Abstract
A high-density linkage map is a valuable tool for functional genomics and breeding. A newly developed sequence-based marker technology, restriction site associated DNA (RAD) sequencing, has been proven to be powerful for the rapid discovery and genotyping of genome-wide single nucleotide polymorphism (SNP) markers and for the high-density genetic map construction. The objective of this research was to construct a high-density genetic map of barley using RAD sequencing. 1894 high-quality SNP markers were developed and mapped onto all seven chromosomes together with 68 SSR markers. These 1962 markers constituted a total genetic length of 1375.8 cM and an average of 0.7 cM between adjacent loci. The number of markers within each linkage group ranged from 209 to 396. The new recessive dwarfing gene btwd1 in Huaai 11 was mapped onto the high density linkage maps. The result showed that the btwd1 is positioned between SNP marks 7HL_6335336 and 7_249275418 with a genetic distance of 0.9 cM and 0.7 cM on chromosome 7H, respectively. The SNP-based high-density genetic map developed and the dwarfing gene btwd1 mapped in this study provide critical information for position cloning of the btwd1 gene and molecular breeding of barley.
SNPs are the most abundant type of genetic markers and ideal for studying the inheritance of genomic regions1. Plant breeders and geneticists have benefited from the availability of tools for the rapid and cost-effective development of molecular marker-based linkage maps2. Linkage maps have proven to be useful for discovering, dissecting and manipulating the genes that determine simple and complex traits in crop plants2,3. Barley (Hordeum vulgare L.) is an important model crop for plant breeding and genetics because it is diploid and has a long history of genetics research. With the development of molecular markers, high density linkage maps of the barley have been constructed using multiple populations since the 21st century. Varshney et al.4 reported a high-density barley microsatellite consensus map with 775 SSR loci. Marcel et al.5 constructed a high-density barley linkage map using 3258 markers (RFLP, AFLP and SSR). Szucs et al.6 reported an integrated 2383-locus linkage map developed in the Oregon Wolfe Barley (OWB) mapping population based on RFLP, SSR and single nucleotide polymorphisms (SNP). SNP markers have recently become increasingly important tools for molecular genetic analysis, as single base-pair changes are the most abundant small-scale genetic variation present between related sequences of DNA2.
Restriction-site Associated DNA (RAD) markers detect genetic variation adjacent to restriction enzyme cleavage sites across a target genome2. More recent efforts have focused on adapting the RAD technique for use in NGS platforms, specifically the Illumina sequencing-by-synthesis method, to enable individual sequence based genotyping of samples2. The sequenced RAD marker system enjoys two favourable characteristics for high-throughput GBS. As previously mentioned, the RAD method uses restriction enzymes as a complexity reduction strategy to reduce the sequenced portion of the genome anywhere from 0.01% to 10%1,2. Furthermore, RAD protocols facilitate the creation of highly multiplexed NGS sequencing formulations. The objective of this study was to construct a RAD marker genetic map in barley for further mapping of dwarf genes.
Dwarfism is a valuable trait in crop breeding, because it increases lodging resistance and decreases damages due to wind and rain7,8. Reducing plant height has played an important role in improving crop yields. Successful use of a dwarfing gene in a breeding program is critical for developing dwarf cultivars8,9. In barley, more than 30 types of dwarf or semi-dwarf genes have been identified8,9,10. However, only a few dwarf genes including uzu, sdw1 and denso have successfully been used in barley breeding program until now8. The uzu, sdw1 and denso genes are located on chromosome 3HL8,9,11,12,13,14.
Huaai 11 is a new source of dwarfism, and consisted of desirable agronomic traits such as shortened stature and early maturity8. This dwarf phenotype was controlled by a new dwarfing gene btwd1 mapped on chromosome 7H. The dwarfing gene btwd1 is non-allelic with the uzu and sdw1, which have widely been used in China. The chromosome location of btwd1 is different from those of the uzu, sdw1/denso, br1, br2 genes and QTL PH-78. It is a new source for broadening the genetic base of dwarfism and provides barley breeders with a new gene in China8. At present, the btwd1 gene has been successfully applied in breeding program by Huazhong Agricultural University. In 2011, new barley cultivar Huadamai 9 was registered in Hubei Province with Huaai 11 as male parent. In 2014, new barley cultivar Huadamai 10 was registered in Anhui, Henan and Hubei Provinces with Huaai 11 as male parent. Both cultivars have high yield and good quality.
In order to efficiently use this new germplasm for barley breeding program, we have constructed a high-density genetic map based on SNP genotyping and further mapping of btwd1 dwarfing gene using a double haploid population derived from a cross of Huadamai 6 and Huaai 11. This information could be useful for position cloning of this gene and developing new varieties for plant breeders using MAS.
Methods
Plant materials and field trial
A DH population including 122 lines was developed from a cross between a common feed barley cultivar Huadamai 6 and Huaai 11 using anther culture in this study. The DH population and parents were planted on the Experimental Farm of Huazhong Agricultural University, Wuhan, China. The field trials were conducted following a randomized complete block design with three replications in 2006–2008. Each of the DH and parental lines were grown in three rows in a plot of 0.6 × 1.5 m2. Height of plants before ripening was measured in the field from soil surface to top of the main culm (with the spike). The height was calculated as the mean of twelve plants8.
Extraction of genomic DNA
The young leaves from each doubled-haploid (DH) lines and parents were collected and frozen for DNA extraction. The CTAB method was used to extract genomic DNA from about 0.6–1.0 g tissues of each accession8,15. DNA samples were electrophoresed on agarose gels for quality assurance purposes. Liquid DNA samples were normalized in water or 1 × TE to a standard concentration of 100–200 ng/μl.
Genotyping
Frozen DNA samples were sent to the Personal Biotechnology Co., Ltd. (Shanghai, China). The libraries were quantified using Qubit fluorometer (Invitrogen), Agilent 2100 (Agilent Technologies) and real-time quantitative PCR, and then submitted for sequencing on the Illumina HiSeq2000 platform. Each marker was required to have an allele present in at least 85% of DH individuals. Marker genotypes not meeting the minimum thresholds were scored as missing data. The information of SSR markers was as previously described16. The segregation data for SSR markers in the same population were detected, and 68 of them were used for constructing an integrated map. The phenotyping data of dwarfing gene btwd1 were from Ren et al.8 and listed in Table 1.
Table 1. Phenotyping data of btwd1 gene.
| line | btwd1 | line | btwd1 | line | btwd1 | line | btwd1 | line | btwd1 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A | 26 | B | 51 | A | 76 | B | 101 | B |
| 2 | A | 27 | B | 52 | B | 77 | B | 102 | B |
| 3 | B | 28 | B | 53 | B | 78 | A | 103 | A |
| 4 | A | 29 | B | 54 | B | 79 | A | 104 | B |
| 5 | A | 30 | A | 55 | A | 80 | A | 105 | A |
| 6 | B | 31 | B | 56 | B | 81 | A | 106 | B |
| 7 | B | 32 | A | 57 | A | 82 | B | 107 | A |
| 8 | A | 33 | B | 58 | B | 83 | B | 108 | B |
| 9 | B | 34 | B | 59 | A | 84 | B | 109 | B |
| 10 | A | 35 | A | 60 | A | 85 | A | 110 | B |
| 11 | B | 36 | B | 61 | B | 86 | B | 111 | A |
| 12 | B | 37 | A | 62 | B | 87 | A | 112 | A |
| 13 | A | 38 | A | 63 | B | 88 | A | 113 | B |
| 14 | B | 39 | A | 64 | B | 89 | A | 114 | A |
| 15 | A | 40 | A | 65 | A | 90 | B | 115 | B |
| 16 | B | 41 | B | 66 | B | 91 | A | 116 | A |
| 17 | A | 42 | B | 67 | B | 92 | A | 117 | B |
| 18 | A | 43 | B | 68 | A | 93 | B | 118 | B |
| 19 | B | 44 | B | 69 | A | 94 | A | 119 | B |
| 20 | A | 45 | A | 70 | B | 95 | B | 120 | A |
| 21 | B | 46 | A | 71 | B | 96 | A | 121 | A |
| 22 | B | 47 | B | 72 | B | 97 | B | 122 | A |
| 23 | B | 48 | A | 73 | B | 98 | A | ||
| 24 | A | 49 | A | 74 | B | 99 | B | ||
| 25 | A | 50 | B | 75 | A | 100 | B |
A: plant height <60 cm; B: plant height ≥60 cm.
Genetic linkage map construction
Before constructing genetic maps, SNPs were filtered by excluding those had poor quality data. Low quality SNP included those with NormR results <0.2 and SNP with large numbers of missing values (15% or more). Markers showing identical segregation patterns were also excluded, with one marker per co-segregating group retained. The DH population, consisting of 122 individuals, was utilized to construct a genetic map. The input datasets were constructed from 1,894 genotyped SNP markers and 68 SSR markers. The program Joinmap 4.0 was used to calculate the marker order and genetic distance17. The marker grouping process used a maximum LOD of 3.0 and 7 linkage groups were created. The Kosambi mapping function was employed for map length estimations. Markers were tested for segregation distortion using the chi-square test. Gene mapping was performed with the computer software MAPMAKER and the genetic distance (centimorgan, cM) was derived using Kosambi function18,19.
Results
Construction of linkage map
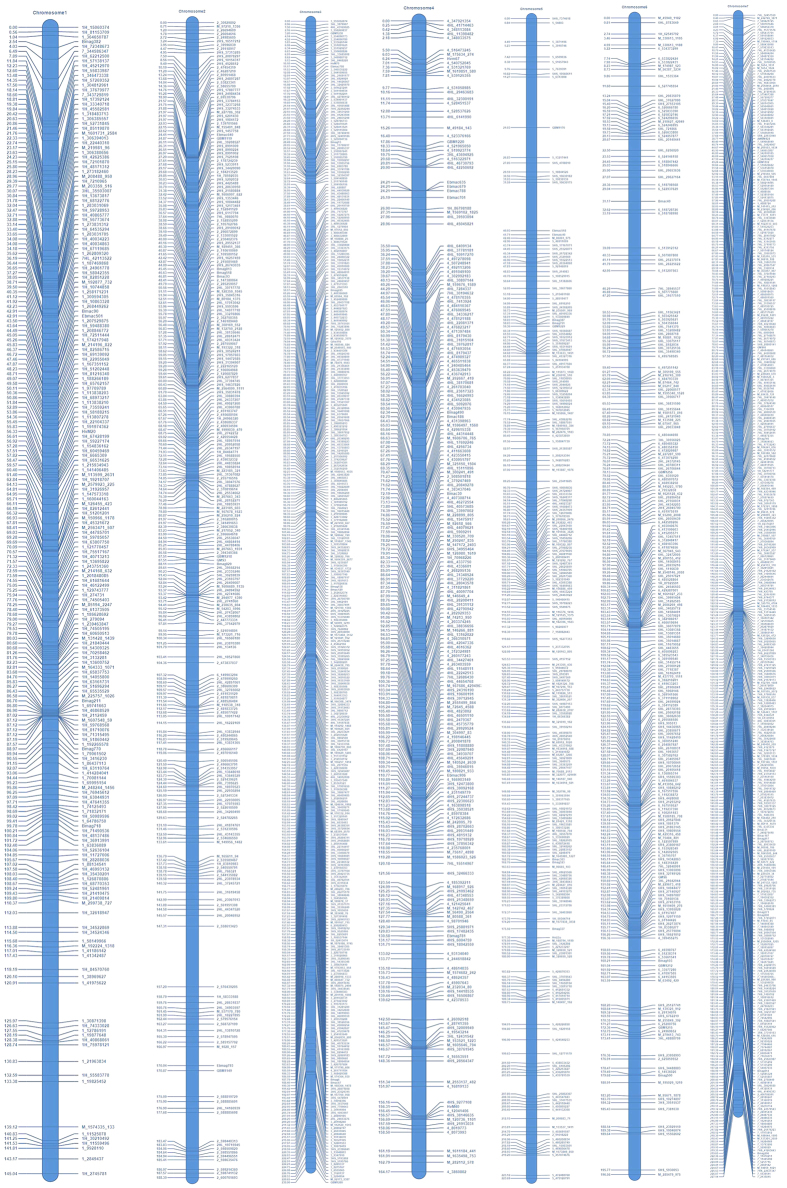
38,268 polymorphic SNPs were detected between the parental lines Huadamai 6 and Huaai 11, of which 10,367 polymorphic SNPs were detected in the mapping population. All 10,367 polymorphic SNP markers showed segregation within the DH population. Low quality SNP markers excluded as a result of filtering processes comprised of 5,375 markers with inconsistent parental scores, monomorphic and more than 15% missing data. The remaining 4992 polymorphic SNP markers and 153 of the obtained before polymorphic SSR marker met the requirements for use in the construction of a genetic map. After removing some of the co-segregated markers and non-linked markers, the remaining 1894 SNP markers and 68 SSR markers were used to create 7 high-density genetic linkage groups. This high-density genetic linkage map was generated from 1,962 markers covering seven linkage groups with a total map distance of 1,375.8 cM. The averaged distance between two positions across the whole map was 0.7 cM. The number of markers on different chromosomes ranged from 209 on 1H to 396 on 7H. The genetic distance on different chromosome ranged from 145.0 cM on 1H to 230.9 cM on 3H (Fig. 1, Table 2, Table S1).
Figure 1. Linkage map of barley based on SNP and SSR markers derived from Huaai 11 × Huadamai 6 DH population.
Table 2. Genetic distance and number of markers from the high-density map.
| Chromosome | Length (cM) | SNP marker | SSR marker | Total no. of markers | Average distance (cM) |
|---|---|---|---|---|---|
| 1H | 145.0 | 202 | 7 | 209 | 0.69 |
| 2H | 188.3 | 239 | 10 | 249 | 0.76 |
| 3H | 230.9 | 374 | 11 | 385 | 0.60 |
| 4H | 164.2 | 218 | 12 | 230 | 0.71 |
| 5H | 223.7 | 235 | 10 | 245 | 0.91 |
| 6H | 196.6 | 241 | 7 | 248 | 0.79 |
| 7H | 227.1 | 385 | 11 | 396 | 0.57 |
| Total | 1375.8 | 1894 | 68 | 1962 | 0.70 |
Molecular mapping of the dwarf gene btwd1
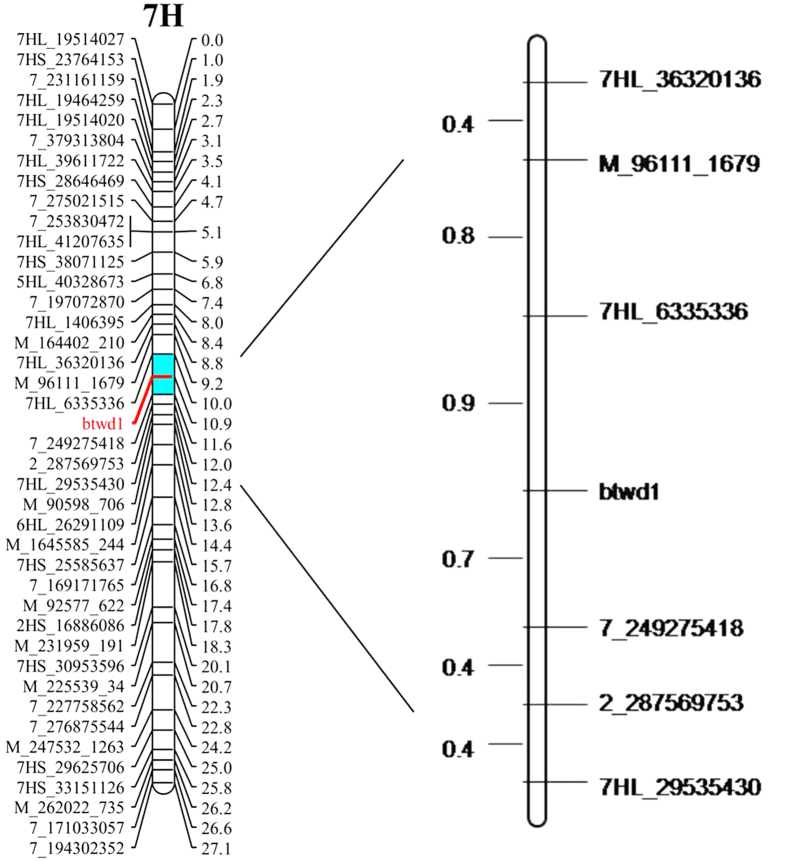
The DH population was constructed from the crosses between Huaai 11 and Huadamai 6 varieties8. Plant height of the DH lines showed a bimodal distribution, the segregation ratio between tall and dwarf is 1:18. Huaai 11 was a six-row new source of dwarf that was controlled by a recessive dwarfing gene btwd1, and Huadamai 6 was a two-row common feed barley cultivar16. Previous linkage analysis between SSR markers and plant height found that the dwarfing gene was located on the long arm of chromosome 7H, associated with the marker Bmac167 and Bmac031 at a genetic distance of 2.2 cM8. A set of 40 SNP markers near the SSR marker Bmac031 and Bmac167 was used to analyze the 122 individuals of DH population. The results showed that the gene btwd1 controlling plant height in Huaai 11 was positioned between SNP marks 7HL_6335336 and 7_249275418 with genetic distance of 0.9 cM and 0.7 cM on chromosome 7HL, respectively (Fig. 2).
Figure 2. Linkage map with the dwarfing gene btwd1 based on SNP markers on barley chromosome 7H.
Discussion
Constructing a high-density linkage map is a vital prerequisite for genetic analysis and efficient molecular breeding. For the past ten years there has been a surge in marker density and convergence toward consensus maps for barley2,4,5,6,20,21,22,23,24,25,26,27,28,29 (Table 3). In the previous published barley genetic maps, limited number of markers were included using traditional marker technologies such as RFLP, RAPD, DArT, SSR and EST based markers4,5,20,21,30. SNP markers have been recognized as important candidate markers due to their high abundance and relatively even distribution in barley genome. Our results demonstrated the value of RAD sequencing for the development of SNP markers and for the production of the most saturated linkage maps. RAD is one such NGS-based method which utilizes the restriction enzyme digestion of genome to reduce the genome complexity1. In RAD, only a small fraction of the genome is sequenced to identify many genetic markers along the genome. It is a simple and more cost-effective than other NGS methods not only for large-scale polymorphism discovery but also for genotyping-by-sequencing approach2. RAD sequencing data are easier to handle because less overall data are detected in comparison with whole-genome sequencing data, and therefore different individuals of populations can be easily genotyped. Using RAD sequencing approach, we identified thousands of SNPs in the Huaai 11 and Huadamai 6 DH population. In this paper, we reported the construction of a new SNP based genetic map of barley from Huaai 11 and Huadamai 6 DH population. The genetic linkage map is composed of 1894 SNP markers and 68 SSR markers. The total length of the map is 1375.8 cM with an average distance of 0.7 cM between loci (Table 2). These markers can be used for genetic diversity analysis, marker–trait association and marker-assisted selection for barley improvement.
Table 3. Recently published genetic linkage maps in barley.
| Population | Size | Years | Location | Types of markers | Number of markers | Map length (cM) | References |
|---|---|---|---|---|---|---|---|
| DH | 150 | 2004 | Dundee, UK | SNP, RFLP, AFLP, SSR | 1237 | 1211 | Rostoks et al.22 |
| DH, RIL | 911 | 2005 | Canberra, Australia | DArT, RFLP, SSR, STS | 2935 | 1161 | Wenzl et al.20 |
| DH, RIL | 683 | 2006 | Wageningen University | RFLP, AFLP, SSR, RAPD | 3258 | 1081 | Marcel et al.5 |
| DH | 315 | 2006 | Gatersleben, Germany | SNP, RFLP, SSR | 1255 | 1118.3 | Stein et al.23 |
| DH, RIL | 654 | 2006 | Wageningen University | SSR | 755 | 1067.8 | Varshney et al.4 |
| DH | 90 | 2006 | Adelaide, Australia | SSR, DArT | 1000 | 1100.1 | Hearnden et al.21 |
| DH | 139 | 2007 | Iowa State University | SNP | 1596 | 1017 | Potokina et al.24 |
| DH | 93 | 2008 | Okayama University | SNP, STS, SSR | 2948 | 2136 | Sato et al.26 |
| DH | 92 | 2008 | Okayama University | SNP | 1116 | 1187.4 | Sato and Takeda.27 |
| DH | 93 | 2008 | University of California | SNP, DArd, STS, RFLP, SSR | 2383 | 1280 | Szucs et al.6 |
| DH | 429 | 2009 | University of California | SNP | 2943 | 1099 | Close et al.25 |
| DH | 93 | 2011 | Oregon State University | SNP | 436 | 1260 | Chutimanitsakun et al.2 |
| RIL | 142 | 2012 | Kannapolis, United States | SNP, DArT, SSR | 297 | 832.1 | Islamovic et al.28 |
| DH | 182 | 2013 | Huangzhou, China | SSR, DArT | 626 | 1081.2 | Wang et al.29 |
We have used these SNP markers to map the dwarfing gene btwd1 of barley in Huaai 11 and Huadamai 6 DH population, which previous mapped using SSR markers8. The results showed that the gene btwd1 controlling plant height of Huaai 11 was positioned between SNP marks 7HL_6335336 and 7_249275418 with a genetic distance of 0.9 cM and 0.7 cM on chromosome 7HL, respectively. Our results are comparable to those in a few previous studies on dwarf and semi-dwarf genes in barley, and corresponded well with our previous result reported by Ren et al.8. The chromosome location of btwd1 is different from the hcm1 gene (2HL)29, sdw3 gene (2HS)31, uzu gene (3HL)8,12,29,32,33, sdw1/denso geng (3HL)8,14,29,34,35, br2 (4H)8,36,37, ari-e gene (5HL)29, br1 gene (7HS)8,36,37, and also different from the plant height QTL on chromosome 1H2,29,38,39,40, 2H2,29,39,41,42, 3H2,28,39,42,43,44, 4H38,39,40,43,45,46, 5H38,39,45, 6H2,45, and 7H29,38,39,42,43,47, which further demonstrated that the btwd1 is a novel gene.
Utilization of dwarfing genes in barley breeding programs has greatly increased barley yields, particularly in Asia and Europe8,47. In barley, only a few dwarf genes have been exploited in barley breeding. The dwarf germplasm Huaai 11 is a new source of dwarfs for broadening the genetic base of dwarfism. Two barley cultivars with high yield and good quality were bred with Huaai 11 as the male parent. The dwarfing gene btwd1 in Huaai 11 has widely been used in China, and provides barley breeders with a new source for barley genetic improvement. In order to efficiently use this new germplasm Huaai 11 for barley breeding program, we will verify the identified SNP markers associated with btwd1 in the lines developed from Huaai 11. We have also constructed a large F2 population including 16000 lines derived from a cross of Huadamai 6 and Huaai 11. We will fine map dwarfing gene btwd1 using this large F2 population and the SNP markers. In near future, we will clone the dwarf gene btwd1 and verify its function.
In conclusion, we have constructed a high-density barley linkage map using SNP markers derived from the DH population RAD sequencing. Our study provides a valuable genetic resource for molecular markers, map-based gene cloning, MAS and the sequence assembly of the barley reference genome. At the same time, the linked SNP markers identified in the present study can provide a useful marker-assisted selection tool to transfer the dwarfing gene btwd1 in barley breeding.
Additional Information
How to cite this article: Ren, X. et al. SNP-based high density genetic map and mapping of btwd1 dwarfing gene in barley. Sci. Rep. 6, 31741; doi: 10.1038/srep31741 (2016).
Supplementary Material
Acknowledgments
Research supported by the National Natural Science Foundation of China (31301310), the Earmarked Fund for China Agriculture Research System (CARS-5) and the Fundamental Research Funds for the Central Universities (2662016PY048).
Footnotes
Author Contributions Conceived and designed the experiments: X.R., D.S. and G.S. Performed the experiments: X.R. and L.L. Analyzed the data: X.R. and J.W. Contributed reagents/materials/analysis tools: X.R., D.S. and C.L. Wrote the paper: X.R., H.L., D.S. and G.S.
References
- Baird N. A. et al. Rapid SNP Discovery and Genetic Mapping Using Sequenced RAD Markers. PloS ONE. 3, e3376 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutimanitsakun Y. et al. Construction and application for QTL analysis of a Restriction Site Associated DNA (RAD) linkage map in barley. BMC Genomics. 12, 4 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanksley S. D. et al. High density molecular linkage maps of the tomato and potato genomes. Genetics. 132, 1141–1160 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney R. K. et al. A high density barley microsatellite consensus map with 775 SSR loci. Theor. Appl. Genet. 114, 1090–1103 (2007). [DOI] [PubMed] [Google Scholar]
- Marcel T. C. et al. High-density consensus map of barley to compare the distribution of QTLs for partial resistance of Puccinia hordei A and of defence gene homologues. Theor. Appl. Genet. 114, 487–500 (2007). [DOI] [PubMed] [Google Scholar]
- Szucs P. et al. An integrated resource for barley linkage map and malting quality QTL alignment. The Plant Genome. 2, 134–140 (2009). [Google Scholar]
- Itoh H. et al. A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol. Biol. 54, 533–547 (2004). [DOI] [PubMed] [Google Scholar]
- Ren X. F. et al. Inheritance and identification of molecular markers associated with a novel dwarfing gene in barley. BMC Genetics. 11, 89 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. & Zhang W. X. Tracing sources of dwarfing genes in barley breeding in China. Euphytica. 131, 285–292 (2003). [Google Scholar]
- Sears R. G. et al. Inheritance of dwarf and semidwarf plant height in barley. Crop Sci. 21, 828–833 (1981). [Google Scholar]
- Hellewell K. B. et al. Enhancing yield of semidwarf barley. Crop Sci. 40, 352–358 (2000). [Google Scholar]
- Zhang J. Inheritance of agronomic traits from the Chinese barley dwarfing gene donors Xiaoshan Lixiahuang and Cangzhou Luodamai. Plant Breed. 119, 523–524 (2000). [Google Scholar]
- Saisho D. et al. Spontaneous brassinolide-insensitive barley mutants ‘uzu’ adapted to east Asia. Breeding Sci. 54, 409–416 (2004). [Google Scholar]
- Jia Q. J. et al. GA-20 oxidase as a candidate for the semidwarf gene sdw1/denso in barley. Funct. Integrat. Genomics. 9, 255–262 (2009). [DOI] [PubMed] [Google Scholar]
- Stein N. et al. A new DNA extraction method for high-throughput marker analysis in a large-genome species such as Triticum aestivum. Plant Breed. 120, 354–356 (2001). [Google Scholar]
- Ren X. F. et al. Molecular detection of QTL controlling plant height components in a doubled haploid barley population. Genet. Mol. Res. 13, 3089–3099 (2014). [DOI] [PubMed] [Google Scholar]
- Van Ooijen J. W. JoinMap 4, Software for the calculation of genetic linkage maps in experimental populations. Kyazma BV, Netherlands, Wageningen (2006).
- Lander E. S. et al. Mapmaker: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1, 174–181 (1987). [DOI] [PubMed] [Google Scholar]
- Liu R. H. & Meng J. L. MapDraw: A microsoft Excel macro for drawing genetic linkage maps based on given genetic linkage data. Hereditas. 25, 317–321 (2003). [PubMed] [Google Scholar]
- Wenzl P. et al. A high-density consensus map of barley linking DArT markers to SSR, RFLP and STS loci and agricultural traits. BMC Genomics. 7, 206 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearnden P. R. et al. A genetic map of 1,000 SSR and DArT markers in a wide barley cross. Theor. Appl. Genet. 115, 383–391 (2007). [DOI] [PubMed] [Google Scholar]
- Rostoks N. et al. Genome-wide SNP discovery and linkage analysis in barley based on genes responsive to abiotic stress. Mol. Genet. Genomics. 274, 515–527 (2005). [DOI] [PubMed] [Google Scholar]
- Stein N. et al. A 1,000-loci transcript map of the barley genome: new anchoring points for integrative grass genomics. Theor. Appl. Genet. 114, 823–839 (2007). [DOI] [PubMed] [Google Scholar]
- Potokina E. et al. Gene expression quantitative trait locus analysis of 16,000 barley genes reveals a complex pattern of genome-wide transcriptional regulation. Plant J. 53, 90–101 (2008). [DOI] [PubMed] [Google Scholar]
- Close T. J. et al. Development and implementation of high-throughput SNP genotyping in barley. BMC Genomics. 10, 582 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K. et al. A high density transcript linkage map of barley derived from a single population. Heredity. 103, 110–117 (2009). [DOI] [PubMed] [Google Scholar]
- Sato K. & Takeda K. An application of high-throughput SNP genotyping for barley genome mapping and characterization of recombinant chromosome substitution lines. Theor. Appl. Genet. 119, 613–619 (2009). [DOI] [PubMed] [Google Scholar]
- Islamovic E. et al. A new genetic linkage map of barley (Hordeum vulgare L.) facilitates genetic dissection of height and spike length and angle. Field Crops Res. 154, 91–99 (2013). [Google Scholar]
- Wang J. M. et al. A new QTL for plant height in barley (Hordeum vulgare L.) showing no negative effects on grain yield. Plos one. 9, e90144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F. et al. Construction of a SNP-based high-density genetic map for pummelo using RAD sequencing. Tree Genet. Genomes. 11, 2 (2015). [Google Scholar]
- Vu G. T. H. et al. Fine mapping and syntenic integration of the semi-dwarfing gene sdw3 of barley. Funct. Integr. Genomics. 10, 509–521 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang J. Allelism tests for the dwarf genes in the three main dwarf sources of barley. Acta. Agronom Sin. 24, 42–46 (1998). [Google Scholar]
- Zhang J. SSR maker tagging of the dwarfing gene uz in barley (Hordeum velgare L.). Acta. Agronom Sin. 29, 637–640 (2003). [Google Scholar]
- Barua U. M. et al. Molecular mapping of genes determining height, time to heading, and growth habit in barley (Hordeum vulgare). Genome 36, 1080–1087 (1993). [DOI] [PubMed] [Google Scholar]
- Laurie D. A. et al. Assignment of the denso dwarfing gene to the long arm of chromosome 3(3H) of barley by use of RFLP markers. Plant Breed. 111, 198–203 (1993). [Google Scholar]
- Tsuchiya T. Allelism testing of genes between brachytic and eractoides mutants. Barley Genet. Newslett. 6, 79–81 (1976). [Google Scholar]
- Li M. et al. Fine mapping of a semi-dwarf gene brachytic 1 in barley. Acta. Genet. Sin. 29, 565–570 (2002). [PubMed] [Google Scholar]
- Pillen K. et al. Advanced backcross QTL analysis in barley (Hordeum vulgare L.). Theor. Appl. Genet. 107, 340–352 (2003). [DOI] [PubMed] [Google Scholar]
- Von Korff M. et al. AB-QTL analysis in spring barley: II. Detection of favourable exotic alleles for agronomic traits introgressed from wild barley. Theor. Appl. Genet. 112, 1221–1231 (2006). [DOI] [PubMed] [Google Scholar]
- Baghizadeh A. et al. QTL analysis for some agronomic traits in barley (Hordeum vulgare L). Int. J. Agr. Biol. 9, 372–374 (2007). [Google Scholar]
- Kjaer B. et al. Quantitative trait loci for heading date and straw characters in barley. Genome 38, 1098–1104 (1995). [DOI] [PubMed] [Google Scholar]
- Hori K. et al. Efficient construction of high-density linkage map and its application to QTL analysis in barley. Theor. Appl. Genet. 107, 806–813 (2003). [DOI] [PubMed] [Google Scholar]
- Sameri M. et al. Quantitative trait loci controlling agronomic traits in recombinant inbred lines from a cross between oriental- and occidental-type barley cultivars. Breed. Sci. 56, 243–252 (2006). [Google Scholar]
- Wang J. M. et al. Identification and molecular mapping of a dwarfing gene in barley (Hordeum vulgare L.) and its correlation with other agronomic traits. Euphytica 175, 331–342 (2010). [Google Scholar]
- Backes G. et al. Localization of quantitative trait loci (QTL) for agronomic important characters by the use of a RFLP map in barley (Hordeum vulgare L.). Theor. Appl. Genet. 90, 294–302 (1995). [DOI] [PubMed] [Google Scholar]
- Dahleen L. S. et al. Quantitative trait loci (QTL) for Fusarium ELISA compared to QTL for fusarium head blight resistance and deoxynivalenol content in barley. Plant Breed. 131, 237–243 (2012). [Google Scholar]
- Yu G. T. et al. A new semi-dwarfing gene identified by molecular mapping of quantitative trait loci in barley. Theor. Appl. Genet. 120, 853–861 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.