Abstract
Of all the aquatic organisms, algae are a good source of biomolecules. Since algae contain pigments, proteins, carbohydrates, fats, nucleic acids and secondary metabolites such as alkaloids, some aromatic compounds, macrolides, peptides and terpenes, they act as reducing agents to produce nanoparticles from metal salts without producing any toxic by-product. Once the algal biomolecules are identified, the nanoparticles of desired shape or size may be fabricated. The metal and metal oxide nanoparticles thus synthesized have been investigated for their antimicrobial activity against several gram-positive and gram-negative bacterial strains and fungi. Their dimension is controlled by temperature, incubation time, pH and concentration of the solution. In this review, we have attempted to update the procedure of nanoparticle synthesis from algae, their characterization by UV-vis, Fourier transform infrared spectroscopy, transmission electron microscopy, scanning electron microscopy, x-ray diffraction, energy-dispersive x-ray spectroscopy, dynamic light scattering and application in cutting-edge areas.
Keywords: Metal and Metal Oxide Nanoparticles, Controlling Factors, Biosynthesis, Characterization and Mechanism, Antimicrobial Activity
Review
Introduction
The nanoparticles are the most fundamental component in the fabrication of a nanostructure. Several synthetic routes are used for the fabrication of nanoparticles of diverse morphology and size. Although these procedures have offered superior quality of nanoparticles, better fabrication procedures are yet to be developed. Currently, scientists have focused their attention on the biosynthesis of nanoparticles involving plant, algae, bacteria, fungi and virus containing proteins, amines, aminoacids, phenols, sugars, ketones and aldehydes which act as reducing agents, capping agents and stabilizers for nanoparticles [1–8].
The use of algae for biogenic synthesis of nanoparticles has become prevalent during these days due to their easy access and efficacy [9–11]. The biomolecules present in the algal extract have relatively been less exploited for nanoparticle synthesis than similar other natural sources such as plants and bacteria [12, 13]. Available functional groups and enzymes in the algal cell walls act as reducing agents, as a consequence of which reduction and fabrication of metal and metal oxide nanoparticles occur at ambient conditions [14, 15]. In recent days, several diverse and potential applications of nanoparticles in crop protection and production, cosmetics, drug delivery, photonic crystals, analysis, food, coatings, paints, bioremediation, catalysis and material science have been applied [4, 7, 16, 17] (Fig. 1). However, the mechanism of interaction of nanoparticles with biological systems at the molecular level is not clearly understood [17–19]. It is essential to understand the intricacies of the various steps involved in the fabrication of nanoparticles from algae, their antimicrobial activity and impact on the environment. Among the transition metal nanoparticles, gold and silver have received more attention than others owing to their application in drug delivery, tumor imaging, identification of pathogens and determination of heavy metals [20–22].
Fig. 1.
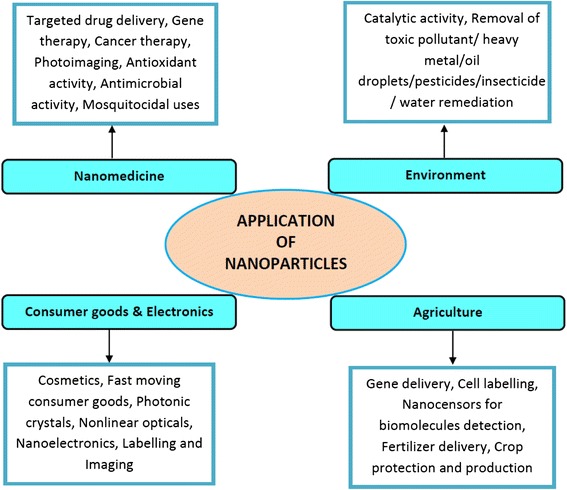
Application of fabricated nanoparticles in cutting-edge areas
Algae are a rich source of biomolecules and frequently used for the extracellular synthesis of nanoparticles [10, 23–25].
Metal nanoparticles have gained extensive attention due to their efficient antimicrobial activities because they can be safely used in human system to inhibit the growth of pathogens without damaging the normal tissues. The mechanism of antimicrobial activity of nanoparticles has been ascribed to the generation of free radical and subsequent damage of the microbial cell wall leading to their death [26]. Besides, the nanoparticles poison the enzyme of single cell pathogens such as bacteria, fungi and viruses for oxygen intake without harming the human enzymes [27]. Algae may produce nanoparticles from any metal salt by extracellular or intracellular pathways involving biochemicals or enzymes present in them. However, enzymes and reducing substances are known to be the main constituents of microorganisms and fungi for the production of metal nanoparticles from metal salts [7, 28–30].
Synthesis of metal and metal oxide nanoparticles of well-defined shape and size depends on the concentration of algal extract/biomass, metal salt, pH of the reaction mixture, temperature and incubation time. They can be characterized by UV-vis, Fourier transform infrared (FTIR), transmission electron microscopy (TEM), scanning electron microscopy (SEM), x-ray diffraction (XRD), energy-dispersive x-ray spectroscopy (EDX) and dynamic light scattering (DLS) (Fig. 2). Biogenic fabrication of metal and metal oxide nanoparticles using various algal species such as Bifurcaria bifurcate, Chlamydomonas reinhardtii, Chlorella vulgaris, Ecklonia cava, Fucus vesiculosus, Oscillatoria willei, Pithophora oedogonia, Sargassum muticum, Sargassum wightii, Spirulina platensis, Stoechospermum marginatum etc. are presented in Table 1. Both freshwater and marine algae have given impetus to the development of industry and technology alike as they prevent pollution in the atmosphere. However, it is quite obvious that nanoparticles may have a positive or negative impact in the living system depending on their shape, size and above all the nature of specific metal ion.
Fig. 2.
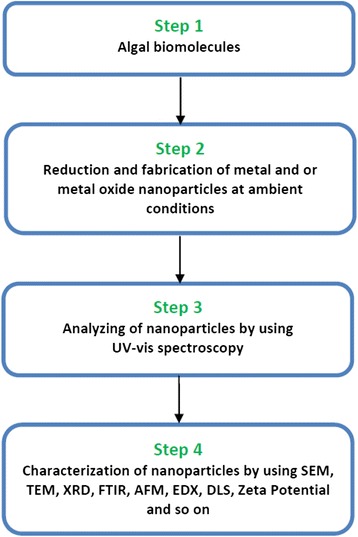
Fabrication/synthesis of nanoparticles from algal molecules and its characterization
Table 1.
Engineered nanoparticles of varying size and shape fabricated from various algal species
| Nanoparticles | Algal Species | Size (nm) | Shape | References |
|---|---|---|---|---|
| Gold | Sargassum wightii | 8–12 | – | Singaravelu et al. [10] |
| Sargassum muticum | 5.42 ± 1.18 | Spherical | Namvar et al. [31] | |
| Spirulina platensis | 6–10 | – | Govindaraju et al. [32] | |
| Spirulina platensis | ~5 | – | Uma Suganya et al. [33] | |
| Stoechospermum marginatum | 18.7–93.7 | Spherical and Hexagonal | Rajathi et al. [34] | |
| Navicula atomus | 9 | – | Schröfel et al. [35] | |
| Cladosiphon okamuranus | 8.54–10.74 | – | Lirdprapamongkol et al. [36] | |
| Tetraselmis kochinensis | 5–35 | Spherical and Triangular | Senapati et al. [37] | |
| Ecklonia cava | 30 ± 0.25 | Spherical and Triangular | Venkatesan et al. [38] | |
| Chlorella vulgaris | 2–10 | Spatial array of Self Assembled Structures | Annamalai and Nallamuthu [39] | |
| Padina gymnospora | 53–67 | Spherical | Singh et al. [40] | |
| Fucus vesiculosus | Varied | Spherical | Mata et al. [41] | |
| Turbinaria conoides | 2–19 | Triangular | Vijayan et al. [42] | |
| Silver | Spirulina platensis | 7–16 | – | Govindaraju et al. [32] |
| Oscillatoria willei | 100–200 | – | Mubarak Ali et al. [43] | |
| Caulerpa racemosa | 5–25 | Spherical and Triangular | Kathiraven et al. [44] | |
| Cystophora moniliformis | 50–100 | Spherical | Prasad et al. [45] | |
| Chlamydomonas reinhardtii | 5–35 | Round and Rectangular | Barwal et al. [46] | |
| Turbinaria conoides | 2–17 | Spherical | Vijayan et al. [42] | |
| Pithophora oedogonia | 25–44 | Cubical and Hexagonal | Sinha et al. [47] | |
| Caulerpa racemosa | 5–25 | – | Kathiraven et al. [44] | |
| Copper Oxide | Bifurcaria bifurcata | 5–45 | Spherical | Abboud et al. [48] |
| Zinc Oxide | Sargassum muticum | 30–57 | Hexagonal | Azizi et al. [49] |
| Iron Oxide | Sargassum muticum | 18 ± 4 | Cubic | Mahdavi et al. [50] |
In this review article, we have discussed the recent advances in nanoparticle fabrication techniques from algae, their characterization by UV-vis, FTIR spectroscopy, TEM, SEM, XRD, AFM, EDX, DLS and application as antimicrobial agents.
Metal Nanoparticles
Silver nanoparticles have been synthesized from Cystophora moniliformis algal extract in aqueous medium at 65 °C [45]. It has been noted that with an increase in temperature, the size of the nanoparticles increases which may be confirmed from their UV-vis spectra (Fig. 3a). The surface plasmon resonance (SPR) peak slowly sharpens with temperature and becomes stable between 65 and 75 °C. It has been suggested that the peak between 450 and 452 nm corresponds to polydispersed spherical silver nanoparticles. Although the SPR peak shifts towards longer wavelength with increasing temperature, the formation of nanoparticles becomes faster but their aggregation occurs between 85 and 95 °C. The size of silver nanoparticles varies between 50 and 100 nm (Fig. 3b).
Fig. 3.
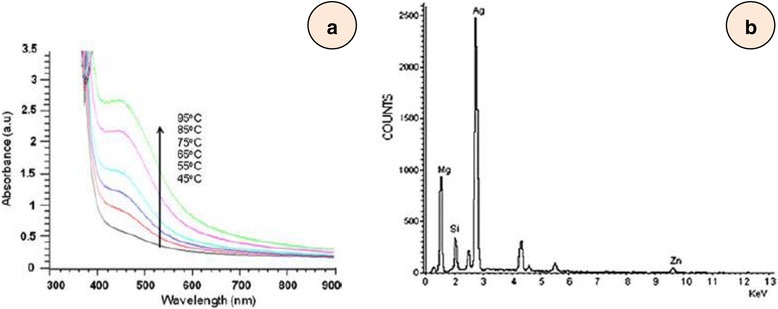
a UV absorption spectra and (b) EDAX analysis of silver nanoparticles synthesized using Cystophora moniliformis [45]
Biosynthesis of silver nanoparticles from polysaccharides extracted from four marine algae namely, Pterocladia capillacae, Jania rubins, Ulva faciata and Colpmenia sinusa has been reported [1]. They were found to be spherical with 7–20 nm diameter. Their antibacterial activity has been ascribed to their attachment to bacterial cell wall inhibiting their vital functions.
Fabrication of silver nanoparticles from Sargassum longifolium alga and their microbial activity against several pathogens have been reported [51]. The yellow reaction mixture comprising of AgNO3 and aqueous algal extract turned brown after 1 h. However, the reaction was completed after 32 h, the intensity of which is time dependent. The absorption peak at 440 nm indicated the formation of polydispersed silver nanoparticles. It has been reported that the pH of the reaction mixture exhibited a significant role in the silver nanoparticle synthesis. The colour change of the reaction mixture was slower at low pH 6.2 than that at high pH 8.4. The colour intensity of the reduction process was increased with the increase of the pH. The antifungal activity against Aspergillus fumigatus, Candida albicans and Fusarium sp. was found to increase with increasing concentration of silver nanoparticles [51].
Biosynthesis and antibacterial activity of silver nanoparticles of 25–44 nm diameter using fresh water green alga, Pithophora oedogonia, has been reported. IR spectrum and quantitative analysis of the extract showed the presence of carbohydrates, saponins, steroids and proteins which reduce AgNO3 to silver nanoparticles. They were found to be more effective against gram negative bacteria than gram positive ones [47].
Kathiraven et al. [44] have also reported the biosynthesis of silver nanoparticles from marine alga, Caulerpa racemosa and their antibacterial activity against human pathogens. They were (silver nanoparticles of 5–25 nm) crystalline with face-centred cubic geometry and effective against Staphylococcus aureus and Proteus mirabilis bacteria at a very low concentration (5–15 μL). Silver nanoparticles were synthesized from 14 bacteria and microalgae. It was observed that the nanoparticles were produced by extracellular polysaccharides even in the dark. Spherical, elongated and irregular silver nanoparticles of different dimensions and morphology were obtained which vary from one species to another [5]. The antibacterial activity was tested against six pathogenic bacteria. The mechanism involves free radical formation which causes damage to the cellular membrane.
Small gold nanoparticles of uniform shape with an average size of ~5 nm were obtained from blue green alga, Spirulina platensis [33]. The protein extract of alga and HAuCl4 in a 1:1 ratio in the presence of NaOH was incubated at room temperature for 48 h. Colour change from green to greyish yellow and eventually to ruby red showed the formation of gold nanoparticles [52]. Three distinct peaks at 685, 524 and 385 nm were observed along with an excitation maximum at 620 nm. The peaks at 685 and 629 nm assigned to HOMO and LUMO charge transfer transitions [53] are the frequencies for secondary amines, OH and COO− groups which would have stabilized the gold nanoparticles. Their antibacterial activity against Bacillus subtilis was examined. The results indicated that nanoparticles caused damage to cells by producing pits in the outer cell wall which disrupt the normal functioning of the bacteria [34]. Since the gold nanoparticles are smaller than the thickness of bacterial cell wall they can easily penetrate into the cell and inhibit their growth.
Extracellular biosynthesis of gold nanoparticles using marine alga Sargassum wightii of 8–12 nm has been reported [10]. The reaction was completed in 15 h with a visibly distinct ruby colour with an absorption maximum at 527 nm. The TEM images showed monodispersed gold nanoparticles, where they are predominantly sphere of 11 nm.
Parial et al. [54] have reported the fabrication of gold nanoparticles from three cynobacteria (Phormidium valderianum, Phormidium tenue, Microcoleus chthonoplastes) and four green algae (Rhizoclonium fontinale, Ulva intestinalis, Chara zeylanica, Pithophora oedogoniana) at different pH at 20 °C. Generally, the gold nanoparticles were spherical at neutral pH and at pH 9 along with hexagonal and triangular ones. At pH 7 and 9, they exhibited a single absorption between 520 and 534 nm, while at about pH 5, two absorption bands at 520 and one ~600–670 were observed. The peaks vary with pH, concentration of the solution and the nature of cynobacteria and algae. These factors also affect the shape and size of the gold nanoparticles. At pH 5, the small spherical particles (15 nm) together with nano rods (411 × 32 nm) with some larger ones (17 nm) are produced. It is, however, noted that all gold nanoparticles are monodispersed with some aggregation.
Dahoumane and co-workers [8] have synthesized gold nanoparticles from living cells of Euglena gracilis microalga. The biomaterial in the alga act as reducing agent, capping agent and catalyst similar to other marine algae [55]. The pH, reaction time, temperature and concentration are controlling factors for the nanoparticles yield. It has been proposed that gold nanoparticle formation and release occur in three steps: (1) uptake of Au+3, (2) reduction of Au+3 to Au0 and (3) release of gold nanoparticles into the solvent. They are well dispersed and do not aggregate. They are spherical in shape whose dimensions vary from 10 nm to several 100 nm. AuCl3 concentration of 10−3 M is lethal to E. gracilis which suggests that all algae have a tolerance limit and certain capacity to reduce metal ions to protect themselves from the toxic influence of Au3+/Au0.
Biogenic fabrication of gold nanoparticles by brown alga, Stoechospermum marginatum biomass, has been reported [34]. The brown colour of extract turned ruby red within 10 min of addition of HAuCl4 exhibiting an absorption at 550 nm in UV-vis spectrum due to SPR [10]. The TEM image revealed that majority of the polydispersed nanoparticles were spherical, hexagonal and triangular with size ranging between 18.7 and 93.7 nm. However, SEM images showed the formation of gold nanoparticles of 40–85 nm. Since the algal extract is known to contain terpenoids and phenols, they reduce the gold ions to gold nanoparticles which are reflected from a change in colour. X-ray diffraction pattern showed face-centred cubic gold structure [56]. Their antimicrobial activity was nearly half of the tetracycline (Table 2) but it is higher than tetracycline against Enterobacter faecalis.
Table 2.
Antibacterial activity of gold nanoparticles (modified, [34])
| Bacterial Pathogens | Gold Nanoparticles | Positive Control (tetracycline) | Negative Control (chloroauric acid) |
|---|---|---|---|
| Pseudomonas aeruginosa | 8 | 13 | 0 |
| Klebsiella oxytoca | 7 | 14 | 0 |
| Enterobacter faecalis | 11 | 9 | 0 |
| Klebsiella pneumoniae | 6 | 12 | 0 |
| Vibrio cholerae | 8 | 15 | 0 |
| Escherichia coli | 0 | 12 | 0 |
| Salmonella typhii | 6 | 13 | 0 |
| Salmonella paratyphi | 8 | 13 | 0 |
| Vibrio parahaemolyticus | 9 | 17 | 0 |
| Proteus vulgaris | 8 | 14 | 0 |
Vijayan et al. [42] have reported the fabrication of gold and silver nanoparticles from a seaweed called Turbinaria conoides. They have been thoroughly characterized, and their antimicrofouling activity has also been evaluated. There are certain microbes which attach themselves to a solid support by producing extracellular polymeric materials in the form of a thin biofilm to which many other fouling agents are attached. In the case of ships, such thin films progressively become thick, increase the weight of the ship, corrode the metal and produce a foul smell. FTIR spectra (Fig. 4) showed peaks corresponding to OH, C=O and C-OH functional groups, but the exact compound containing these groups have not been identified. However, alcohol or ketone may act as a reducing agent but the authors have wrongly taken OH as a hydroxyl group and later identified as an alcoholic group. Likewise, they took the ketonic group C=O as a carboxylic group and suggested them as reductant. Their assignment of the functional groups is based on wrong assumption and is therefore highly dubious. Silver nanoparticles were found to be effective in controlling the bacterial biofilm formation, whereas gold nanoparticles were completely ineffective. Since silver nanoparticles are toxic to many microbes, they can be used to inhibit their growth in vitro and in vivo irrespective of their size, but nontarget organisms may also be affected.
Fig. 4.
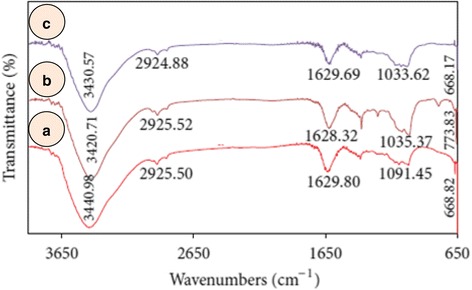
FTIR spectra of (a) Turbinaria conoides extract (b) silver and (c) gold nanoparticles [42]
Nanobiotechnology is extremely useful in exploiting potential of algae and microbes to convert small quantity of metal from huge deposits of ores. Gold and silver nanoparticles were synthesized from red Chondrus crispus and Spyrogira insignis algae [57]. The structure and size were found to be dependent on pH of the solution between 2 and 10. The yield of gold nanoparticles was 70 % at pH 2 but it decreased with increasing pH and, at pH 10, the yield was nearly 60 % only. TEM images revealed that gold nanoparticles produced in acidic medium were polygonal, triangular and hexagonal (Fig. 5). An increase in pH from 2 to 4 showed decrease in size of gold nanoparticles (~30 nm). Formation of spherical nanoparticles was detected from a change in UV-vis absorption spectra which correspond to different shapes. Thus, polygonal nanoparticles or nanosphere may be produced simply by changing the pH of the reaction mixture. However, the UV-vis spectra slightly change due to the colour of the algae too. Kuyucak and Volesk [58] have suggested the following reaction to occur for the reduction of gold ions to gold nanoparticles.
Fig. 5.

TEM images of gold nanostructures synthesized using Chondrus crispus at different initial pH values. a Detail of hexagonal nanoparticles obtained at pH 2. b Detail of a nanotriangle obtained at pH 2, c pH 4 and d pH 10 [57]
This equation is not balanced because it does not account for 3H+ with 4Cl−. It should be written as follows:
Alternatively, it can be written in the following form:
Metal Oxide Nanoparticles
Biosynthesis of zinc oxide nanoparticle from aqueous extract of brown marine macroalga, Sargassum muticum has been reported [49]. The colour of the reaction mixture containing ZnO and algal extract changed from dark brown to a pale white colour indicating the synthesis of zinc oxide nanoparticle. Surface and hydroxyl moieties of polysaccharide present in the extract are involved in the formation of zinc oxide nanoparticles of 30–57 nm. They were agglomerated with hexagonal structure. Authors have concluded that the synthesized zinc oxide nanoparticles prepared from S. muticum is expected to have notable applications in pharmaceutical and biomedical fields and in cosmetic industries.
Abboud et al. [48] have reported the synthesis of copper oxide nanoparticles of 5–45 nm dimension from B. bifurcate algal extract. They were shown to be a mixture of Cu(I) and Cu(II) oxides and were crystalline in nature. Transition metal oxide nanoparticles are an important class of semiconductors and because of incompletely filled d orbitals, they find application in magnetic storage media, energy transformation, electronic and catalysis [59–61]. The formation of copper oxide nanoparticles was confirmed by a change in colour when 1 mM solution of CuSO4 was added to B. bifurcate extract at ambient temperature. Their UV-vis spectra showed distinct change in the absorption peaks owing to the presence of diterpenoids in the extract followed by the formation of cuprous oxide and cupric oxide nanoparticles [62, 63]. The CuSO4 undergoes partial reduction to Cu(I) and Cu(II) oxides which is reflected from the blood red colour exhibiting absorption at 260 and 650 nm. The TEM image showed that majority of the nanoparticles are spherical, although some elongated ones were also observed. Since the nanoparticles are a mixture of cupric oxide and cuprous oxide the XRD pattern showed the presence of two crystalline phases, monoclinic copper(I) oxide and copper(II) oxide with cuprite structure. The antibacterial activity of algal extract and copper oxide nanoparticles was tested against Enterobacter aerogenes and Staphylococcus aureus. It was observed that the algal extract alone was ineffective while copper oxide nanoparticles were significantly active against two bacterial strains.
Iron oxide nanoparticles were synthesized from FeCl3 with an aqueous extract of brown alga Sargassum muticum at 25 °C. The polysaccharides present in the algal extract reduce the FeCl3 to Fe3O4 nanoparticles of 18 ± 4 nm size which are mainly cubic in shape [50].
Very few reports are available on the toxic effects of several metal nanoparticles on marine organisms including algae, bacteria and protozoa in order to have a data bank for risk assessment [64]. Aruoja et al. [65] have synthesized Al2O3, Co3O4, CuO, Fe3O4, MgO, Mn3O4, Sb2O3, SiO2, ZnO, TiO2, WO3 and Pd crystalline nanoparticles. They are 8–21 nm in size. Some of these oxide nanoparticles are acidic, some are basic and others are amphoteric in nature. They give stable suspension in water. Their toxicity has also been investigated against one alga (Pseudokirchneriella subcapitata), three bacteria (Vibrio fischeri, Escherichia coli, Staphylococcus aureus) and one protozoa (Tetrahymena thermophila).
Certain metal containing nanoparticles (Ag, CuO, ZnO) release metal ions and cause toxicity to bacterial cells [64, 66, 67]. Smaller nanoparticles, however, have been shown to exhibit greater toxicity, perhaps due to their penetration into the bacterial cells [68]. Of all the nanoparticles tested for toxicity, CuO was found to be most effective (Fig. 6) against S. aureus and E. coli. The other metal oxide nanoparticles inhibited the growth of these bacteria only at 100 mg L−1 level (Table 3). ZnO and CuO are toxic to T. thermophila at 6 mg L−1 while all other nanoparticles are toxic above 100 mg L−1 level which may not be found in the natural environment except in mining areas only. Since protozoa are small particle feeding organisms, they can be used to remove unwanted particles from waste water. T. thermophila feed on bacteria and metal oxide nanoparticles without making any distinction between the two. They get accumulated in the vacuoles of protozoa [69]. Single-wall carbon nanotubes at a concentration between 3.6 and 6.8 mg L−1 are ingested by T. thermophila after their exposure for 24 h. However, these nanoparticles are toxic above 100 mg L−1. P. subcapitata algal growth inhibition occurs by ZnO and CuO at very low level (0.1 and 0.43 mg L−1). The MgO and SiO2 are least toxic possibly because they are already present in sea water and the algae are accustomed to their presence in level below 100 mg L−1. Toxicity to algae is mainly due to its cells entrapped/enveloped by metal oxide nanoparticles and ROS generation [70].
Fig. 6.
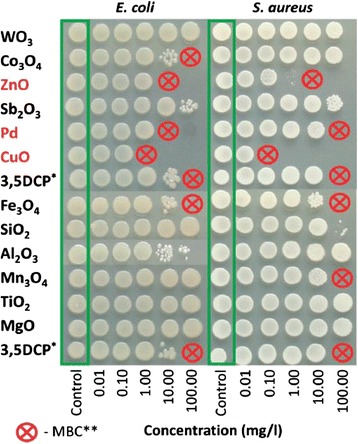
Toxicity of 12 nanoparticles to bacteria Escherichia coli and Staphylococcus aureus. Toxicity was evaluated by determining the colony-forming ability of the bacteria after exposure to nanoparticles in deionized water for 24 h at 25 °C. After exposure, 5 μl of bacterial suspension was transferred onto toxicant-free agarized LB growth medium. The concentrations of the NPs are in mg compound l−1. All concentrations are nominal. *3,5 Dichlorophenol was used as a positive control, **Minimal biocidal concentration [65]
Table 3.
Categorization of nanoparticles based on the toxicity values (EC50 or MBC, mg compound l−1) to bacteria, protozoa and algae. All nanoparticles were tested in nominal concentrations from 0.01 up to 100 mg l−1 [65]
| EC50 or MBC, mg compound l−1 | 72 h EC50 | 24 h EC50 | 30 min EC50 | 24 h MBC | 24 h MBC |
|---|---|---|---|---|---|
| Organisms | Algae | Protozoa | Bacteria | Bacteria | Bacteria |
| Species | Pseudokirchneriella subcapitata | Tetrahymena thermophila | Vibrio fischeri (G−) | Escherichia coli (G−) | Staphylococcus aureus (G+) |
| Exposure Medium | Mineral Medium | DI Water | 2 % NaCl | DI Water | DI Water |
| 0.1–1 | CuO, ZnO, Pd | None | None | CuO | CuO |
| >1–10 | Co3O4, Fe3O4, Mn3O4, TiO2 | CuO, ZnO | CuO | ZnO, Pd | ZnO |
| >10–100 | Al2O3, SiO2, WO3 | Fe3O4, TiO2 | ZnO, Pd, WO3, Sb2O3 | Co3O4, Fe3O4 | Fe3O4, Mn3O4, Pd |
| >100 | MgO, Sb2O3 | Al2O3, Co3O4, MgO, Mn3O4, Pd, Sb2O3, SiO2, WO3 | Al2O3, Co3O4, Fe3O4, MgO, Mn3O4, SiO2, TiO2 | Al2O3, MgO, Mn3O4, Sb2O3, SiO2, TiO2, WO3 | Al2O3, Co3O4, MgO, Sb2O3, SiO2, TiO2, WO |
EC 50 half effective concentration, MBC minimal biocidal concentration, i.e., the lowest tested nominal concentration of nanoparticles which completely inhibited the formation of visible colonies after sub-culturing on toxicant-free agarised growth medium. Prior sub-culturing bacteria were incubated with nanoparticles for 24 h at 25 °C in deionized water
The pH of the suspension containing ZnO and the algae does decrease from 8 to 4, but virtually there is no variation in toxicity as a function of pH [71]. Hartmann et al. [72] have studied the toxicity of TiO2 nanoparticles of 10, 30 and 300 nm against Pseudokirchneriella subcapitata alga. All the three types of particles exhibited algal growth inhibition. The ecotoxicity of Cd to alga, P. subcapitata, in presence of 2 mg L−1 of TiO2 was reduced probably due to non availability of Cd in presence of TiO2 nanoparticles. The toxicity was also found to be dependent on the nanoparticles and their concentration.
Ji and co-workers [73] have studied the toxicity of Al2O3, SiO2, ZnO and TiO2 nanoparticles towards green alga, Chlorella sp. Al2O3, SiO2 and TiO2 (DJ3, rutile) did not show significant toxicity although ZnO and TiO2 (HR3, anatase) inhibited the algal growth in 20 and 30 mg L−1 nanoparticles in aqueous solution.
The ecotoxic effects of oxide nanoparticles are dependent on their size and type. Even at very high concentration (1000 mg L−1), the algal growth did not show any variation from the second day to the sixth day of exposure. Nano Al2O3 showed growth promotion at the fourth day by about 19 %. Lin and Xing [74] have found nano Al2O3 as nontoxic to five plant species. However, at higher concentration of 2000 mg L−1 of Al2O3, root growth is inhibited [75]. Such experimental results may not be applied in the field because such a high concentration is seldom achieved in aquatic system as the algae etc. will dry up due to large accumulation of nanoparticles and other toxic materials.
The toxic effect of the nanoparticle and their bulk material are not the same. For instance, the Chlorella sp. toxicities for different form of Zn follow the order: Zn2+ > nano ZnO > bulk ZnO even when their concentrations are below 50 mg L−1. At higher concentration (>50 mg L−1), the toxicity of ZnO nanoparticles has been shown to be higher than Zn2+. The toxicity also depends on particle size, crystal structure, rutile and anatase. Anatase TiO2 is more toxic than rutile TiO2. Ji et al. [73] have suggested that anatase TiO2 release larger quantity of ROS than rutile TiO2 resulting in an increase toxicity [76]. However, the toxicity of anatase TiO2 nanoparticles decreases if their size increases above 33 nm [77]. Nano TiO2 and nano ZnO can produce photocatalytic ROS in presence of UV light [78], but experimental evidences demonstrated larger production of ROS even in the dark [79]. It is therefore concluded that there are other possible reasons for the toxicity of nanoparticles besides the ROS production.
Conclusions
Algae are considered as significant nanofactories and hold a huge potential as ecofriendly and cost-effective tools, avoiding toxic, harsh chemicals and the high energy demand required for physiochemical fabrication. In the present review, we have discussed the biosynthesis of metal and metal oxide nanoparticles from a variety of algae and their toxicity against several pathogenic gram-positive and gram-negative bacterial strains. The proteins, polysaccharides, amines, amino acids, alcohols, pigments, carboxylic acids carbohydrates and sugars have been shown to act as reducing agents. Also, they act as capping and stabilizing agents for the fabricated nanoparticles. The results suggest that the functionalized metal nanoparticles may be exploited in the treatment of infectious diseases caused by bacteria and fungi. They can also be used in phytomining and sequestering metals from waste disposals by redox process.
Acknowledgements
The authors are thankful to publishers for permission to adopt figures in this review.
Authors’ Contributions
AH gathered the research data. AH and KSS analyzed these data findings and wrote this review paper. Both authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.El-Rafie HM, El-Rafie MH, Zahran MK. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbo Poly. 2013;96:403–410. doi: 10.1016/j.carbpol.2013.03.071. [DOI] [PubMed] [Google Scholar]
- 2.Husen A, Siddiqi KS (2014b) Phytosynthesis of nanoparticles: concept, controversy and application. Nano Res Lett 9:229 [DOI] [PMC free article] [PubMed]
- 3.Husen A, Siddiqi KS (2014c) Plants and microbes assisted selenium nanoparticles: characterization and application. J Nanobiotechnol 12:28 [DOI] [PMC free article] [PubMed]
- 4.Khan M, Al–Marri AH, Khan M, Shaik MR, Mohri N, Adil SF, Kuniyil M, Alkhathlan HZ, Al–Warthan A, Tremel W, Tahir MN, Siddiqui MRH. Green approach for the effective reduction of graphene oxide using Salvadora persica L. root (Miswak) extract. Nano Res Lett. 2015;10:281. doi: 10.1186/s11671-015-0987-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel V, Berthold D, Puranik P, Gantar M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol Rep. 2015;5:112–119. doi: 10.1016/j.btre.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wadhwani SA, Shedbalkar UU, Singh R, Chopade BA. Biogenic selenium nanoparticles: current status and future prospects. Appl Microbiol Biotechnol. 2016;100:2556–2566. doi: 10.1007/s00253-016-7300-7. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqi KS, Husen A. Fabrication of metal nanoparticles from fungi and metal salts: scope and application. Nano Res Lett. 2016;11:98. doi: 10.1186/s11671-016-1311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahoumane SA, Yéprémian C, Djédiat C, Couté A, Fiévet F, Coradin T, Brayner R. Improvement of kinetics, yield, and colloidal stability of biogenic gold nanoparticles using living cells of Euglena gracilis microalga. J Nanopart Res. 2016;18:79. doi: 10.1007/s11051-016-3378-1. [DOI] [Google Scholar]
- 9.Lengke MF, Fleet ME, Southam G. Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold (I)- thiosulfate and gold (III)-chloride complexes. Langmuir. 2006;22:2780–2787. doi: 10.1021/la052652c. [DOI] [PubMed] [Google Scholar]
- 10.Singaravelu G, Arockiamary JS, Kumar VG, Govindaraju K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B Biointerfaces. 2007;57:97–101. doi: 10.1016/j.colsurfb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Ogi T, Saitoh N, Nomura T, Konishi Y. Room temperature synthesis of gold nanoparticles and nanoplates using Shewenella algae cell extract. J Nanopart Res. 2010;12:2531–2539. doi: 10.1007/s11051-009-9822-8. [DOI] [Google Scholar]
- 12.Honary S, Barabadi H, Fathabad EG, Naghibi F. Green synthesis of copper oxide nanoparticles using Penicillium aurantiogriseum, Penicillium citrinum and Penicillium wakasmanii. Digest J Nanomater Biostruct. 2012;7:999–1005. [Google Scholar]
- 13.Rahman A, Ismail A, Jumbianti D, Magdalena S, Sudrajat H. Synthesis of copper oxide nanoparticles by using Phormiium cyanobacterium. Indo J Chem. 2009;9:355–360. [Google Scholar]
- 14.Crookes-Goodson WJ, Slocik JM, Naik RR. Bio-directed synthesis and assembly of nanomaterials. Chem Soc Rev. 2008;37:2403–2412. doi: 10.1039/b702825n. [DOI] [PubMed] [Google Scholar]
- 15.Gade AK, Bonde P, Ingle AP, Marcato PD, Durán N, Rai MK. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mater Bioenergy. 2008;2:243–247. doi: 10.1166/jbmb.2008.401. [DOI] [Google Scholar]
- 16.Lopez-Serrano A, Olivas RM, Landaluze JS, Cámara C. Nanoparticles: a global vision. Characterization, separation, and quantification methods. Potential environmental and health impact. Anal Methods. 2014;6:38–56. doi: 10.1039/C3AY40517F. [DOI] [Google Scholar]
- 17.Husen A, Siddiqi KS (2014a) Carbon and fullerene nanomaterials in plant system. J Nanobiotechnol 12:16 [DOI] [PMC free article] [PubMed]
- 18.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 19.Khot LR, Sankaran S, MariMaja J, Ehsani R, Schuster EW. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot. 2012;35:64–70. doi: 10.1016/j.cropro.2012.01.007. [DOI] [Google Scholar]
- 20.Lin H, Bu Q, Cen X, Zhao YL. Current methods and research progress in nanomaterials risk assessment. Curr Drug Metab. 2012;13:354–63. doi: 10.2174/138920012800166535. [DOI] [PubMed] [Google Scholar]
- 21.Youngjin K, Johnson RC, Hupp JT. Gold nanoparticle-based sensing of “spectroscopically silent” heavy metal ions. Nano Lett. 2001;1:165–167. doi: 10.1021/nl0100116. [DOI] [Google Scholar]
- 22.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Determination of the minimum temperature required for selective photothermal destruction of cancer cells with the use of immunotargeted gold nanoparticles. Photochem Photobiol. 2006;82:412–417. doi: 10.1562/2005-12-14-RA-754. [DOI] [PubMed] [Google Scholar]
- 23.Ghosha P, Hana G, Dea M, Kima CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Del Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Davis TA, Volesky B, Mucci A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003;37:4311–4330. doi: 10.1016/S0043-1354(03)00293-8. [DOI] [PubMed] [Google Scholar]
- 25.Vijayaraghavan K, Mahadevan A, Sathishkumar M, Pavagadhi S, Balasubramanian R. Biosynthesis of Au (0) from Au(III) via biosorption and bioreduction using brown marine alga Turbinaria conoides. Chem Eng J. 2011;167:223–227. doi: 10.1016/j.cej.2010.12.027. [DOI] [Google Scholar]
- 26.Ghodake G, Lee DS. Biological synthesis of gold nanoparticles using the aqueous extract of the brown algae Laminaria japonica. J Nanoelectron Optoelectron. 2011;6:1–4. doi: 10.1166/jno.2011.1166. [DOI] [Google Scholar]
- 27.Kim JS, Kuk E, Yu KN, Kim J, Park SJ, Lee HJ. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Papaspyrides CD, Pavlidou S, Vouyiouka SN. Development of advanced textile materials: natural fibre composites, antimicrobial, and flame-retardant fabrics. J Mat Design Appl. 2009;223:91–102. [Google Scholar]
- 29.Riddin TL, Govender Y, Gericke M, Whiteley CG. Two different hydrogenase enzymes from sulphate-reducing bacteria are responsible for the bioreductive mechanism of platinum into nanoparticles. Enzyme Micro Technol. 2009;45:267–273. doi: 10.1016/j.enzmictec.2009.06.006. [DOI] [Google Scholar]
- 30.Zhang X, Yan S, Tyagi RD, Surampalli RY. Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere. 2011;82:489–494. doi: 10.1016/j.chemosphere.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 31.Namvar F, Azizi S, Ahmad M, Shameli K, Mohamad R, Mahdavi M, Tahir P. Green synthesis and characterization of gold nanoparticles using the marine macroalgae Sargassum muticum. Res Chem Intermed. 2015;41:5723–5730. doi: 10.1007/s11164-014-1696-4. [DOI] [Google Scholar]
- 32.Govindaraju K, Khaleel Basha S, Ganesh Kumar V, Singaravelu G. Silver, gold and bimetallic nanoparticles production using single cell protein (Spirulina platensis) Geitler. J Mater Sci. 2008;43:5115–5122. doi: 10.1007/s10853-008-2745-4. [DOI] [Google Scholar]
- 33.Uma Suganya KS, Govindaraju K, Ganesh Kumar V, Stalin Dhas T, Karthick V, Singaravelu G, Elanchezhiyan M. Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against gram positive organisms. Mat Sci Eng C. 2015;47:351–356. doi: 10.1016/j.msec.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 34.Rajathi FAA, Parthiban C, Ganesh Kumar V, Anantharaman P. Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermum marginatum (kützing) Spectrochim Acta A Mol Biomol Spectrosc. 2012;99:166–173. doi: 10.1016/j.saa.2012.08.081. [DOI] [PubMed] [Google Scholar]
- 35.Schröfel A, Kratošova G, Bohunická M, Dobročka E, Vávra I. Biosynthesis of gold nanoparticles using diatoms–silicagold and EPS-gold bionanocomposite formation. J Nanopart Res. 2011;13:3207–3216. doi: 10.1007/s11051-011-0221-6. [DOI] [Google Scholar]
- 36.Lirdprapamongkol K, Warisnoicharoen W, Soisuwan S, Svasti J. Eco-friendly synthesis of fucoidan-stabilized gold nanoparticles. Am J Appl Sci. 2011;7:1038–1042. doi: 10.3844/ajassp.2010.1038.1042. [DOI] [Google Scholar]
- 37.Senapati S, Syed A, Moeez S, Kumar A, Ahmad A. Intracellular synthesis of gold nanoparticles using alga Tetraselmis kochinensis. Mater Lett. 2012;79:116–118. doi: 10.1016/j.matlet.2012.04.009. [DOI] [Google Scholar]
- 38.Venkatesan J, Manivasagan P, Kim SK, Kirthi AV, Marimuthu S, Rahuman AA. Marine algae-mediated synthesisn of gold nanoparticles using a novel Ecklonia cava. Bioprocess Biosyst Eng. 2014;37:1591–1597. doi: 10.1007/s00449-014-1131-7. [DOI] [PubMed] [Google Scholar]
- 39.Annamalai J, Nallamuthu T. Characterization of biosynthesized gold nanoparticles from aqueous extract of Chlorella vulgaris and their anti-pathogenic properties. Appl Nanosci. 2015;5:603–607. doi: 10.1007/s13204-014-0353-y. [DOI] [Google Scholar]
- 40.Singh M, Kalaivani R, Manikandan S, Sangeetha N, Kumaraguru AK. Facile green synthesis of variable metallic gold nanoparticle using Padina gymnospora, a brown marine macroalga. Appl Nanosci. 2013;3:145–151. doi: 10.1007/s13204-012-0115-7. [DOI] [Google Scholar]
- 41.Mata YN, Torres E, Blazquez ML, Ballester A, Gonzalez F, Munoz JA. Gold(III) biosorption and bioreduction with the brown alga Fucus vesiculosus. J Hazard Mater. 2009;166:612–618. doi: 10.1016/j.jhazmat.2008.11.064. [DOI] [PubMed] [Google Scholar]
- 42.Vijayan SR, Santhiyagu P, Singamuthu M, Ahila NK, Jayaraman R, Ethiraj K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. ScientificWorldJournal. 2014;938272:10. doi: 10.1155/2014/938272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mubarak Ali D, Sasikala M, Gunasekaran M, Thajuddin N. Biosynthesis and characterization of silver nanoparticles using marine cyanobacterium, Oscillatoria willei NTDM01. Dig J Nanomater Biostruct. 2011;6:385–390. [Google Scholar]
- 44.Kathiraven T, Sundaramanickam A, Shanmugam N, Balasubramanian T. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl Nanosci. 2015;5:499–504. doi: 10.1007/s13204-014-0341-2. [DOI] [Google Scholar]
- 45.Prasad TNVKV, Kambala VSR, Naidu R. Phyconanotechnology: synthesis of silver nanoparticles using brown marine algae Cystophora moniliformis and their characterisation. J Appl Phycol. 2013;25:177–182. doi: 10.1007/s10811-012-9851-z. [DOI] [Google Scholar]
- 46.Barwal I, Ranjan P, Kateriya S, Yadav S. Cellular oxidoreductive proteins of Chlamydomonas reinhardtii control the biosynthesis of silver nanoparticles. J Nanobiotechnol. 2011;9:56. doi: 10.1186/1477-3155-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinha SN, Paul D, Halder N, Sengupta D, Patra SK. Green synthesis of silver nanoparticles using fresh water green alga Pithophora oedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Appl Nanosci. 2015;5:703–709. doi: 10.1007/s13204-014-0366-6. [DOI] [Google Scholar]
- 48.Abboud Y, Saffaj T, Chagraoui A, El Bouari A, Brouzi K, Tanane O, Ihssane B. Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata) Appl Nanosci. 2014;4:571–576. doi: 10.1007/s13204-013-0233-x. [DOI] [Google Scholar]
- 49.Azizi S, Ahmad MB, Namvar F, Mohamad R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater Lett. 2014;116:275–277. doi: 10.1016/j.matlet.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahdavi M, Namvar F, Ahmad MB, Mohamad R. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules. 2013;18:5954–5964. doi: 10.3390/molecules18055954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajeshkumar S, Malarkodi C, Paulkumar K, Vanaja M, Gnanajobitha G, Annadurai G. Algae mediated green fabrication of silver nanoparticles and examination of its antifungal activity against clinical pathogens. Int J Met. 2014;2014:692643. [Google Scholar]
- 52.Mulvaney P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir. 1996;12:788–800. doi: 10.1021/la9502711. [DOI] [Google Scholar]
- 53.Leelavathi A, Rao TUB, Pradeep T. Supported quantum clusters of silver as enhanced catalysts for reduction. Nanoscale Res Lett. 2011;6:1–9. doi: 10.1186/1556-276X-6-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parial D, Patra HK, Dasgupta AKR, Pal R. Screening of different algae for green synthesis of gold nanoparticles. Euro J Phyco. 2012;47:22–29. doi: 10.1080/09670262.2011.653406. [DOI] [Google Scholar]
- 55.Liu B, Xie J, Lee JY, Ting YP, Chen JP. Optimization of high-yield biological synthesis of single-crystalline gold nanoplates. J Phys Chem B. 2005;109:15256–15263. doi: 10.1021/jp051449n. [DOI] [PubMed] [Google Scholar]
- 56.Shankar SS, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog. 2003;19:1627–1631. doi: 10.1021/bp034070w. [DOI] [PubMed] [Google Scholar]
- 57.Castro L, Blázquez ML, Muñoz JA, González F, Ballester A (2013) Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol 1–8 doi: 10.1049/iet-nbt.2012.0041 [DOI] [PubMed]
- 58.Kuyucak N, Volesky B. Accumulation of gold by algal biosorbent. Biorecovery. 1989;1:189–204. [Google Scholar]
- 59.Ramgir N, Datta N, Kaur M, Kailasaganapathi S, Debnath AK, Aswal DK, Gupta SK. Metal oxide nanowires for chemiresistive gas sensors: issues, challenges and prospects. Colloids Surf A Physicochem Eng Asp. 2013;439:101–116. doi: 10.1016/j.colsurfa.2013.02.029. [DOI] [Google Scholar]
- 60.Jani AMM, Losic D, Voelcker NH. Nanoporous anodic aluminium oxide: advances in surface engineering and emerging applications. Prog Mater Sci. 2013;58:636–704. doi: 10.1016/j.pmatsci.2013.01.002. [DOI] [Google Scholar]
- 61.Ahmadi SJ, Outokesh M, Hosseinpour M, Mousavand T. A simple granulation technique for preparing high-porosity nano copper oxide(II) catalyst beads. Particuology. 2011;9:480–485. doi: 10.1016/j.partic.2011.02.010. [DOI] [Google Scholar]
- 62.Yin M, Wu CK, Lou Y, Burda C, Koberstein JT, Zhu Y, O’Brien S. Copper oxide nanocrystals. J Am Chem Soc. 2005;127:9506–9511. doi: 10.1021/ja050006u. [DOI] [PubMed] [Google Scholar]
- 63.Borgohain K, Murase N, Mahamuni S. Synthesis and properties of Cu2O quantum particles. J Appl Phys. 2002;92:1292–1297. doi: 10.1063/1.1491020. [DOI] [Google Scholar]
- 64.Ivask A, Juganson K, Bondarenko O, Mortimer M, Aruoja V, Kasemets K, Blinova I, Heinlaan M, Slaveykova V, Kahru A (2014a) Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: a comparative review. Nanotoxicology 8:57–71 [DOI] [PubMed]
- 65.Aruoja V, Pokhrel S, Sihtmäe M, Mortimer M, Mädler L, Kahru A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ Sci Nano. 2015;2:630. doi: 10.1039/C5EN00057B. [DOI] [Google Scholar]
- 66.Heinlaan M, Ivask A, Blinova I, Dubourguier HC, Kahru A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere. 2008;71:1308–1316. doi: 10.1016/j.chemosphere.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 67.Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol. 2013;87:1181–1200. doi: 10.1007/s00204-013-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ivask A, Kurvet I, Kasemets K, Blinova I, Aruoja V, Suppi S, Vija H, Kaekinen A, Titma T, Heinlaan M, Visnapuu M, Koller D, Kisand V, Kahru A (2014b) Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS One 9:e102108 [DOI] [PMC free article] [PubMed]
- 69.Mortimer M, Kahru A, Slaveykova VI. Uptake, localization and clearance of quantum dots in ciliated protozoa Tetrahymena thermophila. Environ Pollut. 2014;190:58–64. doi: 10.1016/j.envpol.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 70.Zhao JY, Riediker M. Detecting the oxidative reactivity of nanoparticles: a new protocol for reducing artifacts. J Nanopart Res. 2014;16:13. doi: 10.1007/s11051-014-2493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aruoja V, Dubourguier HC, Kasemets K, Kahru A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ. 2009;407:1461–1468. doi: 10.1016/j.scitotenv.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 72.Hartmann NB, Kammer FV, Hofmann T, Baalousha M, Ottofuelling S, Baun A. Algal testing of titanium dioxide nanoparticles—testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology. 2010;269:190–197. doi: 10.1016/j.tox.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Ji J, Long Z, Lin D. Toxicity of oxide nanoparticles to the green algae Chlorella sp. Chem Eng J. 2011;170:525–530. doi: 10.1016/j.cej.2010.11.026. [DOI] [Google Scholar]
- 74.Lin DH, Xing BS. Phytotoxicity of nanoparticles: inhibition of seed germination and root elongation. Environ Pollut. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 75.Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett. 2005;158:122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 76.Sayes CM, Wahi R, Kurian PA, Liu YP, West JL, Ausman KD, Warheit DB, Colvin VL. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci. 2006;92:174–185. doi: 10.1093/toxsci/kfj197. [DOI] [PubMed] [Google Scholar]
- 77.Barnard AS. One-to-one comparison of sunscreen efficacy, aesthetics and potential nanotoxicity. Nat Nanotechnol. 2010;5:271–274. doi: 10.1038/nnano.2010.25. [DOI] [PubMed] [Google Scholar]
- 78.Choi O, Hu ZQ. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol. 2008;42:4583–4588. doi: 10.1021/es703238h. [DOI] [PubMed] [Google Scholar]
- 79.Adams LK, Lyon DY, Alvarez PJJ. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006;40:3527–3532. doi: 10.1016/j.watres.2006.08.004. [DOI] [PubMed] [Google Scholar]


