Abstract
Mucopolysaccharidosis type I (MPS I) is a lysosomal disease caused by α-l-iduronidase (IDUA) deficiency and accumulation of glycosaminoglycans (GAG). Lentiviral vector encoding correct IDUA cDNA could be used for treating MPS I. To optimize the lentiviral vector design, 9 constructs were designed by combinations of various promoters, enhancers, and codon optimization. After in vitro transfection into 293FT cells, 5 constructs achieved the highest IDUA activities (5613 to 7358 nmol/h/mg protein). These 5 candidate vectors were then tested by injection (1 × 107 TU/g) into neonatal MPS I mice. After 30 days, one vector, CCEoIDW, achieved the highest IDUA levels: 2.6% of wildtype levels in the brain, 9.9% in the heart, 200% in the liver and 257% in the spleen. CCEoIDW achieved the most significant GAG reduction: down 49% in the brain, 98% in the heart, 100% in the liver and 95% in the spleen. Further, CCEoIDW had the lowest transgene frequency, especially in the gonads (0.03 ± 0.01 copies/100 cells), reducing the risk of insertional mutagenesis and germ-line transmission. Therefore, CCEoIDW is selected as the optimal lentiviral vector for treating MPS I disease and will be applied in large animal preclinical studies. Further, taken both in vitro and in vivo comparisons together, codon optimization, use of EF-1α promoter and woodchuck hepatitis virus posttranscriptional response element (WPRE) could enhance transgene expression. These results provided a better understanding of factors contributing efficient transgene expression in lentiviral gene therapies.
Keywords: Lysosomal disease, Hurler syndrome, Mucopolysaccharidosis, Lentivirus, Gene therapy
1. Introduction
Mucopolysaccharidosis type I (MPS I) is an autosomal recessive disease that leads to neurodegeneration, mental retardation and death in early age. MPS I results from deficiency of α-l-iduronidase (IDUA, E.C.3.2.1.76), which degrades the glycosaminoglycans (GAG). The neurological pathology of MPS I is extremely difficult to treat, because the blood–brain-barrier (BBB) blocks the entry of enzyme into the brain [1], [2], [3]. Currently, MPS I is treated by hematopoetic stem cell transplantation (HSCT) and often in conjunction with enzyme replacement therapy (ERT). However, HSCT has a high rate of mortality (10–15%) and severe morbidity. While ERT alone is thought to have negligible therapeutic impacts at the brains, a high-dose infusion of IDUA can lead to significant increase of enzyme activity in the brain cortex [4]. Given that a small amount of enzyme is sufficient to degrade GAG storage [5], [6], [7], it should be possible to achieve improved neurological outcomes. However, high dose treatment is not feasible for clinical application due to the immune response against human recombinant enzyme [8].
Previously, gene therapy with different vectors in animal models has been used to treat MPS I disease, including retrovirus [9], [10], lentivirus [8], [11], [12], adeno-associated virus [3], [13], [14], [15], Sleeping Beauty transposon [16] and minicircles [17]. It has been shown that injection of lentiviral vector into MPS I mice can achieve metabolic correction and neurological improvements [8], [11], [12]. However, it is difficult to apply the dose (1.65 to 4.5 × 108 TU/g) used into clinical trials. Therefore, optimization of lentiviral vector design is essential for advancing lentiviral gene therapy protocols.
Promoters and enhancers are essential vector components for optimization of transgene expression. It has been shown that the human phosphoglycerate kinase 1 (PGK) promoter leads to moderate transgene expression of lentiviral vector [18], [19], [20]. Lentiviral vector with the human elongation factor 1α (EF-1α) promoter has been used for treating Fabry disease in a murine model [21]. Additionally, a hybrid promoter consisting of the enhancer of the murine cytomegalovirus (CMV) immediate-early gene and human EF-1α promoter was shown to achieve high transgene expression of lentivital vector [22]. However, the effects of different promoters on transgene expression are still not elucidated. Moreover, the use of woodchuck hepatitis virus (WHV) posttranscriptional response element (WPRE) has been found to enhance transgene expression and titers of therapeutic vectors [23], [24], [25]. The enhancing ability of WPRE depends on target cells, the type of viral vector context and its sequence [26], [27], [28], [29]. However, WHV X protein is implicated in the development of liver tumors [30], which raises the safety concern about use of WPRE in vectors for gene therapy. Herein, we designed constructs with full-length WPRE, truncated WPRE (tWPRE) and depleted WPRE for a side-by-side comparison. In this study, an initial in vitro screening of 9 plasmids identified 5 candidates with the highest IDUA transgene expression. Then, the efficacy of these 5 candidate lentiviral vectors in neonatal MPS I mice was comparatively evaluated. This allowed us to determine which lentiviral constructs yielded the highest IDUA levels and the most efficient GAG reduction in vivo.
2. Materials & methods
2.1. Plasmid construction
The human IDUA cDNA generated by reverse transcription PCR from total mRNA of an unaffected individual was inserted into the multi-cloning sites of pHIV-CS (CMV promoter upstream of 5′ LTR). The IDUA expression was under the control of PGK promoter and named as pCPGKID. Then, codon optimization of IDUA cDNA sequence was performed, resulting in what we named the oIDUA sequence. Similar techniques were used to generate similar variants with different promoters (hybrid promoter named as CE, PGK and EF-1α) and variants of WPRE, resulting in 8 more plasmids as followed: pCEFIDW, pCEFoIDW, pCPGKoIDW, pCEoIDW, pCEFoID-tWPRE, pCEFoID, pCEID and pCCEIDW. All plasmids were confirmed by sequencing.
2.2. In vitro plasmid transfection
For each transfection, 25 μg of candidate plasmid and 25 μg of HIV CMVeGFP plasmid were mixed with 133 μL 2.5 M CaCl2 (25 °C) and 1.33 mL RNase/DNase free sterile H2O. After adding 1.33 mL of 2 × HEPES buffered saline (pH 7.1), 7 mL serum free medium was added to the mixture. Then, the HEK 293FT cells were incubated with this transfection solution for 4 to 6 h (37 °C, 5% CO2). After removing the transfection solution, cells were incubated with 9 mL of 10% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO), Dulbecco's modified eagle medium (DMEM) (Sigma-Aldrich, St. Louis, MO) for another 48 h. Finally, cells and medium were collected by centrifuge and processed for biochemical assays.
2.3. Lentiviral vector production
The candidate plasmid was co-transfected with three additional helper plasmids, pLp1 (gag/pol), pLp2 (Rev) and VSVG envelope plasmid into HEK 293FT cells [31]. Vector-containing medium was collected 24, 40 and 64 h after transfection and concentrated. After ultracentrifugation at 7000 rpm overnight (4 °C), the vector pellet was resuspended in 40 mg/mL of lactose/PBS buffer and was stored at − 80 °C. The titer of vector preparations was determined by QPCR.
2.4. MPS I mice and injection
MPS I knockout mice (idua −/−), a kind gift from Dr. Elizabeth Neufeld, UCLA, were generated by insertion of neomycin resistance gene into exon 6 of the 14-exon IDUA gene on the C57BL/6 background [32]. The colony was housed in a pathogen-free facility on a 12-hour light/dark cycle. Newborn mice (1–2 days old) were injected with concentrated lentiviral vector through the superficial temporal vein. All mouse care and handling procedures were in compliance with the rules of the Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota.
2.5. IDUA enzyme assay
IDUA enzyme assay was conducted as previously described [33]. IDUA activity was determined by a fluorometric assay using 4-methylumbelliferyl α-l-iduronide (4-MU iduronide) (Glycosynth, Cheshire, UK) as the substrate, which was diluted with sodium formate buffer (0.4 M, pH 3.5). Then, 25 μL aliquots (360 μM) of substrate were mixed with 25 μL aliquots of tissue homogenates (diluted with 0.2% bovine serum albumin in phosphate buffered saline). The mixture was incubated at 37 °C for 30 min, and 200 μL glycine carbonate buffer (pH 10.4) was added to stop the reaction. IDUA catalyzed the cleavage of the non-fluorescent substrate (4-MU iduronide) and released a fluorescent product (4-MU). 4-Methylumbelliferone (4-MU) (Sigma-Aldrich, St. Louis, MO) was used to generate the standard curve. The resulting fluorescence was measured with excitation at 355 nm and emission at 460 nm. IDUA enzyme activity was expressed in units (nmol converted to product per hour) per mg protein as determined with a Pierce protein assay kit (Fisher, Waltham, MA) or per mL plasma. Then, IDUA enzyme activity was adjusted by Michaelis–Menten equation as described previously [33]. All reactions were performed in triplicate.
2.6. Tissue GAG assay
Tissue GAG assays were conducted as described previously [4]. The supernatants of tissue homogenates were treated by proteinase K (ProK) (NEB, Ipswich, MA) with the ratio of 3(Pro K):1(sample), incubated at 55 °C overnight, and boiled for 10 min to inactivate the enzyme. Samples were incubated with 2.5 μg RNase (Sigma-Aldrich, St. Louis, MO) and 250 U DNase (Sigma-Aldrich, St. Louis, MO) at room temperature overnight. After boiling for 10 min to inactivate the enzymes, GAG levels were determined by the Blyscan Sulfated Glycosaminoglycan Assay (Biocolor, Carrickfergus, UK). Tissue GAG levels were expressed as μg GAG/mg protein.
2.7. Quantitative PCR
DNA was extracted from tissues with QIAGEN DNA mini kit (QIAGEN # 51306). Primers (forward primer: 5′-CGACTGGTGAGTACGCCAAA-3′; reverse primer: 5′- CGCACCCATCTCTCTCCTTCT-3′) and the probe (5′-FAM-ATTTTGACTAGCGGAGGC-TAMRA-3′) targeted the lentiviral psi (Ψ) packaging signal region. Amplicon size was 61 bp. Each reaction contained 2 × TaqMan universal PCR master mix (Life Technologies, Carlsbad, CA), primers (0.008 nmol each), TaqMan probe (0.008 nmol), and 100 ng of sample DNA in a final volume of 10 μL. Real-time PCR was also performed using the mouse apolipoprotein B (ApoB) gene as an internal control, using the following primers (forward primer, 5′-CGTGGGCTCCAGCATTCTA-3′; reverse primer, 5′-TCACCAGTCATTTCTGCCTTTG-3′) and probe (5′-FAM-CCTTGAGCAGTGCCCGACCATTC-TAMRA-3′). Amplification conditions were 2 min at 50 °C and 10 min at 95 °C for the first cycle, followed by 50 cycles of 95 °C for 15 s and 60 °C for 1 min. A standard curve was established from a series of genomic DNA mixtures derived from plasmid with Ψ packaging signal region sequence (1copy per genome). Unknown samples were run in triplicate, while standards and internal controls were performed in duplicate.
2.8. Statistical analysis
Data were represented as mean ± standard errors. For evaluation of differences between samples, Student's T test for comparisons between paired samples and one-way analysis of variance (ANOVA) for comparisons between three or more samples were performed. Statistical significant level was set at p < 0.05. Data analysis was conducted with SAS 9.3 (Cary, NC).
3. Results
3.1. Design of 9 lentiviral constructs
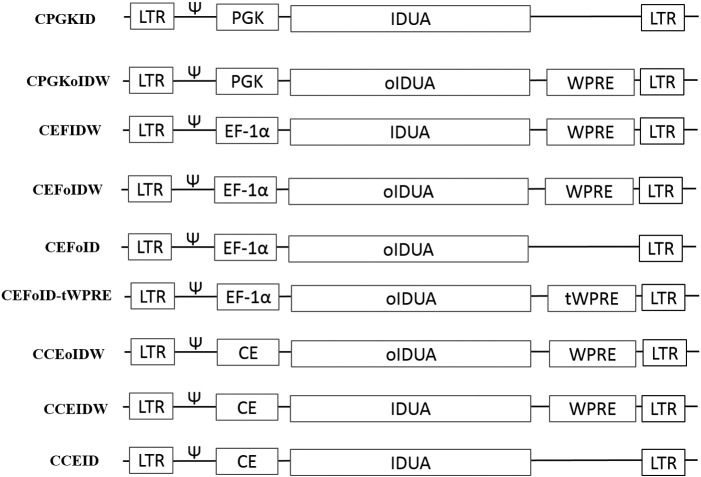
A plasmid CPGKID was constructed using the human PGK promoter to drive expression of human IDUA cDNA in the genome of a self-inactivating (SIN) lentiviral vector. Through codon optimization, optimized IDUA (oIDUA) sequence was obtained and used in 5 constructs. EF-1α promoter and the hybrid promoter containing CMV enhancer and EF-1α were engineered into 4 and 3 different constructs, respectively. Full length of WPRE, which contains three sub-elements: α, β and γ, was introduced into 5 different constructs. It is known that sub-elements γ and α contribute to most of the enhancing function, while β contains WHV X protein [34]. Due to safety concern about the WHV X sequence, CEFoID-tWPRE is designed by deleting β sub-element. The sequence information of these 9 lentiviral constructs was represented in Fig. 1.
Fig. 1.
Graphical representation of 9 lentiviral vectors. The lentiviral backbone is derived from HIV-1 with self-inactivating (SIN) LTRs. Rev. response element (RRE), central polypurine tract (cPPT) and central termination sequence (CTS) are in all constructs but not shown here. LTRs: long terminal repeats; Ψ: HIV-1 packaging signal; oIDUA stands for codon optimized version of human IDUA cDNA sequence; PGK: human phosphoglycerate kinase 1 promoter; EF-1α: human elongation factor 1α promoter; CE: mouse CMV enhancer and human EF- 1α promoter; IDUA: human IDUA cDNA; oIDUA: codon optimized human IDUA cDNA; WPRE: WHV posttranscriptional response element; tWPRE: truncated WPRE by deleting β sub-element.
3.2. Comparison by in vitro transfection
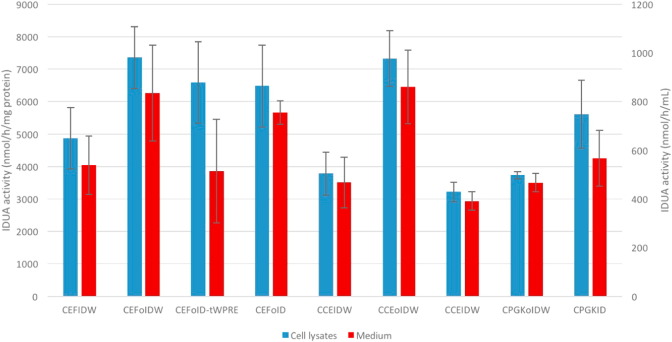
Each of these 9 plasmids was transfected into HEK 293FT cells, and three independent transfection experiments were conducted. The transfection efficiency of each plasmid was similar, shown by co-transfection of a plasmid encoding GFP (data not shown). A total of 5 constructs (CEFoIDW, CEFoID-tWPRE, CEFoID, CCEoIDW and CPGKID) yielded the highest IDUA levels in cell lysates (Fig. 2). Also, these 5 constructs had the highest IDUA levels in supernatants, which confirmed the results seen in the cell lysates (Fig. 2). These 5 constructs were selected as candidates and packaged into lentiviral vectors for in vivo assessment. When comparing constructs with or without codon optimization, CEFoIDW yielded higher IDUA levels than CEFIDW (7359 ± 956 vs 4879 ± 947 nmol/h/mg protein, p < 0.05), while CCEoIDW achieved higher IDUA levels than CCEIDW (7334 ± 858 vs 3784 ± 656 nmol/h/mg protein, p < 0.05). As to comparison between constructs with different promoters, CEFoIDW and CCEoIDW had IDUA levels at 7359 ± 956 and 7334 ± 858 nmol/h/mg protein, respectively, which is higher than that of CPGKoIDW (3732 ± 106 nmol/h/mg, p < 0.05). To determine the effects of WPRE, side-by-side comparisons (CEFoIDW vs CEFoID-tWPRE vs CEFoID) were conducted, and CEFoID had slightly lower IDUA levels than the other constructs (not statistically significant). This observation was confirmed when comparing CCEIDW (3784 ± 656 nmol/h/mg protein) and CCEID (3221 ± 302 nmol/h/mg protein). Notably, the ratio between IDUA levels in supernatants and cell lysates was approximately 11% in all 9 plasmids, indicating that the overexpression of IDUA did not affect the normal secretion of IDUA.
Fig. 2.
IDUA enzyme levels in cell lysates and supernatants 48 h after transfection of candidate plasmids. IDUA enzyme activity in HEK 293 FT cells without transfection is < 0.01 nmol/h/mL in supernatants, and 0.97 ± 0.34 nmol/h/mg protein in cell lysates, while IDUA enzyme activity in cultured medium without cells is 0. Data are mean ± standard errors (n = 3).
A previous study with retroviral gene therapy observed the inclusion-cell (I-cell) phenotype when IDUA levels were extremely high within cells [35]. It was proposed that due to saturation of M6P mediated lysosome-targeting pathway by overexpressed IDUA, other lysosomal enzymes could not be efficiently delivered to the lysosome. To test this possibility, enzyme activity of iduronate-2-sufatase (IDS) in transfected cells and supernatants were assessed. With high IDUA levels, IDS levels in cell lysates decreased to between 2.5 and 3.5 nmol/h/mg protein (wildtype level: 5.5 nmol/h/mg protein), and IDS levels in supernatants increased to approximately 1 nmol/h/mL (wildtype level: 0.89 nmol/h/mL). These results showed that targeting of IDS to the lysosome is only slightly affected by overexpressed IDUA.
3.3. In vivo comparison in neonatal MPS I mice
Vector particles were produced by co-transfection in HEK 293FT cells using a third-generation (4-plasmid) packaging system. Although a previous study [25] found that WPRE can enhance titers of retroviral vector, no significant difference in titers between lentiviral vectors with or without WPRE was identified in our three independent batches of virus production (data not shown). The 5 candidate lentiviral vectors were separately injected into newborn MPS I litters through the temporal facial vein at a dose of 1 × 107 TU/g (injection volume: 40 to 50 μL). Each group enrolled a litter of MPS I pups from MPS I parents (n = 4 to 7).
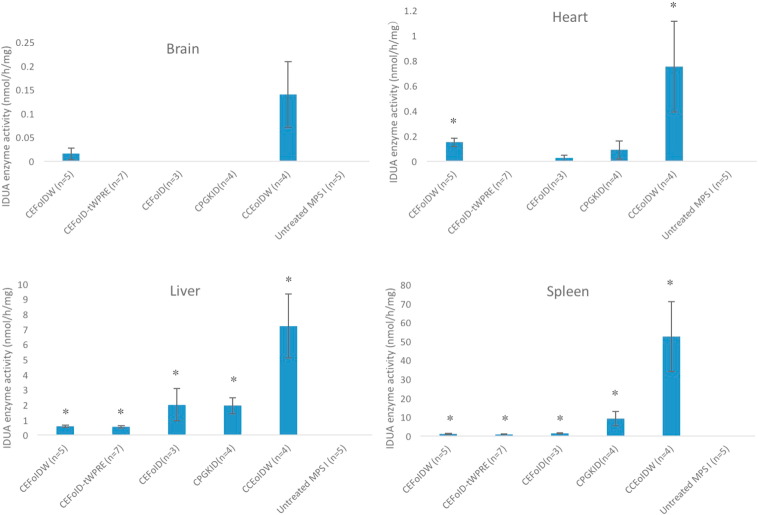
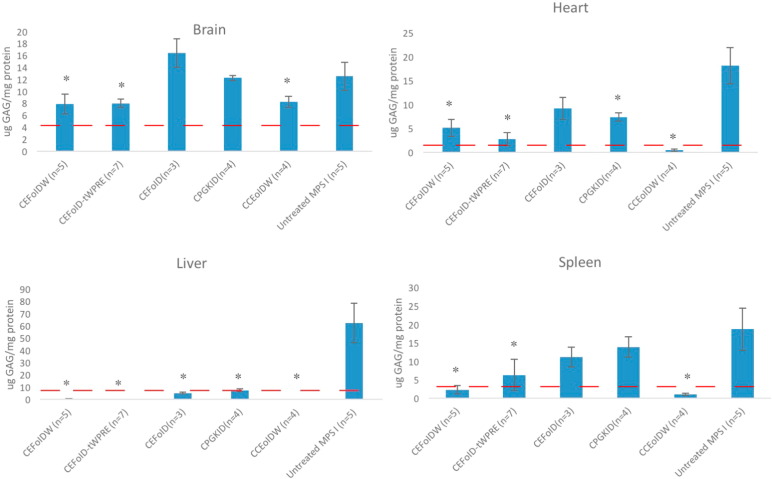
All mice including untreated MPS I controls (n = 5) were sacrificed at the age of 30 days. To determine the biodistribution of lentiviral vectors, genomic DNA from the brain, gonad, liver and spleen were extracted and assessed by quantitative PCR (QPCR) (see details in Table 1). The highest levels of IDUA were observed in the liver ranging from 2.6 to 9.8% (i.e., 2.6 to 9.8 IDUA copies per 100 cells), with 1.5 to 6.2% in the spleen. Of the tissues surveyed, the lowest transgene levels were observed in the gonads, ranging from 0.03 to 0.31%. Of all 5 candidate vectors, CCEoIDW achieved the lowest transgene frequency in the brain (0.06%), gonad (0.03%) and spleen (1.5%), indicating less risk for insertional mutagenesis and germ-line transmission. All 5 candidate vectors achieved significant IDUA enzyme activity in the liver (0.54 ± 0.07 to 7.2 ± 2.1 nmol/h/mg protein) and spleen (0.9 ± 0.18 to 52.5 ± 18.5 nmol/h/mg protein) (data summarized in Fig. 3). CEFoIDW and CCEoIDW achieved significant IDUA activity (0.15 ± 0.03 to 0.75 ± 0.36 nmol/h/mg protein) in the heart. Only CCEoIDW achieved higher IDUA activity in the brain (0.14 ± 0.07 nmol/h/mg protein, p = 0.053). CCEoIDW had the highest IDUA enzyme activity in all tested tissues. Notably, no correlation between transgene frequency and IDUA levels were observed. All 5 candidate vectors achieved significant GAG reduction in the liver (by 88% to 100%, p < 0.05) (summarized in Fig. 4). Of the 5 vectors, all except CEFoID achieved significant GAG reduction in the heart (by 59% to 98%, p < 0.05). CEFoIDW and CCEoIDW achieved significant GAG reduction in the spleen (by 88% to 95%, p < 0.05). CEFoIDW and CCEoIDW achieved significant GAG reduction in the brain (by 34% to 38%, p < 0.05). Interestingly, although no significant IDUA levels were observed in the brain, CEFoID-tWPRE achieved significant GAG reduction (by 36%, p < 0.05). Of 5 candidate vectors, only CCEoIDW and CEFoIDW achieved significant GAG reduction in all tested organs, and CCEoIDW has the lowest GAG levels in the liver, spleen and heart. Taken together, with the lowest transgene frequency, the highest IDUA levels and the most significant GAG reduction, CCEoIDW emerges as the optimal lentiviral vector for treating MPS I disease.
Table 1.
Quantitative measurement of lentiviral DNA in tissues of MPS I mice with 5 candidate lentiviral vectors. The untreated MPS I mice had 0.014 copies/100 cells in the liver, non-detectable (ND, < 0.004%) to 0.023 in the spleen, ND in the brain, and ND to 0.006 in the gonad. Data are mean ± standard errors.
| Copies/100 cells |
||||
|---|---|---|---|---|
| Brain | Gonad | Liver | Spleen | |
| CEFoIDW (n = 5) | 0.16 ± 0.06 | 0.15 ± 0.1 | 6.4 ± 0.42 | 2.4 ± 0.78 |
| CEFoID-tWPRE (n = 7) | 0.24 ± 0.09 | 0.1 ± 0.04 | 9.8 ± 3.3 | 3.7 ± 1 |
| CCEoIDW (n = 4) | 0.06 ± 0.02 | 0.03 ± 0.01 | 4.9 ± 2.5 | 1.5 ± 0.76 |
| CPGKID (n = 4) | 0.25 ± 0.16 | 0.03 ± 0.01 | 2.6 ± 0.64 | 2 ± 0.77 |
| CEFoID (n = 3) | 0.43 ± 0.16 | 0.31 ± 0.16 | 5.7 ± 1.9 | 6.2 ± 1.7 |
Fig. 3.
IDUA transgene expression in tissues of MPS I mice treated with 5 candidate lentiviral vectors. Newborn MPS I pups were injected with 1 × 107 TU/g lentiviral vectors and evaluated after 1 month. Normal heterozygous mice had IDUA levels: 2.7 ± 0.26 nmol/h/mg protein in the brain, 3.8 ± 0.06 in the heart, 1.8 ± 0.1 in the liver and 10.2 ± 0.23 in the spleen. Data are mean ± standard errors. *The difference between treated mice and untreated MPS I mice was significant, with a p value of < 0.05.
Fig. 4.
GAG levels in tissues of MPS I mice treated with 5 candidate lentiviral vectors. Normal heterozygous mice had 4.3 ± 0.4 μg GAG/mg protein in the brain, 1.3 ± 1.3 in the heart, 7.4 ± 3.4 in the liver and 3.6 ± 1.0 in the spleen (shown by red dash lines). Data are mean ± standard errors. *The difference between treated mice and untreated MPS I mice was significant, with a p value of < 0.05.
3.4. Effects of dose and injection volume
To determine the effects of dose on treatment efficacy, a single dose of CPGKID (5 × 106 TU/g) was injected into neonatal MPS I mice (n = 8). The brain, heart, liver and spleen were collected for biochemical assays 30 days after the injection. Compared with a high dose of CPGKID (1 × 107 TU/g), the low dose group led to significantly lower IDUA levels in the liver (0.25 ± 0.05 vs 2 ± 0.54 nmol/h/mg protein, p < 0.05) and spleen (0.41 ± 0.08 vs 9.1 ± 3.7 nmol/h/mg protein, p < 0.05) (Table 2). There was no significant difference in GAG levels in the liver and spleen between these two groups. However, the low dose group led to significantly higher GAG levels (27.1 ± 2.9 vs 7.4 ± 0.84 μg GAG/mg protein) (Table 3). These results showed that the higher dose (1 × 107 TU/g) achieved more significant IDUA transgene expression and GAG reduction.
Table 2.
IDUA transgene expression in tissues of MPS I mice treated with different doses and volumes of lentiviral vector. High dose: 1 × 107 TU/g; low dose: 5 × 106 TU/g. ND: non-detectable (< 0.002 nmol/h/mg). Normal heterozygous mice had IDUA levels: 2.7 ± 0.26 nmol/h/mg protein in the brain, 3.8 ± 0.06 in the heart, 1.8 ± 0.1 in the liver and 10.2 ± 0.23 in the spleen. Data are mean ± standard errors. ⁎The difference between treated mice and untreated MPS I mice was significant, with a p value of < 0.05.
| IDUA activity (nmol/h/mg protein) |
|||||
|---|---|---|---|---|---|
| Volume (μL) | Brain | Heart | Liver | Spleen | |
| CPGKID (high dose, n = 4) | 50 | ND | 0.09 ± 0.07 | 2 ± 0.54⁎ | 9.1 ± 3.7⁎ |
| CPGKID (low dose, n = 8) | 50 | 0.006 ± 0.01 | 0.01 ± 0.01 | 0.25 ± 0.05⁎ | 0.41 ± 0.1⁎ |
| CPGKID (high dose, n = 5) | 100 | ND | 0.34 ± 0.13⁎ | 1.7 ± 0.36⁎ | 2.1 ± 0.47⁎ |
| Untreated MPS I (n = 5) | N/A | ND | ND | ND | ND |
Table 3.
Tissue GAG levels of MPS I mice treated with different doses and volumes of lentiviral vector. Normal heterozygous mice had 4.3 ± 0.4 μg GAG/mg protein in the brain, 1.3 ± 1.3 in the heart, 7.4 ± 3.4 in the liver and 3.6 ± 1.0 in the spleen. ⁎The difference between treated mice and untreated MPS I mice was significant, with a p value of < 0.05.
| μg GAG/mg protein |
|||||
|---|---|---|---|---|---|
| Volume (μL) | Brain | Heart | Liver | Spleen | |
| CPGKID (high dose, n = 4) | 50 | 12.2 ± 0.39 | 7.4 ± 0.84⁎ | 7.2 ± 1.1⁎ | 13.9 ± 2.8 |
| CPGKID (low dose, n = 8) | 50 | 15.7 ± 2 | 27.1 ± 2.9⁎ | 9.6 ± 4.1 | 11.1 ± 1.8 |
| CPGKID (high dose, n = 5) | 100 | 11.3 ± 0.47 | 6.9 ± 1.3⁎ | 6.5 ± 0.5⁎ | 8 ± 1.1 |
| Untreated MPS I (n = 5) | N/A | 12.5 ± 2.3 | 18.1 ± 3.8 | 62.3 ± 16.1 | 18.7 ± 5.7 |
Previously, one study showed that when injecting gene vector into neonatal mice, the injection volume significantly affects transgene expression [36]. To determine the effects of injection volume on transgene expression, a single dose of CPGKID (1 × 107 TU/g) with the volume of 100 μL was injected into neonatal MPS I mice (n = 5). Mice injected with the same dose of CPGKID but at the volume of 50 μL were used as the low volume control group (n = 4). Compared with untreated MPS I mice (n = 5), both the high volume and low volume groups achieved significant IDUA levels in the liver and spleen (p < 0.05). Between these two groups, there was no significant difference in IDUA levels in all tested tissues (Table 2). Compared with untreated MPS I mice, both high volume and low volume groups achieved significant GAG reduction in the heart and liver (p < 0.05). However, there was no significant difference in GAG levels between these two groups (Table 3). Collectively, the higher injection volume did not achieve more significant IDUA levels and GAG reduction.
4. Discussion
4.1. Inference for lentiviral design
In this study, constructs with codon optimization achieved higher IDUA levels in vitro than their counterparts without codon optimization. Since there is no in vivo pairwise comparison, codon optimization can at least enhance transgene expression in vitro. In terms of promoters, EF-1α and the hybrid promoter achieved significantly higher IDUA levels than PGK in vitro. CCEoIDW achieved significantly the highest IDUA levels and efficient GAG reduction in vivo. There was no direct in vivo comparison between constructs with difference only in promoter choices (PGK or EF-1α). Taken together, the hybrid promoter containing CMV enhancer and EF-1α promoter performs better than PGK promoter both in vivo and in vitro. We also compared constructs with variants of WPRE (CEFoIDW vs CEFoID-tWPRE vs CEFoID, CCEIDW vs CCEID), there were no significant effects in vitro. In in vivo experiments, CEFoIDW achieved higher IDUA levels in the heart and more efficient GAG reduction than CEFoID. Similarly, when comparing with constructs with CEFoID-tWPRE, CEFoID had less efficient GAG reduction in the brain, heart and liver (p < 0.05). These results showed that although there were no significant effects in in vitro conditions, WPRE enhanced transgene expression and boosted GAG reduction in vivo.
4.2. IDUA activity and GAG reduction in the brain
Although the transgene frequency of all 5 lentiviral vectors in the brain is extremely low (0.06 to 0.43 copies/100 cells), some lentiviral vectors showed significant GAG reduction in the brain. This observation further confirmed the hypothesis derived from our high dose ERT study [4]: when plasma IDUA level is high enough, a small amount of IDUA can cross the blood–brain-barrier and degrade GAG storage in the brain. Another interesting observation is that CEFoID-tWPRE had undetectable IDUA levels in the brain, but achieved significant GAG reduction. Therefore, it appears that even a non-detectable amount of IDUA could significantly degrade GAG storage. This is consistent with previous findings in pseudo-deficient patients who have extremely low, undetectable IDUA levels, but with normal phenotype.
4.3. Clinical applications
Previous studies using lentiviral vector to treat MPS I disease is of limited of use due to relatively low transgene expression, making it difficult for translation into human clinical trials. Two studies [8], [11] intravenously injected up to approximately 4.5 × 108 TU/g of lentiviral vector into adult MPS I mice. The liver had the most significant effects: only 1% of wildtype levels and 69% of GAG reduction. There were no IDUA activity nor GAG reduction in the brain. Another lentiviral gene therapy study [12] with neonatal MPS I mice used the dose of 1.65 × 108 TU/g, and achieved 27.4% of wildtype IDUA levels in the liver. However, for a 3 kg human baby, this dose means infusing approximately 2.5 L of lentiviral vector (set the vector titer as 2 × 108 TU/mL). In our study, we used the dose of 1 × 107 TU/g, namely 150 mL for human babies, which remarkably improved the ease of viral vector production. Moreover, CCEoIDW achieved more significant IDUA activity and efficient GAG reduction in all tested tissues including the brain.
In this study, with all 5 lentiviral vectors, the gonads always had the lowest transgene frequency (as low as 0.03 copies/100 cells) of all tested tissues. Similar to what a previous study showed [37], these results also indicated that the gonads are relatively isolated from intravenously administered vector, which remarkably reduced the risk of germ-line transmission. Another major safety concern about lentiviral vector is the oncogenesis by insertional activation. In this study, out of all tested tissues with all candidate vectors, the highest transgene frequency is 9.8 copies/100 cells 1 month after treatment (CEFoID-tWPRE in the liver). It is significantly lower than that in another lentiviral gene therapy study at the same time-point, which is 83 copies/100 cells [11]. Moreover, the optimal vector, CCEoIDW had the lowest transgene frequency: 0.06 in the brain, 0.03 in the gonad, 4.9 in the liver and 1.5 in the spleen. This makes CCEoIDW more appealing for significantly reducing risk of insertional mutagenesis. The optimal construct CCEoIDW has both CMV enhancer and WPRE, which may activate adjacent oncogenes and pose risks for insertional mutagenesis. In spite of these possibilities, these enhancer elements have been widely used in gene therapy without being linked to tumorigenesis.
Further, for future clinical trials, different routes of administration including in utero, intravenous, intranasal and intraventricular should be comparatively evaluated.
5. Conclusions
In conclusion, of 9 constructs, CCEoIDW emerges as the optimal lentiviral vector for treating MPS I mice and can be forwarded into preclinical studies. At the dose of 1 × 107 TU/g, CCEoIDW achieved supra-normal IDUA levels and significant GAG reduction in tissues, and importantly in the brain. Considering the relatively low transgene frequency, this dose may be applicable to human clinical trials for neonatal injection. Further, inferences derived from this study about lentiviral vector design will be valuable for gene therapy studies treating other lysosomal diseases.
Acknowledgments
Paul Score, Ph.D. constructed the lentiviral plasmids used in this study. The authors would like to thank Brenda Diethelm-Okita (University of Minnesota) for managing IACUC protocols, Bryan Hall (University of Minnesota) for advising on QPCR experiments, the University of Iowa Vector Core for vector production and in vitro transfection, and Aldevron (Fargo, ND) for sequencing and plasmid manufacturing services. This work was supported by NIH grant “Gene Therapy for Metabolic Diseases” 5P01HD032652. The authors have no conflicts of interest to declare. Dr. Ou is a fellow of the Lysosomal Disease Network.
References
- 1.Begley D.J., Pontikis C.C., Scarpa M. Lysosomal storage diseases and the blood–brain barrier. Curr. Pharm. Des. 2008;14:1566–1580. doi: 10.2174/138161208784705504. [DOI] [PubMed] [Google Scholar]
- 2.Enns G.M., Huhn S.L. Central nervous system therapy for lysosomal storage disorders. Neurosurg. Focus. 2008;24:3–4. doi: 10.3171/FOC/2008/24/3-4/E11. [DOI] [PubMed] [Google Scholar]
- 3.Wolf D.A., Lenander A.W., Nan Z., Belur L.R., Whitley C.B., Gupta P. Direct gene transfer to the CNS prevents emergence of neurologic disease in a murine model of mucopolysaccharidosis type I. Neurobiol. Dis. 2011;43:123–133. doi: 10.1016/j.nbd.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ou L., Herzog T., Koniar B.L., Gunther R., Whitley C.B. High-dose enzyme replacement therapy in murine Hurler syndrome. Mol. Genet. Metab. 2014;11:116–122. doi: 10.1016/j.ymgme.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guffon N., Souillet G., Maire I., Straczek J., Guibaud P. Follow-up of nine patients with Hurler syndrome after bone marrow transplantation. J. Pediatr. 1998;133:119–125. doi: 10.1016/s0022-3476(98)70201-x. [DOI] [PubMed] [Google Scholar]
- 6.Whitley C.B., Ramsay N.K., Kersey J.H., Krivit W. Bone marrow transplantation for Hurler syndrome: assessment of metabolic correction. Birth Defects Orig. Artic. Ser. 1986;22:7–24. [PubMed] [Google Scholar]
- 7.Whitley C.B., Belani K.G., Chang P.N., Summers C.G., Blazar B.R., Tsai M.Y. Long-term outcome of Hurler syndrome following bone marrow transplantation. Am. J. Med. Genet. 1993;46:209–218. doi: 10.1002/ajmg.1320460222. [DOI] [PubMed] [Google Scholar]
- 8.Di Domenico C., Villani G.R., Di Napoli D., Reyero E.G., Lombardo A., Naldini L. Gene therapy for a mucopolysaccharidosis type I murine model with lentiviral-IDUA vector. Hum. Gene Ther. 2005;16:81–90. doi: 10.1089/hum.2005.16.81. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y., Xu L., Hennig A.K., Kovacs A., Fu A., Chung S. Liver-directed neonatal gene therapy prevents cardiac, bone, ear, and eye disease in mucopolysaccharidosis I mice. Mol. Ther. 2005;11:35–47. doi: 10.1016/j.ymthe.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Traas A.M., Wang P., Ma X., Tittiger M., Schaller L., O'donnell P. Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy. Mol. Ther. 2007;15:1423–1431. doi: 10.1038/sj.mt.6300201. [DOI] [PubMed] [Google Scholar]
- 11.Di Domenico C., Di Napoli D., Gonzalez Y., Reyero E., Lombardo A., Naldini L., Di Natale P. Limited transgene immune response and long-term expression of human alpha-l-iduronidase in young adult mice with mucopolysaccharidosis type I by liver-directed gene therapy. Hum. Gene Ther. 2006;17:1112–1121. doi: 10.1089/hum.2006.17.1112. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi H., Carbonaro D., Pepper K., Petersen D., Ge S., Jackson H. Neonatal gene therapy of MPS I mice by intravenous injection of a lentiviral vector. Mol. Ther. 2005;11:776–789. doi: 10.1016/j.ymthe.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Desmaris N., Verot L., Puech J.P., Caillaud C., Vanier M.T., Heard J.M. Prevention of neuropathology in the mouse model of Hurler syndrome. Ann. Neurol. 2004;56:68–76. doi: 10.1002/ana.20150. [DOI] [PubMed] [Google Scholar]
- 14.Ellinwood N.M., Ausseil J., Desmaris N., Bigou S., Liu S., Jens J.K. Safe, efficient, and reproducible gene therapy of the brain in the dog models of Sanfilippo and Hurler syndromes. Mol. Ther. 2011;19:251–259. doi: 10.1038/mt.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciron C., Desmaris N., Colle M.A., Raoul S., Joussemet B., Vérot L. Gene therapy of the brain in the dog model of Hurler's syndrome. Ann. Neurol. 2006;60:204–213. doi: 10.1002/ana.20870. [DOI] [PubMed] [Google Scholar]
- 16.Aronovich E.L., Bell J.B., Khan S.A., Belur L.R., Gunther R., Koniar B. Systemic correction of storage disease in MPS I NOD/SCID mice using the sleeping beauty transposon system. Mol. Ther. 2009;17:1136–1144. doi: 10.1038/mt.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborn M.J., McElmurry R.T., Peacock B., Tolar J., Blazar B.R. Targeting of the CNS in MPS-IH using a nonviral transferrin-alpha-l-iduronidase fusion gene product. Mol. Ther. 2008;16:1459–1466. doi: 10.1038/mt.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim B., Williams D.A., Orkin S.H. Retrovirus-mediated gene transfer of human adenosine deaminase: expression of functional enzyme in murine hematopoietic stem cells in vivo. Mol. Cell. Biol. 1987;7:3459–3465. doi: 10.1128/mcb.7.10.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farina S.F., Girard L.J., Vanin E.F., Nienhuis A.W., Bodine D.M. Dysregulated expression of GATA-1 following retrovirus-mediated gene transfer into murine hematopoietic stem cells increases erythropoiesis. Blood. 1995;86:4124–4133. [PubMed] [Google Scholar]
- 20.Maze R., Kapur R., Kelley M.R., Hansen W.K., Oh S.Y., Williams D.A. Reversal of 1,3-bis(2-chloroethyl)-1-nitrosoureainduced severe immunodeficiency by transduction of murine long-lived hemopoietic progenitor cells using O6-methylguanine DNA methyltransferase complementary DNA. J. Immunol. 1997;158:1006–1013. [PubMed] [Google Scholar]
- 21.Lee C.J., Fan X., Guo X., Medin J.A. Promoter-specific lentivectors for long-term, cardiac-directed therapy of Fabry disease. J. Cardiol. 2011;57:115–122. doi: 10.1016/j.jjcc.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 22.de la Garza-Rodea A.S., van der Velde I., Boersma H., Gonçalves M.A., van Bekkum D.W., de Vries A.A. Long-term contribution of human bone marrow mesenchymal stromal cells to skeletal muscle regeneration in mice. Cell Transplant. 2011;20:217–231. doi: 10.3727/096368910X522117. [DOI] [PubMed] [Google Scholar]
- 23.Kraunus J., Schaumann D.H., Meyer J., Modlich U., Fehse B., Brandenburg G. Self-inactivating retroviral vectors with improved RNA processing. Gene Ther. 2004;11:1568–1578. doi: 10.1038/sj.gt.3302309. [DOI] [PubMed] [Google Scholar]
- 24.Werner M., Kraunus J., Baum C., Brocker T. B-cell-specific transgene expression using a self-inactivating retroviral vector with human CD19 promoter and viral post-transcriptional regulatory element. Gene Ther. 2004;11:992–1000. doi: 10.1038/sj.gt.3302255. [DOI] [PubMed] [Google Scholar]
- 25.Schambach A., Bohne J., Baum C., Hermann F.G., Egerer L., von Laer D. Woodchuck hepatitis virus post-transcriptional regulatory element deleted from X protein and promoter sequences enhances retroviral vector titer and expression. Gene Ther. 2006;13:641–645. doi: 10.1038/sj.gt.3302698. [DOI] [PubMed] [Google Scholar]
- 26.Schambach A., Wodrich H., Hildinger M., Bohne J., Krausslich H.G., Baum C. Context dependence of different modules for posttranscriptional enhancement of gene expression from retroviral vectors. Mol. Ther. 2000;2:435–445. doi: 10.1006/mthe.2000.0191. [DOI] [PubMed] [Google Scholar]
- 27.Salmon P., Kindler V., Ducrey O., Chapuis B., Zubler R.H., Trono D. High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood. 2000;96:3392–3398. [PubMed] [Google Scholar]
- 28.Higashimoto T., Urbinati F., Perumbeti A., Jiang G., Zarzuela A., Chang L.J. The woodchuck hepatitis virus post-transcriptional regulatory element reduces readthrough transcription from retroviral vectors. Gene Ther. 2007;14:1298–1304. doi: 10.1038/sj.gt.3302979. [DOI] [PubMed] [Google Scholar]
- 29.Schambach A., Galla M., Maetzig T., Loew R., Baum C. Improving transcriptional termination of self-inactivating gamma-retroviral and lentiviral vectors. Mol. Ther. 2007;15:1167–1173. doi: 10.1038/sj.mt.6300152. [DOI] [PubMed] [Google Scholar]
- 30.Kingsman S.M., Mitrophanous K., Olsen J.C. Potential oncogene activity of the woodchuck hepatitis post-transcriptional regulatory element (WPRE) Gene Ther. 2005;12:3–4. doi: 10.1038/sj.gt.3302417. [DOI] [PubMed] [Google Scholar]
- 31.Naldini L., Blomer U., Gage F.H., Trono D., Verma I.M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohmi K., Greenberg D.S., Rajavel K.S., Ryazantsev S., Li H.H., Neufeld E.F. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ou L., Herzog T.L., Wilmot C.M., Whitley C.B. Standardization of α-l-iduronidase enzyme assay with Michaelis–Menten kinetics. Mol. Genet. Metab. 2014;111:113–115. doi: 10.1016/j.ymgme.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donello J.E., Loeb J.E., Hope T.J. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J. Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anson D.S., Bielicki J., Hopwood J.J. Correction of mucopolysaccharidosis type I fibroblasts by retroviral-mediated transfer of the human alpha-l-iduronidase gene. Hum. Gene Ther. 1992;3:371–379. doi: 10.1089/hum.1992.3.4-371. [DOI] [PubMed] [Google Scholar]
- 36.Yan S., Fu Q., Zhou Y., Wang J., Liu Y., Duan X. High levels of gene expression in the hepatocytes of adult mice, neonatal mice and tree shrews via retro-orbital sinus hydrodynamic injections of naked plasmid DNA. J. Control. Release. 2012;161:763–771. doi: 10.1016/j.jconrel.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Pan D., Aronovich E., McIvor R.S., Whitley C.B. Retroviral vector design studies toward hematopoietic stem cell gene therapy for mucopolysaccharidosis type I. Gene Ther. 2000;7:1875–1883. doi: 10.1038/sj.gt.3301298. [DOI] [PubMed] [Google Scholar]