Abstract
The concept of gene therapy was introduced in the 1970s after the development of recombinant DNA technology. Despite the initial great expectations, this field experienced early setbacks. Recent years have seen a revival of clinical programs of gene therapy in different fields of medicine. There are many promising targets for genetic therapy as an adjunct to cardiac surgery. The first positive long-term results were published for adenoviral administration of vascular endothelial growth factor with coronary artery bypass grafting. In this review we analyze the past, present, and future of gene therapy in cardiac surgery. The articles discussed were collected through PubMed and from author experience. The clinical trials referenced were found through the Wiley clinical trial database (http://www.wiley.com/legacy/wileychi/genmed/clinical/) as well as the National Institutes of Health clinical trial database (Clinicaltrials.gov).
My hour is almost come.—Hamlet, Act I.
The concept of gene therapy was introduced in the 1970s after the development of recombinant DNA technology. Despite great expectations, the early days of gene therapy are known for setbacks including the failures of initial clinical trials, reports of well-known complications, and safety and ethical concerns. These early issues seriously hampered the development of this scientific field and engendered a lot of skepticism, which to a certain degree persists to this day. However, gene therapy has advantages over other therapies in the ability to change the structure and function of the cell at the molecular level as well as the ability to directly target intracellular signaling pathways. Moreover, gene therapy is currently experiencing a revival in different medical disciplines. Recent major clinical successes in gene therapy include improvement in visual function in patients with Leber’s congenital amaurosis after subretinal delivery of recombinant adeno-associated viral (AAV) vector carrying the gene RPE65 [1], termination of cerebral demyelination in patients with X-linked adrenoleukodystrophy treated with CD34 cells genetically corrected ex vivo with a lentiviral vector encoding the ABCD1 gene [2], complete remission in chemotherapy-refractory B-cell lymphoma after injection of genetically modified T cells [3], the use of autologous genetically modified epidermal stem cells for epidermolysis bullosa, a devastating genetic skin disease, shown to be safe and stable long-term (6.5 years) [4], and the approval by the European Medicines Agency of the first gene therapy product, Glybera (alipogen tiparvovec), consisting of AAV-mediated lipoprotein lipase gene for the treatment of lipoprotein lipase deficiency [5]. In our opinion these achievements are explained by progress in molecular biology and valuable lessons learned from the past.
Cardiac gene transfer received an impetus to its development in 1990 with the surgical direct intramyocardial injection of β-galactosidase/plasmid DNA construct into the left ventricle (LV) of beating rat hearts. Gene activity was demonstrated 4 weeks after delivery, direct evidence that the gene was taken up and expressed in myocytes [6]. In the next 15 years, many cardiac surgeons in the United States and abroad have made significant contributions to the further development of gene-based therapy. However, progress to clinical trials for gene therapy in cardiac surgery has been lacking. Cardiovascular applications account for 7.8% of gene therapy clinical trials, and cardiac surgery accounts for only 6.2% of these—or 0.48% of all gene therapy clinical trials. It is also interesting that provided an explanation of gene therapy, 80% of patients surveyed who underwent cardiac surgery would accept gene therapy as a concomitant treatment [7].
The primary existing clinical trial approaches in cardiac surgery are therapeutic angiogenesis for coronary artery disease as well as the pretreatment of vein grafts before coronary artery bypass grafting (CABG) to prevent graft failure. Promising directions include concomitant genetic treatment with cardiac surgery for low ejection fraction, as well as advances in cardiac transplantation and correction of atrial fibrillation and other types of arrhythmias.
In this review, we will (1) describe and analyze prospective or unfinished gene therapy clinical trials in cardiac surgery, (2) compare and contrast cardiac surgical clinical trials with cardiology clinical trials in their most investigated application, therapeutic angiogenesis, and (3) summarize the hurdles and prospects of current cardiac surgery clinical trials.
Clinical Trials in Cardiac Surgery
The vast majority of gene therapy clinical trials in cardiac surgery are devoted to the stimulation of angiogenesis.
Stimulation of Angiogenesis
Angiogenesis, the formation of new vessels from existing endothelium, has an important role in tissue perfusion, collateral growth, and contractile function. Angiogenesis can be initiated by stimulation of angiogenic growth factors through recombinant or purified proteins that regulate endothelial cell activation and migration, the secretion of plasminogen activators and proteolytic enzymes, and endothelial permeability, and that eventually affect myocyte survival [8]. Gene therapy has an advantage over protein delivery owing to its more sustained therapeutic effect [9]. Exogenous overexpression of genes coding for proangiogenic factors offers an attractive solution in patients for whom full revascularization is impossible. The first gene therapy clinical trial in cardiac surgery was performed in 1997 through 1999 (Table 1) [10–17]. On completion of the CABG procedure, adenoviral (Ad) vector expressing vascular endothelial growth factor (VEGF) 121 (Ad.VEGF 121) was administered by direct myocardial injection into the areas of myocardium that demonstrated reversible ischemia by perfusion scan. The injections were done in 10 sites per patient in the left anterior descending coronary artery and circumflex artery areas with a maximal dose of 4 × 1010 vector genomes. All patients reported improvement in angina class after therapy, and gene administration was well tolerated [10]. Long-term follow-up (median, 11.8 years) showed improved 5- and 10-year survival relative to comparable groups with coronary artery disease treated with medical therapy as well as the safety of Ad.VEGF gene therapy [11]. A later trial reviewed the injection of plasmid vector encoding VEGF165 into ischemic myocardium that could not be surgically revascularized during CABG. Left ventricular function values improved, and the majority of patients were free from angina 6 months after surgery. Patients reported improved quality of life and a reduction in nitroglycerin usage. A reduction in the ischemic defects detected by single-photon emission computed tomography was also observed [12]. A multicenter, randomized, double-blind, placebo-controlled study was performed using a single Ad delivery of an endogenous transcription factor, termed hypoxia inducible factor (HIF-1α), which initiates the expression of multiple proangiogenic genes. Patients who could not undergo complete revascularization in all myocardial areas received 10 injections of Ad.HIF-1 during CABG. At 1-year follow-up, all patients had uncomplicated postoperative courses, and various imaging techniques revealed improved perfusion in the Ad.HIF-injected areas in selected patients and a trend toward improvements in ventricular function in the targeted areas [13]. The randomized, double-blind, placebo-controlled Endothelial Modulation in Angiogenic Therapy trial (ClinicalTrials.gov NCT00134433) was performed with intramyocardial injection of VEGF-165 plasmid (10 injections at a dose 200 μg before release of the cross clamp) and L-arginine (substrate for nitric oxide production) during CABG [14]. End points included 3-month changes versus baseline in myocardial perfusion and contractility. The authors indicated that the patients who received VEGF and L-arginine had improved anterior wall perfusion based on positron emission tomography scan and had better anterior wall contractility [14]. Moreover, we found two more approved trials without published results: intramyocardial injection of Ad.HIF during CABG and intramyocardial injection of Ad.VEGF121 during off-pump CABG (Wiley clinical trial identifier numbers US-0374 and US-0442, respectively). A phase 1, dose-escalation, single-center study assessed the safety and tolerability of the plasmid VM202 encoding hepatocyte growth factor in subjects with ischemic heart disease as an adjunct therapy to CABG. VM202 contains a genomic cDNA hybrid of the human hepatocyte growth factor gene that can express multiple isoforms of hepatocyte growth factor through alternative splicing. Injection was performed in 9 patients into the right coronary artery territory. Authors demonstrated that intramyocardial injection of VM202 can be safely used in patients undergoing CABG and that improved regional myocardial perfusion and wall thickness were observed in the injected region at the 6-month follow-up (ClinicalTrials.gov NCT01422772) [15].
Table 1.
Gene Therapy Clinical Trials in Cardiac Surgery
| Therapeutic Target | Transgene | Route | Vector | Number of Patients | Phase | Procedure | Status | Results | End Points | Follow-Up | Reference/Identifier |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiogenesis | VEGF 121 | intramyocardial | adenovirus | 31 | 1 | CABG + minithoracotomy | completed | positive | angina class, survival, safety | 11.8 years | [10, 11] |
| Angiogenesis | VEGF 165 | intramyocardial | plasmid | 22 | 1 | CABG + minithoracotomy | completed | positive | EF, myocardial perfusion, angiogram | short term | [12] |
| Angiogenesis | HIF-1 | intramyocardial | adenovirus | 13 | 1 | CABG | completed | positive in selected patients | SPECT perfusion, ECHO,MRI, PET | 1 year | [13] |
| Angiogenesis | VEGF 165+arginine | intramyocardial | plasmid | 19 | 1 | CABG | completed | positive | PET perfusion, ECHO | 3 mo | [14] NCT00134433 |
| Angiogenesis | HGF (VM 202RY) | intramyocardial | plasmid | 9 | 1 | CABG | completed | positive | Myocardial perfusion, ECHO, MRI | 6 mo | [15] NCT01422772 |
| Angiogenesis | HIF-1 | intramyocardial | adenovirus | N/A | 1 | CABG | withdrawn | N/A | N/A | N/A | US-0374 |
| Angiogenesis | VEGF 121 | intramyocardial | adenovirus | 1, 2 | off-pump CABG | closed | N/A | N/A | N/A | US-0442 | |
| Vein graft failure | Edifoligide | preimplantation | ODN | 200 | 2 | CABG | completed | positive in selected patients | vein graft failure | 1 year | [16] |
| Vein graft failure | Edifoligide | preimplantation | ODN | 2400 | 3 | CABG | completed | no effect | vein graft failure | 18 mo | [17] NCT00042081 |
| Vein graft failure | AV1–5126 | preimplantation | ODN | n/a | 1, 2 | CABG | terminated | N/A | vein graft failure | 1 year | NCT00451256 |
AAV = adeno-associated virus; CABG = coronary artery bypass grafting; ECHO = echocardiography; EF = ejection fraction; HGF = hepatocyte growth factor; HIF-1 = hypoxia inducible factor; MRI = magnetic resonance imaging; N/A = not available; NCT = clinicaltrials.gov identifier; ODN = oligodeoxynucleotides; PET = positron emission tomography; SPECT = single-photon emission computed tomography; US-0374,0442 =http://www.wiley.com//legacy/wileychi/genmed/clinical/identifier; VEGF = vascular endothelial growth factor.
Comments
On the basis of reviewed completed and pending clinical trials, the following may be concluded: (1) all trials indicate that gene therapy is safe, feasible, and potentially efficacious; (2) growth factor gene therapy demonstrated evidence of improved myocardial perfusion in treated versus control groups; (3) plasmid DNA may not transfect or express as efficiently as viral vectors; (4) single intracoronary or intravenous administration may not deliver sufficient amounts of the vector to the target tissue; and (5) despite several encouraging clinical trials, especially the first, further trials have slowed down. In our view this is because of concerns about side effects of angiogenesis, controversial results after cardiology trials, difficulties in obtaining US Food and Drug Administration permission, and finding the funds for testing.
COMPARATIVE EVALUATION OF THERAPEUTIC ANGIOGENESIS CLINICAL TRIALS IN CARDIOLOGY AND CARDIAC SURGERY
Despite the focus of this review on cardiac surgery, a comparative assessment with cardiology trials is provided because of overlap in therapeutic agents, vector of administration, therapeutic targets, end points, and outcome, as well as the additive value of the results of the two fields. Moreover, many clinical trials in cardiology have used surgical methods of gene transfer, an ongoing trial in the United Kingdom used surgical patients with LV assist devices, and some cardiology trials were performed by surgeons and cardiologists together; thus it is impossible to fully cover cardiac surgery trials without discussing cardiology trials.
Route of Delivery
The first clinical trial using gene therapy for therapeutic angiogenesis in cardiology was started almost at the same time as in cardiac surgery, using surgically assisted minithoracotomy to inject naked plasmid DNA encoding VEGF165 directly into ischemic myocardium not amenable to revascularization [18]. Minithoracotomy with intramyocardial (transepicardial) gene delivery was used several more times in clinical trials in patients who were not candidates for future direct revascularization because of diffuse disease with poor runoff vessels, lack of available conduits, or high operative risks [19–23]. The next step was intramyocardial (transendocardial) injections performed percutaneously in conjunction with the catheter-based LV electromechanical three-dimensional mapping system NOGA [24–27] or a Myostar mapping injection catheter [28]. Catheter-based antegrade intracoronary gene administration allows the possibility of repeated procedures delivering homogeneously to the whole myocardium, representing an advantage compared with intramyocardial transfer. Several phase 1 and 2 clinical trials have been carried out using intracoronary infusion [29–33].
Vectors
The efficacy and duration of gene expression are to a large extent determined by vector type. Despite the diversity of available vectors, all clinical trials were performed with either plasmid DNA [18, 24–27] or replication-deficient adenovirus [22, 29–32]. Viral vectors, in particular adenovirus, have much higher transduction efficiencies than plasmid DNA owing to the ability of a virus to deliver DNA to the nucleus [34]. However, plasmids are easy to isolate, can be produced in large quantities, and have no toxicity or immunogenicity [35].
Therapeutic Agent
Clinical trials for angiogenesis in cardiology included VEGF-A165, VEGF-121, VEGF-C, and fibroblast growth factor. In cardiac surgery, VEGF-A165, αVEGF-121, Ad.HIF-1, and hepatocyte growth factor were investigated. The role of VEGF in the regulation of angiogenesis has been the object of intense investigation. Five members of the VEGF family have been identified (A through D and placental growth factor). The predominant isoform is VEGF-165, and it has been used most frequently in clinical trials [36].
Therapeutic Outcome
The assessment of therapeutic outcome after gene therapy clinical trials usually includes exercise stress testing to estimate functional capacity, echocardiography, nuclear scanning, computed tomography, magnetic resonance imaging, and cardiac catheterization [22, 28, 29, 31, 37]. Significant improvement in angina symptoms and exercise tolerance tests were found in many trials [10, 11, 21, 25, 32, 37, 38]. Improvement of regional wall motion was also noted [26, 39]. Administration of Ad.VEGF121 by direct intramyocardial injections resulted in objective improvement in exercise-induced ischemia in patients with refractory ischemic heart disease [22]. However, a multicenter phase 3 AGENT-4 (Angiogenic Gene Therapy) found no effect on exercise tolerance at 12 weeks in 116 patients [32]. In the Euroinject One Study, no differences were found regarding angina class or size of the perfusion defect after gene transfer [26]. The phase 2 Genetic Angiogenic Stimulation Investigational Study (GENASIS) Trial was stopped because of a lack of positive impact on the primary end point.
Clinical Trial Design
Several small nonrandomized trials dedicated to safety and tolerance were performed both in cardiology and cardiac surgery [18, 19, 24, 33, 38]. These trials provide limited evidence about the efficacy, durability, and stability of the therapeutic effect. It is clear that it is necessary to conduct more randomized, double-blinded, placebo-controlled trials. The phase 2 Kuopio Angiogenesis Trial study (n = 103) was randomized with a placebo-treatment group [29]. Euroinject ONE was a randomized, double-blind, placebo-controlled trial with VEGF-165 plasmid [26]. Other randomized, double-blind, placebo-controlled studies include VEGF-C [25], AGENT-3 and AGENT-4 [32], and the REVASC (Randomized Evaluation of VEGF for Angiogenesis) study [22].
Comments
In general, it should be noted that results of trials in cardiac surgery have been better than those in cardiology. From our point of view this is attributable to three main factors. First, the patient population: most of the clinical trials in cardiology involve patients with severe end-stage disease and no other treatment options, limiting the efficacy of gene transfer. In cardiac surgery trials patients undergoing CABG without severe LV dysfunction are most commonly enrolled. Second, the cardiology trials using intracoronary administration have proved less effective than the epicardial route used in cardiac surgery trials [40]. Third, the intramyocardial injection route of delivery during cardiac surgery provides much more control than a catheter-based navigation system that has been shown to risk injection in the wrong locations, partial injections, intrapericardial delivery, and so forth [27, 39].
Prevention of Vein Graft Failure
The success of CABG depends on the continued patency of the grafts. Given that more than 60% of vein grafts are occluded or damaged 10 years after CABG, the clinical significance of this problem continues to grow [41]. Gene therapy is attractive as an ex vivo method to genetically manipulate the conduit before grafting. Smooth muscle cell proliferation is a key feature of neointimal hyperplasia leading to vein graft failure. It was theorized that genetic inhibition of the transcription factor E2F could prevent smooth muscle cell proliferation [42]. Two basic gene therapy strategies are currently used in cardiac disease. The first strategy is exogenous overexpression through the introduction of DNA encoding a biologically active transgene into cells. The second strategy is the inactivation or silencing of target genes through transfection of short chains of nucleic acids known as oligodeoxynucleotides. Oligodeoxynucleotides bind to specific target mRNA transcripts and serve as a competitive inhibitor to E2F, preventing gene translation. Vein grafts treated with the E2F decoy demonstrated mitigation of intimal hyperplasia and resistance to graft atherosclerosis in the animal model [43]. Phase 1 and 2 studies (PREVENT II) conducted in coronary artery bypass settings showed that oligodeoxynucleotides edifoligide, decoy for E2F treatment of the vein grafts, was safe and feasible. 75 treated patients received a total of 309 grafts. The E2F decoy was associated with a 30% relative reduction in a composite index of vein graft failure [16]. A phase 3 multicenter randomized, double-blind, placebo-controlled trial of 3,014 patients (PREVENT IV) was designed to test the efficacy of edifoligide in preventing angiographic vein graft failure and the occurrence of major adverse cardiac events after CABG surgery. However, edifoligide was found to be no more effective than placebo in these events after CABG (ClinicalTrials.gov NCT00042081) [17].
A growing body of evidence indicates that the c-Myc signaling network is involved in pathogenesis of vein intimal hyperplasia [44]. Downregulation of c-Myc by antisense technology may protect the integrity of the venous wall. It was suggested that pretreatment of venous grafts before CABG with the oligodeoxynucleotide AVI-5126 may block the genes responsible for vein stenosis and occlusion. A randomized, double-blind, placebo-controlled study was initiated in patients undergoing CABG. However this study was terminated and the clinical trial was stopped (ClinicalTrials.gov NCT00451256).
Comments
There are several considerations that need to be addressed about clinical trials studying the prevention of vein graft failure. First, the lack of a direct measure of graft intimal hyperplasia and problems with the use of complicated pressurized devices for gene delivery make trial assessment difficult. Second, there is a significant difference between results in animal models and clinical trials. Third, vein graft failure has a number of underlying mechanisms and genetic intervention in one pathway probably is not enough to prevent disease progression. Identification of new molecular targets involved in smooth muscle cell proliferation as well as the use of new vectors that provide transgene overexpression increase the potential of vein graft gene therapy. Up to now, most of the gene therapy clinical trials in cardiac surgery and cardiology are focused on therapeutic angiogenesis and an antirestenosis strategy. However, it should be noted that during the last decade significant progress in animal models of ischemic heart disease has been achieved concerning the identification of genetic molecular targets in the field of ischemia-reperfusion injury and vascular endothelial dysfunction (Fig 1).
Fig 1.
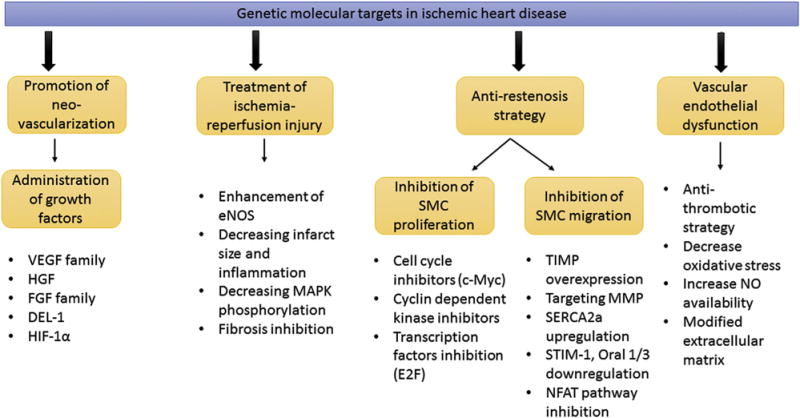
Genetic molecular targets in ischemic heart disease. (c-Myc = proto-oncogene protein; DEL-1 = developmental endothelial locus 1; eNOS = endothelial nitric oxide synthase; E2F = transcription factor; FGF = fibroblast growth factor; HGF = hepatocyte growth factor; HIF-1α = hypoxia inducible factor 1α; MAPK = mitogen-activated protein kinase; MMP = matrix metalloproteinases; NFAT = nuclear factor of activated T cells; NO = nitric oxide; Oral 1/3 = ORAI calcium release-activated calcium modulator 1/3; SERCA2a = sarcoplasmic reticulum adenosine triphosphatase isoform 2a; SMC = smooth muscle cells; STIM-1 = stromal interaction molecule-1; TIMP = tissue inhibitor metalloproteinases; VEGF = vascular endothelial growth factor.)
Prospective Clinical Trials in Cardiac Surgery
Heart Failure Treatment
Findings from cardiology trials led to recommendations supporting the use of gene therapy in patients with LV dysfunction and heart failure (Table 2) [45–49]. Common targets for gene therapy in patients with heart failure include improvement of excitation-contraction coupling to increase myocyte contractility through calcium-cycling proteins and β-adrenergic receptor signaling, inhibition of postinfarction apoptosis and fibrosis, cytoprotective effect, and endogenous stem cell repair (Fig 2). The first clinical trial of gene therapy on patients with heart failure (CUPID) was launched in 2007 to 2008 [45]. The CUPID trial was a multicenter trial designed to evaluate the effects of gene transfer of the SERCA2a cDNA by delivering a single intracoronary infusion of AAV1. Twelve-month follow-up demonstrated improved symptoms, LV function, and biomarkers and reduced cardiovascular events in several patients (ClinicalTrials.gov NCT00454818). The CUPID2 trial is a phase 2b multicenter study enrolling up to 250 patients with moderate-to-severe heart failure and New York Heart Association functional class II to IV despite optimal treatment. The primary end point is time to recurrent event (heart failure–related hospitalization in the presence of terminal events; ClinicalTrials.gov NCT0164330) [46]. The official results of this trial have not been published yet.
Table 2.
Gene Therapy Clinical Trials for Heart Failure
| Therapeutic Target | Transgene | Route | Vector | Number of Patients | Phase | Procedure | Status | Results | End Points | Follow-Up | Reference/Identifier |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart failure | SERCA2a | intracoronary | AAV1 | 9 | 1,2 | primary treatment | completed | positive | safety, walk test, angina class | 1 year | [45] |
| Heart failure | SERCA2a | intracoronary | AAV1 | 39 | 2 | primary treatment | completed | positive | walk test, hospitalizations, angina class | 3 years | [46] NCT00454818 |
| Heart failure | SERCA2a | intracoronary | AAV1 | 250 | 2b | primary treatment | completed | N/A | time to recurrent event | 1 year | [47] NCT01643330 |
| Heart failure | SERCA2a | intracoronary | AAV1 | 24 | 1,2 | LVAD | enrolling | N/A | safety, walk test, angina class | N/A | NCT00534703 |
| Heart failure | AC6 | intracoronary | adenovirus | 72 | 1,2 | primary treatment | enrolling | N/A | treadmill test, ECHO | N/A | [48] NCT00787059 |
| Heart failure | SDF-1 | intramyocardial | plasmid | 93 | 2 | primary treatment | completed | no effect | walk test, angina class | 4 mo | [49] NCT01643590 |
AAV = adeno-associated virus; AC6 = adenylyl cyclase type 6; ECHO = echocardiography; LVAD = left ventricular assist device; N/A = not available; NCT = clinicaltrals.gov.identifier; SDF-1 = stromal-derived factor 1; SERCA2a = sarcoplasmic reticulum adenosine triphosphatase isoform 2a.
Fig 2.
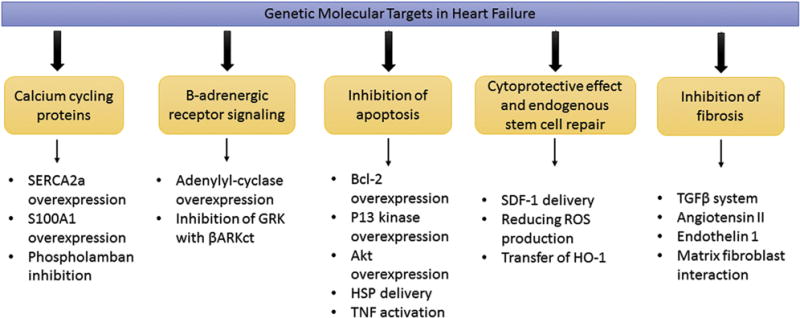
Genetic molecular targets in heart failure. (Akt = serine/threonine kinase; βARKct = βAR kinase carboxy terminus; Bcl-2 = B-cell lymphoma; GRK = G-protein-coupled receptor kinase; HO-1 = heme oxygenase enzyme-1; HSP = heat shock protein; P13 = phosphoinositide 3-kinase; ROS = reactive oxidative species; S100A1 = S100 calcium-binding protein A1; SDF-1 = stromal-derived factor 1; SERCA2a = sarcoplasmic reticulum adenosine triphosphatase isoform 2a; TGF-β = transforming growth factor; TNF = tumor necrosis factor.)
Heart Transplantation
Genetic modification of the heart is an attractive approach to modulating the immune response in cases of acute or chronic rejection after allogeneic transplantation because therapeutic gene can be administered into the donor graft under ex vivo conditions [47]. Transplant immunology has made great advances in the past years. Most notably, transfer of immunosuppressing cytokine genes, such as transforming growth factor-beta and interleukin 10, prolonged allograft survival and delivery of suicide gene therapy to manipulate donor T-cell immunity and enforced the graft-versus-tumor and the graft-versus-infection effects. However, it should be noted that the greatest progress in this field has been made in hematology and ophthalmology. A promising phase 1 clinical trial was initiated in the United Kingdom recently on potential heart transplant patients. Twenty-four cardiac surgical patients undergoing LV assist device insertion as a destination therapy or a bridge to heart transplantation received intracoronary infusion with AAV1/SERCA2a 1 month after LV assist device placement (ClinicalTrials.gov NCT00534703).
Comments
Patients with LV dysfunction caused by coronary artery disease represent approximately 25% of patients in coronary surgery and invasive cardiology units today [50]. It was demonstrated that surgical revascularization is accompanied by a higher survival rate than other treatments. However, only a small number of these patients demonstrated improvement of LV function and LV ejection fraction [51]. The potential molecular targets that have proven to be effective in preclinical gene therapy studies and advanced to clinical trials include calcium cycling proteins (SERCA2a [46], ClinicalTrials.gov NCT 01643330), βAR (Beta Adrenergic Receptors) signaling (AC6 [52], ClinicalTrials.gov NCT00787059), and stromal cell-derived factor 1 (ClinicalTrials.gov NCT01643590).
Despite the clinical opportunity, there are no clinical trials in heart transplantation. Contemporary pharmacologic agents have failed to prevent allograft vasculopathy and chronic rejection. As well, drug-based immunosuppression is associated with major side effects including renal failure, hypertension, leukopenia, a high incidence of infectious complications, and skin cancer. In addition it should be noted that gene administration into the donor organ may be carried out ex vivo under the best conditions for gene transfer with graft protection and no systemic side effects.
Limitations of the Trials and Problems Facing the Implementation of the Gene Therapy in Cardiac Surgery
Transient Gene Expression
One of the main factors limiting the positive effect of gene therapy is transient therapeutic gene expression [48]. This problem is multifaceted. First, many clinical trials were carried out with nonviral or short-term efficacy viral vectors, and the protocols used a single-dose method of delivery. However, new serotypes of AAV vectors have much stronger cardiac tropism, leading to significantly higher and longer-term cardiac transgene expression [49]. Second, techniques of gene delivery applied in clinical trials have been relatively simple, without attempts to limit collateral expression in other organs, extend vector residence time in myocytes, enhance transfer across the endothelial barrier, or use retrograde transcoronary transfer. New techniques including a closed-loop recirculatory system with decreased immune response could offer a solution for clinical therapy [53, 54]. Importantly, evaluation of gene expression was commonly performed on the basis of functional cardiac factors, not sensitive biopsy techniques with reverse transcriptase real-time polymerase chain reaction—in most cases it is unknown what proportion of cells were actually transfected. And finally, many authors believe that a strong placebo effect in several clinical trials complicates the true assessment of gene therapy. Collectively, these concerns underscore the need to conduct randomized, blinded, placebo-controlled studies in large-scale trials.
Correct Selection of Patients and Clinical End Points
All cardiac surgery clinical trials performed to date have lacked the number of patients needed to quantify the potential benefit of gene therapy. A group of selected patients should be standardized. In particular this applies to the stage of disease, pharmacologic treatment, angiographic treatment, and comorbidities. Additionally, many trials have used variable and often subjective factors for end points of therapy such as duration of exercise before angina, ejection fraction determined by echocardiogram, perfusion measured by single-photon emission computed tomography, exercise treadmill time, change of coronary luminal diameter, or diameter stenosis after VEGF therapy [29]. Moreover, changes in variables such as LV wall motion and ejection fraction may be mediated by confounding factors other than gene therapy. It is best practice to apply techniques such as cardiac magnetic resonance perfusion imaging and positron emission tomography that are much more sensitive and precise in determining variables such as LV systolic and diastolic volumes, infarct size, and perfusion.
Dose Effect
All conducted clinical trials clearly found that biologic effects depend on the dose. The optimal dose of gene therapy should be standardized. A maximal tolerated dose that does not cause side effects still has not been identified. The proportion of target cardiomyocytes transfected at different doses is still relatively unknown; moreover, the proportion needed to be transfected to achieve a positive effect is not known.
Lack of Appropriate Clinical Trials Design
Cardiac surgery with gene therapy clinical trials brought many positive hints that gene transfer may eventually be effective. However, it is a best practices tenet to conduct clinical trials under double-blinded, placebo-controlled, randomized conditions with a sufficient number of patients [48]. This is the only way to answer the question of how efficacious gene therapy is during cardiac surgery.
Safety and Tolerability
All clinical trials in cardiac surgery and cardiology have shown short- and long-term safety. Researchers have learned some important lessons from the early clinical trials. Unfortunately, certain properties of Ad vectors administered through the vascular system in large doses make them hazardous. On the other hand, questionable results in some angiogenic trials may indicate that an effective dose was not achieved. In any case, analysis of the safety of gene therapy must take into account off-target transfection and collateral organ issues, the possibility for neoplastic growth, the inflammatory response caused by the viral vector, and the risk for an increase in atherogenesis with plaque destabilization.
Compound Effect of Coronary Artery Bypass Grafting Procedure
The role of CABG in the treatment of patients with coronary artery disease and LV dysfunction is still not clearly established [55]. Extensive experience accumulated with CABG showed that LV dysfunction in patients with ischemic heart disease is not always an irreversible process. So far there is not a strong explanation for why CABG can improve LV function only in some patients [52]. We trust that the delivery of gene therapy may benefit in combination with CABG surgery. For example, angiogenic therapy seeks to enlarge cardiac capillary beds, and targeting of gene-mediated Ca2+ handling proteins or β-adrenergic system proteins seeks to enhance contractility in failing myocytes. Both of these therapies may be enhanced with CABG. We cannot ignore that the benefits of CABG and those of gene therapy are hard to distinguish. Therefore we strongly believe that there is a great need for large double-blind, placebo-controlled, randomized clinical trials combining cardiac surgery and gene therapy in ischemic heart disease and heart failure.
Summary
During the past several years, substantial progress has been made in cardiac gene therapy research. First, many studies have used adeno-associated virus, which is much more effective than plasmid DNA and yields much less immune-inflammatory response than adenovirus. The AAV serotypes, especially 6 and 9 but also 1, were shown to have strong heart tissue tropism and high transduction efficacy in cardiomyocytes and coronary smooth muscle cells. Taken with the lack of human disease, AAV was licensed as the first clinically available gene therapy product, Glybera.
Second, the main requirements for cardiac gene delivery systems have been identified. The ideal method of gene transfer (direct intramyocardial or transvascular) must be safe and translatable, allow for extended vector residence time in the heart, and minimize collateral organ expression. Several groups of researchers have already created acceptable gene delivery technologies including a catheter-based one and a cardiopulmonary bypass–based methodology [53, 54].
Third, new molecular therapeutic targets have been identified in failing cardiac myocytes during ischemia. In addition to stimulation of angiogenesis, promising molecular targets include calcium cycling proteins, the β-adrenergic system, stem cell localization signals, and myocardial apoptosis signaling.
Fourth, the long-term results of cardiac surgery clinical trials have shown a good safety record. Moreover, they have generally demonstrated equal or superior clinical benefits and success compared with those observed in cardiology trials.
Cardiac gene therapy, like every field in medicine, has many unsolved issues: identification of therapeutic genes specific for heart disease and progression, optimal transgene overexpression necessary for an effect, and delivery of gene product to the target tissue without collateral expression, among others. It is impossible to know how long it will take to answer these questions. However, the fact that only 0.5% of all gene therapy clinical trials involve cardiac surgery does not add any optimism. On the other hand, given that more than 30% of patients with multivessel disease cannot undergo optimal revascularization, each patient and surgeon must ask whether it would be better to use adjunctive gene therapy or to leave areas of ischemic myocardium untreated.
Acknowledgments
The authors wish to acknowledge the Gene Therapy Resource Program (GTRP). This review was supported by NIH grant 2-R01 HL083078-05 and a grant from the James H. Heineman Foundation.
References
- 1.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 2.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. He-matopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–23. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 3.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Rosa L, Carulli S, Cocchiarella F, et al. Long-term stability and safety of transgenic cultured epidermal stem cells in gene therapy of junctional epidermolysis bullosa. Stem Cell Rep. 2014;2:1–8. doi: 10.1016/j.stemcr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ylä-Herttuala S. Endgame: glybera finally recommended for approval as the first gene therapy drug in the European union. Mol Ther. 2012;20:1831–2. doi: 10.1038/mt.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin H, Parmacek MS, Morle G, Bolling S, Leiden JM. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990;82:2217–21. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- 7.Bonatti J, Haeusler C, Klaus A, Fink M, Hammerer-Lercher A, Laufer G. Acceptance of gene therapy by the heart surgery patient. Eur J Cardiothorac Surg. 2002;21:981–6. doi: 10.1016/s1010-7940(02)00174-4. [DOI] [PubMed] [Google Scholar]
- 8.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105:724–36. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lassaletta AD, Chu LM, Sellke FW. Therapeutic neo-vascularization for coronary disease: current state and future prospects. Basic Res Cardiol. 2011;106:897–909. doi: 10.1007/s00395-011-0200-1. [DOI] [PubMed] [Google Scholar]
- 10.Rosengart TK, Lee LY, Patel SR, et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999;100:468–74. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- 11.Rosengart TK, Bishawi MM, Halbreiner MS, et al. Long-term follow-up assessment of a phase 1 trial of angiogenic gene therapy using direct intramyocardial administration of an adenoviral vector expressing the VEGF121 cDNA for the treatment of diffuse coronary artery disease. Hum Gene Ther. 2013;24:203–8. doi: 10.1089/hum.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko1sut P, Ma1ecki M, Zelazny P, et al. Gene therapy of coronary artery disease with phvegf165—early outcome. Kardiol Pol. 2003;59:373–84. [PubMed] [Google Scholar]
- 13.Kilian EG, Sadoni S, Vicol C, et al. Myocardial transfection of hypoxia inducible factor-1alpha via an adenoviral vector during coronary artery bypass grafting—a multicenter phase I and safety study. Circ J. 2010;74:916–24. doi: 10.1253/circj.cj-09-0594. [DOI] [PubMed] [Google Scholar]
- 14.Ruel M, Beanlands RS, Lortie M, et al. Concomitant treatment with oral L-arginine improves the efficacy of surgical angiogenesis in patients with severe diffuse coronary artery disease: the Endothelial Modulation in Angiogenic Therapy randomized controlled trial. J Thorac Cardiovasc Surg. 2008;135:762–70. doi: 10.1016/j.jtcvs.2007.09.073. 770.e1. [DOI] [PubMed] [Google Scholar]
- 15.Kim JS, Hwang HY, Cho KR, et al. Intramyocardial transfer of hepatocyte growth factor as an adjunct to CABG: phase I clinical study. Gene Ther. 2013;20:717–22. doi: 10.1038/gt.2012.87. [DOI] [PubMed] [Google Scholar]
- 16.Grube E, Felderhoff T, Fitzgerald P, et al. Phase II trial of the E2F decoy in coronary bypass grafting (abstract). Presented at the American Heart Association Annual Meeting; Anaheim, CA. 2001. [Google Scholar]
- 17.Alexander JH, Hafley G, Harrington RA, et al. PREVENT IV Investigators Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 18.Losordo DW, Vale PR, Symes JF, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–4. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 19.Symes JF, Losordo DW, Vale PR, et al. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease. Ann Thorac Surg. 1999;68:830–6. doi: 10.1016/s0003-4975(99)00807-3. [DOI] [PubMed] [Google Scholar]
- 20.Vale PR, Losordo DW, Milliken CE, et al. Left ventricular electromechanical mapping to assess efficacy of phVEGF(165) gene transfer for therapeutic angiogenesis in chronic myocardial ischemia. Circulation. 2000;102:965–74. doi: 10.1161/01.cir.102.9.965. [DOI] [PubMed] [Google Scholar]
- 21.Reilly JP, Grise MA, Fortuin FD, et al. Long-term (2-year) clinical events following transthoracic intramyocardial gene transfer of VEGF-2 in no-option patients. J Interv Cardiol. 2005;18:27–31. doi: 10.1111/j.1540-8183.2005.04026.x. [DOI] [PubMed] [Google Scholar]
- 22.Stewart DJ, Hilton JD, Arnold JM, et al. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF121) versus maximum medical treatment. Gene Ther. 2006;13:1503–11. doi: 10.1038/sj.gt.3302802. [DOI] [PubMed] [Google Scholar]
- 23.Kalil RA, Salles FB, Giusti II, et al. VEGF gene therapy for angiogenesis in refractory angina: phase I/II clinical trial. Rev Bras Cir Cardiovasc. 2010;25:311–21. doi: 10.1590/s0102-76382010000300006. [DOI] [PubMed] [Google Scholar]
- 24.Vale PR, Isner JM, Rosenfield K. Therapeutic angiogenesis in critical limb and myocardial ischemia. J Interv Cardiol. 2001;14:511–28. doi: 10.1111/j.1540-8183.2001.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 25.Losordo DW, Vale PR, Hendel RC, et al. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105:2012–8. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- 26.Kastrup J, Jørgensen E, Rück A, et al. Euroinject One Group Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris: a randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005;45:982–8. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 27.Stewart DJ, Kutryk MJ, Fitchett D, et al. NORTHERN Trial Investigators VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol Ther. 2009;17:1109–15. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ripa RS, Wang Y, Jørgensen E, Johnsen HE, Hesse B, Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J. 2006;27:1785–92. doi: 10.1093/eurheartj/ehl117. [DOI] [PubMed] [Google Scholar]
- 29.Hedman M, Hartikainen J, Syväanne M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–83. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 30.Grines CL, Watkins MW, Helmer G, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–7. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 31.Grines CL, Watkins MW, Mahmarian JJ, et al. Angiogene GENe Therapy (AGENT-2) Study Group A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol. 2003;42:1339–47. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- 32.Henry TD, Grines CL, Watkins MW, et al. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials. J Am Coll Cardiol. 2007;50:1038–46. doi: 10.1016/j.jacc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Laitinen M, Hartikainen J, Hiltunen MO, et al. Catheter-mediated vascular endothelial growth factor gene transfer to human coronary arteries after angioplasty. Hum Gene Ther. 2000;11:263–70. doi: 10.1089/10430340050016003. [DOI] [PubMed] [Google Scholar]
- 34.Eckhouse SR, Jones JA, Spinale FG. Gene targeting in ischemic heart disease and failure: translational and clinical studies. Biochem Pharmacol. 2013;85:1–11. doi: 10.1016/j.bcp.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz MG, Fargnoli AS, Williams RD, Bridges CR. Gene therapy delivery systems for enhancing viral and nonviral vectors for cardiac diseases: current concepts and future applications. Hum Gene Ther. 2013;24:914–27. doi: 10.1089/hum.2013.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 37.Kuku1a K, Chojnowska L, Dabrowski M, et al. Intramyocardial plasmid-encoding human vascular endothelial growth factor A165/basic fibroblast growth factor therapy using percutaneous transcatheter approach in patients with refractory coronary artery disease (VIF-CAD) Am Heart J. 2011;161:581–9. doi: 10.1016/j.ahj.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 38.Rosengart TK, Lee LY, Patel SR, et al. Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA. Ann Surg. 1999;230:466–72. doi: 10.1097/00000658-199910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gyöngyösi M, Khorsand A, Zamini S, et al. NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocardial injections of plasmid encoding vascular endothelial growth factor A-165 in patients with chronic myocardial ischemia: subanalysis of the EUROINJECT-ONE multicenter double-blind randomized study. Circulation. 2005;112(9 Suppl):I157–65. doi: 10.1161/01.CIRCULATIONAHA.105.525782. [DOI] [PubMed] [Google Scholar]
- 40.Lee LY, Patel SR, Hackett NR, et al. Focal angiogen therapy using intramyocardial delivery of an adenovirus vector coding for vascular endothelial growth factor 121. Ann Thorac Surg. 2000;69:14–24. doi: 10.1016/s0003-4975(99)01102-9. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Stawinski GV, Lytle BW. Coronary artery reoperations. In: Cohn LN, editor. Cardiac surgery in the adult. 3rd. Vol. 2007. New York, NY: McGraw-Hill; pp. 711–32. [Google Scholar]
- 42.Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation. 1998;98:82–9. doi: 10.1161/01.cir.98.1.82. [DOI] [PubMed] [Google Scholar]
- 43.Mann MJ, Gibbons GH, Tsao PS, et al. Cell cycle inhibition preserves endothelial function in genetically engineered rabbit vein grafts. J Clin Invest. 1997;99:1295–301. doi: 10.1172/JCI119288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Florea V, Bhagavatula N, Simovic G, Macedo FY, Fock RA, Rodrigues CO. c-Myc is essential to prevent endothelial proinflammatory senescent phenotype. PloS One. 2013;8:e73146. doi: 10.1371/journal.pone.0073146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hajjar RJ, Zsebo K, Deckelbaum L, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14:355–67. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Greenberg B, Yaroshinsky A, Zsebo KM, et al. Design of phase 2b trial of intracoronary administration of AAV1/SERCA2a in patients with advanced heart failure: the CUPID2 trial (calcium up-regulation by percutaneous administration of gene therapy in cardiac disease phase 2b) JACC Heart Fail. 2014;1:84–92. doi: 10.1016/j.jchf.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Vassalli G, Roehrich ME, Vogt P, et al. Modalities and future prospects of gene therapy in heart transplantation. Eur J Cardiothorac Surg. 2009;35:1036–44. doi: 10.1016/j.ejcts.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 48.Sellke FW. Gene therapy in cardiac surgery: is there a role? J Thorac Cardiovasc Surg. 2003;125:994–7. doi: 10.1067/mtc.2003.264. [DOI] [PubMed] [Google Scholar]
- 49.Fang H, Lai NC, Gao MH, et al. Comparison of adeno-associated virus serotypes and delivery methods for cardiac gene transfer. Hum Gene Ther Methods. 2012;23:234–41. doi: 10.1089/hgtb.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marui A, Kimura T, Nishiwaki N, et al. CREDO-Kyoto PCI/CABG Registry Cohort-2 Investigators Comparison of five-year outcome of coronary artery bypass grafting versus percutaneous coronary intervention in patients with left ventricular ejection fractions ≤50% versus >50% (from the CREDO-Kyoto PCI/CABG Registry Cohort-2) Am J Cardiol. 2014;114:988–96. doi: 10.1016/j.amjcard.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Bonow RO, Maurer G, Lee KL, et al. STICH Trial Investigators Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–25. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang T, Gao MH, Hammond HK. Prospects for gene transfer for clinical heart failure. Gene Ther. 2012;19:606–12. doi: 10.1038/gt.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katz MG, Fargnoli AS, Williams RD, Steuerwald NM, Isidro A, Ivanina AV, et al. Safety and efficacy of high-dose adeno-associated virus 9 encoding sarcoplasmic reticulum Ca(2+) adenosine triphosphatase delivered by molecular cardiac surgery with recirculating delivery in ovine ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2014;148:1065–73. 1073e1–2, n1072–3. doi: 10.1016/j.jtcvs.2014.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15:1550–7. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 55.Velazquez EJ, Lee KL, Deja MA, et al. STICH Investigators Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–16. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]


