Provide a brief summary of your article (100 to 150 words; no references or figures/tables). The synopsis appears only in the table of contents and is often used by indexing services such as PubMed
Hermansky-Pudlak Syndrome (HPS) is an autosomal recessive disorder that is associated with oculocutaneous albinism, bleeding diatheses, granulomatous colitis, and highly penetrant pulmonary fibrosis in some subtypes, including HPS-1, HPS-2, and HPS-4. HPS pulmonary fibrosis exhibits many of the clinical, radiologic, and histologic features found in Idiopathic Pulmonary Fibrosis (IPF), but occurs at a younger age. Despite knowledge of the underlying genetic defects, there are currently no definitive therapeutic or preventative approaches for HPS pulmonary fibrosis other than lung transplantation.
Funding:
NIH/NHLBI R01 HL119503 (LRY) and NIH/NCATS/NHLBI U54 HL127672 (LRY) and ATS
Foundation/American Lung Association Research Grant (SE-C)
Abbreviations
- BAL
bronchoalveolar lavage
- CHS
Chediak–Higashi Syndrome
- CT
computed tomography
- FVC
forced vital capacity
- DLCO
diffusing capacity for carbon monoxide
- HPS
Hermansky-Pudlak Syndrome
- HPSPF
HPS pulmonary fibrosis
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- OCA
oculocutaneous albinism
- PFT
pulmonary function testing
- UIP
usual interstitial pneumonia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The Authors have nothing to disclose.
References
- 1.Gahl WA, Huizing M, Pagon RA, Adam MP, Ardinger HH, et al. Hermansky-Pudlak Syndrome. GeneReviews(R); Seattle (WA): 1993. [Google Scholar]
- 2.Cullinane AR, Curry JA, Carmona-Rivera C, et al. A BLOC-1 mutation screen reveals that PLDN is mutated in Hermansky-Pudlak Syndrome type 9. Am J Hum Genet. 2011;88:778–87. doi: 10.1016/j.ajhg.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Cullinane AR, Curry JA, Golas G, et al. A BLOC-1 mutation screen reveals a novel BLOC1S3 mutation in Hermansky-Pudlak Syndrome type 8. Pigment Cell Melanoma Res. 2012;25:584–91. doi: 10.1111/j.1755-148X.2012.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badolato R, Prandini A, Caracciolo S, et al. Exome sequencing reveals a pallidin mutation in a Hermansky-Pudlak-like primary immunodeficiency syndrome. Blood. 2012;119:3185–7. doi: 10.1182/blood-2012-01-404350. [DOI] [PubMed] [Google Scholar]
- 5.Jones ML, Murden SL, Brooks C, et al. Disruption of AP3B1 by a chromosome 5 inversion: a new disease mechanism in Hermansky-Pudlak syndrome type 2. BMC Med Genet. 2013;14:42. doi: 10.1186/1471-2350-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmona-Rivera C, Golas G, Hess RA, et al. Clinical, molecular, and cellular features of non-Puerto Rican Hermansky-Pudlak syndrome patients of Hispanic descent. J Invest Dermatol. 2011;131:2394–400. doi: 10.1038/jid.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanazu M, Arai T, Sugimoto C, et al. An intractable case of Hermansky-Pudlak syndrome. Intern Med. 2014;53:2629–34. doi: 10.2169/internalmedicine.53.2446. [DOI] [PubMed] [Google Scholar]
- 8.Oh J, Bailin T, Fukai K, et al. Positional cloning of a gene for Hermansky-Pudlak syndrome, a disorder of cytoplasmic organelles. Nat Genet. 1996;14:300–6. doi: 10.1038/ng1196-300. [DOI] [PubMed] [Google Scholar]
- 9.Witkop CJ, Almadovar C, Pineiro B, Nunez Babcock M. Hermansky-Pudlak syndrome (HPS)An epidemiologic study. Ophthalmic Paediatr Genet. An epidemiologic study. Ophthalmic Paediatr Genet. 1990;11:245–50. doi: 10.3109/13816819009020986. [DOI] [PubMed] [Google Scholar]
- 10.Anikster Y, Huizing M, White J, et al. Mutation of a new gene causes a unique form of Hermansky-Pudlak syndrome in a genetic isolate of central Puerto Rico. Nat Genet. 2001;28:376–80. doi: 10.1038/ng576. [DOI] [PubMed] [Google Scholar]
- 11.Santiago Borrero PJ, Rodriguez-Perez Y, Renta JY, et al. Genetic testing for oculocutaneous albinism type 1 and 2 and Hermansky-Pudlak syndrome type 1 and 3 mutations in Puerto Rico. J Invest Dermatol. 2006;126:85–90. doi: 10.1038/sj.jid.5700034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huizing M, Anikster Y, Fitzpatrick DL, et al. Hermansky-Pudlak syndrome type 3 in Ashkenazi Jews and other non-Puerto Rican patients with hypopigmentation and platelet storage-pool deficiency. Am J Hum Genet. 2001;69:1022–32. doi: 10.1086/324168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gahl WA, Brantly M, Kaiser-Kupfer MI, et al. Genetic defects and clinical characteristics of patients with a form of oculocutaneous albinism (Hermansky-Pudlak syndrome) N Engl J Med. 1998;338:1258–64. doi: 10.1056/NEJM199804303381803. [DOI] [PubMed] [Google Scholar]
- 14.Gochuico BR, Huizing M, Golas GA, et al. Interstitial lung disease and pulmonary fibrosis in Hermansky-Pudlak syndrome type 2, an adaptor protein-3 complex disease. Mol Med. 2012;18:56–64. doi: 10.2119/molmed.2011.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ammann S, Schulz A, Krageloh-Mann I, et al. Mutations in AP3D1 associated with immunodeficiency and seizures define a new type of Hermansky-Pudlak syndrome. Blood. 2016 doi: 10.1182/blood-2015-09-671636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Pietro SM, Dell'Angelica EC. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic. 2005;6:525–33. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 17.Dell'Angelica EC. AP-3-dependent trafficking and disease: the first decade. Curr Opin Cell Biol. 2009;21:552–9. doi: 10.1016/j.ceb.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Rouhani FN, Brantly ML, Markello TC, et al. Alveolar macrophage dysregulation in Hermansky-Pudlak syndrome type 1. Am J Respir Crit Care Med. 2009;180:1114–21. doi: 10.1164/rccm.200901-0023OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cullinane AR, Yeager C, Dorward H, et al. Dysregulation of galectin-3. Implications for Hermansky-Pudlak syndrome pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;50:605–13. doi: 10.1165/rcmb.2013-0025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trimble A, Gochuico BR, Markello TC, et al. Circulating fibrocytes as biomarker of prognosis in Hermansky-Pudlak syndrome. Am J Respir Crit Care Med. 2014;190:1395–401. doi: 10.1164/rccm.201407-1287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshioka Y, Kumasaka T, Ishidoh K, et al. Inflammatory response and cathepsins in silica-exposed Hermansky-Pudlak syndrome model pale ear mice. Pathol Int. 2004;54:322–31. doi: 10.1111/j.1440-1827.2004.01626.x. [DOI] [PubMed] [Google Scholar]
- 22.Young LR, Gulleman PM, Bridges JP, et al. The alveolar epithelium determines susceptibility to lung fibrosis in Hermansky-Pudlak syndrome. Am J Respir Crit Care Med. 2012;186:1014–24. doi: 10.1164/rccm.201207-1206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young LR, Pasula R, Gulleman PM, Deutsch GH, McCormack FX. Susceptibility of Hermansky-Pudlak mice to bleomycin-induced type II cell apoptosis and fibrosis. Am J Respir Cell Mol Biol. 2007;37:67–74. doi: 10.1165/rcmb.2006-0469OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, He CH, Herzog EL, et al. Chitinase 3-like-1 and its receptors in Hermansky-Pudlak syndrome-associated lung disease. J Clin Invest. 2015;125:3178–92. doi: 10.1172/JCI79792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahavadi P, Korfei M, Henneke I, et al. Epithelial stress and apoptosis underlie Hermansky-Pudlak syndrome-associated interstitial pneumonia. Am J Respir Crit Care Med. 2010;182:207–19. doi: 10.1164/rccm.200909-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atochina-Vasserman EN, Bates SR, Zhang P, et al. Early alveolar epithelial dysfunction promotes lung inflammation in a mouse model of Hermansky-Pudlak syndrome. Am J Respir Crit Care Med. 2011;184:449–58. doi: 10.1164/rccm.201011-1882OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young LR, Borchers MT, Allen HL, Gibbons RS, McCormack FX. Lung-restricted macrophage activation in the pearl mouse model of Hermansky-Pudlak syndrome. J Immunol. 2006;176:4361–8. doi: 10.4049/jimmunol.176.7.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahuja S, Knudsen L, Chillappagari S, et al. MAP1LC3B overexpression protects against Hermansky-Pudlak syndrome type - 1 induced defective autophagy in vitro. Am J Physiol Lung Cell Mol Physiol. 2015 doi: 10.1152/ajplung.00213.2015. ajplung 00213 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seward SL, Jr., Gahl WA. Hermansky-Pudlak syndrome: health care throughout life. Pediatrics. 2013;132:153–60. doi: 10.1542/peds.2012-4003. [DOI] [PubMed] [Google Scholar]
- 30.Ramsay M, Colman MA, Stevens G, et al. The tyrosinase-positive oculocutaneous albinism locus maps to chromosome 15q11.2-q12. Am J Hum Genet. 1992;51:879–84. [PMC free article] [PubMed] [Google Scholar]
- 31.Witkop CJ, Krumwiede M, Sedano H, White JG. Reliability of absent platelet dense bodies as a diagnostic criterion for Hermansky-Pudlak syndrome. Am J Hematol. 1987;26:305–11. doi: 10.1002/ajh.2830260403. [DOI] [PubMed] [Google Scholar]
- 32.Brantly M, Avila NA, Shotelersuk V, Lucero C, Huizing M, Gahl WA. Pulmonary function and high-resolution CT findings in patients with an inherited form of pulmonary fibrosis, Hermansky-Pudlak syndrome, due to mutations in HPS-1. Chest. 2000;117:129–36. doi: 10.1378/chest.117.1.129. [DOI] [PubMed] [Google Scholar]
- 33.Avila NA, Brantly M, Premkumar A, Huizing M, Dwyer A, Gahl WA. Hermansky-Pudlak syndrome: radiography and CT of the chest compared with pulmonary function tests and genetic studies. AJR Am J Roentgenol. 2002;179:887–92. doi: 10.2214/ajr.179.4.1790887. [DOI] [PubMed] [Google Scholar]
- 34.Thomas de Montpreville V, Mussot S, Dulmet E, Dartevelle P. Pulmonary fibrosis in Hermansky-Pudlak syndrome is not fully usual. Ann Pathol. 2006;26:445–9. doi: 10.1016/s0242-6498(06)70753-2. [DOI] [PubMed] [Google Scholar]
- 35.El-Chemaly S, Malide D, Yao J, et al. Glucose transporter-1 distribution in fibrotic lung disease: association with [(1)(8)F]-2-fluoro-2-deoxyglucose-PET scan uptake, inflammation, and neovascularization. Chest. 2013;143:1685–91. doi: 10.1378/chest.12-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelil T, Shen J, O'Neill AC, Howard SA. Hermansky-pudlak syndrome complicated by pulmonary fibrosis: radiologic-pathologic correlation and review of pulmonary complications. J Clin Imaging Sci. 2014;4:59. doi: 10.4103/2156-7514.143437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gahl WA, Brantly M, Troendle J, et al. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genet Metab. 2002;76:234–42. doi: 10.1016/s1096-7192(02)00044-6. [DOI] [PubMed] [Google Scholar]
- 38.O'Brien K, Troendle J, Gochuico BR, et al. Pirfenidone for the treatment of Hermansky-Pudlak syndrome pulmonary fibrosis. Mol Genet Metab. 2011;103:128–34. doi: 10.1016/j.ymgme.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lederer DJ, Kawut SM, Sonett JR, et al. Successful bilateral lung transplantation for pulmonary fibrosis associated with the Hermansky-Pudlak syndrome. J Heart Lung Transplant. 2005;24:1697–9. doi: 10.1016/j.healun.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Schinella RA, Greco MA, Cobert BL, Denmark LW, Cox RP. Hermansky-Pudlak syndrome with granulomatous colitis. Ann Intern Med. 1980;92:20–3. doi: 10.7326/0003-4819-92-1-20. [DOI] [PubMed] [Google Scholar]
- 41.Hazzan D, Seward S, Stock H, et al. Crohn's-like colitis, enterocolitis and perianal disease in Hermansky-Pudlak syndrome. Colorectal Dis. 2006;8:539–43. doi: 10.1111/j.1463-1318.2006.01046.x. [DOI] [PubMed] [Google Scholar]
- 42.Hussain N, Quezado M, Huizing M, et al. Intestinal disease in Hermansky-Pudlak syndrome: occurrence of colitis and relation to genotype. Clin Gastroenterol Hepatol. 2006;4:73–80. doi: 10.1016/s1542-3565(05)00858-x. [DOI] [PubMed] [Google Scholar]
- 43.Uhlig HH. Monogenic diseases associated with intestinal inflammation: implications for the understanding of inflammatory bowel disease. Gut. 2013;62:1795–805. doi: 10.1136/gutjnl-2012-303956. [DOI] [PubMed] [Google Scholar]