Abstract
Tupistra chinensis is widely distributed in southwestern China and its rhizome is a famous folk medicine for the treatment of carbuncles and pharyngitis. Its chemical identity of potent antiproliferative and anti-inflammatory constituents has been carried out in this study. Twenty-three polyhydroxylated spirostanol saponins, including nine novels, were isolated and identified. The new spirostanol saponins were elucidated as spirost-25(27)-en-1β,2β,3β,4β,5β-pentol-2-O-β-D-xylopyranoside (1), spirost-25(27)- en-1β,2β,3β,4β,5β-pentol-2-O-α-L-arabinopyranoside (2), spirost-25(27)-en- 1β,3α,5β-triol (12), spirost-25(27)-en-1β,3α,4β,5β,6β-pentol (13), spirost-25(27)-en- 1β,2β,3β,5β-tetraol-5-O-β-D-glucopyranoside (16), 5β-spirost-25(27)-en-1β,3β-diol- 3-O-β-D-glucopyranosyl-(1 → 4)-β-D-glucopyranoside (17), (25R)-5β-spirostan- 1β,3β-diol-3-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranoside (18), (25R)-5β- spirostan-1β,3β-diol-3-O-β-D-fructofuranosyl-(2 → 6)-β-D-glucopyranoside (19), 5β-spirost-25(27)-en-3β-ol-3-O-β-D-glucopyranosyl-(1 → 4)-β-D-glucopyranoside (20). The antiproliferative effects against seven human cancer cell lines and inhibitory activities on nitric oxide (NO) production induced by lipopolysaccharide (LPS) in a macrophage cell line RAW 264.7 were assayed for all the isolated compounds. Compounds 17, 19 and 21 exhibited potential antiproliferative activities against all of human cancer cell lines tested. Compounds 21 showed significant inhibition on NO production with IC50 values of 11.5 μM. These results showed that the spirostanol saponins isolated from the dried rhizomes of T. chinensis have potent antiproliferative and anti-inflammatory activities and T. chinensis might be used as anticancer and.anti-inflammatory supplement.
Medicinal plants have been used for treating human diseases and they produced a large variety of secondary metabolites such as alkaloids, flavonoids, triterpenoids and steroidal glycosides1. Steroidal glycosides as the important secondary metabolites of medicinal plants have been reported to possess a wide range of biological activities including anticancer, anti-inflammatory, platelet aggregation inhibition, antihypertensive, cholesterol lowering, antifungal and antiviral2. Additionally, steroidal glycosides have a wide variety of commercial uses such as surfactants, foaming agents and precursors for the industrial production of pharmaceutical drugs3,4.
A series of widespread chronic diseases such as cancer are now the leading causes of morbidity and mortality worldwide. Many cancers arise from sites of infection, chronic irritation and inflammation. Recent data have expanded the concept that inflammation is a critical component of tumor progression. Anti-inflammatory therapy is efficacious towards early neoplastic progression and malignant conversion5,6. Many studies have showed that biological activities of phytochemicals are comparable to previous synthetic compounds, and they have low side effects. Therefore, recent interests in medicinal plants have been focused on identifying active compounds and elucidating underlying molecular mechanisms of action7,8.
The genus Tupistra (Liliaceae) has 12 species in southern China. These species possess similar morphologic characteristics and some can be substituted for each other as a folk medicine to treat pharyngolaryngitis, rheumatic diseases and snake-bite9. Tupistra chinensis is widely distributed in southwestern China and its dried rhizome is a famous folk medicine for the treatment of carbuncles and pharyngitis. Previous phytochemical investigations on T. chinensis have led to the isolation of a variety of biologically active compounds, including steroidal sapogenins and their glycosides10,11,12,13,14,15, cardenolides16, a pregnane genin and its glycoside17,18, flavonoids17,18,19, which have anti-inflammatory20, cytotoxicity21,22,23 and antifungal activities24,25. Steroidal saponins that consist of spirostanol saponins and furostanol saponins were the most abundant active constituents in T. chinensis.
In the course of our continuing search for bioactive leading compounds from the medicinal herbs, we focused on steroidal saponins from the dried rhizomes of T. chinensis and obtained twenty-three polyhydroxylated spirostanol saponins including nine new compounds. The structures of all the isolated compounds were elucidated on the basis of spectroscopic data and chemical methods, including IR, NMR, MS, and GC analysis. Moreover, all of the isolated compounds were evaluated for their antiproliferative activity against seven human cancer cell lines and the inhibitory activities on NO production induced by LPS in a macrophage cell line RAW 264.7.
Results and Discussion
Structure identification
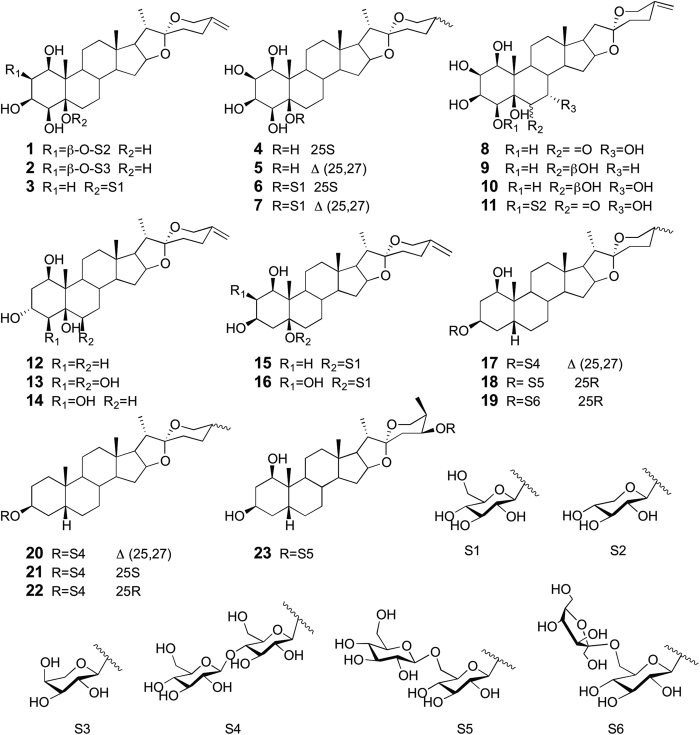
Nine new spirostanol saponins (1, 2, 12, 13, 16–20) and fourteen congeners (3–11, 14, 15, 21–23) were obtained from the 60% ethanol extract of the rhizomes of T. chinensis. Their chemical structures are shown in Fig. 1. The structures of all the isolated compounds were elucidated on the basis of spectroscopic data and chemical methods, including IR, NMR, MS, and GC analysis. The fourteen known spirostanols were identified by comparison of their reported spectroscopic data as spirost-25(27)-en-1β,3β,4β,5β-tetrol-5-O-β-D-glucopyranoside (3)23, neopentrogenin (4)26, Δ25(27)-pentrogenin (5)12, neopentrogenin-5-O-β-D- glucopyranoside (6)26, spirost-25(27)-en-1β,2β,3β,4β,5β-pentol-5-O-β-D- glucopyranoside (7)26, spirost-25(27)-en-1β,2β,3β,4β,5β,7α-hexaol-6-one (8)15, wattigenin A (9)27, spirost-25(27)-en-1β,2β,3β,4β,5β,6β,7α-heptol (10)28, spirost-25(27)-en-1β,2β,3β,4β,5β,7α-hexaol-6-one-4-O-β-D-xylopyranoside (11)29, tupichigenin D (14)18, spirost-25(27)-en-1β,3β,5β-triol-5-O-β-D-glucopyranoside (15)23, (25S)-5β-spirostan-3β-ol-3-O-β-D-glucopyranosyl-(1 → 4)-β-D- glucopyranoside (21)30, (25R)-5β-spirostan-3β-ol-3-O-β-D-glucopyranosyl-(1 → 4)- β-D-glucopyranoside (22)30, wattoside I (23)9. Compounds 4, 6, 7, 21 and 22 are reported for the first time from T. chinensis, to our knowledge. The NMR data of the known compounds could be found in the supplementary material.
Figure 1. Structures of isolated compounds 1–23.
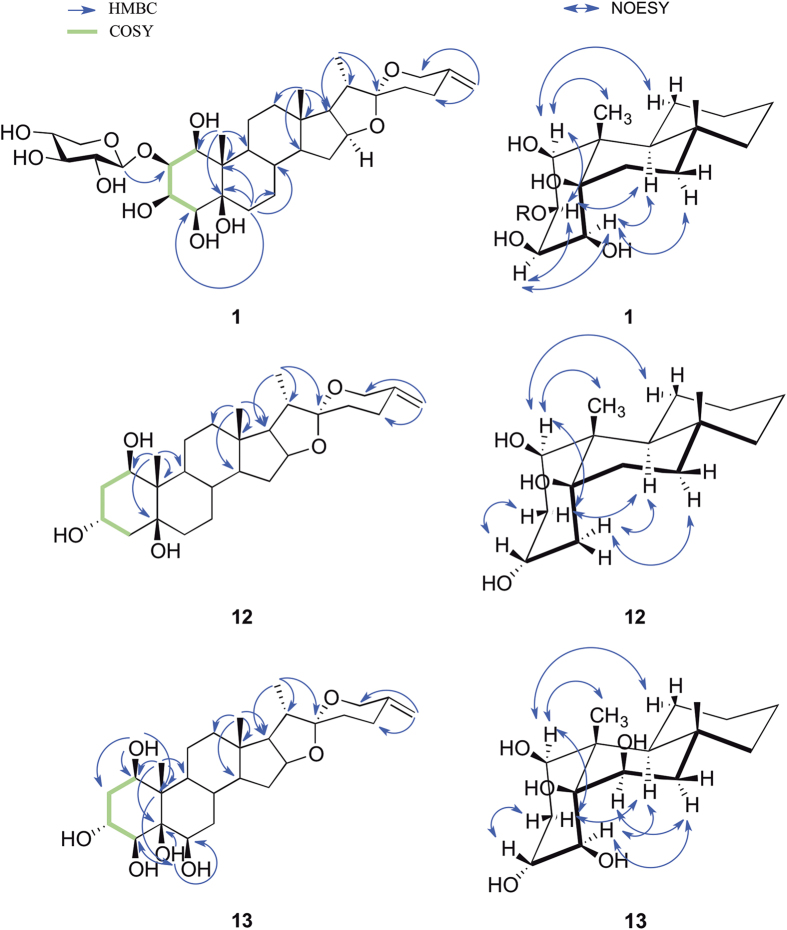
Compound 1 was isolated as white amorphous powder, 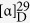 −43.3 (c 0.25, MeOH). The molecular formula was inferred as C32H50O11 according to the positive-ion HRESI-MS peak at m/z [M + H]+ 611.3437 (calcd for C32H51O11 [M + H]+, 611.3431). The IR spectrum showed a strong absorption band at 3364 cm−1, ascribable to hydroxyl functionalities. Acid hydrolysis and GC analysis of 1 gave D-xylose. The 1H NMR spectrum showed three typical steroidal methyl signals at δH 1.59 (3H, s, Me-19), 1.09 (3H, d, J = 6.9 Hz, Me-21) and 0.84 (3H, s, Me-18) (Table 1). Four methane protons indicative of secondary alcoholic functions at δH 4.57 (1H, brs, H-1), 4.41 (1H, m, H-2), 5.03 (1H, brs, H-3) and 4.31 (1H, brs, H-4) were observed. The germinal protons at C-27 were observed at δH 4.57 and 4.82 as two singlets, which are characteristic of an exocyclic methylene. The 13C NMR spectrum exhibited characteristic spirostanol carbon signal at δC 109.8 (C-22) and three methyl (δC 16.8, 13.9, and 15.3), and two olefinic carbons δC 144.7 and 109.1 were assigned to the C-25 and C-27 positions, respectively, diagnostic of C-25 (27)-unsaturated sapogenin. The NMR spectrum of 1 showed an anomeric proton signal at δH 5.15 (1H, d, 7.5 Hz), corresponding to an anomeric carbon at δC 103.5 in the HSQC spectrum, indicated the presence of one sugar moiety. Unambiguous complete assignments for the 1H and 13C NMR signals were made by combination of DEPT, 1H − 1H COSY, HSQC, HMBC, and NOESY spectra. The 1H − 1H COSY spectrum showed that the oxymethine proton δH 4.41 (H-2) was coupled to δH 4.57 (1H, brs, H-1) and 5.03 (1H, brs, H-3), and the oxymethine proton δH 5.03 (H-3) was coupled to the oxymethine proton at δH 4.31 (H-4). These findings supported the location of the hydroxyl groups at C-1, C-2, C-3, C-4, and C-5, together with the long-range correlations observed in the HMBC spectrum (Fig. 2). The HMBC correlations of H-1 (δH 5.15) of xylose with C-2 (δC 74.4) of the aglycon revealed the sugar residue attached to C-2 of the aglycon. The stereochemistry of the ring junctions and substituents of the aglycon of 1 were determined via cross-peaks observed in a NOESY spectrum according to the refs 12,15 and 23 (Fig. 2). The key correlations between H-4α and H-7α/H-9α, and between H-2α/H-9α, supported the A/B cis ring junction pattern. Thus, the hydroxyl groups at C-5 and C-2 have a β-orientation. Additional NOE correlations between H-1α and Me-19/H-11, and between H-3α and H-2α/H-4α, were indicative of β-orientations for OH-1, OH-3 and OH-4. Thus, compound 1 was elucidated as spirost-25(27)-en-1β,2β,3β,4β,5β-pentol-2-O-β-D-xylopyranoside.
−43.3 (c 0.25, MeOH). The molecular formula was inferred as C32H50O11 according to the positive-ion HRESI-MS peak at m/z [M + H]+ 611.3437 (calcd for C32H51O11 [M + H]+, 611.3431). The IR spectrum showed a strong absorption band at 3364 cm−1, ascribable to hydroxyl functionalities. Acid hydrolysis and GC analysis of 1 gave D-xylose. The 1H NMR spectrum showed three typical steroidal methyl signals at δH 1.59 (3H, s, Me-19), 1.09 (3H, d, J = 6.9 Hz, Me-21) and 0.84 (3H, s, Me-18) (Table 1). Four methane protons indicative of secondary alcoholic functions at δH 4.57 (1H, brs, H-1), 4.41 (1H, m, H-2), 5.03 (1H, brs, H-3) and 4.31 (1H, brs, H-4) were observed. The germinal protons at C-27 were observed at δH 4.57 and 4.82 as two singlets, which are characteristic of an exocyclic methylene. The 13C NMR spectrum exhibited characteristic spirostanol carbon signal at δC 109.8 (C-22) and three methyl (δC 16.8, 13.9, and 15.3), and two olefinic carbons δC 144.7 and 109.1 were assigned to the C-25 and C-27 positions, respectively, diagnostic of C-25 (27)-unsaturated sapogenin. The NMR spectrum of 1 showed an anomeric proton signal at δH 5.15 (1H, d, 7.5 Hz), corresponding to an anomeric carbon at δC 103.5 in the HSQC spectrum, indicated the presence of one sugar moiety. Unambiguous complete assignments for the 1H and 13C NMR signals were made by combination of DEPT, 1H − 1H COSY, HSQC, HMBC, and NOESY spectra. The 1H − 1H COSY spectrum showed that the oxymethine proton δH 4.41 (H-2) was coupled to δH 4.57 (1H, brs, H-1) and 5.03 (1H, brs, H-3), and the oxymethine proton δH 5.03 (H-3) was coupled to the oxymethine proton at δH 4.31 (H-4). These findings supported the location of the hydroxyl groups at C-1, C-2, C-3, C-4, and C-5, together with the long-range correlations observed in the HMBC spectrum (Fig. 2). The HMBC correlations of H-1 (δH 5.15) of xylose with C-2 (δC 74.4) of the aglycon revealed the sugar residue attached to C-2 of the aglycon. The stereochemistry of the ring junctions and substituents of the aglycon of 1 were determined via cross-peaks observed in a NOESY spectrum according to the refs 12,15 and 23 (Fig. 2). The key correlations between H-4α and H-7α/H-9α, and between H-2α/H-9α, supported the A/B cis ring junction pattern. Thus, the hydroxyl groups at C-5 and C-2 have a β-orientation. Additional NOE correlations between H-1α and Me-19/H-11, and between H-3α and H-2α/H-4α, were indicative of β-orientations for OH-1, OH-3 and OH-4. Thus, compound 1 was elucidated as spirost-25(27)-en-1β,2β,3β,4β,5β-pentol-2-O-β-D-xylopyranoside.
Table 1. NMR spectroscopic data for compounds 1 and 2 (pyridine-d5).
| Position | 1 |
2 |
||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 77.8 | 4.57, brs | 77.8 | 4.58, m |
| 2 | 74.4 | 4.41, m | 74.0 | 4.41, m |
| 3 | 72.8 | 5.03, brs | 72.8 | 5.03, brs |
| 4 | 68.7 | 4.31, brs | 68.7 | 4.31, brs |
| 5 | 78.2 | 78.2 | ||
| 6 | 30.5 | 2.48, d (13.2) | 30.5 | 2.48, d (13.2) |
| 1.67, m | 1.67, m | |||
| 7 | 28.8 | 1.54, m | 28.8 | 1.50, m |
| 1.14, m | 1.10, m | |||
| 8 | 35.2 | 1.71, m | 35.2 | 1.71, m |
| 9 | 45.7 | 1.22, m | 45.7 | 1.20, m |
| 10 | 45.5 | 45.5 | ||
| 11 | 21.9 | 1.58, m | 21.9 | 1.57, m |
| 1.45, m | 1.41, m | |||
| 12 | 40.2 | 1.59, m | 40.2 | 1.58, m |
| 1.03, m | 1.01, m | |||
| 13 | 40.9 | 40.9 | ||
| 14 | 56.4 | 1.03, m | 56.4 | 1.01, m |
| 15 | 32.5 | 2.03, m | 32.5 | 2.02, m |
| 1.44, m | 1.43, m | |||
| 16 | 81.7 | 4.59, m | 81.8 | 4.58, m |
| 17 | 63.3 | 1.81, m | 63.4 | 1.80, m |
| 18 | 16.8 | 0.84, s | 16.8 | 0.83, s |
| 19 | 13.9 | 1.59, s | 13.9 | 1.58, s |
| 20 | 42.2 | 1.96, m | 42.2 | 1.95, m |
| 21 | 15.3 | 1.09, d (7.0) | 15.3 | 1.09, d (7.0) |
| 22 | 109.8 | 109.8 | ||
| 23 | 33.5 | 1.81, m | 33.5 | 1.76–1.79, m |
| 24 | 29.3 | 2.70, m | 29.3 | 2.70, m |
| 2.25, d (11.5) | 2.24, d (11.5) | |||
| 25 | 144.7 | 144.7 | ||
| 26 | 65.4 | 4.47, d (12.1) | 65.4 | 4.47, d (12.1) |
| 4.04, d (12.1) | 4.04, d (12.1) | |||
| 27 | 109.1 | 4.82, s | 109.1 | 4.81, s |
| 4.78, s | 4.78, s | |||
| 2-O-Xyl | 2-O-Ara | |||
| 1 | 103.5 | 5.15, d (7.5) | 103.4 | 5.13, d (7.0) |
| 2 | 75.4 | 4.08, m | 72.8 | 4.56, m |
| 3 | 78.7 | 4.18, m | 74.8 | 4.22, m |
| 4 | 71.4 | 4.24, m | 69.7 | 4.36, m |
| 5 | 67.7 | 4.40, m | 67.3 | 4.38, m |
| 3.76, m | 3.85, m | |||
Figure 2. Selected 1H − 1H COSY, HMBC and NOESY correlations of compounds 1, 12 and 13.
Compound 2 was isolated as white amorphous powder, with the same molecular formula of C32H50O11 as 1 on the basis of HRESIMS (m/z 611.3412 [M + H]+). Acid hydrolysis of 2 gave L-arabinose. From a comparison of 1H and 13C NMR data of 2 with those of 1 (Table 1), it was apparent that 2 contained the same aglycon as 1, except for a little different in the monosaccharide chain. Instead of the signals for a xylopyranosyl moiety, signals assignable to an α-L-arabinopyranosyl residue were observed at δH 5.13 (1H, d, J = 7.0 Hz, H-1), 4.56 (1H, m, H-2), 4.22 (1H, m, H-3), 4.36 (1H, m, H-4), 4.38 (1H, m, H-5a) and 3.85 (1H, m, H-5b), and δC 103.4 (C-1), 72.8 (C-2), 74.8 (C-3),69.7 (C-4), 67.3 (C-5). The linkage position of the arabinosyl moiety to C-2 of the aglycone was confirmed by an HMBC correlation between the anomeric proton at δH 5.13 and the C-2 carbon of the aglycone at δC 74.0. Therefore, the structure of 2 was elucidated as spirost-25(27)-en-1β,2β,3β,4β,5β-pentol- 2-O-α-L-arabinopyranoside.
Compound 12 was isolated as white amorphous powder, 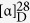 −33.1 (c 0.25, MeOH). The HRESIMS gave an ion at m/z 447.3112 [M + H]+ (calcd for C27H43O5, 447.3110), consistent with the molecular formula C27H42O5. The 1H NMR spectrum showed three typical steroidal methyl signals at δH 1.50 (3H, s, Me-19), 1.12 (3H, d, J = 7.0 Hz, Me-21) and 0.87 (3H, s, Me-18) (Table 2). The 13C NMR spectrum exhibited characteristic spirostanol carbon signal at δC 109.8 (C-22) and three methyl (δC 16.9, 13.7, and 15.4), and two olefinic carbons δC 144.8 and 109.1 were observed, diagnostic of C-25 (27)-unsaturated sapogenin. The 1H − 1H COSY spectrum showed that two methylene protons at δ 2.12 (H-2α) and 2.61 (H-2β) were coupled to δ 4.31 (H-1) and 5.13 (H-3), and the oxymethine proton δ 5.13 (H-3) was coupled to two methylene protons at δ 2.26 (H-4β) and 2.61 (H-4α). These findings indicated the location of the hydroxyl groups at C-1, C-3, and C-5, together with the long-range correlations observed in the HMBC spectrum (Fig. 2). The key NOESY correlations (Fig. 2) between H-4α and H-7α/H-9α, and between H-2α and H-9α, supported the A/B cis ring junction pattern. Thus, the hydroxyl group at C-5 has a β-orientation. Additional NOE correlations between H-1α and Me-19/H-11, and between H-3β and H-2β/H-4β, were indicative of β-orientation for OH-1 and α-orientation for OH-3. On the basis of the above analysis, compound 12 was finally assigned to be spirost-25(27)-en-1β,3α,5β-triol.
−33.1 (c 0.25, MeOH). The HRESIMS gave an ion at m/z 447.3112 [M + H]+ (calcd for C27H43O5, 447.3110), consistent with the molecular formula C27H42O5. The 1H NMR spectrum showed three typical steroidal methyl signals at δH 1.50 (3H, s, Me-19), 1.12 (3H, d, J = 7.0 Hz, Me-21) and 0.87 (3H, s, Me-18) (Table 2). The 13C NMR spectrum exhibited characteristic spirostanol carbon signal at δC 109.8 (C-22) and three methyl (δC 16.9, 13.7, and 15.4), and two olefinic carbons δC 144.8 and 109.1 were observed, diagnostic of C-25 (27)-unsaturated sapogenin. The 1H − 1H COSY spectrum showed that two methylene protons at δ 2.12 (H-2α) and 2.61 (H-2β) were coupled to δ 4.31 (H-1) and 5.13 (H-3), and the oxymethine proton δ 5.13 (H-3) was coupled to two methylene protons at δ 2.26 (H-4β) and 2.61 (H-4α). These findings indicated the location of the hydroxyl groups at C-1, C-3, and C-5, together with the long-range correlations observed in the HMBC spectrum (Fig. 2). The key NOESY correlations (Fig. 2) between H-4α and H-7α/H-9α, and between H-2α and H-9α, supported the A/B cis ring junction pattern. Thus, the hydroxyl group at C-5 has a β-orientation. Additional NOE correlations between H-1α and Me-19/H-11, and between H-3β and H-2β/H-4β, were indicative of β-orientation for OH-1 and α-orientation for OH-3. On the basis of the above analysis, compound 12 was finally assigned to be spirost-25(27)-en-1β,3α,5β-triol.
Table 2. NMR spectroscopic data for compounds 12 and 13 (pyridine-d5).
| Position | 12 |
13 |
||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 75.9 | 4.31, brs | 76.2 | 4.19, brs |
| 2 | 38.9 | 2.61, m | 36.6 | 2.62, m |
| 2.12, t (11.1) | 2.23, m | |||
| 3 | 63.7 | 5.13, m | 69.2 | 4.85, m |
| 4 | 44.0 | 2.61, m | 75.9 | 4.34, d (9.1) |
| 2.26, m | ||||
| 5 | 77.4 | 80.7 | ||
| 6 | 36.7 | 1.98, m | 69.5 | 4.94, s |
| 1.58, d (13.2) | ||||
| 7 | 29.2 | 1.49, m | 35.4 | 2.07, m |
| 1.07, m | 1.55, m | |||
| 8 | 35.4 | 1.66, m | 30.7 | 2.38, d (8.4) |
| 9 | 45.8 | 1.45, m | 45.9 | 1.50, m |
| 10 | 43.3 | 44.9 | ||
| 11 | 21.7 | 1.38, m | 21.4 | 1.50, m |
| 1.40, m | ||||
| 12 | 40.3 | 1.66, m | 40.1 | 1.66, m |
| 0.99, m | 1.00, m | |||
| 13 | 41.1 | 41.1 | ||
| 14 | 56.6 | 0.97, m | 56.3 | 1.02, m |
| 15 | 32.5 | 2.05, dd (12.1, 5.5) | 32.5 | 2.07, m |
| 1.46, m | 1.43, m | |||
| 16 | 81.8 | 4.65, dd (14.6, 7.7) | 81.8 | 4.62, dd (14.5, 7.6) |
| 17 | 63.4 | 1.85, m | 63.4 | 1.85, m |
| 18 | 16.9 | 0.87, s | 16.9 | 0.87, s |
| 19 | 13.7 | 1.50, s | 16.9 | 1.86, s |
| 20 | 42.3 | 1.98, m | 42.2 | 1.96, m |
| 21 | 15.4 | 1.12, d (7.0) | 15.4 | 1.10, d (7.0) |
| 22 | 109.8 | 109.8 | ||
| 23 | 33.6 | 1.80, m | 33.6 | 1.77, m |
| 24 | 29.3 | 2.73, td (13.2, 5.8) | 29.3 | 2.69, td (13.6, 5.5) |
| 2.26, m | 2.23, m | |||
| 25 | 144.8 | 144.8 | ||
| 26 | 65.4 | 4.51, d (12.0) | 65.4 | 4.47, d (12.1) |
| 4.06, d (12.0) | 4.03, d (11.9) | |||
| 27 | 109.1 | 4.83, s | 109.1 | 4.81, s |
| 4.80, s | 4.78, s | |||
Compound 13 was isolated as white amorphous powder, 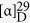 −34.9 (c 0.25, MeOH). The molecular formula, C27H42O7, was deduced from the HRESI-MS peak (m/z [M + H]+ 479.3018, calcd for C27H43O7, 479.3009). The 1H NMR spectrum showed three typical steroidal methyl signals at δH 1.86 (3H, s, Me-19), 1.10 (3H, d, J = 7.0 Hz, Me-21) and 0.87 (3H, s, Me-18) (Table 2). The down-field shifted hydrogen chemical shift of Me-19 in combination with an oxymethine proton (δH 4.94) at B ring confirmed the presence of the OH group attached at C-6 in compound 13, which was consistent with previous reports12. The 13C NMR spectrum exhibited characteristic spirostanol carbon signal at δC 109.8 (C-22) and three methyl (δC 16.9, 16.9, and 15.4), and two olefinic carbons δC 144.8 and 109.1 were observed, diagnostic of C-25 (27)-unsaturated sapogenin. These 1H-NMR data and 13C-NMR signals suggested that 13 was a C-25(27) unsaturated spirostane type steroidal sapogenin. The 1H-1H COSY spectrum showed that two methylene protons at δ 2.23 (H-2α) and 2.62 (H-2β) were coupled to δ 4.19 (H-1) and 4.85 (H-3), and the oxymethine proton δ 4.34 (H-4) was in turn coupled with the oxygenated methane proton at δ 4.85 (H-3). The oxygenated methane proton at δ 4.94 (H-6) was coupled with two methylene protons at δ 1.55 (H-7α) and 2.07 (H-7β). These findings supported the location of the hydroxyl groups at C-1, C-3, C-4, and C-6. The key NOESY correlations (Fig. 2) between H-4α and H-7α/H-9α, and between H-2α and H-9α, supported the A/B cis ring junction pattern. Thus, the hydroxyl groups at C-4 and C-5 have β-orientation. Additional NOE correlations between H-1α and Me-19/H-11, and between H-3β and H-2β, between H-6α and H-7α, were indicative of β-orientation for OH-1, OH-6 and α-orientation for OH-3. On the basis of the above analysis, compound 13 was formulated as spirost-25(27)-en-1β,3α,4β,5β,6β-pentol.
−34.9 (c 0.25, MeOH). The molecular formula, C27H42O7, was deduced from the HRESI-MS peak (m/z [M + H]+ 479.3018, calcd for C27H43O7, 479.3009). The 1H NMR spectrum showed three typical steroidal methyl signals at δH 1.86 (3H, s, Me-19), 1.10 (3H, d, J = 7.0 Hz, Me-21) and 0.87 (3H, s, Me-18) (Table 2). The down-field shifted hydrogen chemical shift of Me-19 in combination with an oxymethine proton (δH 4.94) at B ring confirmed the presence of the OH group attached at C-6 in compound 13, which was consistent with previous reports12. The 13C NMR spectrum exhibited characteristic spirostanol carbon signal at δC 109.8 (C-22) and three methyl (δC 16.9, 16.9, and 15.4), and two olefinic carbons δC 144.8 and 109.1 were observed, diagnostic of C-25 (27)-unsaturated sapogenin. These 1H-NMR data and 13C-NMR signals suggested that 13 was a C-25(27) unsaturated spirostane type steroidal sapogenin. The 1H-1H COSY spectrum showed that two methylene protons at δ 2.23 (H-2α) and 2.62 (H-2β) were coupled to δ 4.19 (H-1) and 4.85 (H-3), and the oxymethine proton δ 4.34 (H-4) was in turn coupled with the oxygenated methane proton at δ 4.85 (H-3). The oxygenated methane proton at δ 4.94 (H-6) was coupled with two methylene protons at δ 1.55 (H-7α) and 2.07 (H-7β). These findings supported the location of the hydroxyl groups at C-1, C-3, C-4, and C-6. The key NOESY correlations (Fig. 2) between H-4α and H-7α/H-9α, and between H-2α and H-9α, supported the A/B cis ring junction pattern. Thus, the hydroxyl groups at C-4 and C-5 have β-orientation. Additional NOE correlations between H-1α and Me-19/H-11, and between H-3β and H-2β, between H-6α and H-7α, were indicative of β-orientation for OH-1, OH-6 and α-orientation for OH-3. On the basis of the above analysis, compound 13 was formulated as spirost-25(27)-en-1β,3α,4β,5β,6β-pentol.
Compound 16 was isolated as white amorphous powder. The molecular formula of C33H52O11 was determined by the positive-ion HRESIMS peak at m/z [M + H]+ 625.3552 (calcd for C33H53O11, 625.3588). The 1H NMR spectrum showed three typical steroidal methyl signals at δH 1.62 (3H, s, Me-19), 1.08 (3H, d, J = 7.0 Hz, Me-21) and 0.83 (3H, s, Me-18) (Table 3). The 1H NMR spectrum of 16 was similar to that of 1, except for the absence of one oxymethine proton. This suggests that both compounds possessed the same C-25(27) unsaturated spirostane type skeleton. The 13C NMR spectrum exhibited characteristic spirostanol carbon signal at δC 109.8 (C-22) and three methyl (δC 16.8, 14.1, and 15.3), and two olefinic carbons δC 144.8 and 109.1 were assigned to the C-25 and C-27 positions, respectively. Compared the NMR data with those of spirost-25(27)-en-1β,2β,3β,5β-tetraol-5-O-β-D-galactopyranoside31, the NMR features of those two compounds were almost same except for the signals of the sugar moiety. The NMR spectrum of 16 showed an anomeric proton signal at δH 5.29 (1H, d, J = 7.7 Hz, H-1-Glc), corresponding to an anomeric carbon at δC 97.7 in the HSQC spectrum, indicated the presence of one sugar moiety. The sugar was identified as D-glucose by GC analysis of its chiral derivatives after acid hydrolysis. Thus, compound 16 was inferred as spirost-25(27)-en-1β,2β,3β,5β-tetraol-5-O-β-D- glucopyranoside.
Table 3. NMR spectroscopic data for compound 16 (pyridine-d5).
| Position | δC | δH (J in Hz) | Position | δC | δH (J in Hz) |
|---|---|---|---|---|---|
| 1 | 78.0 | 4.20, d (7.8) | 18 | 16.8 | 0.83, s |
| 2 | 68.4 | 3.95, m | 19 | 14.1 | 1.62, s |
| 3 | 71.4 | 4.51, brs | 20 | 42.3 | 1.96, m |
| 4 | 37.0 | 2.50, m | 21 | 15.3 | 1.08, d (7.0) |
| 5 | 83.4 | 22 | 109.8 | ||
| 6 | 31.0 | 2.11, td (13.5) | 23 | 33.6 | 1.82, m |
| 2.03, m | 1.78, m | ||||
| 7 | 29.3 | 1.53, d (13.7) | 24 | 29.3 | 2.72, td (13.4) |
| 0.88, dd (12.7, 3.9) | 2.25, d (11.5) | ||||
| 8 | 34.8 | 1.59, m | 25 | 144.8 | |
| 9 | 45.9 | 1.17, m | 26 | 65.4 | 4.48, d (12.2) |
| 10 | 47.0 | 4.05, d (12.2) | |||
| 11 | 21.8 | 1.42, m | 27 | 109.1 | 4.83, s |
| 12 | 40.2 | 1.59, m | 4.79, s | ||
| 1.02, m | 5-O-Glc | ||||
| 13 | 40.8 | 1 | 97.7 | 5.29, d (7.7) | |
| 14 | 56.3 | 1.02, m | 2 | 75.9 | 3.95, m |
| 15 | 32.5 | 2.03, m | 3 | 79.1 | 4.26, t (8.6) |
| 1.45, m | 4 | 72.0 | 4.08, m | ||
| 16 | 81.8 | 4.61, m | 5 | 79.1 | 4.08, m |
| 17 | 63.3 | 1.84, m | 6 | 63.1 | 4.60, m |
| 4.31, dd (11.5) |
Compound 17 was isolated as white amorphous powder. The HRESIMS of 17 showed the ion at m/z 755.4275 [M + H]+, and its molecular formula was inferred as C39H62O14 on the basis of the analysis of 1H and 13C NMR and DEPT spectra. Acid hydrolysis and GC analysis of 17 gave D-glucose. Compared the 1H and 13C NMR data with those of isorhodeasapogenin 3-O-β-D-glucopyranosyl-(1 → 4)-β-D- glucopyranoside32, the NMR features of those two compounds were almost same except for the signals of the F-ring, which was suggested the same A-E ring substitution in the two compounds. The signals due to the methyl of C-27 in isorhodeasapogenin 3-O-β-D-glucopyranosyl-(1 → 4)-β-D-glucopyranoside were replaced by the signals assigned to the C-25(27) exo-methylene group at δH 4.79 (1H, s) and 4.82(1H, s), and δC 144.8 (C-25) and 109.1 (C-27) in 17. Hence, the structure of 17 was elucidated as 5β-Spirost-25(27)-en-1β,3β-diol-3-O-β-D-glucopyranosyl- (1 → 4)-β-D-glucopyranoside.
Compound 18 was obtained as white amorphous powder. The molecular formula of C39H64O14 was assigned from the positive-ion HRESIMS peak (m/z 757.4403 [M + H]+, calcd for C39H65O14 [M + H]+, 757.4374). The IR spectrum showed the characteristic absorption of hydroxyl group at 3411 cm−1 and the four steroidal sapogenins absorption bands 986, 919, 899, 873 cm−1, and the 899 band was stronger than the 919 band. It was suggested that 18 was a sapogenin with “iso” configuration of the F ring33. Acid hydrolysis and GC analysis of 18 gave D-glucose. From a comparison of 1H and 13C NMR data of 18 (Table 4) with those of isorhodeasapogenin 3-O-glucopyranosyl-(1 → 4)-β-D-glucopyranoside32, it was apparent that 18 contained the same aglycon as isorhodeasapogenin 3-O-glucopyranosyl-(1 → 4)-β-D- glucopyranoside, except for a little different in the saccharide chains. The linkage of the sugar units was established from the following HMBC correlations: H-1 (δH 5.02) of the additional glucose′ with C-6 (δC 70.7) of the glucose, H-1 (δH 4.93) of the glucose with C-3 (δC 76.0) of the aglycon. Thus, compound 18 was elucidated as (25R)-5β-spirostan-1β,3β-diol-3-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranoside.
Table 4. NMR spectroscopic data for compounds 17–20 (pyridine-d5).
| Position | 17 |
18 |
19 |
20 |
||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 72.7 | 3.89, d (9.1) | 72.3 | 3.95, m | 72.3 | 3.94, brs | 31.3 | 1.70, m |
| 1.46, m | ||||||||
| 2 | 32.3 | 2.30, m | 31.7 | 2.35, d (11.3) | 31.8 | 2.39, d (14.7) | 27.3 | 1.91, m |
| 1.84, m | 1.82, m | 1.80, m | 1.52, m | |||||
| 3 | 75.2 | 4.53, m | 76.0 | 4.55, m | 75.6 | 4.52, brs | 74.9 | 4.32, m |
| 4 | 29.5 | 1.90, m | 30.4 | 1.92, m | 30.1 | 1.89, m | 30.9 | 1.79, m |
| 1.82, m | 1.74, m | 1.73, m | 1.71, m | |||||
| 5 | 31.3 | 2.38, d (11.5) | 31.4 | 2.40, d (14.9) | 31.4 | 2.34, d (12.4) | 37.3 | 2.04, m |
| 6 | 26.8 | 1.73, m | 26.8 | 1.72, m | 26.8 | 1.69, m | 27.3 | 1.73, m |
| 1.15, m | 1.10, m | 1.10, m | 1.06, m | |||||
| 7 | 26.8 | 1.28, m | 26.6 | 1.23, m | 26.7 | 1.23, m | 27.1 | 1.24, m |
| 1.01, m | 0.94, m | 0.97, m | 0.96, m | |||||
| 8 | 36.1 | 1.59, m | 36.1 | 1.56, m | 36.1 | 1.56, m | 35.9 | 1.49, m |
| 9 | 42.5 | 1.20, m | 42.2 | 1.15, m | 42.3 | 1.15, m | 40.6 | 1.29, m |
| 10 | 40.8 | 40.6 | 40.6 | 35.6 | ||||
| 11 | 21.4 | 1.28, m | 21.4 | 1.23, m | 21.4 | 1.23, m | 21.5 | 1.32, m |
| 1.19, m | ||||||||
| 12 | 40.5 | 1.66, m | 40.5 | 1.61, m | 40.6 | 1.62, m | 40.6 | 1.68, m |
| 1.06, m | 1.00, m | 1.03, m | 1.08, m | |||||
| 13 | 41.0 | 41.0 | 41.0 | 41.3 | ||||
| 14 | 56.7 | 1.08, m | 56.6 | 1.05, m | 56.6 | 1.06, m | 56.8 | 1.08, m |
| 15 | 32.5 | 2.03, m | 32.5 | 2.02, m | 32.5 | 2.03, m | 32.5 | 2.04, m |
| 1.43, m | 1.42, m | 1.14, m | 1.42, m | |||||
| 16 | 81.8 | 4.60, m | 81.5 | 4.60, m | 81.5 | 4.62, d (7.3) | 81.9 | 4.62, dd (14.7, 7.5) |
| 17 | 63.5 | 1.84, m | 63.5 | 1.83, m | 63.5 | 1.86, m | 63.5 | 1.85, m |
| 18 | 17.0 | 0.84, s | 17.0 | 0.81, s | 17.0 | 0.81, s | 16.9 | 0.82, s |
| 19 | 19.5 | 1.26, s | 19.5 | 1.24, s | 19.4 | 1.23, s | 24.2 | 0.84, s |
| 20 | 42.2 | 1.97, m | 42.3 | 1.95, m | 42.4 | 1.96, m | 42.2 | 1.97, m |
| 21 | 15.4 | 1.10, d (7.0) | 15.4 | 1.14, d (6.9) | 15.4 | 1.14, d (7.0) | 15.4 | 1.10, d (6.9) |
| 22 | 109.8 | 109.6 | 109.6 | 109.8 | ||||
| 23 | 33.6 | 1.78, m | 32.2 | 1.68, m | 32.2 | 1.68, m | 33.6 | 1.78, m |
| 24 | 29.3 | 2.72, m | 29.6 | 1.56, m | 29.6 | 1.56, m | 29.3 | 2.72, td (13.5, 5.4) |
| 2.26, m | ||||||||
| 25 | 144.8 | 30.9 | 1.56, m | 31.0 | 1.56, m | 144.8 | ||
| 26 | 65.4 | 4.48, d (12.1) | 67.2 | 3.58, m | 67.2 | 3.60, m | 65.4 | 4.49, m |
| 4.05, d (12.1) | 3.51, t (10.5) | 3.51, m | 3.51, m | 4.05, m | ||||
| 27 | 109.1 | 4.82, s | 17.7 | 0.68, d (5.6) | 17.7 | 0.69, d (5.6) | 109.1 | 4.82, s |
| 4.79, s | 4.78, s | |||||||
| 3-O Glc | 3-O Glc | 3-O Glc | 3-O Glc | |||||
| 1 | 101.3 | 4.97, d (7.8) | 102.4 | 4.93, d (7.8) | 102.1 | 4.89, d (7.8) | 103.2 | 4.91, d (7.8) |
| 2 | 74.9 | 3.96, m | 75.2 | 3.93, m | 75.3 | 3.91, m | 75.1 | 4.08, m |
| 3 | 77.2 | 4.31, m | 78.8 | 4.20, m | 78.8 | 4.20, m | 77.3 | 4.32, m |
| 4 | 81.5 | 4.33, m | 72.2 | 4.05, m | 72.4 | 4.07, m | 81.7 | 4.39, t (9.1) |
| 5 | 77.0 | 3.96, m | 77.3 | 4.15, m | 77.1 | 4.07, m | 76.8 | 3.92, dt (9.5, 3.2) |
| 6 | 62.4 | 4.58, m | 70.7 | 4.89, d (9.1) | 63.1 | 4.85, d (9.1) | 62.6 | 4.60, m |
| 4.31, m | 4.18, m | 4.55, m | 4.51, m | |||||
| Glc(1 → 4) | Glc(1 → 6) | Fru(2 → 6) | Glc(1 → 4) | |||||
| 1′ | 105.3 | 5.21, d (7.9) | 105.6 | 5.02, d (7.7) | 63.2 | 4.37, m | 105.3 | 5.23, d (7.9) |
| 4.26, d (8.0) | ||||||||
| 2′ | 75.1 | 4.11, m | 75.8 | 4.06, m | 106.2 | 75.2 | 4.13, t (8.3) | |
| 3′ | 78.8 | 4.02, m | 78.7 | 3.93, m | 79.8 | 5.21, m | 78.6 | 4.24, m |
| 4′ | 71.9 | 4.22, m | 71.8 | 4.25, m | 77.3 | 5.01, t (7.9) | 71.8 | 4.23, m |
| 5′ | 78.6 | 4.22, m | 78.8 | 4.24, m | 84.4 | 4.57, m | 78.8 | 4.01, m |
| 6′ | 62.8 | 4.53, m | 63.0 | 4.53, m | 64.4 | 4.37, m | 62.7 | 4.51, m |
| 4.31, m | 4.39, m | 4.32, m | ||||||
Compound 19 was isolated as white amorphous powder. The HRESIMS of 19 showed the ion at m/z 757.4401 [M + H]+, and its molecular formula was inferred as C39H64O14 on the basis of the analysis of 1H and 13C NMR and DEPT spectra. On acid hydrolysis, 19 liberated D-fructose and D-glucose, identified by GC analysis of their chiral derivatives. From comparison of the NMR data of 19 with those of 18, it was indicated that 19 contained the same aglycone as 18. The HMBC correlations between the H-6 (δH 4.85, 4.55) of the glucose and C-2 (δC 106.2) of the fructose indicated the saccharide attached to C-3 of the aglycone was β-D-fructofuranosyl-(2 → 6)-β-D- glucopyranosyl. Thus, compound 19 was elucidated as (25R)-5β-spirostan-1β,3β-diol- 3-O-β-D-fructofuranosyl-(2 → 6)-β-D-glucopyranoside.
Compound 20 was isolated as white amorphous powder. The HRESIMS of 20 showed the ion at m/z 739.4254 [M + H]+, and its molecular formula was inferred as C39H62O13 on the basis of the analysis of 1H and 13C NMR and DEPT spectra. Compared the 1H and 13C NMR data with those of (25R)-5β-spirostan-3β-ol-3-O-β-D- glucopyranosyl-(1 → 4)-β-D-glucopyranoside34, the NMR features of those two compounds were almost same except for the signals of the F-ring, which was suggested the same A-E ring substitution in the two compounds. The signals due to the methyl of C-27 in (25R)-5β-spirostan-3β-ol-3-O-β-D-glucopyranosyl-(1 → 4)-β-D- glucopyranoside were replaced by the signals assigned to the C-25(27) exo-methylene group at δH 4.78 (1H, s) and 4.82(1H, s), and δC 144.8 (C-25) and 109.1 (C-27) in 20. Hence, the structure of 20 was elucidated as 5β-Spirost-25(27)-en-3β-ol-3-O-β-D- glucopyranosyl-(1 → 4)-β-D-glucopyranoside.
Antiproliferative activities
TB, TC, TD and all the isolated compounds were evaluated for antiproliferative activities against seven human tumor cell lines including FaDu (human hypopharyngeal carcinoma), Detroit 562 (human metastatic pharyngeal squamous cell carcinoma), CNE-1 (high differentiation human nasopharyngeal carcinoma), CNE-2 (low differentiation human nasopharyngeal carcinoma), HepG2 (human hepatocellular carcinoma), K562 (human chronic leukemia), SPC-A-1 (human lung adenocarcinoma) with a modified MTT method according to reported protocols, and cis-dichlorodiammineplatinum (II) was used as a positive control. The results were shown in Table 5 and Table S2 for the isolated compound and the crude extract of T. chinensis, respectively. TD showed moderate activity against five out of seven cancer cell lines (Fadu, Detroit 562, HepG2, K562 and SPC-A-1) with IC50 values of 20.9–29.3 μg/mL. TC exhibited selective antiproliferative activity against Fadu, Detroit 562, and K562 with IC50 values of 6.7 ± 0.5, 45.9 ± 1.7, and 6.5 ± 2.3 μg/mL, respectively. Compounds 17, 19 and 21 exhibited moderate antiproliferative effects against all of human cancer cell lines tested. Compound 22 showed moderate inhibitory activity against six out of seven human cancer cell lines tested excepted CNE-2. Compound 18 displayed weak inhibitory activities against Detroit 562, CNE-2 and HepG2 with IC50 values of 39.4 ± 0.7, 40.7 ± 3.1 and 46.5 ± 1.0 μM, respectively. Compound 20 showed moderate antiproliferative activity against FaDu, Detroit 562 and HepG2. Compound 4 exhibited moderate inhibitory activity against FaDu and HepG2 with IC50 values of 27.6 ± 3.6 and 29.7 ± 3.1 μM, respectively. Compounds 3, 5 and 12 exhibited potential antiproliferative activities against FaDu with IC50 values of 28.6 ± 1.6, 41.2 ± 1.8 and 12.1 ± 1.2 μM, respectively. The other compounds exhibited IC50 values larger than 50.0 μM against the seven tested cell lines and were considered to be inactive. Compounds 17–22 showed higher antiproliferative activities against human tumor cell lines than other compounds, suggesting that glycosylation at C-3 of the aglycon play an important role in the growth inhibition on cancer cells of spirostanol saponins. The structure-activity relationship of the isolated compounds (1–23) indicated that the glycosylation at C-3 of the aglycon was the most important structural feature for the high antiproliferative activity and other structural features (e.g., OH position and number) played a modified role in enhancing or reducing the activity. However comparison of antiproliferative activity is only an approximation as there are notable differences in IC50 values between control compounds. A choice of reference and cell lines may lead to various conclusions with respect to the potency of antiproliferative compounds35.
Table 5. Antiproliferative activities of some compounds from T. chinensis against human cancer cell lines.
| Compound | IC50 (μM)a |
||||||
|---|---|---|---|---|---|---|---|
| FaDu | Detroit 562 | CNE-1 | CNE-2 | HepG2 | K562 | SPC-A-1 | |
| 3 | 28.6 ± 1.6* | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 |
| 4 | 27.6 ± 3.6*** | >50.0 | >50.0 | >50.0 | 29.7 ± 3.1*** | >50.0 | >50.0 |
| 5 | 41.2 ± 1.8*** | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 |
| 12 | 12.1 ± 1.2 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 | >50.0 |
| 17 | 20.3 ± 2.1 | 20.2 ± 2.0 | 33.6 ± 0.3*** | 23.6 ± 1.9*** | 28.6 ± 1.0*** | 27.4 ± 0.2*** | 24.8 ± 0.7*** |
| 18 | >50.0 | 39.4 ± 0.7*** | >50.0 | 40.7 ± 3.1*** | 46.5 ± 1.0*** | >50.0 | >50.0 |
| 19 | 61.4 ± 2.5*** | 21.5 ± 1.1*** | 50.9 ± 1.1*** | 29.5 ± 0.9*** | 34.0 ± 0.5*** | 40.2 ± 2.1*** | 39.4 ± 1.0*** |
| 20 | 20.1 ± 0.4 | 20.1 ± 0.6*** | >50.0 | >50.0 | 28.0 ± 0.7*** | >50.0 | >50.0 |
| 21 | 12.7 ± 1.7* | 22.7 ± 0.5** | 17.4 ± 0.5*** | 14.5 ± 1.1** | 14.3 ± 1.5*** | 22.2 ± 0.2*** | 22.2 ± 0.2*** |
| 22 | 20.5 ± 3.4 | 29.1 ± 0.6* | 33.7 ± 0.6*** | >50.0 | 17.7 ± 0.3*** | 33.8 ± 0.1*** | 26.3 ± 1.0*** |
| CISb | 18.1 ± 0.7 | 26.2 ± 1.5 | 10.8 ± 0.4 | 7.9 ± 1.1 | 6.5 ± 0.3 | 13.5 ± 0.8 | 3.8 ± 0.3 |
aValues are presented as means ± SD (n = 3).
bPositive control.
*p < 0.05, **p < 0.01, ***p < 0.001 versus positive control.
Anti-inflammatory activities
Nitric oxide (NO) is a signaling molecule that plays a key role in the pathogenesis of inflammation36. NO production by immune cells has been used as an indicator of the presence and extent of inflammation as well as the effectiveness of anti-inflammatory agents37.
Compounds 1–23 were tested for their inhibitory effects on NO production induced by LPS in a macrophage cell line RAW 264.7. Cell viability was first determined by the MTT method to find whether inhibition of NO production was due to the cytotoxicity of the tested samples. The anti-inflammatory activities were summarized in Table 6. All the tested samples exhibited no cytotoxicity against RAW 264.7 macrophage cells at their effective concentrations. The crude extracts of T. chinensis TB, TC and TD showed moderate inhibition on NO production with IC50 values of 32.6 ± 3.7, 37.5 ± 5.4 and 36.6 ± 1.8 μg/mL (see Table S3 in the supporting information). Compound 21 showed significant inhibition on NO production with IC50 values of 11.5 μM, comparable to that of the positive control indomethacin.at 47.4 μM38. Compounds 1, 4, 9, 10, 13, 14, 17 and 20 exhibited moderate inhibition with IC50 values between 21.7 and 33.6 μM. Compounds 5, 7, 15, 18 and 19 displayed inhibition activity, but quite limited with IC50 values of 45.9 ± 3.1, 45.2 ± 5.7, 46.1 ± 13.1, 41.6 ± 13.5 and 45.4 ± 11.2 μM, respectively. Other compounds showed weak activity with IC50 value above than 50 μM. The structure-activity relationship of the isolated spirostanol saponins shows that small changes such as different positions or the number of the hydroxyl groups, R/S configuration on the aglycone led to changes in activity. The most active compound is 21, and comparison with 22 shows that change in the orientation or of the methyl group at C-25 from axial in 21 to equatorial in 22 diminishes the activity39. Comparison of the activity of 4 and 5 shows that 4 is more active than 5 due to the exocyclic double in 5, which can be illustrated by the relative higher IC50 value of compound 20 and the relative lower IC50 value of compound 21. The kinds, the number and the linkage of sugar also play important roles in their anti-inflammatory activity40.
Table 6. Inhibitory effects of some compounds from T. chinensis on NO production induced by LPS in macrophagesac.
| Compound | IC50 (μM)a | Compound | IC50 (μM)a |
|---|---|---|---|
| 1 | 28.7 ± 2.1 | 15 | 46.1 ± 13.1 |
| 4 | 21.7 ± 1.3 | 17 | 21.0 ± 0.8 |
| 5 | 45.9 ± 3.1 | 18 | 41.6 ± 13.5 |
| 7 | 45.2 ± 5.7 | 19 | 45.4 ± 11.2 |
| 9 | 33.6 ± 1.7 | 20 | 22.6 ± 5.0 |
| 10 | 28.7 ± 4.5 | 21 | 11.5 ± 1.3 |
| 13 | 26.1 ± 0.5 | Indomethacinb | 47.4 ± 4.5 |
| 14 | 29.1 ± 0.6 |
aValues are presented as means ± SD (n = 3).
bPositive control.
cCompounds 2, 3, 6, 8, 11, 12, 16, 19, 22, 23 with IC50 > 50.0 μM.
Conclusion
Twenty-three polyhydroxylated spirostanol saponins, including nine new compounds (1, 2, 12, 13, 16–20) were isolated from the rhizomes of T. chinensis and their structures were determined by spectroscopic methods. Among the isolated compounds, Compounds 17, 19 and 21 exhibited potential antiproliferative activities against all of human cancer cell lines tested. Compound 21 showed significant inhibition on NO production with IC50 values of 11.5 μM. The present investigation suggested that T. chinensis can be a potential source of natural antiproliferative and anti-inflammatory agents and its spirostanol saponins may be responsible for the biological activity. However, further studies are required, not only to assess the bioactivities of the isolates in vivo, but also to investigate the mechanisms underlying the observed biological activities of isolated compounds.
Materials and Methods
Chemicals
HPLC-grade methanol was purchased from Oceanpak Chemical Co. (Gothenburg, Sweden). D101 macroporus resin (Xi′an Lanxiao Resin Corporation Ltd., Xi′an, China), silica gel (200–300 mesh, Anhui Liangchen Silicon Material Co. Ltd., Lu’an, China) and ODS (40–60 μm, Merck KGaA, Darmstadt, Germany) were used for column chromatography. All solvents used for column chromatography were of analytical grade and purchased from Sinopharm Chemical Reagent Co. (Shanghai, China). Foetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from HyClone Laboratories (Logan, UT, USA). Sugar reagents used for GC analysis, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), cis-dichlorodiammineplatinum (II) and indomethacin were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
General experimental procedures
Optical rotations were measured with a JASCO P-1020 digital polarimeter. IR spectra were recorded on a PerkinElmer 100 IR spectrometer with KBr. NMR spectra were obtained on a Bruker Ultrashield 500 Plus instrument, using tetramethylsilane as internal standard. Waters AQUITY UPLC/Q-TOF mass spectrometer was used to record HRESIMS spectra. Semi-preparative HPLC was performed using a RAININ pump equipped with a Gilson 133 refractive index detector. A semi-preparative column (COSMOSIL-Pack 5C18-MS-II, 10ID × 250 mm, 5 μm) was used for HPLC. Microplate reader (Kehua Technologies, Inc., Shanghai, China) was used for bioassay.
Plant material
The rhizomes of T. chinensis Baker was purchased from Shennongjia Forest District (Shennongjia, China), and identified by Prof. Xiangjiu He, the School of Pharmacy, Guangdong Pharmaceutical University. A voucher specimen (No. GDPU-NPR-2013002) was deposited in the Department of Medicinal Chemistry, Guangdong Pharmaceutical University, Guangzhou, China.
Extraction and isolation
The air-dried rhizomes of T. chinensis (17.0 kg) were extracted four times with 60% EtOH (each 70 L × 4 h) under reflux. The combined EtOH extracts were evaporated under reduced pressure (for complete removal of organic solvent) to yield an aqueous suspension (20 L), and then partitioned between H2O (20 L) and EtOAc (20 L × 4) to give an EtOAc fraction (218.0 g). The aqueous fraction was applied to a D101 macroporous resin column, eluted with H2O (50 L), 20% EtOH (50 L), 60% EtOH (50 L) and 80% EtOH (50 L), repectively. The solvents were evaporated under vacuum to yield 20% EtOH eluted fraction (TB, 194.5 g) and 60% EtOH eluted fraction (TC, 458 g) and 80% EtOH eluted fraction (TD, 83.8 g).
A half part of fraction TC (200.0 g) was subjected to silica gel column chromatography (200 ~ 300 mesh, 3 kg, 95 × 1140 mm) eluted with a gradient of CHCl3/MeOH (20:1 to 2:1, followed by MeOH) to yield 18 fractions (C1-C18). Fraction C3 (108.2 mg) was further purified by ODS and semi-preparative HPLC (3.0 mL/min, 85% MeOH in H2O isocratic elution) to afford compound 12 (2.7 mg). Fraction C4 (157.5 mg) was separated by an ODS column eluted with a gradient of MeOH-H2O (3:7 to 5:5, v/v) to afford compound 14 (23.0 mg). Fraction C5 (625.8 mg) was separated by an ODS column eluted with a gradient of MeOH-H2O (1:9 to 2:8, v/v) to afford compound 13 (20.9 mg). Fraction C12 (5.1 g) was subjected to ODS eluted with a gradient of MeOH-H2O (3:7 to 8:2, v/v) to afford eight fractions (C12-1 to C12-8). Subfraction C-12-3 (335.5 mg) was further purified by semi-preparative HPLC (3.0 mL/min, 60% MeOH in H2O isocratic elution) to afford compound 7 (194.7 mg).
Fraction TD (83.3 g) was separated by silica gel column chromatography (200 ~ 300 mesh, 2 kg, 95 × 745 mm) eluted with a gradient of CHCl3/MeOH (100:1 to 1:1, v/v) to obtain twenty-one fractions (D1-D21). Fraction D13 (15.8 g) was separated by an ODS column eluted with a gradient of MeOH-H2O (1:9 to 7:3, v/v) to afford nine fractions (D13-1 to D13-9). Compound 5 (15.3 mg) was crystallized from subfraction D13-4 (90.2 mg) in MeOH. Fraction D14 (21.5 g) was subjected to ODS eluted with a gradient of MeOH-H2O (1:9 to 8:2, v/v) to afford nine fractions (D14-1 to D14-9). Compound 8 (18.6 mg) was crystallized from subfraction D14-5 in MeOH. Subfraction D-14-7 (335.5 mg) was further purified by semi-preparative HPLC (3.0 mL/min, 70% MeOH in H2O isocratic elution) to afford compound 4 (14.3 mg) and compound 15 (32.4 mg). Fraction D15 (1.8 g) was purified by ODS to obtain eleven fractions (D15-1 to D15-11). Subfraction D15-6 (87.4 mg) was purified by semi-preparative HPLC (3.0 mL/min, 80% MeOH in H2O isocratic elution) to yield compound 3 (51.8 mg). Fraction D16 (1.5 g) was purified by ODS to obtain three fractions (D16-1 to D16-3). Subfraction D16-3 (1.18 g) was purified by semi-preparative HPLC (3.0 mL/min, 75% MeOH in H2O isocratic elution) to yield compounds 20 (17.2 mg), 21 (14.2 mg) and 22 (23.7 mg). Fraction D18 (11.4 g) was subjected to ODS to obtain seventeen fractions (D18-1 to D18-17). Subfraction D18-9 (1.3 g) was further purified by semi-preparative HPLC (3.0 mL/min, 60% MeOH in H2O isocratic elution) to yield compounds 9 (87.3 mg). Subfraction D18-10 (649.5 mg) was further purified by semi-preparative HPLC (3.0 mL/min, 60% MeOH in H2O isocratic elution) to yield compounds 6 (55.3 mg) and 16 (25.5 mg). Subfraction D18-14 (243.8 mg) was further purified by semi-preparative HPLC (3.0 mL/min, 75% MeOH in H2O isocratic elution) to yield compounds 17 (27.7 mg), 18 (22.0 mg) and 19 (58.1 mg). Fraction D19 (3.6 g) was subjected to silica gel column chromatography (300 ~ 400 mesh, 150 g, 95 × 745 mm) eluted with a gradient of CHCl3/MeOH (10:1 to 3:1, v/v) to obtain eight fractions (D19-1 to D19-8). Subfraction D19-7 was further purified by semi-preparative HPLC (3.0 mL/min, 70% MeOH in H2O isocratic elution) to yield compounds 1 (45.4 mg) and 23 (10.1 mg). Fraction D20 (4.9 g) was subjected to ODS eluted with a gradient of MeOH-H2O (1:9 to 8:2, v/v) to obtain ten fractions (D20-1 to D20-10). Subfraction D20-6 was further purified by semi-preparative HPLC (3.0 mL/min, 70% MeOH in H2O isocratic elution) to yield compound 2 (28.1 mg). Fraction D21 (4.6 g) was subjected to ODS eluted with a gradient of MeOH-H2O (1:9 to 7:3, v/v) to obtain eleven fractions (D21-1 to D21-11). Subfraction D21-6 (1.2 g) was further purified by semi-preparative HPLC (3.0 mL/min, 60% MeOH in H2O isocratic elution) to yield compounds 10 (62.0 mg) and 11 (29.2 mg).
Spirost-25(27)-en-1β,2β,3β,4β,5β-pentol-2-O-β-D-xylopyranoside (1) White amorphous powder; 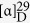 −43.3 (c 0.25, MeOH); IR (KBr) νmax 3364, 2940, 1645, 1454, 1380, 1045, 920cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z [M + H]+ 611.3437 (calcd for C32H51O11, 611.3431).
−43.3 (c 0.25, MeOH); IR (KBr) νmax 3364, 2940, 1645, 1454, 1380, 1045, 920cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z [M + H]+ 611.3437 (calcd for C32H51O11, 611.3431).
Spirost-25(27)-en-1β,2β,3β,4β,5β-pentol-2-O-α-L-arabinopyranoside (2) White amorphous powder; 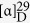 −49.2 (c 0.25, MeOH); IR (KBr) νmax 3422, 2949, 1650, 1452, 1380, 1047, 923 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z [M + H]+ 611.3412 (calcd for C32H51O11, 611.3431).
−49.2 (c 0.25, MeOH); IR (KBr) νmax 3422, 2949, 1650, 1452, 1380, 1047, 923 cm−1; 1H and 13C NMR data, see Table 1; HRESIMS m/z [M + H]+ 611.3412 (calcd for C32H51O11, 611.3431).
Spirost-25(27)-en-1β,3α,5β-triol (12) White amorphous powder; 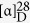 −33.1 (c 0.25, MeOH); IR (KBr) νmax 3300, 2950, 1650, 1592, 1455, 1381, 1046, 926, 899, 878 cm−1; 1H and 13C NMR data, see Table 2; HRESIMS m/z [M + H]+ 447.3112 (calcd for C27H43O5, 447.3110).
−33.1 (c 0.25, MeOH); IR (KBr) νmax 3300, 2950, 1650, 1592, 1455, 1381, 1046, 926, 899, 878 cm−1; 1H and 13C NMR data, see Table 2; HRESIMS m/z [M + H]+ 447.3112 (calcd for C27H43O5, 447.3110).
Spirost-25(27)-en-1β,3α,4β,5β,6β-pentol (13) White amorphous powder; 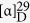 −34.9 (c 0.25, MeOH); IR (KBr) νmax 3398, 2954, 1717, 1655, 1450, 1381, 1043, 924 cm−1; 1H and 13C NMR data, see Table 2; HRESIMS m/z [M + H]+ 479.3018 (calcd for C27H43O7, 479.3009).
−34.9 (c 0.25, MeOH); IR (KBr) νmax 3398, 2954, 1717, 1655, 1450, 1381, 1043, 924 cm−1; 1H and 13C NMR data, see Table 2; HRESIMS m/z [M + H]+ 479.3018 (calcd for C27H43O7, 479.3009).
Spirost-25(27)-en-1β,2β,3β,5β-tetraol-5-O-β-D-glucopyranoside (16) White amorphous powder; 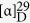 −44.9 (c 0.50, MeOH); IR (KBr) νmax 3424, 2941, 1651, 1452, 1372, 1046, 922 cm−1; 1H and 13C NMR data, see Table 3; HRESIMS m/z [M + H]+ 625.3552 (calcd for C33H53O11, 625.3588).
−44.9 (c 0.50, MeOH); IR (KBr) νmax 3424, 2941, 1651, 1452, 1372, 1046, 922 cm−1; 1H and 13C NMR data, see Table 3; HRESIMS m/z [M + H]+ 625.3552 (calcd for C33H53O11, 625.3588).
5β-Spirost-25(27)-en-1β,3β-diol-3-O-β-D-glucopyranosyl-(1 → 4)-β-D-glucopyranoside (17) White amorphous powder; 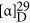 −52.9 (c 0.25, MeOH); IR (KBr) νmax 3419, 2921, 1645, 1452, 1047, 920 cm−1; 1H and 13C NMR data, see Table 4; HRESIMS m/z [M + H]+ 755.4275 (calcd for C39H63O14, 755.4218).
−52.9 (c 0.25, MeOH); IR (KBr) νmax 3419, 2921, 1645, 1452, 1047, 920 cm−1; 1H and 13C NMR data, see Table 4; HRESIMS m/z [M + H]+ 755.4275 (calcd for C39H63O14, 755.4218).
(25R)-5β-Spirostan-1β,3β-diol-3-O-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranoside (18) White amorphous powder; 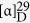 −27.0 (c 0.12, MeOH); IR (KBr) νmax 3411, 2935, 1641, 1451, 1050, 986, 919, 899, 873 cm−1; 1H and 13C NMR data, see Table 4; HRESIMS m/z [M + H]+ 757.4403 (calcd for C39H65O14, 757.4374).
−27.0 (c 0.12, MeOH); IR (KBr) νmax 3411, 2935, 1641, 1451, 1050, 986, 919, 899, 873 cm−1; 1H and 13C NMR data, see Table 4; HRESIMS m/z [M + H]+ 757.4403 (calcd for C39H65O14, 757.4374).
(25R)-5β-Spirostan-1β,3β-diol-3-O-β-D-fructofuranosyl-(2 → 6)-β-D-glucopyranoside (19) White amorphous powder; 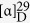 −66.8 (c 0.50, MeOH); IR (KBr) νmax 3391, 2929, 1632, 1451, 1053, 984, 918, 899, 865 cm−1; 1H and 13C NMR data, see Table 4; HRESIMS m/z [M + H]+ 757.4401 (calcd for C39H65O14, 757.4374).
−66.8 (c 0.50, MeOH); IR (KBr) νmax 3391, 2929, 1632, 1451, 1053, 984, 918, 899, 865 cm−1; 1H and 13C NMR data, see Table 4; HRESIMS m/z [M + H]+ 757.4401 (calcd for C39H65O14, 757.4374).
5β-Spirost-25(27)-en-3β-ol-3-O-β-D-glucopyranosyl-(1 → 4)-β-D- glucopyranoside (20) White amorphous powder; 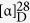 −30.6 (c 0.50, MeOH); IR (KBr) νmax 3423, 2925, 1634, 1452, 1043, 921, 896 cm−1; 1H and 13C NMR data, see Table 4; HRESIMS m/z [M + H]+ 739.4254 (calcd for C39H63O13, 739.4269).
−30.6 (c 0.50, MeOH); IR (KBr) νmax 3423, 2925, 1634, 1452, 1043, 921, 896 cm−1; 1H and 13C NMR data, see Table 4; HRESIMS m/z [M + H]+ 739.4254 (calcd for C39H63O13, 739.4269).
Acid hydrolysis and GC analysis
Compounds 1, 2, 12, 13 and 16–20 (1–2 mg) were dissolved in 5 mL 2 M HCl and heated at 90 °C for 8 h. The reaction mixture was extracted with EtOAc (5 mL × 3) and the aqueous residue was evaporated under vacuum at 60 °C. Then 5 mg NH2OH.HCl was added to the residue and the mixture was dissolved in 600 μL pyridine. After heating at 90 °C for 30 min, 300 μL Ac2O was added. After homogenized, the mixture was heated at 90 °C for another 1 h. The reaction mixture was analyzed by GC using standard aldononitrile peracetates as reference samples41.
Measurement of inhibition activity on tumor cell proliferation
Antiproliferative activities against human nasopharyngeal cancer cells (CNE-1, CNE-2, FaDu, Detroit 562), human liver cancer cells (HepG2), human chronic leukemia cells (K562) and human lung adenocarcinoma cells (SPC-A-1) of the pure spirostanol saponins isolated from T. chinensis were measured by the modified MTT assay as described previously42.
Measurement of anti-inflammatory activity
Determination of NO production was performed by measuring the accumulation of nitrite in the culture supernatant using the Griess reagent, as previously described43.
Statistical analysis
All the experiments were conducted for three independent replicates, and data were expressed as mean ± SD. Statistical analyses were performed by one-way ANOVA. Dunnett’s Multiple Comparison Test was used to determine the significance of differences between the groups. Differences at P < 0.05 were considered statistically significant.
Additional Information
How to cite this article: Xiang, L. et al. Antiproliferative and anti-inflammatory polyhydroxylated spirostanol saponins from Tupistra chinensis. Sci. Rep. 6, 31633; doi: 10.1038/srep31633 (2016).
Supplementary Material
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 81573303), the fund of Guangdong provincial department of science and development in the field of social development (No. 2015A020211027), and the starting fund of Guangdong Pharmaceutical University.
Footnotes
Author Contributions L.X. and X.Y. carried out the experiments and L.X. prepared the manuscript. X.H. and Y.W. conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript. All authors reviewed the manuscript.
References
- Newman D. J. & Cragg G. M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661 (2016). [DOI] [PubMed] [Google Scholar]
- Sparg S. G., Light M. E. & van Staden J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 94, 219–243 (2004). [DOI] [PubMed] [Google Scholar]
- Munafo J. P. Jr. & Gianfagna T. J. Chemistry and biological activity of steroidal glycosides from the Lilium genus. Nat. Prod. Rep. 32, 454–477 (2015). [DOI] [PubMed] [Google Scholar]
- de Costa F., Yendo A. C. A., Fleck J. D., Gosmann G. & Fett-Neto A. G. Immunoadjuvant and anti-inflammatory plant saponins: characteristics and biotechnological approaches towards sustainable production. Mini-Rev. Med. Chem. 11, 857–880 (2011). [DOI] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sica A. & Balkwill F. Cancer-related inflammation. Nature 454, 436–444 (2008). [DOI] [PubMed] [Google Scholar]
- Coussens L. M. & Werb Z. Inflammation and cancer. Nature 420, 860–867 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasannan R. et al. Key cell signaling pathways modulated by zerumbone: role in the prevention and treatment of cancer. Biochem. Pharmacol. 84, 1268–1276 (2012). [DOI] [PubMed] [Google Scholar]
- Efferth T. & Koch E. Complex interactions between phytochemicals. the multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 12, 122–132 (2011). [DOI] [PubMed] [Google Scholar]
- Shen P. et al. Steroidal saponins from rhizomes of Tupistra wattii Hook. f. Chem. Pharm. Bull. 51, 305–308 (2003). [DOI] [PubMed] [Google Scholar]
- Guo Z. et al. Structural elucidation and NMR spectral assignment of three new furostanol saponins from the roots of Tupistra chinensis. Magn. Reson. Chem. 47, 613–616 (2009). [DOI] [PubMed] [Google Scholar]
- Liu C. et al. New furostanol saponins from the rhizomes of Tupistra chinensis. Nat. Prod. Res. 27, 123–129 (2013). [DOI] [PubMed] [Google Scholar]
- Pan W. B., Chang F. R. & Wu Y. C. Spirostanol sapogenins from the underground parts of Tupistra chinensis. Chem. Pharm. Bull. 48, 1350–1353 (2000). [DOI] [PubMed] [Google Scholar]
- Zou K., Wang J., Du M., Li Q. & Tu G. A pair of diastereoisomeric steroidal saponins from cytotoxic extracts of Tupistra chinensis rhizomes. Chem. Pharm. Bull. 54, 1440–1442 (2006). [DOI] [PubMed] [Google Scholar]
- Zou K. et al. Structural elucidation of four new furostanol saponins from Tupistra chinensis by 1D and 2D NMR spectroscopy. Magn. Reson. Chem. 47, 87–91 (2009). [DOI] [PubMed] [Google Scholar]
- Pan W. B., Chang F. R. & Wu Y. C. Tupichigenin A, a new steroidal sapogenin from Tupistra chinensis. J. Nat. Prod. 63, 861–863 (2000). [DOI] [PubMed] [Google Scholar]
- Pan Z. H. et al. A cytotoxic cardenolide and a saponin from the rhizomes of Tupistra chinensis. Fitoterapia 83, 1489–1493 (2012). [DOI] [PubMed] [Google Scholar]
- Pan W. B., Wei L. M., Wei L. L. & Wu Y. C. Chemical constituents of Tupistra chinensis rhizomes. Chem. Pharm. Bull. 54, 954–958 (2006). [DOI] [PubMed] [Google Scholar]
- Pan W. B., Chang F. R., Wei L. M. & Wu Y. C. New flavans, spirostanol sapogenins, and a pregnane genin from Tupistra chinensis and their cytotoxicity. J. Nat. Prod. 66, 161–168 (2003). [DOI] [PubMed] [Google Scholar]
- Xiao Y. H. et al. Three spirostanol saponins and a flavane-O-glucoside from the fresh rhizomes of Tupistra chinensis. Fitoterapia 102, 102–108 (2015). [DOI] [PubMed] [Google Scholar]
- Xu L. L. et al. New polyhydroxylated furostanol saponins with inhibitory action against NO production from Tupistra chinensis rhizomes. Molecules 12, 2029–2037 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. X. et al. Five new furostanol saponins from the rhizomes of Tupistra chinensis. Fitoterapia 83, 323–328 (2012). [DOI] [PubMed] [Google Scholar]
- Luo H. J., Wang J. Z., Deng W. Q. & Zou K. Induced-fit docking and binding free energy calculation on furostanol saponins from Tupistra chinensis as epidermal growth factor receptor inhibitors. Med. Chem. Res. 22, 4970–4979 (2013). [Google Scholar]
- Song X. et al. Two new spirostanol saponins from the the roots and rhizomes of Tupistra chinensis. Phytochem. Lett. 13, 6–10 (2015). [Google Scholar]
- Shi W. P., Zhang Y. X., Lin Q. S., Wang M., Wang H. & Zhao C. J. Study on antioxidant and antimicrobial activities of methanol extract from Tupistra chinensis Bak. Food Res. Dev. 34, 22–24 (2013). [Google Scholar]
- Wu G. X., Liu A. Y. & Chen W. X. Effects of extracts of Tupistra chinensis on mycelia growth of Peronophythora litchii and storage life of litchi fruit. Scientia Agricultura Sinica 39, 1703–1708 (2006). [Google Scholar]
- Yang Q. X. & Yang C. R. Steroidal constituent of Aspidistra elatior from Yongshan, Yunnan. Acta Botanica Yunnanica 22, 109–115 (2000). [Google Scholar]
- Wang Y. F., Li X. C., Liu Y. Q., Wang J. J. & Yang C. R. A steroidal sapogenin from Tupistra wattii. Acta Botanica Yunnanica 17, 341–344 (1995). [Google Scholar]
- Wu G. X., Liu A. Y., Wei X. Y. & Chen W. X. Isolation and structural elucidation of sapogenins from Tupistra chinensis and their antifungal activity against Peronophythora litchii. J. Wuhan Botanical Res. 25, 89–92 (2007). [Google Scholar]
- Liu C. X. et al. Tupisteroide A–C, three new polyhydroxylated steroidal constituents from the roots of Tupistra chinensis. Magn. Reson. Chem. 50, 320–324 (2012). [DOI] [PubMed] [Google Scholar]
- Sharma S. C. & Sharma H. C. Oligofurostanosides from Asparagus curillus leaves. Phytochemistry 33, 683–686 (1993). [DOI] [PubMed] [Google Scholar]
- Higano T., Kuroda M., Jitsuno M. & Mimaki Y. Polyhydroxylated steroidal saponins from the rhizomes of Convallaria majalis. Nat. Prod. Commun. 2, 531–536 (2007). [Google Scholar]
- Xiang L. M., Wang Y. H., Yi X. M., Zheng G. J. & He X. J. Bioactive spirostanol saponins from the rhizome of Tupistra chinensis. Steroids 108, 39–46 (2016). [DOI] [PubMed] [Google Scholar]
- Eddy C. R., Wall M. E. & Scott M. K. Catalog of infrared absorption spectra of steroidal sapogenin acetates. Anal. Chem. 25, 266–271 (1953). [Google Scholar]
- Yokosuka A., Suzuki T., Tatsuno S. & Mimaki Y. Steroidal glycosides from the underground parts of Yucca glauca and their cytotoxic activities. Phytochemistry 101, 109–115 (2014). [DOI] [PubMed] [Google Scholar]
- Podolak I., Galanty A. & Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem. Rev. 9, 425–474 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J. N., Al-Omran A. & Parvathy S. S. Role of nitric oxide in inflammatory diseases. Inflammopharmacol. 15, 252–259 (2007). [DOI] [PubMed] [Google Scholar]
- Zhong Y., Chiou Y. S., Pan M. H. & Shahidi F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 134, 742–748 (2012). [DOI] [PubMed] [Google Scholar]
- Jiang K. et al. Anti-inflammatory terpenoids from the leaves and twigs of Dysoxylum gotadhora. J. Nat. Prod. 78, 1037–1044 (2015). [DOI] [PubMed] [Google Scholar]
- Gonzalez M., Zamilpa A., Marquina S., Navarro V. & Alvarez L. Antimycotic spirostanol saponins from Solanum hispidum leaves and their structure-activity relationships. J. Nat. Prod. 67, 938–941 (2004). [DOI] [PubMed] [Google Scholar]
- Man S. L., Gao W. Y., Zhang Y. J., Huang L. Q. & Liu C. X. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 81, 703–714 (2010). [DOI] [PubMed] [Google Scholar]
- Xiang L. M., Wang Y. H., Yi X. M., Feng J. Y. & He X. J. Furospirostanol and spirostanol saponins from the rhizome of Tupistra chinensis and their cytotoxic and anti-inflammatory activities. Tetrahedron 72, 134–141 (2016). [Google Scholar]
- Septisetyani E. P., Ningrum R. A., Romadhani Y., Wisnuwardhani P. H. & Santoso A. Optomization of sodium dodecyl sulphate as a formazan solvent and comparison of 3-(4,-5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay with WST-1 assay in MCF-7 cells. Indonesian J. Pharm. 25, 245 (2014). [Google Scholar]
- Thambiraj S. R., Phillips M., Koyyalamudi S. R. & Reddy N. Antioxidant activities and characterisation of polysaccharides isolated from the seeds of Lupinus angustifolius. Ind. Crops and Prod. 74, 950–956 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.