Abstract
Medullary thyroid carcinoma (MTC) is a rare form of malignancy, having an intermediate prognosis. Controversies exist regarding the best surgical approach. The aim of the study was to analyze the outcome in a group of patients with MTC, diagnosed and followed up in a single care center. We performed a retrospective analysis of all the patients diagnosed with MTC in the Department of Endocrinology from the County Emergency Hospital Timisoara between 1992 and 2012. The study group included 19 patients, 6 men (31.6 %), mean age 41.2 ± 12.5 years (20–72 years). The preoperative diagnosis was based on the protocol for nodular thyroid disease. Total or near-total thyroidectomy was performed in 10 out of 16 patients who could be operated. Postoperative follow-up included repeated measurements of serum calcitonin and imaging investigations. Nine out of the total of 19 (47.3 %) patients had hereditary forms of MTC. Most of the cases (84.2 %) were submitted to surgery. The median duration of follow-up was 84 months. The pTNM staging indicated that the majority of the patients with hereditary MTC were diagnosed in an earlier stage. Disease remission was achieved in 7 cases (43.8 %). Four patients, all with sporadic forms, died. Survival rates at 1, 5 and 10 years were significantly higher (p = 0.048) in patients with hereditary MTC. An early diagnosis of MTC allows a better surgical approach and an improved survival rate. We support the general recommendation that modified radical neck dissection is not necessary for all the patients with MTC.
Keywords: Medullary thyroid carcinoma, Multiple endocrine neoplasia, Modified radical neck dissection, Total thyroidectomy, Prognosis
Introduction
Medullary thyroid carcinoma (MTC) is a rare form of malignancy that accounts for about 3–10 % of all thyroid carcinomas. It is derived from the parafollicular cells (C cells) of the thyroid and occurs as sporadic or hereditary (inherited) forms, displaying several clinical behaviors. Sporadic MTC is usually unilateral and represents 75 % of the cases. Inherited forms (25 %) are most often bilateral and multicentric. The hereditary variety can be transmitted as a single entity, familial MTC, or it can occur in association with other malignancies, constituting multiple endocrine neoplasia (MEN) type 2A and 2B syndromes (MEN-2A and MEN-2B) [1].
Several MEN-2A variants have been described. In addition to MTC, the patients with this syndrome present with pheochromocytoma (50 %), parathyroid neoplasia (10–35 %), cutaneous lichen amyloidosis (<10 %), or Hirschprung’s disease (rarely) [2].
MEN-2B syndrome consists in a combination of MTC with pheochromocytoma (50 %) or intestinal ganglioneuromatosis and mucosal neuromas (>98 %). The patients have a marfanoid habitus (>95 %) and lack parathyroid disease [2].
The prognosis of MTC is intermediate between the anaplastic and the differentiated thyroid cancer. It is known that the clinical course of the disease varies from case to case. Some patient- and disease-related factors, such as age at diagnosis, extent of disease, and type of surgical intervention, may influence the clinical outcome, although these data are still controversial [3].
The potentially curative treatment for MTC is surgery, consisting in total thyroidectomy with dissection of lymph nodes in the central compartment of the neck [1, 3]. According to the current guidelines of the American Thyroid Association and European Thyroid Association, the recommendations are for a modified radical neck dissection of the ipsilateral and contralateral compartment in cases with evidence of suspicious or metastatic nodes at preoperative imaging [4–6]. There are some authors who advocate the necessity of modified radical neck dissection (ipsilateral or bilateral) at the initial operation even in patients without any evidence of suspicious lymph nodes [7, 8]. This more aggressive approach, which significantly raises the morbidity related to the intervention [9, 10], is justified, in their opinion, as the tumor has early lymphatic metastasis.
The aim of the present study was to evaluate the clinical outcome of MTC in a group of patients treated and followed up in a single care center over a 20-year interval and to assess their long-term disease-free survival according to the form of the disease (hereditary or sporadic) and the type of surgery.
Material and Methods
Subjects
This was a retrospective study that included 19 patients, 6 men (31.6 %) and 13 women (68.4 %), male/female ratio 1:2.1, having mean age at diagnosis 41.2 ± 12.5 years (median 42 years, range 20–72 years). They were diagnosed with MTC in the Department of Endocrinology from the County Hospital Timisoara, Romania, between years 1992 and 2012. The data for all the patients were collected from the day of the diagnosis to the last visit in the clinic (for those who are alive) or until their death.
Diagnosis and Therapy of MTC
Data about the personal and family history, biochemical evaluation, surgical intervention, pathological result, and long-term outcome were collected retrospectively from the medical records of the patients. The family history was reviewed in order to determine if the disease was sporadic or hereditary. A case was considered hereditary based on a family history of MEN-2 syndrome, on the association with other neoplasia, and/or on the finding of the RET mutation at genetic testing.
The diagnosis of MTC was based on clinical picture, biochemical tests (elevated basal serum calcitonin and carcinoembryonic antigen—CEA), and imaging (ultrasonography of the cervical region, CT scans and/or MRI). Genetic testing for RET mutations has been available since the year 2004 and has been performed in all the 11 cases diagnosed subsequently.
Calcitonin was determined by a chemiluminiscence immunoassay method (DiaSorin, Stillwater, MN). The normal reference value is <18.2 pg/mL for men and <11.5 pg/mL for women. Serum CEA levels were measured with a commercial chemiluminiscent microparticle immunoassay (Axsym Abbott Laboratories, Tokyo, Japan). The normal reference value is <10 ng/mL. The genetic tests were performed in two centers, one in Germany and the other in Sweden, based on an international collaboration.
All the surgical interventions were carried on in our hospital (most of them in the Second Department of Surgery).
The confirmation of the diagnosis was based on the pathological examination, with the classical hematoxylin-eosin staining, performed in all operated patients, and, in some cases, on immunohistochemistry that indicated a positive reaction to calcitonin.
After the surgical intervention, a pTNM staging could be performed, according to the last recommendations from 2003 [11, 12].
Follow-Up
The protocol for surveillance was not standardized. In general, the patients were evaluated for the first time between 1 and 2 months after surgery, when basal serum calcitonin and, in some cases, serum CEA were measured, and imaging tests were performed. Patients with postoperative high values of calcitonin (n = 6) were submitted to various imaging investigations, in order to detect metastases: I131 or Tc99 thyroid scintiscans, bone scintigraphy, CT, or MRI for cervical region, mediastinum, thorax, and abdomen.
Patients were considered in complete remission if the calcitonin levels returned to normal range following surgery. Biochemically active disease was diagnosed based on postoperative elevated basal calcitonin levels. Clinically persistent MTC was defined as the presence of a detectable local disease or of metastases after surgery, in addition to elevated basal calcitonin levels.
Ethics
All the procedures related to the study were approved by the Local Ethics Committee of the hospital.
Statistical Analysis
Data were collected and analyzed using SPSS v.17 statistical suite (SPSS Inc, Chicago, IL). Results were expressed as median and interquartile range (values without Gaussian distribution) or mean values ± standard deviations (values Gaussian distributed), after testing for normality with Shapiro-Wilk test. The significance of differences between groups was analyzed with Fisher’s exact test (percentages), Mann-Whitney U test (non-Gaussian variables), and unpaired Student’s t test (Gaussian variables). Survival was evaluated with the product-limit estimate method (Kaplan-Meier), and survival curves were compared with the log-rank test. The threshold for significance was set at p < 0.05.
Results
Baseline Data
From the entire study group, 10 patients (52.7 %) had the sporadic form of MTC (Table 1). All the patients diagnosed with the hereditary form had MEN-2A syndrome. Family history was positive in 8 of these cases; 5 patients presented other neoplasia (unilateral or bilateral pheochromocytoma). The genetic test was performed in 7 cases with hereditary MTC (77.8 %). The RET mutations detected were in the 11th exon, codon 634, represented by a Cys634Phe substitution (C634F) in 3 cases and a Cys634Trp substitution (C634W) in 4 patients. The mutations found are specific for the MEN-2A phenotype.
Table 1.
Baseline characteristics of the study group
| All patients | Sporadic forms (n = 10; 52.7 %) | Hereditary forms (n = 9; 47.3 %) | p a | |
|---|---|---|---|---|
| Age at diagnosis (years) | 41.2 ± 12.5 | 37.8 ± 11.8 | 44.2 ± 13 | NS |
| Male/female (n) | 6/13 | 4/6 | 2/7 | NS |
| Local disease (n, %) | 14 (73.7) | 6 (60) | 8 (88.9) | NS |
| Disseminated disease (n, %) | 5 (26.3) | 4 (40) | 1 (11.1) | |
| RET mutation | 7/11 | 0/4 | 7/7 | 0.003 |
NS not significant
a p between sporadic and hereditary forms
Basal or preoperative serum calcitonin level was measured in 8 patients (42.1 %): 5 with hereditary disease and 3 with sporadic forms. The values ranged between 57.6 and 12,468 pg/mL.
Surgical Therapy
Sixteen out of 19 (84.2 %) patients were submitted to surgery. The intervention was represented by total thyroidectomy with central (levels VI and VII) and bilateral neck (levels II, III, IV, and V) lymph nodes dissection in 2 cases (10.5 %), total or near-total thyroidectomy in 10 patients (52.7 %), subtotal followed by total thyroidectomy in 2 cases (10.5 %) and subtotal, palliative surgery in 2 patients (10.5 %). The remaining 3 patients (15.8 %) were not operated: 1 (5.3 %) patient was due to a very poor general condition and multiple metastases at diagnosis and 2 patients (10.5 %) recently diagnosed through genetic testing are still on the waiting list.
pTNM Staging
The distribution of the 16 operated cases, according to the last pTNM staging, is presented in Table 2. This indicates that most of the patients with hereditary forms were diagnosed and operated in an earlier stage of the disease, in comparison with sporadic MTC cases.
Table 2.
Distribution of the patients according to the pTNM staging (2009)
| Parameter | All cases (n = 16) | Sporadic forms (n = 9) | Hereditary forms (n = 7) | p a |
|---|---|---|---|---|
| T1 (n, %) | 5 (31.2) | 1 (11.1) | 4 (57.1) | NS |
| T2 (n, %) | 6 (37.5) | 4 (44.5) | 2 (28.6) | NS |
| T3 (n, %) | 3 (18.8) | 2 (22.2) | 1 (14.3) | NS |
| T4a-b (n, %) | 2 (12.5) | 2 (22.2) | 0 (0) | NS |
| N1a (n, %) | 1 (6.3) | 1 (11.1) | 0 (0) | NS |
| N1b (n, %) | 2 (12.5) | 1 (11.1) | 1 (14.3) | NS |
| M1 (n, %) | 1 (6.3) | 1 (11.1) | 0 (0) | NS |
| Stage I (n, %) | 5 (31.2) | 1 (11.1) | 4 (57.1) | NS |
| Stage II (n, %) | 7 (43.8) | 5 (55.6) | 2 (28.6) | NS |
| Stage III (n, %) | 1 (6.3) | 1 (11.1) | 0 (0) | NS |
| Stage IVa–c (n, %) | 3 (18.8) | 2 (22.2) | 1 (14.3) | NS |
NS not significant
a p between sporadic and hereditary forms
Clinical Evolution of the MTC
The median duration of follow-up was of 84 months (range 6–168 months). Disease remission was achieved in 7 out of the 16 cases submitted to surgery (43.8 %): 3 (18.8 %) with hereditary and 4 (25 %) with sporadic form of MTC. Biochemically active disease was detected in 9 patients (56.3 %), 6 females and 3 males. From these, 6 cases were labeled as having clinically persistent MTC. Two of these patients were operated again, 5 and 8 years later, respectively, for local metastases in cervical lymph nodes.
Until the end of year 2012, 4 patients (21.1 %), all diagnosed with sporadic forms of MTC, died due to complications of the primary tumor or the metastases. One of them (who was diagnosed in an advanced stage and could not be operated) died 1.5 years later. The other 3 fatal cases were represented by the 2 patients who underwent only palliative surgery and by 1 patient who developed, in time, multiple distal metastases (the last case died 13 years after the first surgical intervention). The median survival period for these 4 patients was 2.75 years (range between 1.5 and 13 years).
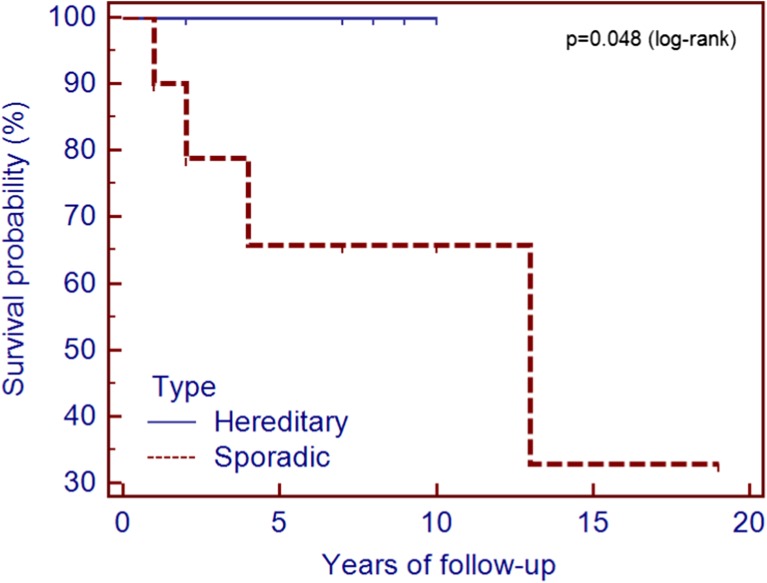
The 1, 5, and 10 years survival rates for the whole group were 95, 78, and 62 %, respectively. For the cases with hereditary disease, the survival rates were 100 % at 1, 5, and 10 years. All these patients are in good general condition. In the group of the patients with sporadic form, the survival rates were significantly lower (p = 0.048): 90, 63, and 57 %, respectively (Fig. 1).
Fig. 1.
Survival rates in patients with sporadic and hereditary forms of medullary thyroid carcinoma
Discussion
MTC is an uncommon form of thyroid cancer and has a variable clinical presentation, evolution, and response to therapy. Several studies that analyzed the prognostic factors, progression of the disease, and survival rates have yielded contradictory results. Some of the differences could be explained by the rarity of this cancer, difficulties in the grouping, and the follow-up of many patients in a single center, heterogeneous collection and reporting of the data, and a continuous improvement in laboratory diagnosis and imaging investigations.
According to our knowledge, this is the first study that has analyzed the outcome of the patients with this type of thyroid cancer followed up in a single endocrine care center in Romania. In addition, the majority of the patients underwent surgery in a single clinic from the same hospital.
Total thyroidectomy with bilateral neck lymph nodes dissection was performed only in 25 % of the operated cases, but total or near-total thyroidectomy was performed in the majority of the patients, where it was feasible (in 2 cases only palliative surgery could be performed).
The overall survival rate in our study was 95 % at 1 year, 78 % at 5 years, and 62 % at 10 years, similar to other published data (Table 3). It was significantly better in cases with hereditary forms of MTC, probably due to a better awareness of the disease and, consequently, an earlier diagnosis (Table 2). All the four patients who died had sporadic forms of MTC and were diagnosed in an advanced stage (three in stage IV, including the patient who could not be operated, and one in stage III). From the patients who died, two benefitted only from palliative surgery and one could not be operated. One can state that proper surgical intervention performed in an early stage of the disease ensures a prolonged survival of these cases.
Table 3.
Comparison of the characteristics and evolution of patients with MTC in different single centers
| Vlad et al. | Grozinsky-Glasberg et al. [3] | Gülben et al. [13] | Clark et al. [14] | Rendl et al. [15] | Hyer et al. [16] | Dottorini et al. [17] | Kebebew et al. [18] | Ito et al. [19] | |
|---|---|---|---|---|---|---|---|---|---|
| Study period | 1992–2012 | 1970–2006 | 1993–2003 | 1969–2000 | 1996–2008 | 1949–1998 | 1970–1992 | 1960–1998 | 1975–2005 |
| Number | 19 | 51 | 32 | 30 | 32 | 162 | 53 | 104 | 118 |
| Mean age (years) | 41 | 47 | 45 (median) | 38 (median) | 40; 42 (median) | 44 (median) | 47 (median) | 38 | – |
| Women (%) | 68.4 | 57 | 59 | 56.6 | 62.5 | 54 | 60.3 | 53 | – |
| Hereditary forms (%) | 47.3 | 20 | 0 | 30 | 34 | 32 | 17 | 44 | 39 |
| Distant metastases (%) | 26.3 | 27.4 | 28 | 40 | 18.8 | 48 | – | – | 2.5 |
| Follow-up (years) | 7 (median) | 9.5 (median) | 4 (median) | 12.4 (median) | 9.5 (median) | 9 (median) | 4 (median) | 5 (median) | 11.75 (mean) |
| Survival (%) | |||||||||
| 1 year | 95 | – | – | – | – | – | – | – | – |
| 5 years | 78 | 88 | 51 | 97 | 96 | 72 | 74.4 | 89.3 | – |
| 10 years | 62 | 85 | – | 88 | 91 | 56 | 71 | 86.5 | 96.6 |
By comparing the patients’ data with those reported by other center studies (Table 3), the percentage of women was higher in our group, without having a clear explanation for this finding.
The hereditary forms of the disease represented almost half of the cases. This high percentage could be a consequence of the active screening of MTC among the relatives of the patients with familial forms, the underdiagnosis of the sporadic forms or the reduced frequency of sporadic forms in Romania (or, at least, in our region), taking into account that in the last years, the measurement of calcitonin became a routine for the evaluation of nodular goiter.
We mention that all the cases with hereditary MTC belonged to MEN-2A syndrome, which is known to have a better survival rate in comparison to other hereditary forms [4, 20, 21].
The strengths of our study are follow-up of the patients in a single endocrine care unit that allowed access to all the available data and the possibility to perform genetic testing in more than half of the patients. This allowed us to confirm the diagnosis of the hereditary forms of MTC and facilitated the diagnosis in other family members. The weaknesses of our work are represented by the retrospective character of the study, so that the protocol for surveillance of the cases was not uniform, by the lack of stimulatory tests for calcitonin and by the fact that preoperative serum calcitonin level was determined in less than half of the patients. The lack of systematic measurement of calcitonin in the past in patients with nodular goiter might have led to a late diagnosis in some cases.
To sum up, our data demonstrate that an early diagnosis of MTC (usually in patients with the hereditary form) allows a better surgical approach and an improved survival rate and supports the general recommendation that modified radical neck dissection is not necessary for all the patients with MTC.
Compliance with Ethical Standards
This study received no financial support. For this type of study, formal consent is not required. All the procedures related to the study were approved by the local ethics committee of the hospital.
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Matias-Guiu X, De Lellis R. Medullary thyroid carcinoma: a 25-year perspective. Endocr Pathol. 2014;25:21–29. doi: 10.1007/s12022-013-9287-2. [DOI] [PubMed] [Google Scholar]
- 2.Schlumberger MJ, Filetti S, Hay ID. Nontoxic diffuse and nodular goiter and thyroid neoplasia. In: Kronenberg HM, Melmed S, Polonsky KS, Reed Larsen P, editors. Williams textbook of endocrinology. 11. Philadelphia: Saunders Elsevier; 2008. pp. 411–442. [Google Scholar]
- 3.Grozinsky-Glasberg S, Benbassat CA, Tsvetov G, Feinmesser R, Hava Peretz H, Shimon I, Lapidot M. Medullary thyroid cancer: a retrospective analysis of a cohort treated at a single tertiary care center between 1970 and 2005. Thyroid. 2007;17:549–556. doi: 10.1089/thy.2006.0229. [DOI] [PubMed] [Google Scholar]
- 4.American Thyroid Association Guidelines Task Force. Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA., Jr Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 5.Schlumberger M, Bastholt L, Dralle H, Jarzab B, Pacini F, Smit JWA, The European Thyroid Association Task Force 2012 European Thyroid Association guidelines for metastatic medullary thyroid cancer. Eur Thyroid J. 2012;1:5–14. doi: 10.1159/000336977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duntas LH. Clinical comments related to medullary thyroid cancer diagnosis and management. Thyroid Res. 2013;6(Suppl 1):S6. doi: 10.1186/1756-6614-6-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen TW, Shapiro SE, Gagel RF, Sherman SI, Lee JE, Evans DB. Medullary thyroid carcinoma: results of a standardized surgical approach in a contemporary series of 80 consecutive patients. Surgery. 2003;134:890–899. doi: 10.1016/S0039-6060(03)00408-2. [DOI] [PubMed] [Google Scholar]
- 8.Moley JF, DeBenedetti MK. Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg. 1999;229:880–887. doi: 10.1097/00000658-199906000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. 2007;245:604–610. doi: 10.1097/01.sla.0000250451.59685.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong KP, Lang BH. The role of prophylactic central neck dissection in differentiated thyroid carcinoma: issues and controversies. J Oncol. 2011;2011:127929. doi: 10.1155/2011/127929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene FL, Page DL, Fleming ID, Fritz A, Balch DM. AJCC cancer staging manual. 6. Chicago: Springer Verlag; 2003. [Google Scholar]
- 12.Boostrom SY, Grant CS, Thompson GB, Farley DR, Richards ML, Hoskin TL, Hay ID. Need for a revised staging consensus in medullary thyroid carcinoma. Arch Surg. 2009;144:663–669. doi: 10.1001/archsurg.2009.122. [DOI] [PubMed] [Google Scholar]
- 13.Gülben K, Berberoğlu U, Boyabatli M. Prognostic factors for sporadic medullary thyroid carcinoma. World J Surg. 2006;30:84–90. doi: 10.1007/s00268-005-7949-z. [DOI] [PubMed] [Google Scholar]
- 14.Clark JR, Fridman TR, Odell MJ, Brierley J, Walfish PG, Freeman JL. Prognostic variables and calcitonin in medullary thyroid cancer. Laryngoscope. 2005;115:1445–1450. doi: 10.1097/01.mlg.0000168114.90852.a6. [DOI] [PubMed] [Google Scholar]
- 15.Rendl G, Manzl M, Hitzl W, Sungler P, Pirich C. Longterm prognosis of medullary thyroid carcinoma. Clin Endocrinol. 2008;69:497–505. doi: 10.1111/j.1365-2265.2008.03229.x. [DOI] [PubMed] [Google Scholar]
- 16.Hyer SL, Vini L, A’Hern R, Harmer C. Medullary thyroid cancer: multivariate analysis of prognostic factors influencing survival. Eur J Surg Oncol. 2000;26:686–690. doi: 10.1053/ejso.2000.0981. [DOI] [PubMed] [Google Scholar]
- 17.Dottorini ME, Assi A, Sironi M, Sangalli G, Spreafico G, Colombo L. Multivariate analysis of patients with medullary thyroid carcinoma. Prognostic significance and impact on treatment of clinical and pathologic variables. Cancer. 1996;77:1556–1565. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1556::AID-CNCR20>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 18.Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88:1139–1148. doi: 10.1002/(SICI)1097-0142(20000301)88:5<1139::AID-CNCR26>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y, Miyauchi A, Yabuta T, Fukushima M, Inoue H, Tomoda C, Uruno T, Kihara M, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F. Alternative surgical strategies and favorable outcomes in patients with medullary thyroid carcinoma in Japan: experience of a single institution. World J Surg. 2009;33:58–66. doi: 10.1007/s00268-008-9795-2. [DOI] [PubMed] [Google Scholar]
- 20.de Groot JW, Plukker JT, Wolffenbuttel BH, Wiggers T, Sluiter WJ, Links TP. Determinants of life expectancy in medullary thyroid cancer: age does not matter. Clin Endocrinol (Oxf) 2006;65:729–736. doi: 10.1111/j.1365-2265.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- 21.O’Riordain DS, O’Brien T, Weaver AL, Gharib H, Hay ID, Grant CS, van Heerden JA. Medullary thyroid carcinoma in multiple endocrine neoplasia types 2A and 2B. Surgery. 1994;116:1017–1023. [PubMed] [Google Scholar]