Abstract
Purpose
Perceived stress is associated with temporomandibular disorder (TMD), but whether cortisol levels are elevated in individuals with TMD is unknown. We hypothesized that cortisol concentration, a biomarker of hypothalamic-pituitary-adrenal (HPA) axis function, was elevated in TMD cases relative to controls, and that perceived stress was positively correlated with cortisol concentration.
Methods
In this case control study, TMD case status was determined by examiners using TMD Research Diagnostic Criteria. Participants (n=116) aged 18 to 59 years were recruited from within a 50 mile radius of the University of North Carolina at Chapel Hill. Following examination, cases (n=45) and controls (n=71) completed the 14-item Perceived Stress Scale using a reference interval of the past 3 months. Approximately 100 strands of hair were cut from the posterior vertex segment of their scalp. The 3 centimeters of hair most proximal to the scalp was analyzed with a commercially available salivary cortisol enzyme immunoassay adapted for hair cortisol. This length corresponds to the last 3 months of systemic HPA axis activity.
Results
TMD cases perceived higher stress than controls (p=0.001). However, hair cortisol concentration was lower in TMD cases than controls (p<0.001). The correlation coefficient revealed a weak negative relationship (r=−0.188) between perceived stress and hair cortisol concentration (p=0.044). In analysis stratified by case status, the relationship of perceived stress and hair cortisol concentration was non-significant for cases (p=0.169) and controls (p=0.498).
Conclusion
Despite greater perceived stress, TMD cases had lower hair cortisol concentrations than controls and the 2 measures of stress were weakly and negatively correlated.
Keywords: Temporomandibular joint disorders, Epidemiology, Factor, psychosocial, Hormones, hypothalamic pituitary regulating
Introduction
One of the most fundamental physiological responses to stress is activation of the hypothalamic-pituitary-adrenocortical (HPA) axis. The end product of HPA axis activation is stimulation of the adrenal cortex to increase secretion of the glucocorticoid cortisol. While protective in the short term, sustained activation of this hormonal response system is theorized to lead to tissue damage and subsequent dysregulation of biological systems.1 Since the 1960s, investigators have measured cortisol levels in blood, saliva or urine to understand how stress increases vulnerability to disease.
Well before the role of HPA axis was theorized, stress was recognized to contribute to acute necrotizing ulcerative gingivitis, so-called “trench-mouth,” among WWI soldiers. Today, stress has salience to oral health research because it is implicated in the pathogenesis of several dental conditions that have relevance to dental hygiene clinical practice. Heightened levels of stress are associated with oral mucosal lesions such as oral lichen planus2,3 and recurrent aphthous stomatitis.4 Among middle-aged adults, those with greater perceived stress were less likely to have retained 20 teeth,5 the minimum number required for adequate function.6 Psychosocial stress is believed to increase susceptibility to gingival infection and depress immune responsiveness to periodontal patho gens.7,8 A cross-sectional study of 1,426 adults found that financial strain was associated with greater clinical attachment loss and alveolar bone loss.9
Perhaps the strongest evidence for a putative role of stress in oral disorders comes from studies of the onset, severity and chronicity of temporomandibular disorders (TMD). TMD is the most common form of chronic orofacial pain, affecting 5% of the U.S. population.10 Sanders et al demonstrated a strong dose-dependent relationship between severity of perceived stress and odds of examiner-determined TMD.11 Baseline findings from the OPPERA prospective cohort study investigating risk factors for TMD found that compared with controls, TMD cases reported higher levels of psychosocial symptoms, affective distress, somatic awareness and pain catastrophizing.12 Longitudinal research that followed healthy adults with no prior history of TMD found that those with greater perceived stress were more likely to experience first-onset TMD than adults with less perceived stress.13
It is perhaps surprising that cortisol measurement does not feature more prominently in oral health research as a biomarker of stress. New protocols for salivary cortisol collection offer advantages over blood and urine sampling protocols in terms of cost and simplicity. Yet major difficulties remain in obtaining valid and reliable measurements of cortisol in observational studies. Firstly, cortisol secretion follows a robust 24 hour rhythm, peaking around 8:00 with a nadir between 20:00 and 24:00.14 Overlying this daily pattern is a series of 8 to 10 pulses. Such variation means that exact timing of specimen collection is critical if cortisol concentrations are to be meaningfully compared, and multiple measures per subject are often required. The United States National Longitudinal Study of Adolescent Health recently reported its decision to drop salivary cortisol measurement from its protocol because responses and protocol adherence were inadequate.15
A second limitation of cortisol measurement in blood, saliva and urine is that each of these fluids provides a very limited temporal window of cortisol activity. Levels of cortisol in blood and saliva reflect average hormone levels in the past 1 hour while cortisol in urine captures a slightly longer interval of up to 24 hours. None of these are able to measure chronic stress exposure which is thought to pose a greater threat to health than the short-term physiologic responses to acute stress.16,17
An important breakthrough was the development of an assay to measure endogenous concentrations of cortisol in human scalp hair,18 permitting a reliable measurement of the stress response over a prolonged period, (e.g., chronic stress exposure).19 Cortisol is thought to be incorporated into hair through diffusion from body secretions of sweat and sebum during formation of the hair shaft.20 Since hair grows at a precise rate of 0.35 mm per day, equivalent to 1 cm per month,21 hair length is an accurate index of exposure to stress over time. Thus hair cortisol promises a new, simple and noninvasive way in epidemiologic research to examine the role of stress.
To clarify the role of stress in TMD, the first aim of this study was to confirm the well-documented association between perceived stress and TMD. Once established, the second aim was to determine the relationship between hair cortisol concentration and TMD status. The third aim was to examine the correlation between perceived stress and hair cortisol concentration. The authors tested the hypotheses that both perceived and biologic measures of stress were elevated among TMD cases and that perceived stress was positively correlated with hair cortisol concentration.
Methods and Materials
This study was approved by the University of North Carolina Biomedical Institutional Review Board. All participants gave written informed consent before their inclusion in the study. In this case control study, cases had examiner-diagnosed TMD. Controls were also examined and found not to have this condition.
Setting
During the period July 2010 to October 2011, potential participants were recruited by advertisements placed in brochures, on the internet, radio and newspapers within a 50 mile radius of the Center for Neurosensory Disorders, School of Dentistry at the Center for Neurosensory Disorders, the University of North Carolina at Chapel Hill.
Inclusion and Exclusion Criteria
Criteria eligible participants were males and females between 18 to 60 years of age with scalp hair at least 3 cm in length. Respondents were first screened in a telephone interview to exclude those with conditions known to influence cortisol levels. Exclusionary criteria were diagnoses of any one of Cushing's syndrome or Addison's disease, diabetes, heart trouble or disease, hypertension that was not well controlled with medication, hyperthyroidism, major psychiatric disorder requiring hospitalization within the previous 6 months, chronic respiratory disease not controlled with medication, seizures, renal failure or dialysis. Also excluded were those who were pregnant, nursing, undergoing orthodontic treatment, radiation or chemotherapy, as well as persons with drug or alcohol abuse, trauma or surgery on the head, face or neck within the last 6 months. Persons having used corticosteroid treatment in the last 12 months (including cortisol containing creams, lotions and nasal spray) were likewise excluded. Finally, those having used permanent or semi-permanent hair color within 3 months were excluded since cortisol levels are lower in artificially colored hair.18
TMD Case Classification
A medical history was recorded for all screened participants prior to the clinical examination. Examinations were performed by 6 dental hygiene examiners trained in the examination protocol and calibrated for reliability and validity of their diagnostic decisions every 6 months. The standardized physical examination of the head and neck followed the research diagnostic criteria for TMD.22 In summary, TMD cases were people who reported a 6 month history of pain in the temporomandibular structures, with at least 5 days of such pain in the month preceding the examination and where the examiner found at least 3 muscle groups in the temporomandibular region that were tender to palpation or jaw maneuver. Controls reported no history of orofacial pain within the preceding 6 months and no prior diagnosis for TMD. Additionally, their examination confirmed that they did not have TMD, arthralgia or myalgia.
Hair Sampling
A hair sample (approximately 100 strands, ≥20 mg of hair) of at least 3 cm in length was collected by study personnel. The sample was cut using fine scissors from as close as possible to the scalp from the vertex posterior region. Intra-individual variation in cortisol content is less in this region (coefficient of variation=15.6%), as compared to hair sampled from other than in the posterior vertex, anterior vertex, nape, temporal and frontal regions (coefficient of variation=30.5%).18 Because scalp hair grows 1 cm per month on average,23 analysis of 3 cm of hair most proximal to the scalp provides information about 3 months of systemic cortisol exposure. Hair samples were attached to a sheet of paper using Millipore tape (Billerica, Mass.), the scalp end was marked and the collection date and participant identification number were recorded. The paper was then enclosed in an envelope sealed with identification number and date on outside of envelope and stored at room temperature. Within 6 months of collection, samples were sent by mail to the laboratory at the University of Western Ontario, London, Ontario where cortisol levels were analyzed.
Hair Sample Preparation and Quantification of Hair Cortisol
In preparation for analysis, hair samples were measured and the length and color of the hair recorded. The most proximal 3 cm hair segment was cut, placed into a glass vial, labeled and weighed to ensure a minimal weight for analysis of 10 to 15 mg. Hair was then washed twice by immersing the segments in 3 ml of isopropanol, followed by a 3 minute incubation on a shaker at 0.11 g (100 rpm) at room temperature. Laboratory analysis was performed using a commercially available salivary cortisol enzyme immunoassay kit from Alpco Diagnostics (Salem, NH). Details of the laboratory procedures are reported fully elsewhere.24
Perceived Stress
Perceived stress was measured using the psychometrically-validated and widely used 14-item Perceived Stress Scale (PSS).25 Summary scores from this instrument and its shorter 10-item subset are shown in previous studies to be positively associated with TMD.13,26 The PSS was developed to evaluate the theoretical construct of stress proposed by Lazarus and Folkman27 that a stimulus is stressful when perceived as both threatening and exceeding one's coping resources. The PSS takes into account these appraisals by measuring the degree to which respondents consider their lives to be unpredictable, uncontrollable and overloaded.25 In each question, respondents were asked to indicate how often they felt or thought a certain way. The conventional 1 month reference interval was extended in this study to 3 months. This was considered to better represent exposure to chronic stress than the 1 month interval, without being so long that recall bias would limit the interpretation of findings. Responses were recorded on a 5-point ordinal scale coded: never=0, almost never=1, sometimes=2, fairly often=3 and very often=4. In computing a summary score, positively worded items were reverse coded, consistent with recommended scoring methods.25
Covariates
Covariates were sex, age in years, race, ethnicity, educational attainment, annual household income and cigarette smoking status. This information was obtained by questionnaire at the time of the physical examination.
Statistical Analysis
Participants with hair cortisol concentrations >1500 ng/g were excluded from analysis on the basis of possible contamination due to use of creams or ointments containing hydrocortisone.28 Initial exploration using histograms and qnorm diagnostic plots showed that PSS scores were normally distributed, and cortisol concentrations were skewed towards higher values. Therefore log10 transformed cortisol values were modeled when the continuous values were analyzed. To account for the potential effect of confounding, analyses were repeated after stratifying on TMD case status.
The Pearson's product moment correlation coefficient was used to determine the strength and direction of the relationship between PSS scores and cortisol concentration. A scatter plot was fitted to graphically depict this relationship. Fisher's exact test was used to compare dichotomous variables and the independent samples t-test (2-sided) compared differences in mean log10 cortisol concentration between TMD cases and controls.
Results
Data were analyzed for 45 TMD cases and 71 controls after omitting 3 subjects whose cortisol concentrations exceeded 1,500 ng/g. The age of participants ranged from 18 to 59 years (mean=29.9 years) and the sample was predominantly female (80.2%) and Caucasian (84.2%).
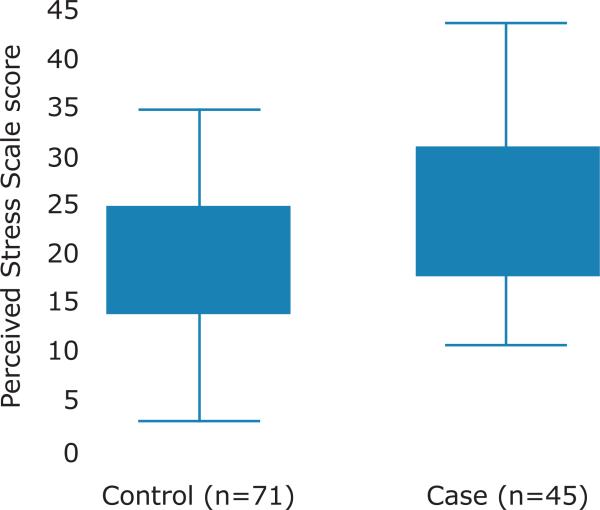
TMD cases and controls did not differ on the basis of socio-demographic characteristics or smoking status. However, compared with controls, TMD cases perceived significantly higher levels of stress in their daily lives (p<0.001, Figure 1, Table I).
Figure 1. Box and Whisker Plot of the Distribution of Perceived Stress Scores for TMD Controls and TMD Cases.
The horizontal line within the box is the median value while the lower and upper hinges are the 25th percentile and 75th percentile, respectively. The ends of the whiskers represent the minimum and maximum values. A 2-group mean comparison t-test indicates the mean value for controls (19.7, s.e. 0.0) is statistically significant from that of cases (24.8, s.e. 1.2), p=0.0007.
Table I.
Distribution of Mean PSS Scores and Mean Log10 Hair Cortisol Concentration
| Perceived Stress score | Log10 Cortisol concentration | |||||
|---|---|---|---|---|---|---|
| Mean | SD | p-value | Mean | SD | p-value | |
| TMD status | ||||||
| Control | 19.69 | 7.24 | 0.001 | 2.38 | 0.24 | <0.001 |
| Case | 24.80 | 8.27 | – | 2.19 | 0.32 | – |
|
Sex | ||||||
| Female | 22.27 | 7.89 | 0.108 | 2.29 | 0.30 | 0.495 |
| Male | 19.26 | 8.25 | – | 2.34 | 0.26 | – |
|
Age group (years) | ||||||
| <25 | 21.02 | 6.13 | 0.723 | 2.31 | 0.26 | 0.618 |
| 25-34 | 22.41 | 8.60 | – | 2.27 | 0.29 | – |
| 35-60 | 21.50 | 9.52 | – | 2.34 | 0.33 | – |
|
Race | ||||||
| White | 21.58 | 8.24 | 0.842 | 2.30 | 0.29 | 0.771 |
| Not white | 22.00 | 7.37 | – | 2.32 | 0.28 | – |
|
Educational attainment | ||||||
| ≤High school graduation | 20.62 | 7.25 | 0.364 | 2.37 | 0.31 | 0.127 |
| Some college or higher | 22.11 | 8.32 | – | 2.28 | 0.28 | – |
|
Household income (USD) | ||||||
| <$40,000 | 22.59 | 8.18 | 0.414 | 2.29 | 0.27 | 0.946 |
| $40,000–<$100,000 | 21.11 | 8.77 | – | 2.31 | 0.27 | – |
| ≥$100,000 | 19.50 | 5.87 | – | 2.29 | 0.37 | – |
|
Smoking status | ||||||
| Current | 23.38 | 6.44 | 0.729 | 2.24 | 0.26 | 0.271 |
| Former | 20.65 | 10.05 | – | 2.40 | 0.32 | – |
| Never | 21.71 | 7.78 | – | 2.29 | 0.28 | – |
Perceptions of stress and levels of hair cortisol did not differ significantly between participants on the basis of age, sex, race, smoking or socioeconomic status (Table I). Despite perceiving higher levels of stress, cortisol concentrations were significantly lower in TMD cases than in controls (p<0.001).
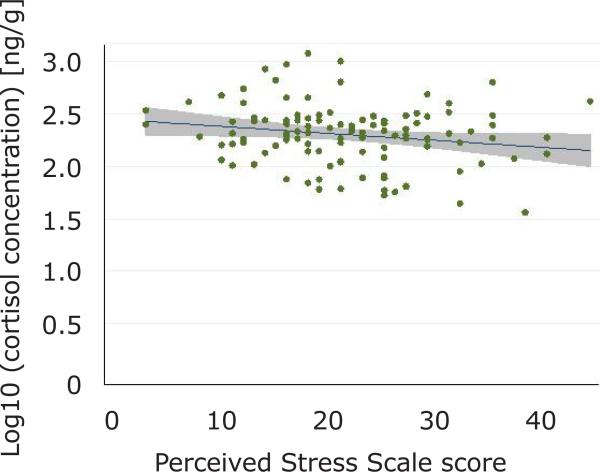
Examination of the cloud of observations on the scatter plot revealed a weak, negative relationship but statistically significant relationship between perceived stress and cortisol concentration (r=−0.188, p=0.044, Figure 2). When examined in separate strata of case status, the relationship was negative in each stratum, but failed to reach statistical significance for cases (r=−0.111, p=0.169) and controls (r=−0.082, p=0.498). Examination of the stratum-specific odds ratios and their confidence intervals suggested that the relationship between perceived stress and hair cortisol concentration was similar in TMD cases and controls.
Figure 2. Scatter Plot of the Relationship Between Perceived Stress Score (x-axis) and log10 Cortisol Concentration.
(Y-axis) showing the fitted line and 95% confidence interval (n=116 observations). The Pearson correlation coefficient for this relationship is −0.188, p=0.044.
Discussion
Key Findings
In this study, TMD cases perceived significantly more stress than controls over the preceding 3 months, confirming a well-established relationship between psychosocial stress and TMD. Our expectation that higher stress perception in cases would correspond with elevated cortisol production was not supported. In fact, cortisol production was significantly lower in cases than controls. Among all subjects combined, perceived stress and cortisol concentration were significantly and negatively related, albeit in a weak relationship. When examined in stratum-specific analyses, perceived stress and cortisol concentration were negatively associated for both cases and controls, but non-significantly. In summary, individuals with higher perceived stress had lower hair cortisol concentration, and this effect was more pronounced among cases than controls.
Comparison with Previous Studies
This study is not the first to find an inverse or null association between perceived stress and hair cortisol concentration. A study that administered the PSS with a 3 month reference interval to university students reported a weak negative correlation with hair cortisol content (r=−0.061, p=0.025).29 Another study compared long-term unemployed individuals with people in stable employment. The study found that the unemployed reported higher PSS scores, and the hair cortisol concentration was not associated with perceived stress.30 Likewise, PSS scores and hair cortisol concentration were not associated among patients attending a cardiac rehabilitation program.31 Elsewhere, a study comparing adults with severe chronic pain with healthy controls found a weak positive correlation between PSS scores and hair cortisol that failed to reach statistical significance (r=0.24, p=0.08, Spearman).32 Similarly, the correlation between PSS scores and hair cortisol concentration was weakly positive but did not reach statistical significance (r=0.2, p=0.06) for subjects in a case control study where cases were patients with adrenal insufficiency who were on hydrocortisone replacement therapy.24 These findings differ from another conducted with pregnant women that reported a positive relationship between PSS scores and hair cortisol concentration.33
Few epidemiologic studies have measured hair cortisol in stress-related disorders. In these few studies, divergent findings report that cortisol is elevated in some disorders while lower in others. A pilot study compared hair cortisol concentration in severe chronic pain patients recruited from a chronic pain clinic who had received opioid treatment for at least 1 year (n=15), with pain-free control group recruited from the community (n=39). Perceived stress and cortisol levels were both higher in the opioid-treated chronic pain group with cortisol being almost elevated two-fold in the pain group (83.1 [33.0 to 204.9] pg/mg) relative to controls (46.1 [27.2 to 199.9] pg/mg).32
Consistent with findings from the severe chronic pain study, a study of men hospitalized following acute myocardial infarction found significantly higher median hair cortisol levels over the 3 months preceding the event (295.3 ng/g [105.4 to 809.3]) than hospitalized men admitted for other conditions (224.9 ng/g [76.58 to 949.9]).34 By contrast to these 2 studies, in a case control study in which cases had generalized anxiety disorder, hair cortisol concentrations were 50 to 60% lower in cases than in healthy-age and sex-matched controls - a result that contradicted earlier research using short terms measures of cortisol.35
A study that might shed light on these differential patterns examined hair cortisol levels in female adolescents at multiple time points following the 2008 Wenchuan earthquake in China.36 Subjects were classified into 1 of 3 groups: those who experienced the earthquake and developed posttraumatic stress disorder (PTSD), those who experienced the earthquake and did not develop PTSD and a group of non-PTSD controls from a different region that was unaffected by the earthquake. Hair segments corresponding to time before and several occasions after the earthquake were compared for cortisol concentration in all 3 groups. Hair cortisol concentrations were similar in all groups before the earthquake suggesting no difference in HPA axis activity at baseline. In the first 2 months following the earthquake, cortisol levels were significantly higher in both groups exposed to the earthquake compared with the control group. Then, at 2 to 4 months after the earthquake, and again at 5 to 7 months after the earthquake, the non-PTSD group exposed to the earthquake had significantly higher cortisol concentration than both the exposed PTSD group and the control group. The authors interpreted this as a blunted HPA response in the PTSD group.36 The important finding was the change in cortisol secretion over time in the PTSD group from elevated initially, relative to controls, to suppressed.
Possible Mechanisms and Explanations
The noteworthy finding of the study of stress-responsive physiology to the earthquake is that timing since onset of chronic stress is important. It is possible that chronic stress elicits both an increased and a decreased production in cortisol, at different stages following onset of stress. In fact, this explanation was a major finding of a meta-analysis of 107 studies published between 1950 to 2005 that examined the relationship between chronic stress and HPA axis activity.37 The meta-analysis concluded that exposure to chronic stress initially activates the HPA axis producing elevated secretion of cortisol. Over time HPA activity subsides and cortisol secretion rebounds to below normal levels.37 The rebound may be a consequence of a cumulative stress burden. This is consistent with the concept of allostatic load that posits that overuse of systems designed to manage transient stress leads to impairment of the HPA function including a decrease in responsiveness to novel stressors and disturbance in the regulation of the key mediators.38
Applied to the present study, it is possible that prolonged or repeated perceptions of stress reported by TMD cases lead to blunted HPA activity and deficient cortisol signaling. In support of this idea are findings from a study of working women where high scores on the PSS were associated with an 11% attenuation in diurnal variation of salivary cortisol characterized as a pronounced reduction in cortisol awakening response.39
Strengths and Limitations
Strengths of the study relate to the rigor of the measurement protocols. The quantification of hair cortisol was conducted in laboratories in the Department of Physiology and Pharmacology, University of Western Ontario, an internationally prominent center for hair cortisol research. The Research Diagnostic Criteria for TMD case classification are standardized criteria that reliably ascertain TMD case classification. The PSS is widely used and has well established reliability and validity. Our findings are the first in the oral health literature to investigate hair cortisol as a systemic biomarker of long-term exposure stress. While our results did not support our hypothesis, the findings serve to challenge an over-simplistic view of psychoneuroimmunology in TMD and other stress-related disorders.
There are several limitations to this study. Firstly, the expectation of a strong correlation between perceived stress and hair cortisol concentration rests on an erroneous assumption that these factors are 2 measures of the same phenomenon. However, one is a cognitive appraisal of stress and the other is the physiologic response to stress. Secondly, since information regarding the duration of TMD in the cases is not available, it was not possible to determine whether chronic cases were more likely than recent-onset cases to have a lower cortisol concentration. Information on other variables that may influence cortisol, such as alcohol use and body mass index, was not collected.
Implications for Dental Hygiene Practice
Psychosocial stress contributes to the etiology of several disorders that dental hygienists evaluate in clinical practice. Patients may be unaware that their orofacial muscle or joint pain has dental relevance. Likewise, the patient may not recognize that stress might be a contributing factor to their symptoms. Dental hygienists are well positioned to observe, discuss and evaluate potential TMD and its risk factors in the course of their intraoral and extraoral examinations. This is consistent with the American Dental Hygienists’ Association Standards for Clinical Dental Hygiene Practice that hygienists perform an individualized assessment that includes interpretation of symptoms and clinical signs while systematically taking account of the general health status, history and needs of the patient.40 In discussing the patient's oral status, the dental hygienist may inform the patient that stress is a common factor in TMD since this may be taken into consideration in formulating a patient-centered and evidence-based treatment plan.
This project was completed in partial fulfillment of the Masters of Science degree in Dental Hygiene Education at the University of North Carolina at Chapel Hill.
Conclusion
Measurement of hair cortisol in epidemiologic studies is still in its infancy and the mixed findings make interpretations difficult. Our understanding will be improved with prospective cohort studies that collect hair samples before and after first-onset of TMD.
Acknowledgments
This study was supported by the North Carolina Translational and Clinical Sciences Institute grant 10KR30904 and National Institutes of health grants U01De017018 and P01NS045685.
This study supports the NDHRA priority area, Clinical Dental Hygiene Care: Investigate the links between oral and systemic health.
Footnotes
The Journal of Dental Hygiene Best Paper Award was created this year to recognize the most outstanding research paper published from the previous year (2013). All original research papers published in 2013 were evaluated by a panel of judges, using specific criteria, to make the final selection. This manuscript first appeared in Volume 87, Issue Number 2 of the April 2013 issue of the Journal of Dental Hygiene.
References
- 1.McEwen BS. Protective and damaging effects of stress mediators: the good and bad sides of the response to stress. Metabolism. 2002;51(6 Suppl 1):2–4. doi: 10.1053/meta.2002.33183. [DOI] [PubMed] [Google Scholar]
- 2.Ivanovski K, Nakova M, Warburton G, et al. Psychological profile in oral lichen planus. J Clin Periodontol. 2005;32(10):1034–1040. doi: 10.1111/j.1600-051X.2005.00829.x. [DOI] [PubMed] [Google Scholar]
- 3.Koray M, Dülger O, Ak G, et al. The evaluation of anxiety and salivary cortisol levels in patients with oral lichen planus. Oral Dis. 2003;9(6):298–301. doi: 10.1034/j.1601-0825.2003.00960.x. [DOI] [PubMed] [Google Scholar]
- 4.McCartan BE, Lamey PJ, Wallace AM. Salivary cortisol and anxiety in recurrent aphthous stomatitis. J Oral Pathol Med. 1996;25(7):357–359. doi: 10.1111/j.1600-0714.1996.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 5.Sanders AE, Slade GD, Turrell G, Spencer AJ, Marcenes W. Does psychological stress mediate social deprivation in tooth loss? J Dent Res. 2007;86(12):1166–1170. doi: 10.1177/154405910708601205. [DOI] [PubMed] [Google Scholar]
- 6.Gotfredsen K, Walls AW. What dentition assures oral function? Clin Oral Implants Res. 2007;18(Suppl 3):34–45. doi: 10.1111/j.1600-0501.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- 7.Moss ME, Beck JD, Kaplan BH, et al. Exploratory case-control analysis of psychosocial factors and adult periodontitis. J Periodontol. 1996;67(10 Suppl):1060–1069. doi: 10.1902/jop.1996.67.10s.1060. [DOI] [PubMed] [Google Scholar]
- 8.LeResche L, Dworkin SF. The role of stress in inflammatory disease, including periodontal disease: review of concepts and current findings. Periodontol 2000. 2002;30:91–103. doi: 10.1034/j.1600-0757.2002.03009.x. [DOI] [PubMed] [Google Scholar]
- 9.Genco RJ, Ho AW, Kopman J, Grossi SG, Dun-ford RG, Tedesco LA. Models to evaluate the role of stress in periodontal disease. Ann Periodontol. 1998;3(1):288–302. doi: 10.1902/annals.1998.3.1.288. [DOI] [PubMed] [Google Scholar]
- 10.Isong U, Gansky SA, Plesh O. Temporomandibular joint and muscle disorder-type pain in U.S. adults: the National Health Interview Survey. J Orofac Pain. 2008;22(4):317–322. [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders AE, Maixner W, Nackley AG, et al. Excess risk of temporomandibular disorder associated with cigarette smoking in young adults. J Pain. 2012;13(1):21–31. doi: 10.1016/j.jpain.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fillingim RB, Ohrbach R, Greenspan JD, et al. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 2011;12(11 Suppl):T46–60. doi: 10.1016/j.jpain.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slade GD, Diatchenko L, Bhalang K, et al. Influence of psychological factors on risk of temporomandibular disorders. J Dent Res. 2007;86(11):1120–1125. doi: 10.1177/154405910708601119. [DOI] [PubMed] [Google Scholar]
- 14.Liddle GW. Analysis of circadian rhythms in human adrenocortical secretory activity. Arch Intern Med. 1966;117(6):739–743. [PubMed] [Google Scholar]
- 15.Halpern CT, Whitsel EA, Wagner B, Harris KM. Challenges of measuring diurnal cortisol concentrations in a large population-based field study. Psychoneuroendocrinology. 2012 Apr;37(4):499–508. doi: 10.1016/j.psyneuen.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamashiro KL, Sakai RR, Shively CA, Karatsoreos IN, Reagan LP. Chronic stress, metabolism, and metabolic syndrome. Stress. 2011;14(5):468–474. doi: 10.3109/10253890.2011.606341. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt MV, Sterlemann V, Müller MB. Chronic stress and individual vulnerability. Ann N Y Acad Sci. 2008;1148:174–183. doi: 10.1196/annals.1410.017. [DOI] [PubMed] [Google Scholar]
- 18.Sauvé B, Koren G, Walsh G. Tokmakejian S, Van Uum SH. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 2007;30(5):E183–E191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- 19.Manenschijn L, Koper JW, Lamberts SW, van Rossum EF. Evaluation of a method to measure long term cortisol levels. Steroids. 2011;76(10-11):1032–1036. doi: 10.1016/j.steroids.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Raul JS, Cirimele V, Ludes B, Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem. 2004;37(12):1105–1111. doi: 10.1016/j.clinbiochem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi S, Miyamoto I, Takeda K. Measurement of human hair growth by optical microscopy and image analysis. Br J Dermatol. 1991;125(2):123–129. doi: 10.1111/j.1365-2133.1991.tb06058.x. [DOI] [PubMed] [Google Scholar]
- 22.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6(4):301–355. [PubMed] [Google Scholar]
- 23.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Sci Int. 2000;107(1-3):5–12. doi: 10.1016/s0379-0738(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 24.Gow R, Koren G, Rieder M, Van Uum S. Hair cortisol content in patients with adrenal insufficiency on hydrocortisone replacement therapy. Clin Endocrinol (Oxf) 2011;74(6):687–693. doi: 10.1111/j.1365-2265.2011.04001.x. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 26.Sanders AE, Maixner W, Nackley AG, et al. Excess risk of temporomandibular disorder associated with cigarette smoking in young adults. J Pain. 2012;13(1):21–31. doi: 10.1016/j.jpain.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarus RS, Folkman S. Stress, appraisal, and coping. Springer; New York (NY): 1984. [Google Scholar]
- 28.Gow R, Thomson S, Rieder M, Van Uum S, Koren G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int. 2010;196(1-3):32–37. doi: 10.1016/j.forsciint.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Karlén J, Ludvigsson J, Frostell A, Theodorsson E, Faresjö T. Cortisol in hair measured in young adults - a biomarker of major life stressors? BMC Clin Pathol. 2011;11(1):12. doi: 10.1186/1472-6890-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology. 2010;35(9):1404–1409. doi: 10.1016/j.psyneuen.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Dowlati Y, Herrmann N, Swardfager W, et al. Relationship between hair cortisol concentrations and depressive symptoms in patients with coronary artery disease. Neuropsychiatr Dis Treat. 2010;6:393–400. [PMC free article] [PubMed] [Google Scholar]
- 32.Van Uum SH, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, Koren G. Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress. 2008;11(6):483–488. doi: 10.1080/10253890801887388. [DOI] [PubMed] [Google Scholar]
- 33.Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med. 2007;30(2):E103–E107. doi: 10.25011/cim.v30i2.986. [DOI] [PubMed] [Google Scholar]
- 34.Pereg D, Gow R, Mosseri M, et al. Hair cortisol and the risk for acute myocardial infarction in adult men. Stress. 2011;14(1):73–81. doi: 10.3109/10253890.2010.511352. [DOI] [PubMed] [Google Scholar]
- 35.Steudte S, Stalder T, Dettenborn L, et al. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res. 2011;186(2-3):310–314. doi: 10.1016/j.psychres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Luo H, Hu X, Liu X, et al. Hair Cortisol level as a bio-marker for altered hypothalamic-pituitary-adrenal activity in female adolescents with posttraumatic stress disorder after the 2008 Wenchuan earthquake. Biol Psychiatry. 2012;72(1):65–69. doi: 10.1016/j.biopsych.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 37.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 38.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 39.Farag NH, Moore WE, Lovallo WR, Mills PJ, Khandrika S, Eichner JE. Hypothalamic-pituitary-adrenal axis function: relative contributions of perceived stress and obesity in women. J Womens Health (Larchmt) 2008;17(10):1647–1655. doi: 10.1089/jwh.2008.0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Dental Hygienists’ Association [2012 Mar 23];Standards of Clincal Dental Hygiene Practice. ADHA [Internet] 2008 Available from: http://www.adha.org/resources-docs/7261_Standards_Clinical_Practice.pdf.