Abstract
A new Cu current collector was prepared by introducing a mussel-inspired polydopamine coating onto a Cu foil surface to improve the electrochemical performance of a Si electrode. The polydopamine coating covalently bonded the polymeric binder (with hydroxyl functional groups) via a condensation reaction. The coating improved the adhesion strength between the Si composite electrode and the Cu current collector (245.5 N m−1, 297.5 N m−1, and 353.2 N m−1 for the Si electrodes based on bare Cu, polydopamine-treated Cu without thermal treatment, and polydopamine-treated Cu with thermal treatment, respectively). We demonstrate that the detachment between the Si composite electrode and the current collector plays an important role in determining the electrochemical performance of the Si electrode. The cycle life and rate capability of the Si electrode improved when the polydopamine surface-treated Cu current collector was used (963.9 mAh g−1, 1361.1 mAh g−1, and 1590.0 mAh g−1 for the Si electrodes based on bare Cu, polydopamine-treated Cu without thermal treatment, and polydopamine-treated Cu with thermal treatment, respectively, at C/2 after 500 cycles).
Lithium-ion batteries (LIBs) are used to power portable electric devices (e.g., cell phones, laptops, and cameras), but their application in electric vehicles (EVs) and energy storage systems (ESSs) requires significant increases in volumetric and gravimetric capacities, longer cycle lives, and lower costs1,2,3. Thus, electrodes that can deliver a larger amount of lithium ions than the current electrodes must be developed because the current electrodes have nearly reached their practical capacity limits4,5,6,7.
Si is a promising candidate for next-generation anode materials because of its extremely high theoretical specific capacity (approximately 4200 mAh g−1), relatively low operating potential, worldwide abundance, and environmentally friendly nature4. However, Si suffers from huge volume changes (approximately 400%) during the Li+ insertion and extraction processes, which causes the pulverization and cracking of the Si particles4,8. These morphological changes continually consume a large amount of Li ions and electrolytes to form a solid electrolyte interphase (SEI) layer on the newly exposed Si surface9,10. Furthermore, these changes break the electrical contact between the Si particles and the conductive additives9,11,12,13. The detached Si particles, which are called dead Si, are the major reason for the capacity loss during cycling11,12,13,14. Consequently, these factors result in a short shelf life for Si-based batteries, mainly because of the fast capacity decay, which impedes the successful implementation of Si-based anodes in LIBs.
The dimensional modification of Si active materials has been a major tactic to improve the cycle life of Si electrodes by reducing the dimensional stress that accompanies the Si volume changes. For example, Si does not cause cracking and pulverization below a critical size15. Consequently, many types of Si active materials have been developed, such as nanowires, nanotubes, nanoparticles, and nanosheets16,17. However, nano-sized materials are unfavorable to use in commercial electrode production processes because they cause low tap densities, handling difficulties, and safety problems18,19. Furthermore, the high surface area of nano-sized materials consumes a larger amount of Li ions to form the SEI layer in the formation step. Although nano/micro-hierarchical structured Si may be a good alternative16,20, this option does not appear appropriate for mass production because of the complexity of the structures and high production costs.
Developing a polymeric binder for Si electrodes is a practical and efficient approach to improve the cycle life of Si electrodes. Numerous alternative polymeric binders, such as poly (acrylic acid) (PAA)11,21,22, carboxymethyl cellulose23,24, alginate25, polyamide-imide, polyimide26, and polymers that form crosslinking networks27,28, have been proposed. These binders significantly improve the cycle life of Si electrodes by maintaining a tighter connection between the Si particles and the conductive additives than polyvinylidene difluoride, which is the conventional polymeric binder.
Detachment between the current collector and the composite electrodes, which consist of active materials, polymeric binders, and conductive additives, is considered another important electrode failure mechanism in conventional LIBs (their active materials (LiNi0.5Mn1.5O429 and graphite30) are based on intercalation chemistry). The adhesion property in Si electrodes, where large volume changes occur, plays a more important role in the electrode performance considering the negligible volume changes of intercalation-based active materials. Despite numerous attempts to develop alternative Cu current collector systems, previous studies mainly focused on the structural modification of conventional current collectors (e.g., surface-roughened current collectors or porous current collectors31,32,33,34) to simply increase the contact area between the composite electrode and the current collectors.
We modified the surface properties of a conventional Cu current collector by using a mussel-inspired polydopamine coating technique to improve the inherent adhesion property between the composite electrode and the current collectors by altering the adhesion mechanism. Polydopamine can be used to modify the surface of a wide range of inorganic and organic materials and even metals35,36,37. This approach is efficient and economical because we use the conventional Cu current collectors, and it is environmentally friendly because water is the major solvent. We investigated the effect of polydopamine-modified Cu current collectors on the cycle performance of Si electrodes and quantitatively analyzed the adhesion property using a surface and interfacial cutting analysis system (SAICAS).
Results
Characterization of the polydopamine film on the Cu current collectors
After the polydopamine coating process, the polydopamine-treated Cu current collectors (PD-treated Cu) was more reddish-brown than the bare Cu current collectors (bare Cu) (Fig. 1a). The surfaces of the Cu current collectors were examined using X-ray photoelectron spectroscopy (XPS) to investigate the existence of polydopamine. As shown in Fig. 1b, the bare Cu provided a sharp O 1s peak, which was mainly attributed to the oxidized Cu and residual contaminants. After the polydopamine treatment, the XPS spectrum showed a new N 1s peak and a small O 1s peak. We infer that the polydopamine film was well formed on the Cu surfaces because polydopamine contains a large number of nitrogen-containing moieties35,36. See Supplementary Information for more detailed information (Figs S1 and S2).
Figure 1. Preparation of the polydopamine-treated Cu current collector.
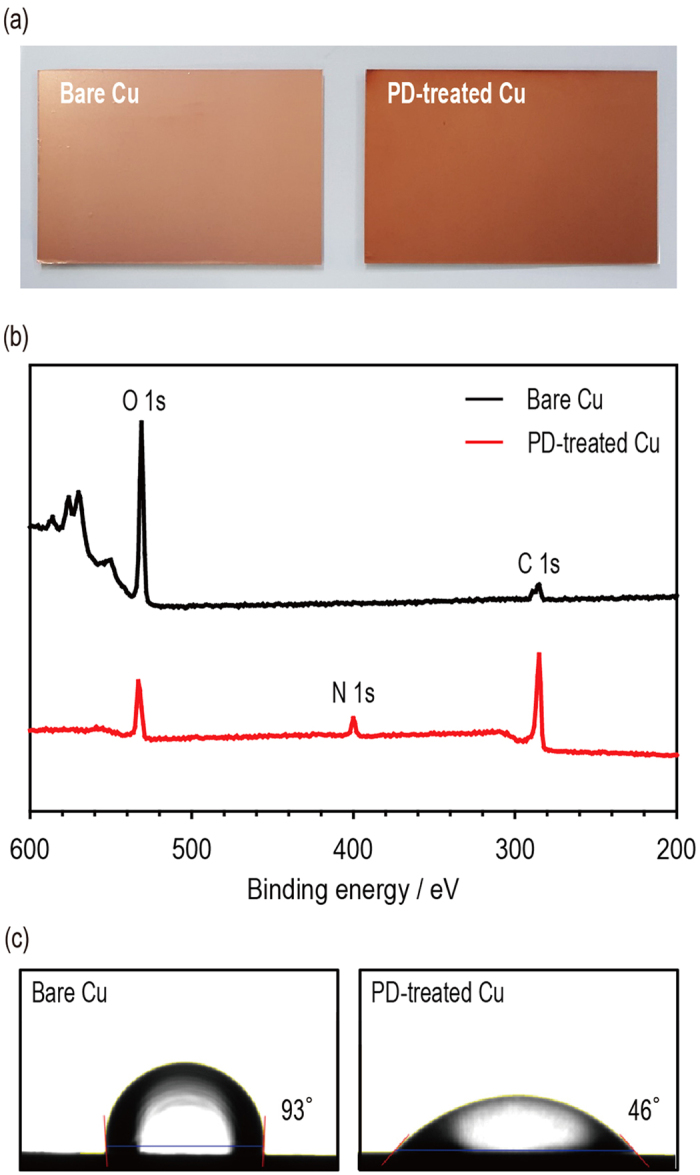
(a) OM images of the bare Cu (left) and the polydopamine-treated Cu (right). (b) XPS spectra of the bare Cu (top) and polydopamine-treated Cu (bottom). (c) Contact angle images of the bare Cu (left) and the polydopamine-treated Cu (right).
In general, polydopamine can alter the surface from hydrophobic to hydrophilic. The surface properties of the bare Cu and PD-treated Cu were investigated by dropping a water droplet onto the surface of each current collector. As shown in Fig. 1c, the PD-treated Cu had a much smaller contact angle (46°) than did bare Cu (93°). Again, we attribute this result to the polar functional groups in polydopamine, which contains nitrogen and oxygen35,36.
Preparation and characterization of the crosslinked PD-treated Cu
The amino groups (N–H) can form covalent amide bonds with hydroxyl groups (O–H) via a condensation reaction38,39. Based on this reaction, we can chemically connect the polydopamine coating layer with a large number of amino groups (N–H) to the PAA polymeric binders, as illustrated in Fig. 2a. The covalent bonds help increase the adhesion strength between the polydopamine coating layer and the Si composite electrode.
Figure 2. Characterization of the crosslinked PD-treated Cu.
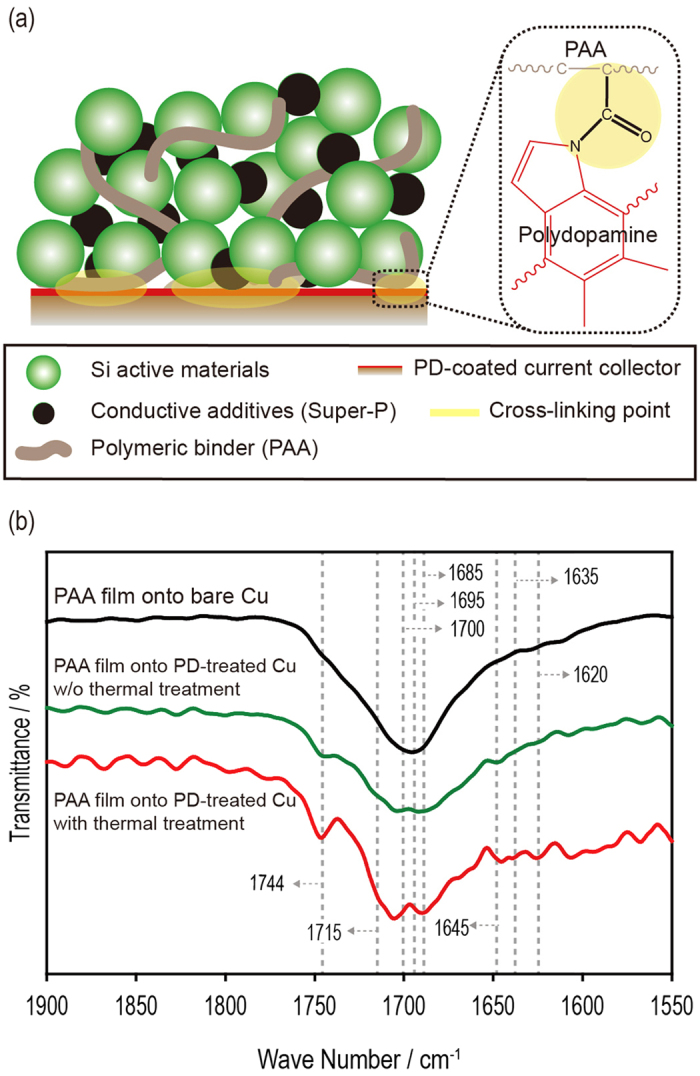
(a) Schematic for the covalent bonds between the PD-treated CU(X) -> Cu and the PAA binder in the Si composite electrode. (b) FTIR spectra of a PAA film coated onto bare Cu, a PAA film coated onto PD-treated Cu without thermal treatment, and a PPA film coated onto PD-treated Cu with thermal treatment (stored in a vacuum oven at 80 °C overnight).
Using FTIR, we investigated the PAA films that were coated onto bare Cu, PD-treated Cu without thermal treatment, and PD-treated Cu with thermal treatment to verify the presence of covalent bonding between the polydopamine coating layer and the PAA polymeric binder. For the thermal treatment, the sample was stored in a vacuum oven at 80 °C overnight. As shown in Fig. 2b, the PAA film on the Cu current collector showed a strong absorption peak at approximately 1695 cm–1, which is related to the carboxylate anion40,41 of the PAA polymeric binder. The PAA film on the PD-treated Cu had an absorption peak at 1645 cm–1, which is related to the N–H deformation vibration of polydopamine42. After the thermal treatment, the spectrum for the PAA film on the PD-treated Cu contained new peaks near 1744, 1715, 1700, 1685, 1635, and 1620 cm−1, which are related to the changes of the C = O groups of the amide38,43,44. These results are attributed to covalent bonding between the polydopamine coating layer and the PAA polymeric binder after the thermal treatment.
Effect of PD-treated Cu current collectors on the electrochemical performance of a Si electrode
We initially prepared Si electrodes using bare Cu (Si–bare Cu) and PD-treated Cu (Si–PD-treated Cu). Furthermore, some of the Si–PD-treated Cu received another thermal treatment (crosslinked Si–PD-treated Cu) by storing the sample in a vacuum oven at 80 °C overnight to form crosslinking networks between the polydopamine coating on the Cu current collectors and the PAA in the Si composite electrode. The effect of the PD-treated Cu on the electrochemical performance of the Si electrodes was investigated using 2032 coin-type half-cells (Si/separator/Li metal). As illustrated in Fig. 3a (see also Fig. S3 in Supplementary Information), the crosslinked Si–PD-treated Cu had the best discharge capacity retention ability (1590.0 mAh g−1; 69% of the initial discharge capacity) among the three Si electrodes after 500 cycles (operated at C/2 (1.2 A g−1)). The discharge capacity retention ability of the Si–PD-treated Cu and the Si–bare Cu electrodes were 1361.1 mAh g−1 (61% of the initial discharge capacity) and 963.9 mAh g−1 (44% of the initial discharge capacity), respectively. Similarly, each unit cell revealed the same coulombic efficiency (CE) trend during cycling performance. As illustrated in Fig. 3b, the crosslinked Si–PD-treated Cu had the highest CE among the three Si electrodes that reached 99.5%. This indicates a relatively stable SEI formation and minimal side reaction of the crosslinked Si–PD-treated Cu. See Fig. S4 in Supplementary Information for the cycle performances of Si electrodes having higher loading amount of 1 mg cm−2.
Figure 3. Electrochemical performance of the Si electrode.
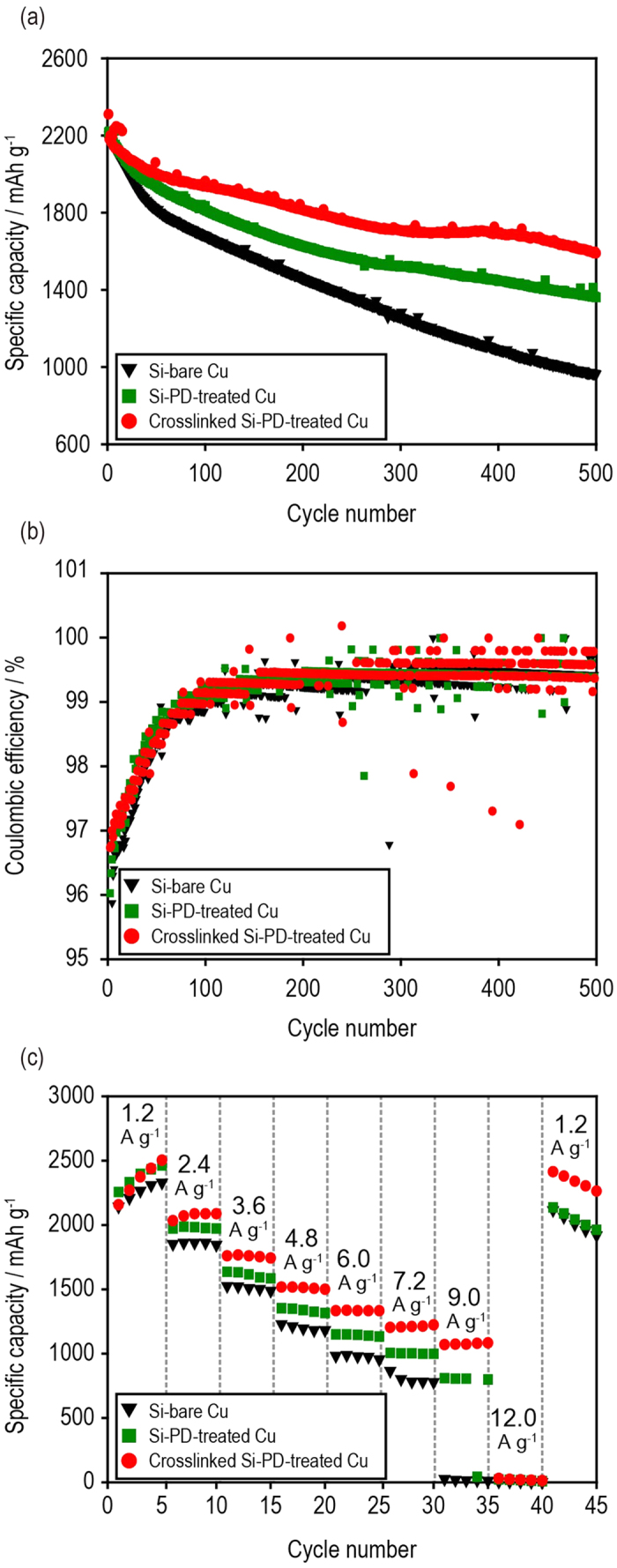
(a) Cycling performances (C/2, 1.2 A g−1), (b) coulombic efficiencies, and (c) the rate capabilities of the unit cells (Si/separator/Li metal, charged and discharged by changing the current density from C/2 (1.2 A g−1) to 5C (12 A g−1)).
The effect of the PD-treated Cu on the rate capability of the Si electrode was also investigated using the identically prepared Si electrodes. The Si electrodes were charged and discharged by changing the current density from C/2 (1.2 A g−1) to 5C (12 A g−1). The experimental rate capability was similar to that obtained during the cycle performance, as shown in Fig. 3a. Again, the crosslinked Si–PD-treated Cu (1083.3 mAh g−1 at 9 A g−1) had a better rate capability than did both the Si–PD-treated Cu (800.0 mAh g−1 at 9 A g−1) and Si–bare Cu electrodes (6.7 mAh g−1 at 9 A g−1) (Fig. 3c).
Morphological changes of the Si electrodes
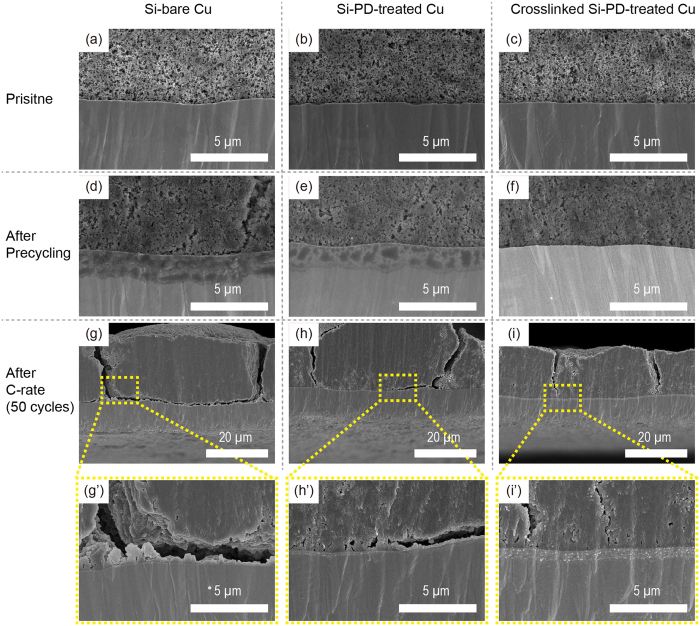
We monitored the cross-sectional view of the Si electrodes using scanning electron microscopy (SEM) to investigate the morphological changes in the Si electrode during cycling. As shown in Fig. 4a–c, all three Si electrodes (Si–bare Cu, Si–PD-treated Cu, and crosslinked Si–PD-treated Cu) had similar morphological features before precycling. However, after precycling, the Si–bare Cu electrode had huge cracks across the Si composite, whereas the others did not show any huge cracks in the electrodes (Fig. 4d–f). The Si particles in all Si electrodes were enlarged after precycling. Several huge cracks were visible for all three Si electrodes after 50 rate-capability test cycles (Fig. 3b). A clear detachment between the Si composite and the Cu current collector appeared for the Si–bare Cu electrode (Fig. 4g,g’). In contrast, the Si–PD-treated Cu provided the Si composite with morphological features that were slightly detached from the PD-treated Cu (Fig. 4h,h’). Furthermore, the crosslinked Si–PD-treated Cu did not show any detachment between the Si composite and the PD-treated Cu (Fig. 4i,i’).
Figure 4. Morphological changes of the Si electrode.
Cross-sectional images of Si-bare Cu, Si–PD-treated Cu, and crosslinked Si–PD-treated Cu (a–c) before cycling, (d–f) after precycling, and (g–i’) after the C-rate test.
Adhesion strength between the Si composite and the Cu current collectors
To investigate the relation between the physical properties of the Si composite and the electrochemical performance, we measured the adhesion strength of the Si electrodes using a SAICAS. Unlike the conventional peel test, a SAICAS can quantitatively measure the adhesion strength of the Si electrode at a specific depth from the surface by cutting the film using a V-shaped microblade26,45.
Using SAICAS, we measured 1) the adhesion strength between the Si composite and the Cu current collector (FSi-Cu) and 2) the adhesion strength of the interlayer (FSi) at a specific depth of the Si composite (4 μm from the surface). As shown in Fig. 5a, the Si–PD-treated Cu had a larger FSi-Cu than did the Si–bare Cu. Thus, the hydrogen bonding between polydopamine and PAA played a key role. In contrast, the crosslinked Si–PD-treated Cu had the highest FSi-Cu (Si–bare Cu = 245.5 N m−1, Si–PD-treated Cu = 297.5 N m−1, and crosslinked Si–PD-treated Cu = 353.2 N m−1). This synergistic adhesion strength improvement may be attributed to the formation of a covalent bond between polydopamine and PAA during the thermal treatment, as previously discussed. In contrast, the Si electrodes had similar FSi values (Si–bare Cu = 219.7 N m−1, Si–PD-treated Cu = 219.8 N m−1, and crosslinked Si–PD-treated Cu = 205.6 N m−1; Fig. 5b). These results are reasonable because the polydopamine coating layer on the Cu current collector rarely encounter O–H functional groups with the PAA in the mid layer of the Si composite electrode.
Figure 5. Adhesion strength between the Si composite and the Cu current collectors.
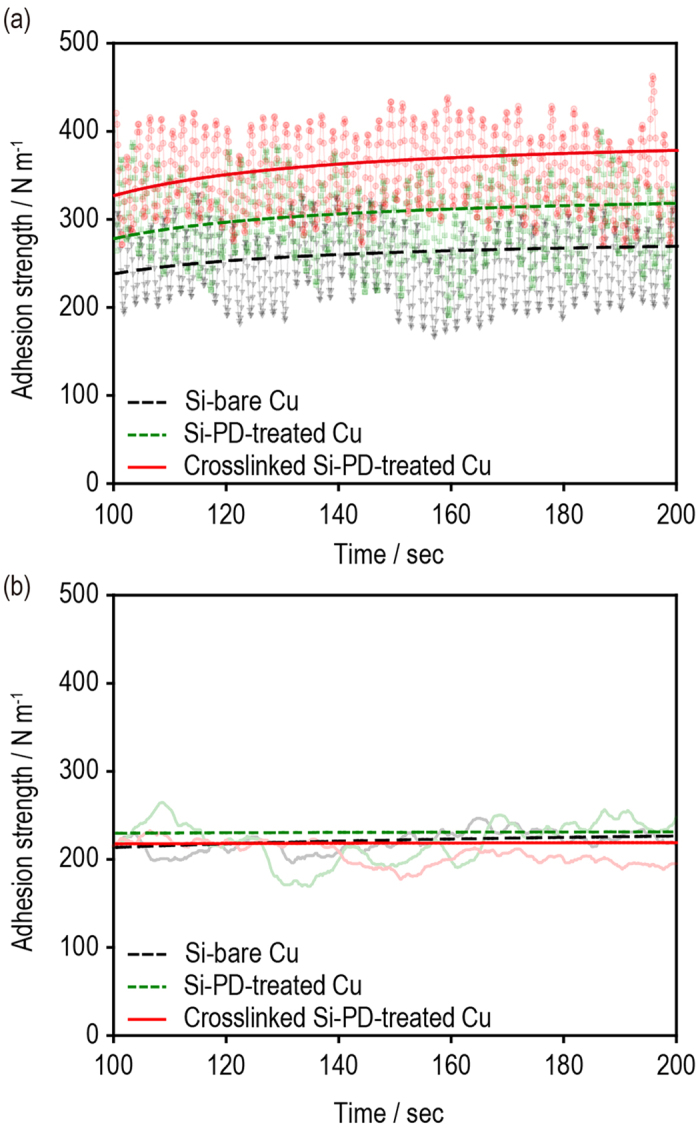
(a) The adhesion strength between the Si composite and Cu current collector (FSi-Cu) and (b) the adhesion strength of the interlayer (FSi) at a specific depth of the Si composite (4 μm from the surface) measured using SAICAS.
Discussion
We demonstrated the feasibility of using a mussel-inspired polydopamine coating on a Cu current collector to improve the electrochemical performance of Si electrodes. Thus, we must consider the significant drawback of using polydopamine for this application because polydopamine is an insulating material, similar to most other polymeric materials. The insulating property of polydopamine may impede the current flow through the current collector and results in a poor electrochemical performance.
To investigate the effect of the polydopamine coating on the surface resistance of the Si electrodes (Si–bare Cu, Si–PD-treated Cu, and crosslinked Si–PD-treated Cu), we measured the resistances of 1) the outer layer of the Si composite electrode (Rout) and 2) the interlayer between the Si composite electrode and the Cu current collector (Rin) (Table 1). As expected, the Si–bare Cu electrode had the smallest Rout and Rin values (0.595 Ω cm and 2.37 mΩ cm2, respectively); the Si–PD-treated Cu (Rout = 3.48 Ω cm and Rin = 91.0 mΩ cm2) and crosslinked Si–PD-treated Cu (Rout = 3.65 Ω cm and Rin = 49.2 mΩ cm2) had higher Rout and Rin than the Si–bare Cu electrode. The easier electron transfer near the electrically conductive current collector makes Rout larger than Rinfor each Si electrode.
Table 1. Resistance of the Si composite.
| Si–bare Cu | Si–PD-treated Cu | Crosslinking Si–PD-treated Cu | |
|---|---|---|---|
| Rout/Ω cm | 0.595 | 3.48 | 3.65 |
| Rin/mΩ cm2 | 2.37 | 91.0 | 49.2 |
It is reasonable that the Si–PD-treated Cu (Rout = 3.48 Ω cm) and the crosslinked Si–PD-treated Cu (Rout = 3.65 Ω cm) have similar Rout values because they have a similar material constitution near the outside of the Si electrode even after the thermal treatment. In contrast, they had different Rin values (91.0 mΩ cm2 for the Si–PD-treated Cu and 49.2 mΩ cm2 for the crosslinked Si–PD-treated Cu). We believe that the reason for this is the change in material constitution in the chemical interaction between the Si composite electrode and the polydopamine layer during the thermal treatment.
In summary, although the polydopamine-related Si electrodes had higher surface resistances than the Si–bare Cu, we observed that the polydopamine insulating layer improved the electrochemical performance of the Si electrodes. We believe that the electrochemical performance of the Si electrode was affected by a combination of factors that accompany a larger volume change. Although the polydopamine coating layer enhanced the surface resistance of the Cu current collectors, it could transform the surface properties of the substrate from hydrophobic to hydrophilic. Moreover, the coating layer could form covalent bonds between the Si composite electrode and the Cu current collectors and improved their adhesion strength. The hydrophilic surface is advantageous when the material is wetted with polar liquid electrolytes, which are commonly used in commercialized LIBs. The improved adhesion strength between the Si composite electrode and the Cu current collector yielded better electrical connections during cycling, which improved the cycle performance and rate capability of the Si electrodes.
Methods
Preparation of the Polydopamine-treated Cu Current Collector
A Cu current collector was coated using the polydopamine (PD) coating process by Ryou et al.37 A mixture of a Tris buffer solution (pH 8.5, 10 mM) and methanol (CH3OH/buffer = 1/1 in wt%) was used to make a dopamine solution (2 mg mL−1).
Electrode Preparation
Si electrodes were prepared from slurries that contained 60 wt% Si activated material (30 nm; Nanostructured & Amorphous Materials, Inc, Houston, USA), 20 wt% conductive carbon (Super-P, Timcal, Switzerland), and 20 wt% poly (acrylic acid) (MW = 450,000; Sigma-Aldrich, South Korea) binder in deionized water. The slurry was cast on two types of Cu current collectors: (i) a bare Cu current collector (8 μm; Iljin Materials, South Korea) and (ii) a Cu current collector that was treated with polydopamine on the surface of a bare Cu current collector (PD-treated Cu) using a doctor blade. The slurries that were cast onto the bare Cu and PD-treated Cu were dried in a convection oven at 80 °C in air for 2 h. In addition, the electrode with the PD-treated Cu was dried overnight in a vacuum oven at 80 °C to induce the crosslinking reaction between the PAA binder in the composite electrode material and the PD layer on Cu; the loading amount of the Si electrode was regulated to 0.53 mg cm−2 using a Mettler Toledo XP26 balance with an accuracy of 1 mg ± 0.001 (Fig. S5, Supplementary Information). The thicknesses of the three types of electrodes were controlled to around 8 μm confirmed by SAICAS (Fig. S5, Supplementary Information).
Cell Assembly
The tested coin-2032 half cells were fabricated using Si anodes (diameter: 12 mm; dried in a vacuum oven at 60 °C for 12 h before use), Li metal as a counter electrode (450 μm; Honjo Metal Co., Japan), and a polyethylene separator (ND420, Asahi Kasei, Japan), which was impregnated with a liquid electrolyte (1.15 M LiPF6 in ethylene carbonate/ethyl methyl carbonate; EC/EMC = 3/7 vol%; Panax Etec, South Korea). The electrolyte contained 5 wt% fluoroethylene carbonate (Panax Etec, South Korea). The cell assembly processes were performed in an argon-filled glove box, where the dew point was maintained at less than −80 °C.
Electrochemical Measurements
After the coin cells were stored for 12 h, a battery tester (PNE Solution, South Korea) was used to evaluate the formation step and cycle performance of the Si-bare Cu, Si–PD-treated Cu, and crosslinked Si–PD-treated Cu. The cells were cycled between 0.05 and 2.0 V at a constant current density of 0.2 A g−1 at room temperature. Then, the corresponding unit cells were cycled (between 0.05 and 2.0 V) at a current density of 1.2 A g−1 for 500 cycles to measure the cycle performance.
Further Analysis
We confirmed that polydopamine was well coated on the surface of the Cu current collector using a contact angle analysis (Surface Electro Optics Co., Ltd., South Korea) and XPS (Sigma probe, Thermo VG Scientific, USA).
The attenuated total reflection FTIR (ATR-FTIR) spectra were recorded at a spectral resolution of 4 cm−1 in the frequency range of 600–4000 cm−1 (FTIR 4200, JASCO Co. Ltd., Japan) to observe the crosslinking reaction between the PD layer on the PD-treated Cu and the PAA binder in the composite electrode.
We cut and prepared three types of composite electrodes to confirm the contact between the electrode materials and the current collectors at three stages: (i) pristine state, (ii) after the formation step, and (iii) after 50 cycles. An ion milling system (E-3500, Hitachi, Japan) was used to cut each electrode at a constant power of 2.1 W (6 kV and 0.35 mA) in vacuum (<2.0 × 10−4 Pa). The cross-sectional morphologies of the electrodes were monitored using field-emission scanning electron microscopy (FE-SEM, S4800, Hitachi, Japan).
The internal adhesion strength in the composite electrode materials and interfacial adhesion strength between the electrodes and the Cu current collectors were measured using a surface and interfacial cutting analysis system (SAICAS, Daipla Wintes Co., Ltd., Japan). A boron nitride blade (blade width: 1 mm), which was fixed at a 45° shear angle, was used for the SAICAS measurements. During the test, the blade moved in the horizontal direction with a velocity of 2.0 μm s−1 while maintaining a vertical force of 0.2 N.
The volume resistances of the Si composite electrodes (outer layer resistances) and interfacial resistances between the composite electrodes and the current collectors (interlayer resistances) were measured using a multi-point-measuring-type electrode resistance meter (HIOKI KOREA Co., ltd., Japan).
Additional Information
How to cite this article: Cho, I. et al. Mussel-inspired Polydopamine-treated Copper Foil as a Current Collector for High-performance Silicon Anodes. Sci. Rep. 6, 30945; doi: 10.1038/srep30945 (2016).
Supplementary Material
Acknowledgments
This research was supported by the international Collaborative Energy Technology R&D Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea. (No. 20158510050020) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2014R1A1A1005861).
Footnotes
Author Contributions I.C. and S.G. conceived the idea, fabricated the material, and performed the electrochemical analysis of the electrodes. D.S. developed the manufacturing processes. Y.L. characterized the materials (FTIR and XPS). M.-H.R. and Y.L. wrote the main manuscript text and guided the experiments.
References
- Armand M. & Tarascon J.-M. Building better batteries. Nature 451, 652–657 (2008). [DOI] [PubMed] [Google Scholar]
- Dunn B., Kamath H. & Tarascon J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011). [DOI] [PubMed] [Google Scholar]
- Scrosati B. & Garche J. Lithium batteries: Status, prospects and future. J. Power Sources 195, 2419–2430 (2010). [Google Scholar]
- Tarascon J.-M. & Armand M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001). [DOI] [PubMed] [Google Scholar]
- Xiao J. et al. Hierarchically porous graphene as a lithium–air battery electrode. Nano Lett. 11, 5071–5078 (2011). [DOI] [PubMed] [Google Scholar]
- Huang X. et al. A hierarchical Tin/Carbon composite as an anode for lithium‐ion batteries with a long cycle life. Angew. Chem. Int. Ed. 54, 1490–1493 (2015). [DOI] [PubMed] [Google Scholar]
- Lee J. S. et al. Metal–air batteries with high energy density: Li–air versus Zn–air. Adv. Energy Mater. 1, 34–50 (2011). [Google Scholar]
- Winter M. & Besenhard J. O. Electrochemical lithiation of tin and tin-based intermetallics and composites. Electrochim. Acta 45, 31–50 (1999). [Google Scholar]
- Wu H. et al. Engineering empty space between Si nanoparticles for lithium-ion battery anodes. Nano Lett. 12, 904–909 (2012). [DOI] [PubMed] [Google Scholar]
- Xu K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004). [DOI] [PubMed] [Google Scholar]
- Ryou M. H. et al. Mussel‐inspired adhesive binders for high‐performance silicon nanoparticle anodes in lithium‐ion batteries. Adv. Mater. 25, 1571–1576 (2013). [DOI] [PubMed] [Google Scholar]
- Wachtler M., Besenhard J. O. & Winter M. Tin and tin-based intermetallics as new anode materials for lithium-ion cells. J. Power Sources 94, 189–193 (2001). [Google Scholar]
- Saint J. et al. Towards a fundamental understanding of the improved electrochemical performance of silicon–carbon composites. Adv. Funct. Mater. 17, 1765–1774 (2007). [Google Scholar]
- Jeena M. et al. Multifunctional molecular design as an efficient polymeric binder for silicon anodes in lithium-ion batteries. ACS Appl. Mater. Inter. 6, 18001–18007 (2014). [DOI] [PubMed] [Google Scholar]
- Kim H., Seo M., Park M. H. & Cho J. A critical size of silicon nano‐anodes for lithium rechargeable batteries. Angew. Chem. Int. Ed. 49, 2146–2149 (2010). [DOI] [PubMed] [Google Scholar]
- Liang B., Liu Y. & Xu Y. Silicon-based materials as high capacity anodes for next generation lithium ion batteries. J. Power Sources 267, 469–490 (2014). [Google Scholar]
- Szczech J. R. & Jin S. Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 4, 56–72 (2011). [Google Scholar]
- Derrien G., Hassoun J., Panero S. & Scrosati B. Nanostructured Sn–C composite as an advanced anode material in high‐performance Lithium‐ion batteries. Adv. Mater. 19, 2336–2340 (2007). [Google Scholar]
- Bruce P. G., Scrosati B. & Tarascon J. M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 47, 2930–2946 (2008). [DOI] [PubMed] [Google Scholar]
- Liu C., Li F., Ma L. P. & Cheng H. M. Advanced materials for energy storage. Adv. Mater. 22, E28–E62 (2010). [DOI] [PubMed] [Google Scholar]
- Magasinski A. et al. Toward efficient binders for Li-ion battery Si-based anodes: polyacrylic acid. ACS Appl. Mater. Inter. 2, 3004–3010 (2010). [DOI] [PubMed] [Google Scholar]
- Erk C., Brezesinski T., Sommer H., Schneider R. & Janek J. r. Toward silicon anodes for next-generation lithium ion batteries: A comparative performance study of various polymer binders and silicon nanopowders. ACS Appl. Mater. Inter. 5, 7299–7307 (2013). [DOI] [PubMed] [Google Scholar]
- Lestriez B., Bahri S., Sandu I., Roué L. & Guyomard D. On the binding mechanism of CMC in Si negative electrodes for Li-ion batteries. Electrochem. Commun. 9, 2801–2806 (2007). [Google Scholar]
- Bridel J.-S., Azais T., Morcrette M., Tarascon J.-M. & Larcher D. Key parameters governing the reversibility of Si/Carbon/CMC electrodes for Li-ion batteries. Chem. Mater. 22, 1229–1241 (2009). [Google Scholar]
- Kovalenko I. et al. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 334, 75–79 (2011). [DOI] [PubMed] [Google Scholar]
- Choi J. et al. Highly Adhesive and Soluble Copolyimide Binder: Improving the Long-Term Cycle Life of Silicon Anodes in Lithium-Ion Batteries. ACS Appl. Mater. Inter. 7, 14851–14858 (2015). [DOI] [PubMed] [Google Scholar]
- Jeong Y. K. et al. Hyperbranched β-cyclodextrin polymer as an effective multidimensional binder for silicon anodes in lithium rechargeable batteries. Nano Lett. 14, 864–870 (2014). [DOI] [PubMed] [Google Scholar]
- Koo B. et al. A highly cross‐linked polymeric binder for high‐performance silicon negative electrodes in lithium ion batteries. Angew. Chem. Int. Ed. 51, 8762–8767 (2012). [DOI] [PubMed] [Google Scholar]
- Yoon T. et al. Failure mechanisms of LiNi0.5Mn1.5O4 electrode at elevated temperature. J. Power Sources 215, 312–316 (2012). [Google Scholar]
- Kim J.-H. et al. Capacity fading mechanism of LiFePO 4-based lithium secondary batteries for stationary energy storage. J. Power Sources 229, 190–197 (2013). [Google Scholar]
- Takamura T., Ohara S., Uehara M., Suzuki J. & Sekine K. A vacuum deposited Si film having a Li extraction capacity over 2000 mAh/g with a long cycle life. J. Power Sources 129, 96–100 (2004). [Google Scholar]
- Lee K.-L., Jung J.-Y., Lee S.-W., Moon H.-S. & Park J.-W. Electrochemical characteristics of a-Si thin film anode for Li-ion rechargeable batteries. J. Power Sources 129, 270–274 (2004). [Google Scholar]
- Takamura T., Uehara M., Suzuki J., Sekine K. & Tamura K. High capacity and long cycle life silicon anode for Li-ion battery. J. Power Sources 158, 1401–1404 (2006). [Google Scholar]
- Reyter D. et al. An electrochemically roughened Cu current collector for Si-based electrode in Li-ion batteries. J. Power Sources 239, 308–314 (2013). [Google Scholar]
- Waite J. H. Surface chemistry: mussel power. Nat. Mater. 7, 8–9 (2008). [DOI] [PubMed] [Google Scholar]
- Lee H., Dellatore S. M., Miller W. M. & Messersmith P. B. Mussel-inspired surface chemistry for multifunctional coatings. science 318, 426–430 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryou M. H., Lee Y. M., Park J. K. & Choi J. W. Mussel‐inspired polydopamine‐treated polyethylene separators for high‐power Li‐ion batteries. Adv. Mater. 23, 3066–3070 (2011). [DOI] [PubMed] [Google Scholar]
- Wang L., Wang D., Dong Z., Zhang F. & Jin J. Interface chemistry engineering for stable cycling of reduced GO/SnO2 nanocomposites for lithium ion battery. Nano Lett. 13, 1711–1716 (2013). [DOI] [PubMed] [Google Scholar]
- Wang L., Wang D., Zhang F. & Jin J. Interface chemistry guided long-cycle-life Li–S battery. Nano Lett. 13, 4206–4211 (2013). [DOI] [PubMed] [Google Scholar]
- Sugama T., Kukacka L. & Carciello N. Nature of interfacial interaction mechanisms between polyacrylic acid macromolecules and oxide metal surfaces. J. Mater. Sci. 19, 4045–4056 (1984). [Google Scholar]
- Luo N., Stewart M. J., Hirt D. E., Husson S. M. & Schwark D. W. Surface modification of ethylene‐co‐acrylic acid copolymer films: Addition of amide groups by covalently bonded amino acid intermediates. J. Appl. Polym. Sci. 92, 1688–1694 (2004). [Google Scholar]
- Bie Y. et al. Polydopamine wrapping silicon cross-linked with polyacrylic acid as high-performance anode for lithium-ion batteries. ACS Appl. Mater. Inter. 8, 2899–2904 (2016). [DOI] [PubMed] [Google Scholar]
- Hellwig P., Soulimane T., Buse G. & Mäntele W. Similarities and dissimilarities in the structure-function relation between the cytochrome c oxidase from bovine heart and from Paracoccus denitrificans as revealed by FT-IR difference spectroscopy. FEBS letters 458, 83–86 (1999). [DOI] [PubMed] [Google Scholar]
- Coleman M. M., Lee K. H., Skrovanek D. J. & Painter P. C. Hydrogen bonding in polymers. 4. Infrared temperature studies of a simple polyurethane. Macromolecules 19, 2149–2157 (1986). [Google Scholar]
- Son B. et al. Measurement and analysis of adhesion property of lithium-ion battery electrodes with SAICAS. ACS Appl. Mater. Inter. 6, 526–531 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.