Abstract
This study aimed to determine effects of rice straw biochar on Pb sequestration in a soil-rice system. Pot experiments were conducted with rice plants in Pb-contaminated paddy soils that had been amended with 0, 2.5, and 5% (w/w) biochar. Compared to the control treatment, amendment with 5% biochar resulted in 54 and 94% decreases in the acid soluble and CaCl2-extractable Pb, respectively, in soils containing rice plants at the maturity stage. The amount of Fe-plaque on root surfaces and the Pb concentrations of the Fe-plaque were also reduced in biochar amended soils. Furthermore, lead species in rice roots were determined using Pb L3-edge X-ray absorption near edge structure (XANES), and although Pb-ferrihydrite complexes dominated Pb inventories, increasing amounts of organic complexes like Pb-pectins and Pb-cysteine were found in roots from the 5% biochar treatments. Such organic complexes might impede Pb translocation from root to shoot and subsequently reduce Pb accumulation in rice with biochar amendment.
Lead (Pb) is a toxic element commonly found in heavy-metal contaminated soils, and it has been one of the major global environmental concerns over the past few decades, especially in developing countries1. Plants play important roles in trapping and removing Pb from contaminated soils and affect its global transport. However, when Pb is excessively accumulated in the edible parts of crops, it can cause harm to human beings who consume the crop. For example, Pb can accumulate in the brain and severely affect the development of intelligence in children2.
Rice is widely cultivated globally, especially in southeastern Asia, and is a staple food for many people. It has been shown that rice accumulates considerable amounts of heavy metals in its grains3,4; therefore rice is assumed to be an important source of dietary heavy metal intake for people who consume it regularly. Indeed, studies have confirmed that Pb pollution in rice grains is common in some regions of China, especially in areas where the soil has been heavily polluted with Pb5.
Immobilization with amendments is a practical and low-cost measure that can be used to inhibit the uptake of heavy metals by crops grown on contaminated agricultural soils, especially in the countries or regions where there is a shortage of arable land. Immobilizing agents, such as Ca-, P-, and Si-containing materials, can selectively reduce the availability or mobility of heavy metals in the soil by adsorbing, complexing or precipitating heavy metals6.
In contrast, biochar is a solid carbonaceous residue produced by burning biomass under oxygen-free to oxygen-deficient conditions, and is increasingly being used to immobilize heavy metals in soils. Immobilization with biochar is considered as a promising method, owing to its distinct physicochemical properties, such as a surface area that is rich in organic groups, a alkaline pH, and a high carbon: nitrogen (C:N) ratio7,8. Biochar made from various raw materials has already been shown to effectively reduce the availability and mobility of heavy metals, such as Cd, Pb, Cu, and Ni, in soils9,10,11. And both Extended x-ray absorption fine structure (EXAFS) spectroscopy and chemical extractions have shown that, when soils are amended with biochar, Pb is transformed into more stable Pb-hydroxide, chloropyromorphite and Pb-phosphate12,13. Moreover, immobilization may also reduce Pb concentrations in the edible parts of crops14.
However, the inhibitory effect of an amendment on the accumulation of Pb in a soil-plant system depends not only on a decrease in the available Pb in soil, but also on a decrease in the translocation of Pb within the plants. Many studies have demonstrated that biochar reduces both the available Pb in soil and the Pb concentration in the edible parts of crops9,11,12,15, although not unanimously14. A few studies have also focused on the transfer characteristics of Pb accumulation in rice grains and revealed potential mechanisms underlying Pb retention in different parts of rice after soil amendment with biochar.
The transformation of Pb species in paddy soils and in different rice tissues after biochar applications remains unclear. Therefore, the objectives of this study were to investigate: (1) the Pb immobilization mechanisms in paddy soils and rice plants, and (2) the relationship between Pb accumulation in rice grains and the Pb transfer factors between different parts of the soil-rice system after biochar application.
Results and Discussion
Effects of biochar on Pb availability in the soil
Significant (One-way analysis of variance (ANOVA), P < 0.05) increases of 86.9% of water-soluble organic carbon (WSOC) and soil pH increased from 5.63 to 6.81 during tillering stage were found with the 5% biochar amendment, but the values of WSOC and soil pH at maturity stage were lower than tillering stage (Table 1). The biochar produced at a pyrolytic temperature of 500 °C had exhibited a higher pH, and the pH increase is due to the release of alkali salts from the feedstock during pyrolysis16. Then, because higher pH favors the dissolution of soil organic matter17, increased WSOC was also observed.
Table 1. CaCl2-and DTPA-extractable Pb in soil amended with biochar (mean ± S.E., n = 3).
| Biochar added | Tillering stage | pH | Maturity stage | pH | ||||
|---|---|---|---|---|---|---|---|---|
| CaCl2-Pb (mg kg−1) | DTPA-Pb (mg kg−1) | WSOC (mg kg−1) | CaCl2-Pb (mg kg−1) | DTPA-Pb (mg kg−1) | WSOC (mg kg−1) | |||
| 0% | 10.04 ± 1.54aa | 616.7 ± 19.76ab | 321.22 ± 20.70b | 5.63 ± 0.24c | 35.29 ± 5.49a | 750.2 ± 13.39a | 180.79 ± 10.63b | 5.31 ± 0.27b |
| 2.50% | 3.30 ± 0.46b | 639.8 ± 14.49a | 426.26 ± 16.31ab | 6.09 ± 0.06b | 12.68 ± 0.83b | 709.1 ± 26.82a | 166.45 ± 4.28b | 5.48 ± 0.10b |
| 5% | 0.33 ± 0.24b | 508.9 ± 46.02b | 599.85 ± 68.03a | 6.81 ± 0.31a | 2.00 ± 0.16b | 625.4 ± 16.87b | 270.57 ± 12.02a | 6.11 ± 0.13a |
*aThe same letter in a column indicates no significant difference at P < 0.05 according to Duncan’s multiple range test.
The CaCl2− and diethylene triamine pentaacetic acid (DTPA)-extractable Pb were significantly (P < 0.05) reduced in soils with biochar amendments at tillering and maturity stages (Table 1). Available soil Pb decreased more significantly with increasing biochar amendments. With 5% biochar amendment, CaCl2-extractable Pb decreased 96.7%, comparing with control (in absence biochar), at the tillering stage and 94.3% at the maturity stage, whereas DTPA-extractable Pb decreased 17.5% at the tillering stage, and 16.6% at the maturity stage. These results agree with a previous study, which found that CaCl2-extractable Pb declined sharply with the addition of biochar, whereas DTPA-extractable Pb fell slowly14. In addition, we also found that CaCl2- and DTPA-extractable Pb increased from the tillering stage to the maturity stage, which could be attributable to the decrease in soil pH.
The mobility and availability of Pb in soils are largely controlled by the chemical forms of Pb present and decrease in the following order: acid soluble forms >reducible forms >oxidizable forms >residual forms18. In our study, when 5% biochar was added, acid soluble Pb (II) was reduced significantly (P < 0.05) from 8.12% (control) to 3.67% at the tillering stage, and from 8.75% (control) to 4.03% at the maturity stage. In contrast, reducible Pb increased by 10.35 and 6.27% at the tillering and maturity stages, respectively (Fig. 1). These results suggested that after biochar application, acid soluble Pb (II) was partly transformed into reducible Pb in the soils, which agreed with a previous study that showed that Pb was immobilized by the formation of surface complexes between Pb2+ and functional groups on biochar when rice straw derived biochar was added to a polluted Ultisols16.
Figure 1.
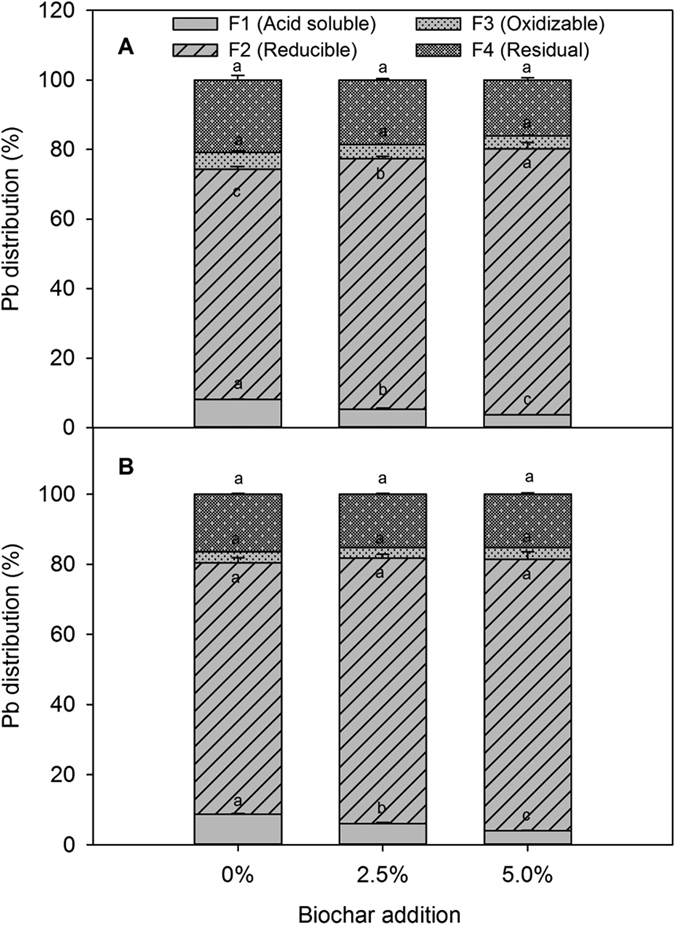
Lead percentage (%) showing the European Community Bureau of Reference sequential fractionations of soils at the tillering (A) and the maturity stages (B).
Soil pH was considered a critical factor in determining Pb availability, and in our study, we found that CaCl2-extractable Pb was negatively correlated with pH (rtillering stage = 0.78, P = 0.012; rmaturity stage = 0.74, P = 0.022; n = 9). This relationship was agreed with previous results that indicated that Pb phytoavailability decreases as the pH increases19. As pH increases, the hydrolysis of Pb is also increased, leading to more specific adsorption of Pb by the variable charge of the soils and more precipitation with phosphate as pyromorphite13,16. In addition, increased pH could cause an increase in Si concentration in pore water and silicon accumulation in rice plant resulted in Pb toxicity alleviation by some physiological mechanisms20,21. Lead can also be immobilized via specific adsorption onto the biochar particles due to the increased density of cation-exchange sites on the biochar surfaces22.
Effects of biochar on the Fe and Pb concentrations in iron plaque
Figure 2 showed the amounts of iron plaque (denoted as dithionite-citrate-bicarbonate (DCB)-extractable Fe) on the root surfaces after the different biochar amendments. With increasing biochar amendments, the DCB-extractable Fe (DCB-Fe) of roots was reduced significantly (one-way ANOVA, P < 0.05) at both tillering and maturity stages, and a similar trend was observed for DCB-extractable Pb (DCB-Pb). The formation of iron plaque is affected by many factors, including soil properties, root oxidation, and Fe (II) concentration in the soil rhizosphere. Therefore, it is likely that higher proportions of biochar resulted in a higher soil pH and lower water-soluble Fe in the soil solution, and ultimately, resulted in reduced Fe-plaque formation.
Figure 2.
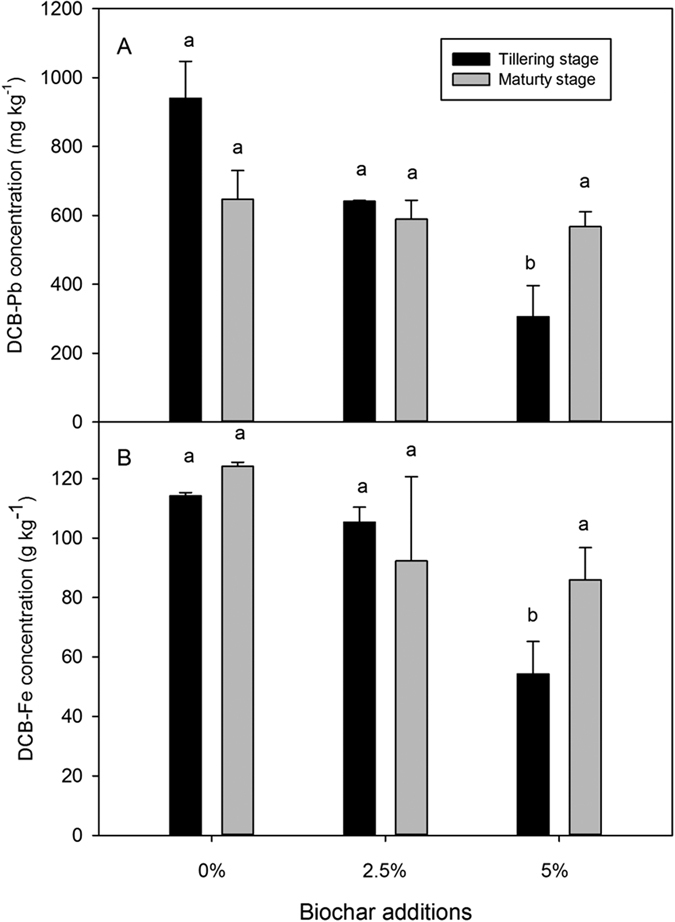
Concentrations of dithionite-citrate-bicarbonate (DCB)-extractable Pb (A) and Fe (B) (g kg−1) in iron plaques as a function of biochar amendment level.
Formation of Fe-plaque is also related to plant growth stage. DCB-Fe decreased from the tillering to the maturity stage. However, the Pb concentration in the roots (Fe-plaque removed) increased from the tillering to the maturity stage (Table 2). Generally, Fe-plaque has a high adsorption capacity for heavy metals due to its iron hydroxide functional groups23,24, and although it has also been reported that Fe-plaque fails to affect heavy metal uptake by plants24, prior research25 demonstrated that the increased capacity of Fe-plaque to retain Pb is the main mechanism for reducing Pb accumulation in rice shoots.
Table 2. Lead concentration in different parts of the rice plant (mg kg−1, mean ± SE, n = 3).
| Biochar added | Tillering stage | Maturity stage | Husk | Brown rice | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Root (without iron plaque) | Root (with iron plaque) | Stem | Leaf | Root (without iron plaque) | Root (with iron plaque) | Stem | Leaf | |||
| 0% | 707.1 ± 42.17aa | 2176 ± 423.9a | 21.56 ± 1.21a | 22.78 ± 2.60a | 870.1 ± 77.42b | 1553 ± 62.17a | 41.73 ± 2.09a | 111.6 ± 14.90a | 2.33 ± 0.39a | 0.40 ± 0.10a |
| 2.50% | 849.2 ± 153.1a | 2104 ± 65.45a | 8.73 ± 0.68b | 6.69 ± 1.01b | 918.9 ± 95.43ab | 1543 ± 50.68a | 13.31 ± 0.92b | 22.67 ± 3.78b | 0.72 ± 0.13b | 0.20 ± 0.03ab |
| 5% | 570.7 ± 132.9a | 1278 ± 26.67b | 4.51 ± 0.62c | 5.55 ± 1.20b | 1194 ± 83.26a | 1722 ± 401.9a | 10.61 ± 0.60b | 21.14 ± 6.22b | 0.47 ± 0.16b | 0.14 ± 0.02b |
*aThe same letter in a column indicates no significant difference at P < 0.05 according to Duncan’s multiple range test.
Effects of biochar on the concentration of Pb in different rice plant tissues
The Pb concentrations of rice shoots were significantly (one-way ANOVA, P < 0.05) reduced by the addition of biochar (Table 2). The Pb concentrations in the stems and leaves at the tillering stage were reduced by 79.08 and 75.64%, respectively, in soils with 5% biochar whereas the Pb concentrations in the stems, leaves, husks, and brown rice were reduced by 74.57, 81.06, 79.83, and 65%, respectively. Moreover, Pb concentration of brown rice was reduced from 0.4 mg kg−1 (control) to 0.14 mg kg−1 (5% biochar), the latter of which was below the hygienic standard for rice in China (0.2 mg kg−1)26. From statistical analysis, the Pb concentration of various parts of rice showed positive correlations with CaCl2-extractable Pb at the maturity stage (rstem = 0.879, Pstem = 0.002; rleaf = 0.913, Pleaf = 0.001; rrice = 0.713, Price = 0.031; rhusk = 0.851, Phusk = 0.004, n = 9) and at the tillering stage (rstem = 0.954, Pstem < 0.001; rleaf = 0.842, Pleaf = 0.004, n = 9). Pb concentrations in different rice tissues were also significantly correlated with DTPA-extractable soil Pb at the maturity stage (rstem = 0.671, Pstem = 0.048; rleaf = 0.695, Pleaf = 0.038; rrice = 0.666, Price = 0.050; rhusk = 0.672, Phusk = 0.047; n = 9), but had no correlation at the tillering stage. In line with a previous report that no significant change in metal extractability by DTPA was observed after biochar application to field soil14, the results indicate that CaCl2-extractable Pb is better than DTPA-extractable Pb at predicting the bioavailability of soil Pb in biochar treated soils.
Effects of biochar on the transfer of Pb between different rice plant tissues
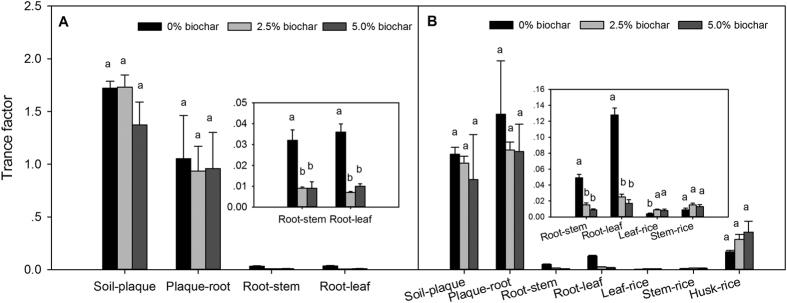
Transfer factors (TF) are used to estimate the transport of elements from soil to plants, or from one part of a plant to another27, and were used in this study to assess the transfer characteristics of Pb among rice tissues (Fig. 3). Biochar amendment significantly (one-way ANOVA, P < 0.05) decreased the root-to-leaf and root-to-stem transfer of Pb at both tillering and maturity stages. A significant reduction in TFroot-leaf (72.22%), and TFroot-stem (71.88%) occurred at the tillering stage of plants from the 5% biochar treatment, and TFroot-leaf and TFroot-stem were also reduced at the maturity stage, by 86.72 and 81.63%, respectively. However, there were no significant changes in TFsoil-iron plaque, TFiron plaque-root, TFstem-rice, and TFleaf-rice in response to biochar amendment.
Figure 3.
Transfer factors for Pb between different parts of the soil-rice system at tillering (A) and the maturity stages (B).
Lead species in roots from the biochar treatments were determined using Pb L3-XANES, and the best fit was derived by incrementally increasing the number of fit components and minimizing the fit residual. The linear combination fitting (LCF) results indicated that ferrihydrite associated with Pb dominated the Pb inventory (47.1–60.8%) in roots with Fe-plaque (Table 3). Pb-pectin and Pb-cysteine complexes ranked as the second dominant forms. The roots from control treatments had more ferrihydrite associated Pb than those from biochar treatments, which could be attributed to the greater accumulation of Fe-plaque on the root surfaces of control plants, compared to plants from 5% biochar treatment group. However, with biochar application, more Pb was combined with pectin and cysteine, which can occur inside root cells. In fact, the Pb-cysteine was up to about 21.6% in the roots of the plants subjected to the 5% biochar treatment, whereas none was found in the control roots.
Table 3. The results of linear combination fitting for the Pb L3-edge XANES spectra of roots (with iron plaque).
| Biochar added | Weighted percentage (%) of the roots | |||||
|---|---|---|---|---|---|---|
| Pb(NO3)2 | Ferrihydrite | Pectin | Cysteine | Pb5PO4Cl | R-factor (×103)b | |
| 0% | 7.4 ± 1.6 | 60.8 ± 3.4 | 21.4 ± 2.1 | 0 | 10.8 ± 2.5 | 0.21 |
| 5% | 11.4 ± 1.0 | 47.1 ± 1.7 | 20.9 ± 1.9 | 21.6 ± 3.1 | 0 | 0.12 |
The data show the proportion (%) of the reference spectra that resulted in the best fit to the sample dataa.
aMean ± standard deviation. Weighting factors on each fit summed to 100 ± 3%.
bNormalized sum of the squared residuals of the fit (R-factor = ∑(data-fit)2/∑data2).
There are two main processes that contributed to Pb translocation from root to shoot; (1) translocation by symplastic passage and active loading into the xylem, and (2) translocation driven by root pressure and the transpiration stream28. For most nonhyperaccumulating plants, Pb mainly accumulates in the roots with only a small portion being transported to shoots via symplastic pathways29,30. In these plants, the cell wall is the first defensive barrier that reduces Pb transfer into root cells and functions by binding Pb with polysaccharides pectins in cell walls31. Accordingly, the complexation of pectin with Pb2+ inside the root reduces the translocation of Pb from the root to the shoot32. Moreover, the retention capacity for Pb is linearly dependent on the number of functional groups such as -COOH, -OH, -SH, in cell walls32. The Pb-cysteine complex was found in the roots of the 5% biochar treatment plants (Fig. 4, Table 3). The formation of Pb-cysteine can also inhibit the translocation of Pb from the root to the shoot, resulting in relatively lower TFroot-leaf and TFroot-stem values for biochar treated rice plants.
Figure 4.
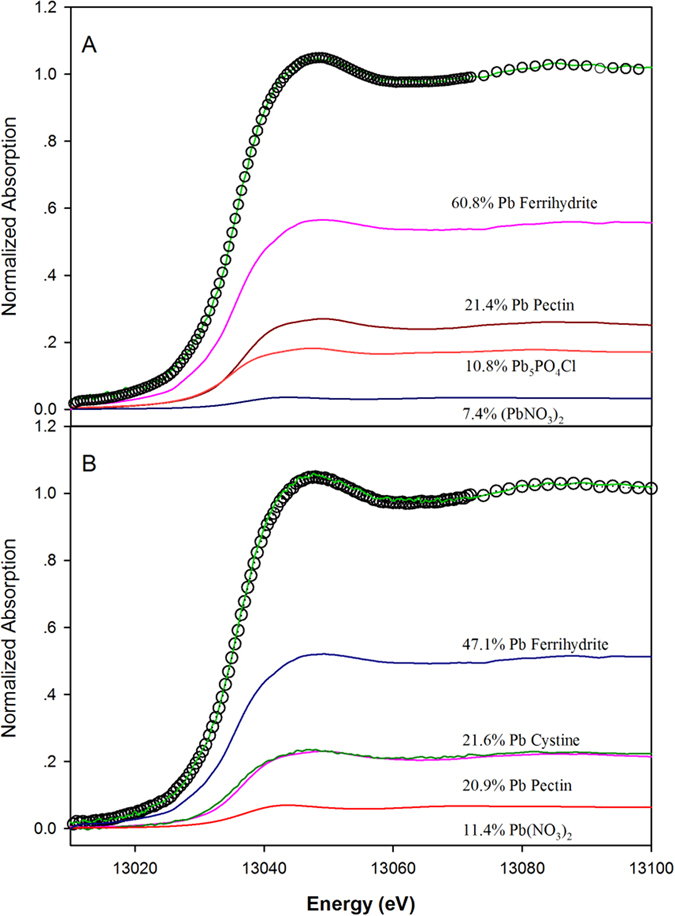
Pb L3 edge XANES spectra for the root samples with 0% (A) and 5% (B) biochar treatments along with the standards used to give the best fit. Solid line = measured; Open circles = best LCF fit.
The Pb concentration in brown rice decreased after biochar application (Table 2), and this was significantly correlated with TFroot-leaf (r = 0.720, P = 0.031, n = 9) and TFroot-stem (r = 0.798, P = 0.010, n = 9). Clearly, the decrease in Pb transfer from root to shoot, with the available soil Pb, is critical for Pb accumulation in rice grains.
Conclusions
The amendment of biochar into a Pb-contaminated soil resulted in a decrease in soil Pb availability and amounts of Fe-plaque on root surfaces of rice. In addition, Pb concentrations in Fe-plaque were decreased after the biochar addition. Both results possibly explained the decreasing amounts of Pb uptake on rice roots. For the rice roots, we found Pb-pectin and Pb-cysteine in samples with biochar amendments. Such organic complexes could impede Pb translocation from root to shoot and subsequently lead to less Pb accumulation in brown rice with biochar addition in soils.
Methods
Preparation of biochar
Rice straw was collected from Minghou Agricultural Experiment Station, Fuzhou City, Fujian Province, China, and to produce biochar, the rice straw was carbonized at a pyrolysis temperature of 500 °C under anaerobic conditions in an oven for 2 h. There was also a continuous flux of nitrogen gas until the biochar had cooled down to 25 °C. The resulting biochar contained 0.37 mg kg−1 Cd, 6.16 mg kg−1 Pb, and 217.39 mg kg−1 Zn, and had a surface area of 18.64 m2 g−1, and a pH (H2O) of 10.3. The biochar was then ground, sifted through a 2-mm sieve, and stored in airtight containers prior to use as a soil amendment.
Soil sampling and analyses
Contaminated soil was collected from the surface horizon (0–20cm) of a paddy field near a smelter (26°15′28.49″N and 118°15′12.78″E) in Youxi County, central Fujian Province, China. The soil samples were air-dried, ground, passed through a 2-mm sieve, and then analyzed (Table 4). Soil pH was determined using a pH meter (SevenCompact; Mettler-Toledo, Greifensee, Switzerland) in 5:1 water/soil suspensions. Cation-exchange capacity (CEC) was determined using 1 M ammonium acetate buffered at pH 7.0. Soil organic matter was determined using the K2Cr2O7 wet oxidation method. Soil particle-size distribution (<2 mm fractions) was determined using the pipette method after hydrogen peroxide treatments33.
Table 4. Physicochemical properties of the Pb-contaminated soil used in this study.
| Parameter | Value |
|---|---|
| pH | 6.05 |
| CEC (cmol+ kg−1) | 17 |
| Organic matter (g kg−1) | 21.86 |
| Metals (mg kg−1) | |
| Cd | 5.88 |
| Pb | 1602 |
| Zn | 2132 |
| Exchangeable cation (cmol+ kg−1) | |
| Ca | 6.94 |
| Mg | 1.06 |
| K | 0.84 |
| Na | 0.51 |
| Soil texture | Silty loam |
| Particle size distribution (%) | |
| Sand | 44.56 |
| Silt | 48.21 |
| Clay | 7.23 |
Growth conditions and seed treatment
All of our pot experiments were conducted in a greenhouse at the College of Resource and Environmental Sciences, Fujian Agriculture and Forestry University, Fuzhou City, Fujian Province, South-eastern China. Rice seeds (Donglian 5 of conventional Oryza sativa L. ssp. indica) were sterilized in a solution containing 1% sodium hypochlorite solution for 15–30 min and germinated in dishes containing moist tissue paper for 3 d. After germination, rice seedlings were selected transferred to a beaker, and grown for 12 d. Subsequently, five homogeneous seedlings were selected transplanted into each pot, and the potted plants were grown in a greenhouse under normal sunlight.
The pots used in this study were 9L ceramic pots that measured 20 cm (bottom) and 28 cm (top) in diameter and 17 cm in height and they will filled with 7.5 kg air-dried soil, 2.1 g urea, 1.2 g NH4H2PO4, and 2.1 g K2SO4. In addition, the soil mixtures were amended with 0% (control, absence of biochar), 2.5%, or 5% biochar by weight. The mixtures were equilibrated 10 d at 70% of water holding capacity. All experiments were performed in triplicates. Each pot was submerged in water, and the water level was maintained at approximately 1–2 cm above the soil surface during the entire growing period.
Sample collection and analysis
Soil samples and rice plants were collected from each pot at the tillering and maturity stages. The soil samples were air-dried, ground with a wood roller, and passed through a 2-mm sieve. A small portion of the soil was further ground with an agate mortar to pass a 0.149-mm sieve and was subsequently used for chemical and physical analyses.
Bioavailable Pb in the soils was extracted using a CaCl2 solution (0.1 M) and a DTPA solution (diethylene triamine pentaacetic acid, 0.005 M; CaCl2, 0.01 M; triethanolamine [TEA], 0.1 M; pH = 7.3), according to the following procedures: 5.00g air-dried soil (<2 mm) was mixed with 25 mL of CaCl2 solution or DTPA solution. The soil suspension was continuous shaken at 25 °C for 2 h and then immediately filtered. Exactly 1.0 g of the air-dried soil was then mixed with 25 mL decarbonized distilled deionized water (DDW) and shaken at 25 °C for 2 h. Then the soil suspension was filtered through a Millipore filter (0.45 μm), and the filtrated was used for water-soluble organic carbon (WSOC) analysis, which was detected using a TOC analyzer (model 5000A; Shimadzu, Tokyo, Japan).
Plant shoot tissues were separated into stems, leaves, husks, and brown rice. The plant tissues were deep-washed in DDW, and after drying at 70 °C until constant weight, the dry weights of the aerial portions of the plants in each pot were recorded.
Iron plaque was extracted from fresh root surfaces using a dithionite-citrate-bicarbonate (DCB) solution, containing 0.03 M sodium citrate (Na3C6H5O7·2H2O), 0.125 M sodium bicarbonate (NaHCO3), and 0.06 M sodium dithionite (Na2S2O4). The rice roots were dipped into the DCB solution (40 mL) at 25 °C for 60 min, and then rinsed three times with DDW. The water used for rinsing was mixed with the DCB extracts for a total volume of up to 100 mL, and the concentrations of Pb and Fe in the diluted DCB extracts were measured. After extraction with the DCB solution, fresh roots were oven dried for 48 h at 70 °C for further analysis.
The dried plant tissues were pulverized into a powder and 0.1–0.2 g of the powder was digested with HNO3/hyperchloric acid in heating blocks34. After digestion, the sample solutions were dried and dissolved in 0.5 M HNO3, filtered through a Millipore filter (0.45 μm), then stored in plastic bottles for subsequent analysis. The Pb concentration was measured using an Induced Couple Plasma-Mass Spectrometer (ICP-MS, NexION 300X; Perkin Elmer, NY).
Sequential extraction of soil Pb
After harvest, soils with and without biochar were subjected to a European Community Bureau of Reference (BCR) sequential extraction, using a three-stage modified procedure recommended by the BCR methodology35. Briefly, four metal fractions, including exchangeable and acid soluble (F1), reducible (F2), oxidizable (F3), and residual (F4) fractions, were determined (see supporting information for details).
Lead L3-edge X-ray absorption spectroscopy (XAS)
The collected root samples were freeze drying for three days, pulverized into powders, and passed through a 0.149-mm mesh sieve. Approximately 0.2 g of the root samples were mounted in acrylic holders, sealed with Kapton tape to avoid desiccation, and stored at 4 °C until analysis. Spectra were collected at room temperature using the Beamline BL12B at Spring-8, Hyogo Prefecture, Japan, where the storage ring was operated at a fixed current of 100 mA. The Si (111) monochromator energy was calibrated to 13035 eV based on the first inflection point in the L3-edge derivative spectra from a Pb foil. Spectra were collected in fluorescence or transmission mode with a Lytle detector or gas ionization chamber at photon energies between −200 to +750 eV relative to the PbL3-edge energy at 13,035 eV, using a step size of 0.5 eV across the absorption edge region (−30 to +40 eV), and a step size of k = 0.06 Å−1 at higher energies.
At least three scans were acquired for each sample. Multiple XAS scans were aligned, merged and processed using the Athena program, an interface to IFEFFIT36,37 The backgrounds of the spectra were corrected using a linear pre-edge function between −200 and −30 eV and a linear or quadratic function between 55 and 400 eV, included a flattening function in the post-edge region for normalization. The distributions of the Pb species in the samples were determined using LCF across the region from 30 eV below to 33 eV above the PbL3 edge.
Reference materials for LCF included Pb complexed with biochar and adsorbed on kaolinite, ferrihydrite, Al-hydroxide, birnessite, humic acid, and root cell walls, which were prepared by mixing 250 mL of 0.5 mM Pb(NO3)2 with 2.5 g of sorbents and incubating the suspensions at pH 5 for 48 h. In addition, the organic Pb complexes of Pb-malate, Pb-citrate, Pb-cysteine, Pb-oxalate, Pb-pectin, Pb-acetate, and Pb-glutathione were prepared by adding 12.5 mL of 100.0 mM malate, citrate, cysteine, oxalate, pectin, acetate, glutathione, respectively, to 1.25 mL of 100 mM Pb(NO3)2, diluting them with 0.5 mM HNO3 to 250 mL and then mixing them at pH 5 for 24 h. The references of PbS, Pb(NO3)2, PbCl2, PbO, PbO2, PbSO4, Pb5(PO4)3Cl, and Pb(OH)2·2PbCO3 were purchased from Sigma-Aldrich whereas Pb3(PO4)2 was synthesized as described by Wruck38.
Calculation of the transfer factor
The transfer factor from part “a” to part “b” was calculated using the following formula:
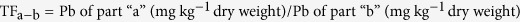 |
Data processing and statistics
One-way analysis of variance (ANOVA) and the Duncan multiple range test (DMRT) were used to test for significant differences between the Pb concentrations of different plant tissues and soils, according to a 5% level of significance. The correlation coefficient was computed using SPSS, version 18.0 (SPSS Institute, Chicago, IL, 2009).
Additional Information
How to cite this article: Li, H. et al. Biochar amendment immobilizes lead in rice paddy soils and reduces its phytoavailability. Sci. Rep. 6, 31616; doi: 10.1038/srep31616 (2016).
Supplementary Material
Acknowledgments
This study was funded by the National Natural Science Foundation of China (grant no. U1305232 and 41301576). The authors thank Huang Bifei for technical assistance in ICP-MS analysis, Bo Xu for his assistance on determination of Fe-plaque, Shouyin Tang, Haixia Dong, Mingliu Zhao for their experimental cooperation.
Footnotes
Author Contributions All authors were involved in the study design and contributed to the writing of the manuscript. G.W. (Guo Wang) designed the experiment(s), H.L. (Honghong Li) and Y.L. (Yuting Liu) conducted the experiment(s) and analyzed experimental results, H.L. wrote the paper with the help of Y.L. (Yuting Liu), Y.C. (Yanhui Chen), S.W. (Shanli Wang), M.W. (Mingkuang Wang), T.X. (TuanhuiXie) G.W.(Guo Wang) revised and finalized the manuscript.
References
- McCann C. M. et al. Remediation of a historically Pb contaminated soil using a model natural Mn oxide waste. Chemosphere 38, 211–217 (2015). [DOI] [PubMed] [Google Scholar]
- Ryan J. A. et al. Reducing children’s risk from lead in soil. Environ Sci Technol 38, 18a–24a (2004). [DOI] [PubMed] [Google Scholar]
- Singh A., Sharma R. K., Agrawal M. & Marshall F. M. Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem Toxicol 48, 611–619 (2010). [DOI] [PubMed] [Google Scholar]
- Yoshihara T., Goto F., Shoji K. & Kohno Y. Cross relationships of Cu, Fe, Zn, Mn, and Cd accumulations in common japonica and indica rice cultivars in Japan. Environ Exp Bot 68, 180–187 (2010). [Google Scholar]
- Williams P. N. et al. Occurrence and Partitioning of Cadmium, Arsenic and Lead in Mine Impacted Paddy Rice: Hunan, China. Environ Sci Technol 43, 637–642 (2009). [DOI] [PubMed] [Google Scholar]
- Udeigwe T. K., Eze P. N., Teboh J. M. & Stietiya M. H. Application, chemistry, and environmental implications of contaminant-immobilization amendments on agricultural soil and water quality. Environ Int 37, 258–267 (2011). [DOI] [PubMed] [Google Scholar]
- Gul S., Whalen J. K., Thomas B. W., Sachdeva V. & Deng H. Y. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agr Ecosyst Environ 206, 46–59 (2015). [Google Scholar]
- Yang Y. et al. Biochar from Alternanthera philoxeroides could remove Pb(II) efficiently. Bioresource Technol 171, 227–232 (2014). [DOI] [PubMed] [Google Scholar]
- Kim H. S. et al. Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environmental Earth Sciences 74, 1249–1259 (2015). [Google Scholar]
- Herath I., Kumarathilaka P., Navaratne A., Rajakaruna N. & Vithanage M. Immobilization and phytotoxicity reduction of heavy metals in serpentine soil using biochar. J Soil Sediment 15, 126–138 (2015). [Google Scholar]
- Puga A. P., Abreu C. A., Melo L. C. A., Paz-Ferreiro J. & Beesley L. Cadmium, lead, and zinc mobility and plant uptake in a mine soil amended with sugarcane straw biochar. Environ Sci Pollut R 22, 17606–17614 (2015). [DOI] [PubMed] [Google Scholar]
- Almaroai Y. A. et al. Effects of biochar, cow bone, and eggshell on Pb availability to maize in contaminated soil irrigated with saline water. Environmental Earth Sciences 71, 1289–1296 (2014). [Google Scholar]
- Ahmad M. et al. Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere 95, 433–441 (2014). [DOI] [PubMed] [Google Scholar]
- Bian R. J. et al. A three-year experiment confirms continuous immobilization of cadmium and lead in contaminated paddy field with biochar amendment. Journal of hazardous materials 272, 121–128 (2014). [DOI] [PubMed] [Google Scholar]
- Zheng R. L. et al. The effects of biochars from rice residue on the formation of iron plaque and the accumulation of Cd, Zn, Pb, As in rice (Oryza sativa L.) seedlings. Chemosphere 89, 856–862 (2012). [DOI] [PubMed] [Google Scholar]
- Jiang T. Y., Jiang J., Xu R. K. & Li Z. Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 89, 249–256 (2012). [DOI] [PubMed] [Google Scholar]
- Curtin D., Peterson M. E. & Anderson C. R. pH-dependence of organic matter solubility: Base type effects on dissolved organic C, N, P, and S in soils with contrasting mineralogy. Geoderma 271, 161–172 (2016). [Google Scholar]
- Zimmerman A. J. & Weindorf D. C. Heavy metal and trace metal analysis in soil by sequential extraction: a review of procedures. International journal of analytical chemistry 2010, 387803 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben D., Evrard L. & Sonnet P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 92, 1450–1457 (2013). [DOI] [PubMed] [Google Scholar]
- Liang Y., Sun W., Zhu Y. G. & Christie P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147, 422–428 (2007). [DOI] [PubMed] [Google Scholar]
- Neumann D. & zur Nieden U. Silicon and heavy metal tolerance of higher plants. Phytochemistry 56, 685–692 (2001). [DOI] [PubMed] [Google Scholar]
- Harvey O. R., Herbert B. E., Rhue R. D. & Kuo L. J. Metal Interactions at the Biochar-Water Interface: Energetics and Structure-Sorption Relationships Elucidated by Flow Adsorption Microcalorimetry. Environ Sci Technol 45, 5550–5556 (2011). [DOI] [PubMed] [Google Scholar]
- Batty L. C., Baker A. J. M., Wheeler B. D. & Curtis C. D. The effect of pH and plaque on the uptake of Cu and Mn in Phragmites australis (Cav.) Trin ex. Steudel. Ann Bot-London 86, 647–653 (2000). [Google Scholar]
- Liu H. J., Zhang J. L., Christie P. & Zhang F. S. Influence of iron plaque on uptake and accumulation of Cd by rice (Oryza sativa L.) seedlings grown in soil. Sci Total Environ 394, 361–368 (2008). [DOI] [PubMed] [Google Scholar]
- Wei W., Cui J. & Wei Z. Effects of low molecular weight organic acids on the immobilization of aqueous Pb(II) using phosphate rock and different crystallized hydroxyapatite. Chemosphere 105, 14–23 (2014). [DOI] [PubMed] [Google Scholar]
- MHPRC (Ministry of Healtlh of the People’s Republic of China). Maximum levels of contaminants in foods (GB2762-2005). Beijing, China: MHPRC. (in Chinese) (2005).
- Al Attar L., Al-Oudat M., Safia B. & Ghani B. A. Transfer factor of 90Sr and 137Cs to lettuce and winter wheat at different growth stage applications. Journal of Environmental Radioactivity 150, 104–110 (2015). [DOI] [PubMed] [Google Scholar]
- Verbruggen N., Hermans C. & Schat H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol 182, 781–781 (2009). [DOI] [PubMed] [Google Scholar]
- Malecka A., Piechalak A., Morkunas I. & Tomaszewska B. Accumulation of lead in root cells of Pisum sativum. Acta Physiol Plant 30, 629–637 (2008). [Google Scholar]
- Shahid M., Pinelli E., Pourrut B., Silvestre J. & Dumat C. Lead-induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotox Environ Safe 74, 78–84 (2011). [DOI] [PubMed] [Google Scholar]
- Krzeslowska M. The cell wall in plant cell response to trace metals: polysaccharide remodeling and its role in defense strategy. Acta Physiol Plant 33, 35–51 (2011). [Google Scholar]
- Pelloux J., Rusterucci C. & Mellerowicz E. J. New insights into pectin methylesterase structure and function. Trends Plant Sci 12, 267–277 (2007). [DOI] [PubMed] [Google Scholar]
- Jackson M. L. Soil chemical analysis. 2nd Ed, Published by Author. University of Wisconsin, Madison, WI (1979).
- Sun Y. B., Zhou Q. X. & Diao C. Y. Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L. Bioresource Technol 99, 1103–1110 (2008). [DOI] [PubMed] [Google Scholar]
- Rauret G. et al. Application of a modified BCR sequential extraction (three-step) procedure for the determination of extractable trace metal contents in a sewage sludge amended soil reference material (CRM 483), complemented by a three-year stability study of acetic acid and EDTA extractable metal content. J Environ Monitor 2, 228–233 (2000). [DOI] [PubMed] [Google Scholar]
- Newville M. IFEFFIT: interactive XAFS analysis and FEFF fitting. J Synchrotron Radiat 8, 322–324 (2001). [DOI] [PubMed] [Google Scholar]
- Ravel B. & Newville M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12, 537–541 (2005). [DOI] [PubMed] [Google Scholar]
- Wruck B., Salje E. K. H., Zhang M., Abraham T. & Bismayer U. On the thickness of ferroelastic twin walls in lead phosphate Pb3(PO4)2-an X-Ray-Diffraction Study. Phase Transitions 48, 135–148 (1994). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.