Abstract
Medication-related osteonecrosis of the jaw (MRONJ) represents a complication of bisphosphonate treatment that responds poorly to standard treatment. In a retrospective cohort study we investigated a possible role of Actinomyces spp. in the pathogenesis of MRONJ. Deep biopsies of necrotic bone were collected during surgical treatment of MRONJ and evaluated by histology and microbiology for the presence of Actinomyces spp. Microbiological, demographic and clinicpathological data were analyzed for risk of Actinomyces-associated MRONJ. Between 2005 and 2014, 111 patients suffering from histologically-confirmed MRONJ were identified. Actinomyces spp. were detected in 99 cases (89%) by histology and in six further patients by microbiological culture. A diverse microbial flora was found in all specimens without association with Actinomyces spp. Demographic and clinicopathological characteristics did not separate significantly Actinomyces-positive from Actinomyces-negative cases. Our observations confirm previous reports of a high prevalence of Actinomyces spp. in MRONJ in the single largest cohort available up to now. The high prevalence of Actinomyces spp. and the lack of clinicopathological risk factors underline the prominent role of Actinomyces spp. in MRONJ and may change the current understanding of MRONJ. Established prolonged antimicrobial treatment regimens against Actinomyces spp. infection could therefore be a mainstay of future MRONJ management.
Medication-related osteonecrosis of the jaw (MRONJ) is a rare but difficult to treat complication of bisphosphonate treatment. Incidence of MRONJ ranges between 0.1% and 10% in patients with cancer with clearly higher rates observed in investigator-initiated, academic studies1. MRONJ is characterized by necrosis of the maxilla and mandible that leads to superficially exposed bone without a tendency of spontaneous healing for more than eight weeks. The diagnosis is established on basis of a history of recent treatment with an antiresorptive drug in absence of a history of radiotherapy to the jaws2. In most cases the onset of MRONJ is triggered by a dental procedure affecting the oral mucosa or alveolar bone3. Signs and symptoms may occur before the development of clinically detectable MRONJ including tooth mobility, prolonged jaw pain, gingival swelling, erythema, and ulceration4. Fistulae may develop upon secondary infection and are associated with significantly increased morbidity and reduced quality of life. Management of MRONJ is based on discontinuation of bisphosphonate administration, antimicrobial treatment and surgical resection of necrotic bone2. Resolution of MRONJ, however, may be achieved only in 30% of patients and clearly underlines the urgent need for more effective prophylactic and therapeutic strategies5.
The causal link between MRONJ and administration of bisphosphonates was established with the rapidly increasing number of case series and observational studies that substantiated early evidence1. Accordingly, warnings about the risk of MRONJ in patients with cancer were issued by health authorities and manufacturers of bisphosphonates. A better understanding of the pathophysiology underlying the evolution of MRONJ, however, would facilitate development of more efficient preventive strategies. Intravenous bisphosphonates are important treatment options for preventing skeletal related events and to prolong survival in a diversity of generalized or disseminated bone diseases including malignancy, osteoporosis, Paget’s disease, and metastatic bone disease of multiple myeloma, breast, lung, and prostate cancer5,6. On the other hand, prolonged and high-dose treatment with intravenous bisphosphonates as well as combination treatment with anti-angiogenetic drugs were identified as significant risk factors for MRONJ7,8.
Still, bisphosphonate treatment may not explain exclusively the observed predilection of the jaw of cancer patients1. Remodeling or oversuppression of bone resorption, inhibition of blood supply, constant microtrauma, dentoalveolar surgery, or local inflammation were proposed in association with bisphosphonate treatment to explain the unique localization to the jaw but none of these hypotheses explain all cases9. Evidence suggesting a role of Actinomyces species in the evolution of MRONJ accumulated over the past years, but these small case series and reviews revealing a high prevalence of Actinomyces spp. in MRONJ did not had impact on current treatment recommendations up to now10,11,12,13.
Actinomyces spp. are Gram-positive, facultative anaerobic, non-spore-forming and commonly filamentous microorganisms. Actinomyces spp. are commensals of the mucosa of oropharynx, gastrointestinal tract and female genital tract. Nevertheless, in case of breaches to the mucosal barrier by trauma, surgical procedures, or foreign bodies, microbes may invade deep tissue structures and cause a difficult to treat, chronic-progressive disease termed Actinomycosis. Standards of treatment for invasive actinomycosis have been developed, validated and adapted during the past five decades and is based on prolonged antimicrobial treatment for 2–6 months combined with surgery. The anatomic region affected (e.g. cervicofacial, pulmonal, abdominal) is not associated with a requirement to adapt antimicrobial treatment duration14,15.
The aim of the present study was to evaluate in a large and well-characterized cohort of patients suffering from MRONJ the incidence of Actinomyces spp. infection and possible risk-factors that may predispose patients to this infection. Contrary to expectation, a large majority of the present MRONJ cases was associated with Actinomyces spp. infection.
Materials and Methods
Study population
The present retrospective study of Actinomycosis associated with MRONJ included all consecutive patients suffering from MRONJ and who were treated at the Department of Oral- and Maxillofacial Surgery, at the Medical University of Vienna, between 2005 and 2014. Inclusion criteria were clinically diagnosed MRONJ according the current guidelines of the American Association of Oral and Maxillofacial Surgeons (AAOMS)2, current or previous treatment of malignancy or osteoporosis with a bisphosphonate. Accordingly, the clinical diagnosis of MRONJ was defined as presence of exposed necrotic bone of maxilla or mandible, with or without pain and signs and symptoms of superinfection, for more than eight weeks without tendency of spontaneous healing. Patients were excluded from the study when having received radiotherapy, bone biopsy was not available for histological evaluation, or the clinically suspected diagnosis could not verified by histology. Staging of confirmed cases of MRONJ was done in respect of clinical symptoms and severity of the disease from Stage 0 to Stage 3 according to AAOMS guidelines2. Stage 0 cases were not included as they are characterized by non-specific symptoms or radiographic findings without featuring exposed necrotic bone. Treatment of the Stage 1 to 3 MRONJ cases followed an established algorithm combining conservative pre-treatment followed by surgical removal of necrotic bone and soft tissue closure. Sample collection for histology was performed in all cases during the surgical standard procedure. According to this standard treatment algorithm, all patients received systemic antibiotic treatment with amoxicillin (2 × 1 g/24 hours)/clavulanic acid (2 × 500 mg/24 hours) or clindamycin (3 × 300 mg/24 hours) for approximately 4 weeks between admittance and surgery16,17.
Demographic, clinicopathological and follow-up data were extracted retrospectively from the Vienna General Hospital Patient Information System (AKIM). The study protocol was approved by the Ethics Committee of the Medical University of Vienna, Austria (ECS 1824/2015) and all study related procedures were done according to the declaration of Helsinki. Due to the retrospective cohort study design, no informed consent had to be obtained by approval of the ethics committee.
Histological and microbiological analysis
The specimens retrieved under surgical treatment of MRONJ comprised of deep bone samples for analysis by histology and microbiology in all cases. The respective specimens were divided into two aliquots and either fixed in 4% phosphate-buffered saline (PBS)-formalin and decalcified with use of ethylenediaminetetraacetic acid (EDTA) for 7 days for histological analysis or immediately transferred into reduced transport medium (Port-A-Cul™, Becton, Dickinson and Company, NJ, USA) and transported to the microbiological laboratory for microbiological culture.
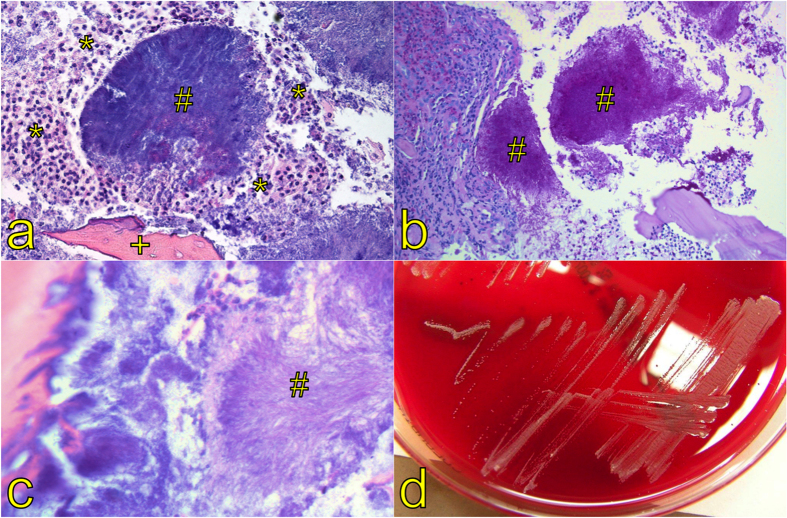
For histological analysis, specimens were then embedded in paraffin, cut into 2 μm thick slices and stained by hematoxylin and eosin (H&E). To highlight Actinomyces spp., the specimens were stained additionally by periodic acid-Schiff (PAS), Gram and Grocott’s methenamine silver stain (GMS). The slides were examined by a trained pathologist for pathognomonic features of actinomycosis (sulfur granules) and photographed by light microscopy (Fig. 1)18,19.
Figure 1.
H&E staining of an aggregate composed of Actinomyces spp. filaments, so called sulfur granules (#), which macroscopically appear as yellow granules, surrounded by neutrophilic granulocytes (*) and a necrotic bone trabecula (+, magnification x100) (a). The granules (#) stain PAS positive (magnification x200) (b). High magnification elucidates the filamentous structure (#, sun-ray morphology) of the organisms (magnification x400) (c). Typical growth pattern of Actinomyces spp. in microbiological culture (d).
For microbiological culture, specimens were streaked to blood agar, chocolate agar, and Sabouraud Dextrose-Agar and were incubated at 29°–31 °C as well as 35°–37 °C. For the growth of anaerobic bacteria, Brucella agar (Becton Dickinson, Heidelberg, Germany) was inoculated and incubated at 35°–37 °C under anaerobic conditions for 21 days in total. In case of growth, identification was performed either biochemically using Vitek® (bioMérieux, Marcy l’Etoile, France) or MALDI-TOF (Bruker, Bremen, Germany). If these methods were unsuccessful, isolates were identified by 16S sequence analysis (Fig. 1).
Statistical analysis
The primary endpoint of this study was the prevalence of MRONJ-related actinomycosis within the study population as confirmed by histology. Secondary objectives were to determine clinicopathological risk factors for the development of MRONJ-related actinomycosis by means of logistic regression models. The models including one factor were compared to the null model using a likelihood ratio test and thus providing the p-values. The distribution of bisphosphonate drugs in the actinomyces positive and the negative group was tested for count distribution by means of a chi-squared test instead of applying logistic regression. Furthermore, a Fisher’s exact test was used to investigate the allocation of microbiological culture results between actinomyces positive and the negative cases in histology. A p-value of <0.05 was defined as statistically significant. All calculations were performed using the statistical programming environment “R” (version 2.15.1, Vienna, Austria). Finally all p-values were corrected for multiple testing using Bonferroni adjustment.
Results
Demographic, clinical and histological results
A total of 150 patients fulfilling the clinical criteria of MRONJ were identified during the study period of 2005 to 2014. Of these patients, 39 cases were excluded from the study because of a previous history of radiotherapy (n = 6), unavailable bone specimen for histological analysis (n = 24), or histological evaluation not supporting the clinical diagnosis (n = 9). The final cohort of 111 patients with clinically diagnosed and histologically confirmed MRONJ were predominantly female, above the age of 65 years, and all were Caucasian (Table 1). The majority of MRONJ patients had a malignancy as underlying disease and most of them suffered from malignant bone disease. MRONJ was diagnosed in most patients at a clinical stage 2 (n = 55) or stage 1 (n = 43). Thirteen patients had stage 3 disease. The predominant location of MRONJ was in the mandibular bone (n = 72), followed by the maxilla (n = 34) or both (n = 5).
Table 1. Demographics of the MRONJ study population.
| Characteristic | Patients with MRONJ |
|---|---|
| (n = 111) | |
| Mean age (SD), years | 69 (10) |
| Gender, no. (%) | |
| Male | 42 (38) |
| Female | 69 (62) |
| Underlying disease, no. (%) | |
| Osteoporosis | 26 (23) |
| Cancer | 85 (77) |
| Malignant bone disease, no. (%) | 74 (87) |
| MRONJ Staging, no. (%) | |
| Stage 0 | 0 (0) |
| Stage 1 | 43 (39) |
| Stage 2 | 55 (49) |
| Stage 3 | 13 (12) |
| Location of MRONJ, no. (%) | |
| Mandible | 72 (64) |
| Maxilla | 34 (31) |
| Both | 5 (5) |
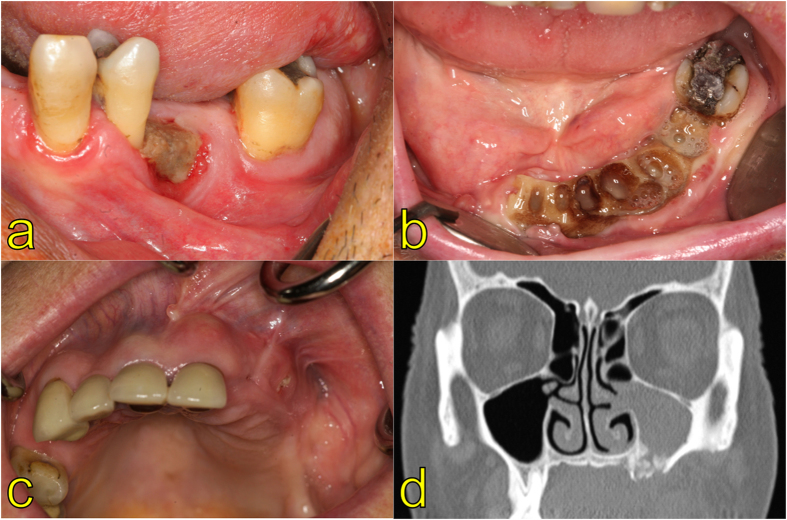
The clinical presentation of MRONJ was uniformly a white-yellowish areas of exposed necrotic bone, sometimes accompanied erythema of the mucosa or gingival tissue surrounding the lesion (Fig. 2). Uncharacteristic for “classical” Actinomycosis, sinus formation with affection of oro-pharyngeal soft tissue was not noticed for the present patients.
Figure 2.
Clinical findings in patients suffering from MRONJ showing necrotic alveolar bone (Stage 1) distal to a premolar tooth (a) or large areas of exposed bone with sings of infection (Stage 2) caused by tooth extractions under bisphosphonate therapy (b). Despite no large portion of necrotic bone in the maxilla (c), Stage 3 is characterized by severe infection leading to maxillary sinusitis in the presented case (d).
Histological evaluation of the bone specimens collected in the course of surgical treatment showed the presence of Actinomyces spp. in 99 of 111 (89%) MRONJ cases. The pathognomonic features of Actinomyces spp. infection was found in all histopathological samples, including aggregates composed of Actinomyces spp. filaments (sulfur granules), sun-ray morphology of these granules and accompanying inflammatory tissue reaction. Microbiological evaluation of the bone specimens collected in parallel was uniformly negative for Actinomyces spp. with only seven exceptions (Table 2). In one MRONJ case with an Actinomyces-negative histology result, the pathogen could be cultured from the bone specimen. A diverse microbial flora could be cultured from all but 7 (6%) of the 111 specimens including apathogenic residential oral flora, anaerobic mixed flora and facultative pathogenic microbes. The most commonly facultative pathogens cultured belonged to the families of Enterobacteriaceae, Neisseriaceae, and Streptococcaceae. The relative frequency of microbes cultured did not differ significantly between Actinomyces spp. positive and negative cases of MRONJ.
Table 2. Microbiological culture results of bone specimens.
| Taxonomic family | Actinomyces spp. detection by histology (n = 111) | OR | p-value* | Adjusted p | |||
|---|---|---|---|---|---|---|---|
| Positive, no. (%) | Negative, no. (%) | ||||||
| Anaerobic mixed flora | 22 | (20) | 5 | (5) | 0.4 | 0.160 | 1.0 |
| Residential oral flora | 25 | (23) | 5 | (5) | 0.5 | 0.301 | 1.0 |
| Actinomycetaceae | 6 | (5) | 1 | (1) | 0.7 | 0.561 | 1.0 |
| Bacteroidaceae | 8 | (7) | 1 | (1) | 1 | 1.000 | 1.0 |
| Clostridiaceae | 4 | (4) | 0 | (0) | Inf | 1.000 | 1.0 |
| Enterobacteriaceae | 26 | (23) | 6 | (5) | 0.4 | 0.101 | 1.0 |
| Enterococcaceae | 5 | (5) | 0 | (0) | Inf | 1.000 | 1.0 |
| Lactobacillaceae | 7 | (6) | 1 | (1) | 0.8 | 1.000 | 1.0 |
| Neisseriaceae | 22 | (20) | 2 | (2) | 1.4 | 1.000 | 1.0 |
| Pasteurellaceae | 9 | (8) | 3 | (3) | 0.3 | 0.120 | 1.0 |
| Prevotellaceae | 6 | (5) | 0 | (0) | Inf | 1.000 | 1.0 |
| Saccharomycetaceae | 18 | (16) | 2 | (2) | 1.1 | 1.000 | 1.0 |
| Staphylococcaceae | 4 | (4) | 1 | (1) | 0.5 | 0.442 | 1.0 |
| Streptococcaceae | 34 | (31) | 3 | (3) | 1.6 | 0.747 | 1.0 |
| Others | 5 | (5) | 0 | (0) | Inf | 1.000 | 1.0 |
| No growth | 7 | (6) | 0 | (0) | Inf | 1.000 | 1.0 |
*Fisher’s Exact.
To evaluate potential risk factors for Actinomyces-associated MRONJ, patients were compared with respect to selected demographic and clinicopathological characteristics (Table 3). In this analysis, none of the parameters evaluated separated patients positive for Actinomyces spp. clearly from those without detectable infection.
Table 3. Risk factors for detection of Actinomyces spp. in MRONJ lesions.
| Characteristic | Actinomyces spp. detection by histology | OR (95%-CI) | p-value* | Adjusted p | |||
|---|---|---|---|---|---|---|---|
| Positive, no. (%) | Negative, no. (%) | ||||||
| Bisphosphonate | — | 0.830# | 1.0 | ||||
| Zoledronate | 58 | (52) | 9 | (8) | |||
| Combination therapy | 19 | (17) | 2 | (2) | |||
| Alendronate | 11 | (10) | 1 | (1) | |||
| Ibandronate | 6 | (5) | 0 | (0) | |||
| Risedronate | 2 | (2) | 0 | (0) | |||
| N/A | 2 | (2) | 0 | (0) | |||
| Pamidronate | 1 | (1) | 0 | (0) | |||
| Administration route | 1.86 (0.22,15.52) | 0.540 | 1.0 | ||||
| intravenous | 83 | (75) | 11 | (10) | |||
| oral | 14 | (12) | 1 | (1) | |||
| N/A | 2 | (2) | 0 | (0) | |||
| Primary disease | 0.63 (0.13,3.05) | 0.545 | 1.0 | ||||
| Osteoporosis | 24 | (21) | 2 | (2) | |||
| Cancer+ | 75 | (68) | 10 | (9) | |||
| Type of malignancy | |||||||
| Breast | 24 | (21) | 5 | (4) | |||
| Multiple Myeloma | 16 | (14) | 5 | (5) | |||
| Prostate | 13 | (11) | 0 | (0) | |||
| Renal | 9 | (8) | 0 | (0) | |||
| Lung | 6 | (5) | 2 | (2) | |||
| Others | 5 | (4) | 0 | (0) | |||
| Malignant bone disease | 63 | (57) | 11 | (10) | 0.16 (0.02,1.28) | 0.031 | 0.744 |
| Chemotherapy | 0.84 (0.09,7.45) | 0.876 | 1.0 | ||||
| Current§ | 7 | (6) | 1 | (1) | |||
| Former$/Never | 92 | (83) | 11 | (10) | |||
| Smoking | 6.29 (0.78,50.7) | 0.031 | 0.744 | ||||
| Current | 36 | (32) | 1 | (1) | |||
| Former$/Never | 63 | (57) | 11 | (10) | |||
| Alcohol | 1.78 (0.37,8.67) | 0.452 | 1.0 | ||||
| Yes | 26 | (23) | 2 | (2) | |||
| No | 73 | (66) | 10 | (9) | |||
| Diabetes | 1.69 (0.35,8.24) | 0.497 | 1.0 | ||||
| Yes | 25 | (22) | 2 | (2) | |||
| No | 74 | (67) | 10 | (9) | |||
| Obesity | 22028341.47 (0,Inf) | 0.018 | 0.450 | ||||
| BMI > 30 | 18 | (16) | 0 | (0) | |||
| BMI ≤ 30 | 63 | (57) | 12 | (11) | |||
| N/A | 18 | (16) | 0 | (0) | |||
N/A, data not available; *logistic regression analysis; #chi squared test; +nine patients with cancer additionally suffered from osteoporosis; §three patients additionally received high dosage corticosteroids; $history of chemotherapy or smoking was defined by patients’ state at diagnosis of MRONJ.
Discussion
MRONJ affects up to 10% of cancer patients treated with antiresorptive drugs2. The disease is associated with significantly increased morbidity and decreased quality of life. Treatment is associated with high rates of treatment failures and recurrences. Prophylactic and therapeutic strategies are impeded by the very limited understanding of pathomechanisms relevant to the evolution of MRONJ3,20,21. In the present study, we substantiate previous evidence that a large majority of MRONJ lesions are infected by Actinomyces spp. We failed to identify clear risk factors for Actinomyces spp. positivity in MRONJ cases – possibly because of the small number of Actinomyces spp. negative cases. As a consequence of these results, current recommendations may have to be revised and adapted to the observation that MRONJ is frequently associated with invasive Actinomyces spp. infection.
The detection of Actinomyces spp. in 89% of bone specimens by histology is remarkable but may still be a significant underestimation of the factual frequency of MRONJ associated with this infection. Sensitivity of histological evaluation of clinical specimens for microbes is almost universally lower than bacterial culture because the latter involves an amplification step that increases the number of diagnostic targets by several log-titers.
Nevertheless, bacterial culture of bone specimens was negative for Actinomyces spp. in all but 6% of samples. Actinomyces spp. are fastidious and successful isolation requires culture of specimens under anaerobic or microaerophilic conditions for prolonged periods22,23. Sensitivity of culture is further reduced significantly by antimicrobial treatment of patients before sample collection24,25, which was the case in most of the present patients. To increase detection rates by microbial culture, antimicrobial therapy should be discontinued for a few weeks before surgery and collection of clinical samples. Nevertheless, cessation of antimicrobial treatment is difficult to be justified giving the risk of progression and the high sensitivity and specificity of histopathology for the diagnosis of actinomycosis. Antimicrobial treatment before sample collection also changes very likely the oropharyngeal microbiome and microbiological culture results have to be interpreted with caution in bone samples collected from MRONJ cases.
In contrast to microbiological culture, detection of the Actinomyces spp. by histology together with pathognomonic sulfur granules and signs of subacute or chronic inflammation, granulation tissue, and osteonecrosis substantiate a possible causal link. Actinomyces spp. may be detected reliably in affected bone specimens because of their morphologic appearance upon staining with PAS, Gram and GMS19. Nevertheless, histology requires at least several days to provide a reliable diagnosis. In addition, large and high-quality bone specimens are required for a reliable histological diagnosis. The focus of the present study on patients from whom a sufficiently large bone specimen was available for histological evaluation may be associated with a selection bias towards cases of more severe disease that more likely are treated by respective surgery. A rapid diagnosis with epidemiological information on antimicrobial resistance patterns of clinical isolates is important for treatment success. Accordingly, novel assays for the fast and reliable diagnosis of Actinomyces-associated MRONJ are urgently required.
The clinical presentation of “classical”, oropharyngeal actinomycosis and MRONJ-associated actinomycosis differ significantly. Classical actinomycosis manifests clinically as chronic, granulomatous abscess with tissue fibrosis and draining sinuses and unresponsiveness to empiric antimicrobial therapy of a presumed abscess. The face and neck are the most common sites of actinomycosis in humans followed by thoracic, abdomino-pelvic, cerebral and skin infections26. Actinomycosis is generally considered an uncommon disease that affects patients of all ages and can appear in both immunocompetent and immunocompromised individuals27. In contrast, MRONJ-associated actinomycosis affects primarily cancer patients and is limited in most cases to the jaw bone. Macroscopically visible sulphur granules that are pathognomonic for classical actinomycosis are not visible in MRONJ-associated actinomycosis. Risk factors identified presently in association with detection of Actinomyces spp. have to be interpreted with caution in view of the small numbers of Actinomyces-negative patients. Hence, MRONJ-associated actinomycosis differs in terms of optimum diagnostic approach and clinical presentation clearly from classical oro-pharyngeal actinomycosis.
To attain an optimum treatment success, the antimicrobial regimen for actinomycosis-associated MRONJ prior to surgery still has to be defined despite the exquisite sensitivity of Actinomyces spp. to betalactam agents. Smith et al. proposed as possible scheme for the antibiotic treatment of cervicofacial actinomycoses may consist of amoxicillin plus clavulanic acid or also ampicillin plus sulbactam based on the frequent detection of mixed infections28. The rational mentioned for this broad-spectrum antimicrobial treatment was poor treatment success achieved in early studies with low-dose penicillin G and a mixed flora of anaerobic and aerobic pathogens commonly recovered along with Actinomyces spp28. In our experience, however, patients with orocervicofacial actinomycosis respond well to antimicrobial therapy with a betalactam agent only indicative of the low relevance of concomitantly isolated facultative pathogens23. Moreover, our present observations may also indicate that cancer patients receiving antiresorptive drugs may benefit from antimicrobial prophylaxis concomitant with dentoalveolar surgery.
Betalactam agents have a high therapeutic index which allows safe administration of high doses of drugs and high therapeutic drug levels are achieved in serum, tissues, bile, and synovial fluid. We found recently that Actinomyces spp. isolates are universally susceptible to betalactam antimicrobial agents15. Treatment without ß-lactamase inhibitor reduces also significantly the rate of gastrointestinal adverse events such as abdominal discomfort or diarrhea for any cause, including Clostridium difficile enterocolitis. Accordingly, we recommend for the treatment of actinomycosis in concordance with others the use of a betalactam antimicrobial agents at high daily doses prior to final surgical treatment of MRONJ23. For patients with penicillin allergy, tetracyclines are good alternative for oral therapy, especially in milder disease presentations. In severe infections, carbapenems or the newer compound tigecyclin may be appropriate therapeutic options15,29.
In conclusion, we found that MRONJ is very frequently associated with the detection of Actinomyces spp. by histological evaluation of specimens from necrotic bone. The generally limited sensitivity of histology for the detection of microbes may have further underestimated the factual incidence of Actinomycosis in these patients. The observation of a frequent infection of necrotic bone by Actinomyces spp. may suggest that standard surgical treatment of MRONJ may be complemented by antimicrobial treatment.
Additional Information
How to cite this article: Russmueller, G. et al. The association of medication-related osteonecrosis of the jaw with Actinomyces spp. infection. Sci. Rep. 6, 31604; doi: 10.1038/srep31604 (2016).
Acknowledgments
The authors would like to thank Ms. Andrea Graf for her work in the microbiological laboratory and figure provision.
Footnotes
Author Contributions G.R.,C.S., C.P. and T.F. wrote the main manuscript. R.S. performed the complete statistical analysis. K.W., V.S. and M.S. were responsible for data acquisition and processing. B.W. and I.S. provided histological and microbiological data and figures. All authors reviewed the manuscript.
References
- Edwards B. J. et al. Pharmacovigilance and reporting oversight in US FDA fast-track process: bisphosphonates and osteonecrosis of the jaw. The Lancet. Oncology 9, 1166–1172 (2008). [DOI] [PubMed] [Google Scholar]
- Ruggiero S. L. et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw-2014 update. J Oral Maxillofac Surg 72, 1938–1956 (2014). [DOI] [PubMed] [Google Scholar]
- Fliefel R., Troltzsch M., Kuhnisch J., Ehrenfeld M. & Otto S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: a systematic review. Int J Oral Maxillofac Surg 44, 568–585 (2015). [DOI] [PubMed] [Google Scholar]
- Estilo C. L. et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. The oncologist 13, 911–920 (2008). [DOI] [PubMed] [Google Scholar]
- Saad F., Chi K. & Fleshner N. The role of bisphosphonates in the management of bone metastases in prostate cancer. The Canadian journal of urology 11, 2376–2382 (2004). [PubMed] [Google Scholar]
- Letocha A. D. et al. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res 20, 977–986 (2005). [DOI] [PubMed] [Google Scholar]
- Bamias A. et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 23, 8580–8587 (2005). [DOI] [PubMed] [Google Scholar]
- Aragon-Ching J. B. et al. Higher incidence of Osteonecrosis of the Jaw (ONJ) in patients with metastatic castration resistant prostate cancer treated with anti-angiogenic agents. Cancer Invest 27, 221–226 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. R. & Burr D. B. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg 67, 61–70 (2009). [DOI] [PubMed] [Google Scholar]
- De Ceulaer J., Tacconelli E. & Vandecasteele S. J. Actinomyces osteomyelitis in bisphosphonate-related osteonecrosis of the jaw (BRONJ): the missing link? European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology 33, 1873–1880 (2014). [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Pien F. D. & Suzuki J. B. Identification and treatment of bisphosphonate-associated actinomycotic osteonecrosis of the jaws. Implant Dent 20, 331–336 (2011). [DOI] [PubMed] [Google Scholar]
- Schipmann S. et al. Osteopathology associated with bone resorption inhibitors - which role does Actinomyces play? A presentation of 51 cases with systematic review of the literature. J Oral Pathol Med 42, 587–593 (2013). [DOI] [PubMed] [Google Scholar]
- Badros A. et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol 24, 945–952 (2006). [DOI] [PubMed] [Google Scholar]
- Moghimi M., Salentijn E., Debets-Ossenkop Y., Karagozoglu K. H. & Forouzanfar T. Treatment of cervicofacial actinomycosis: a report of 19 cases and review of literature. Med Oral Patol Oral Cir Bucal 18, e627–632 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger C. & Willinger B. Resistance patterns in clinical isolates of pathogenic Actinomyces species. J Antimicrob Chemother 71, 422–427 (2016). [DOI] [PubMed] [Google Scholar]
- Wutzl A. et al. Treatment results of bisphosphonate-related osteonecrosis of the jaws. Head Neck 30, 1224–1230 (2008). [DOI] [PubMed] [Google Scholar]
- Holzinger D. et al. Long-term success of surgery in bisphosphonate-related osteonecrosis of the jaws (BRONJs). Oral Oncol 49, 66–70 (2013). [DOI] [PubMed] [Google Scholar]
- Hansen T. et al. Actinomycosis of the jaws--histopathological study of 45 patients shows significant involvement in bisphosphonate-associated osteonecrosis and infected osteoradionecrosis. Virchows Arch 451, 1009–1017 (2007). [DOI] [PubMed] [Google Scholar]
- Lo Muzio L. et al. The contribution of histopathological examination to the diagnosis of cervico-facial actinomycosis: a retrospective analysis of 68 cases. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology 33, 1915–1918 (2014). [DOI] [PubMed] [Google Scholar]
- Migliorati C. A., Siegel M. A. & Elting L. S. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. The Lancet. Oncology 7, 508–514 (2006). [DOI] [PubMed] [Google Scholar]
- Reid I. R. Osteonecrosis of the jaw: who gets it, and why? Bone 44, 4–10 (2009). [DOI] [PubMed] [Google Scholar]
- Holmberg K., Nord C. E. & Dornbusch K. Antimicrobial in vitro susceptibility of actinomyces israelii and arachnia propionica. Scandinavian journal of infectious diseases 9, 40–45 (1977). [DOI] [PubMed] [Google Scholar]
- Smego R. A. Jr. & Foglia G. Actinomycosis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 26, 1255-1261; quiz 1262–1253 (1998). [DOI] [PubMed] [Google Scholar]
- Kononen E. & Wade W. G. Actinomyces and related organisms in human infections. Clinical microbiology reviews 28, 419–442 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody J. W. Jr. & Seabury J. H. Actinomycosis and nocardiosis. A review of basic differences in therapy. Am J Med 28, 99–115 (1960). [DOI] [PubMed] [Google Scholar]
- Nedomansky J., Weiss D., Willinger B., Nickl S. & Steininger C. Acne inversa complicated by Actinomyces neuii. Infection (2015). [DOI] [PubMed] [Google Scholar]
- Wong V. K., Turmezei T. D. & Weston V. C. Actinomycosis. BMJ 343, d6099 (2011). [DOI] [PubMed] [Google Scholar]
- Smith A. J., Hall V., Thakker B. & Gemmell C. G. Antimicrobial susceptibility testing of Actinomyces species with 12 antimicrobial agents. J Antimicrob Chemother 56, 407–409 (2005). [DOI] [PubMed] [Google Scholar]
- Martin M. V. Antibiotic treatment of cervicofacial actinomycosis for patients allergic to penicillin: a clinical and in vitro study. Br J Oral Maxillofac Surg 23, 428–434 (1985). [DOI] [PubMed] [Google Scholar]