Abstract
Toluene diisocyanate (TDI) is a leading cause of chemical-induced occupational asthma which impacts workers in a variety of industries worldwide. Recently, the robust regulatory potential of regulatory T cells (Tregs) has become apparent, including their functional role in the regulation of allergic disease; however, their function in TDI-induced sensitization has not been explored. To elucidate the kinetics, phenotype, and function of Tregs during TDI sensitization, BALB/c mice were dermally exposed (on each ear) to a single application of TDI (0.5–4% v/v) or acetone vehicle and endpoints were evaluated via RT-PCR and flow cytometry. The draining lymph node (dLN) Treg population expanded significantly 4, 7, and 9 days after single 4% TDI exposure. This population was identified using a variety of surface and intracellular markers and was found to be phenotypically heterogeneous based on increased expression of markers including CD103, CCR6, CTLA4, ICOS, and Neuropilin-1 during TDI sensitization. Tregs isolated from TDI-sensitized mice were significantly more suppressive compared with their control counterparts, further supporting a functional role for Tregs during TDI sensitization. Last, Tregs were depleted prior to TDI sensitization and an intensified sensitization response was observed. Collectively, these data indicate that Tregs exhibit a functional role during TDI sensitization. Because the role of Tregs in TDI sensitization has not been previously elucidated, these data contribute to the understanding of the immunologic mechanisms of chemical induced allergic disease.
Keywords: chemical sensitization, isocyanate, TDI, chemical allergy, regulatory T cells, hypersensitivity
Toluene diisocyanate (TDI) is a low-molecular weight, highly reactive chemical utilized in the automobile industry and in the manufacture of polyurethane, foams, paints, and coatings. The U.S. Environmental Protection Agency reports that domestic production and importation of 2,4- and 2,6-TDI isomers rose above 1 billion pounds in 2006 (NTP, 2011). TDI is a potent sensitizer and irritant; repeat dermal and/or inhalation exposure can lead to a variety of allergic diseases including asthma, hypersensitivity pneumonitis, rhinitis, and contact dermatitis (Anderson and Meade, 2014; Bello et al., 2007; Mapp, 2001) These diseases can be disabling and extremely severe, potentially resulting in lifelong illness and/or death (Fabbri et al., 1988). TDI is generally classified as primarily a respiratory, Th2-mediated sensitizer with a Th1 component (Matheson et al., 2005). Although the murine local lymph node assay (LLNA) is validated for the identification of chemicals that preferentially elicit dermal allergic disease, it is not validated for chemicals that preferentially elicit respiratory disease (Anderson et al., 2011). In light of this challenge, further mechanistic insight into the role of immunologically relevant cellular subsets (in addition to Th1 and Th2), involved in respiratory sensitization may ultimately contribute to the development of improved predictive models based on cellular phenotype, secreted cytokine expression, or other related parameters. Recent immunological developments such as the discovery of novel T cell subsets including Th17, T follicular helper, Th22, Th9, and Treg, cells support the potential for the utilization of novel mediators of allergic disease (Liu and Wisnewski, 2003).
Classical regulatory T cells (Tregs) (CD4+ CD25+) were initially identified based on their suppressive capabilities which contributed to the maintenance of immune tolerance in mice (Sakaguchi et al., 1995). Following this discovery, a transcription factor known as forkhead box p3 (Foxp3) was identified as the master transcription factor of Tregs, allowing for their identification and functional manipulation (Hori et al., 2003). Both naturally occurring Tregs (nTregs) that develop in the thymus and inducible Tregs (iTregs) that develop in the periphery are involved in the maintenance of immune tolerance. Functionally, Tregs have demonstrated a critical role in the development of immune tolerance and can serve as effectors helping to prevent overzealous adaptive responses to foreign antigens and allergens (Sojka et al., 2008; Yadav et al., 2012). This suppressive function is mediated by a variety of mechanisms including, but not limited to, the control of conventional T cell proliferation through the inhibition of co-stimulation via cytotoxic T-lymphocyte associated protein 4 (CTLA4) expression and/or IL-2 consumption, immunosuppressive cytokine secretion (IL-10 and Transforming growth factor β(TGF-β), metabolic interference, and disruption of dendritic cell function (Corthay, 2009; Kimber et al., 2012; Sojka et al., 2008).
Although a functional role for Tregs has been suggested in models of chemical-induced contact hypersensitivity (Christensen et al., 2015), this cellular subset has not been investigated in TDI sensitization. Vanoirbeek et al. (2004) observed suspected immune tolerance induced by high dose (3% TDI in acetone-olive oil) dermal sensitization followed by intranasal challenge resulting in the absence of airway hyperreactivity, contrasted with low dose (0.3% TDI in acetone-olive oil) dermal sensitization resulting in airway hyperreactivity following intranasal challenge. Although not explicitly stated in the article, this seminal study suggested a role for Tregs in TDI sensitization. The collection of data regarding Tregs and chemical allergy is growing but remains limited. In order to elucidate the immunologic mechanisms involved in TDI sensitization, the biological functions of pertinent immune cell subsets need to be delineated. It is important to note that although a variety of Tregs may be involved in chemical sensitization, this study focuses on classical Tregs (CD3+ CD4+ CD25+ Foxp3+) due to their well-documented regulatory potential in a variety of disease states, including allergy and asthma (Robinson, 2009), and to the lack of data regarding these cells in models of low molecular weight chemical allergy. Here, we utilize a murine model of dermal TDI sensitization in order to elucidate the expression kinetics, phenotype, and functional role of Tregs during chemical sensitization.
MATERIALS AND METHODS
Mice
Female BALB/c mice (6–8 weeks of age) purchased from Taconic (Germantown, New York) were acclimated for 5 days and then randomly assigned to treatment group. Homogenous weight distribution was insured across treatment groups. BALB/c mice were selected on the basis of their Th2 bias, robust IgE production, and the historical use of these mice in the laboratory to investigate chemical sensitization (Anderson et al., 2010). Mice were housed in ventilated plastic shoebox cages with hardwood chip bedding at a maximum of 5 animals per cage. A NIH-31 modified 6% irradiated rodent diet (Harlan Teklad) and tap water were administered ad libitum. Housing facilities were maintained at 68–72°F and 36–57% relative humidity, and a 12-h light–dark cycle was maintained. All animal experiments were performed in the Association for Assessment and Accreditation of Laboratory Animal Care accredited National Institute for Occupational Safety and Health animal facility in accordance with an Institutional Animal Care and Use Committee-approved protocol.
TDI sensitization model
Toluene 2,4-diisocyanate (TDI, CAS no. 584-84-9) was purchased from Sigma-Aldrich Chemical Company (Milwaukee, Wisconsin). Animals (n = 4–5) were exposed to a single dose of 0.5–4% TDI (v/v) on the dorsal surface of each ear (25 μl per ear). The highest concentration (4% v/v; the maximum sensitizing concentration with minimal toxicity) and dosing regimen was previously shown to induce sensitization (Anderson et al., 2013) and sensitization was confirmed in the lower dose based on total serum IgE levels and dLN allergic cytokine mRNA expression (0.5% v/v; data not shown). Acetone was selected as the vehicle control to minimize chemical reactivity as diisocyanates react with OH groups which are present in other potential vehicles such as olive oil. Ear thickness was measured 4 days following TDI exposure using a modified engineer's micrometer (Mitutoyo Corporation, Japan) and measurements were collected in millimeters (mm). Average ear swelling was calculated as previously described in Anderson et al. (2012). Mice were euthanized via CO2 asphyxiation at time points ranging from 1 to 11 days postchemical exposure.
Euthanasia, tissue collection, and processing
Animals were weighed, euthanized via CO2 asphyxiation, and examined for gross pathology at the designated time point. Left and right auricular draining lymph nodes (dLNs; drain site of chemical application) were collected in sterile phosphate-buffered saline (pH 7.4) and manually dissociated using the frosted ends of 2 microscope slides. dLN cellularity was determined using a Cellometer (Nexcelom Bioscience, Lawrence, Massa chusetts) with size exclusion parameters (3.5–36 μm) and a combined Acridine Orange/Propidium Iodide solution to identify viable cells. Blood was collected via cardiac puncture, placed into serum collection tubes, centrifuged, and serum was removed and stored at −80°C for subsequent total IgE analysis via ELISA. Ears were collected in 1 mL RNALater (Ambion, Pittsburgh, Pennsylvania), stored at −80°C for subsequent gene expression analysis.
RNA isolation and reverse transcription
Ears were processed for RNA isolation using a Tissue Lyser II in Qiagen lysis buffer. Total RNA was isolated from the ears and dLN using the Qiagen RNeasy and miRNeasy kits, respectively. A QiaCube (Qiagen, Hilden, Germany) automated RNA isolation machine was utilized in conjunction with the specified RNA isolation kits. A DNase treatment was performed for removal of residual DNA. The concentration and purity of the RNA was determined using a ND-1000 spectrophotometer (Thermo Scientific Nanodrop, Wilmington, Delaware). First strand cDNA synthesis was performed using a High-Capacity cDNA Synthesis Kit (Applied Biosystems, Carlsbad, California) according to manufacturer recommendations. Ultimately, the cDNA was analyzed for mRNA expression as described in the real-time PCR (RT-PCR) methods section below.
Real-Time PCR
For analysis of mRNA expression, TaqMan Universal Fast master mix (Applied Biosystems, Calsbad, California), cDNA, and mouse-specific mRNA primers (TaqMan Custom PCR Arrays, Carlsbad, California) were combined and PCR was performed according to manufacturer protocol. Primers used include: il-1β, il-6, and tnf-α. Master mix, primers, and cDNA were added to a MicroAmp Fast Optical 96-well reaction plate and analyzed on an Applied Biosystems 7500 Fast RT PCR system using cycling conditions as specified by the manufacturer. β-actin was used as the endogenous reference control gene as expression was determined to be stable following chemical exposure (data not shown). RT-PCR data were collected and represented as relative fold change over vehicle control, calculated by the following formula: 2−ΔΔCt = ΔCtSample – ΔCtControl. ΔCt = CtTarget – Ctβ-ACTIN, where Ct = cycle threshold.
Flow cytometric analysis
Single cell suspensions were prepared from tissues and a minimum of 150 000 dLN cells were aliquoted into 96-well U-bottom plates and washed in staining buffer (PBS + 1% bovine serum albumin + 0.1% sodium azide). Cells were resuspended in staining buffer containing anti-mouse CD16/32 antibody (clone 2.4G2; BD Biosciences, San Jose, California) for blocking of Fc receptors to minimize nonspecific binding. Cells were resuspended in staining buffer containing a cocktail of fluorochrome-conjugated antibodies specific for cell surface antigens including: CCR6 (clone: 29-2L17, fluorophore: BV605, BioLegend), CD3 (500A2, V500, BD), CD4 (RM4-5, AF700, BD), CD8a (53-6.7, AF488, BioLegend), CD25 (PC61, APC Cy7, BioLegend), CD45 (30-F11, PE, BD), CD103 (2E7, PerCP Cy5.5, BioLegend), Inducible T-cell costimulator (ICOS) (C938.4A, PE Cy7, eBioscience), Neu-1 (3E12, PE, BioLegend), and Ter-119 (TER-119, FITC, eBioscience).
Following surface staining, cells were washed in staining buffer and fixed using the Foxp3 fixation buffer set (eBioscience, San Diego, California). After overnight incubation in staining buffer, cells were permeablilized using the Foxp3 fixation buffer set (eBioscience, San Diego, Calfornia) and re-suspended in permeabilization buffer containing a cocktail of fluorochrome-conjugated antibodies specific for intracellular antigens including: CTLA4 (UC10-4B9, BV421, BioLegend), Foxp3 (FLK-16s, eF450, and APC, eBioscience), Gata3 (TWAJ, eF660, eBioscience), Rorγt (Q31-378, PerCP Cy5.5, eBioscience), and Tbet (4B10, PE Cy7, eBioscience). Following staining, cells were re-suspended in staining buffer and analyzed on an LSR II flow cytometer using FacsDiva software (BD Biosciences). Data analysis was performed with FlowJo 10.0 software (TreeStar Inc., Ashland, Oregon). A minimum of 10 000 events were captured for each sample. Leukocytes were first identified by their expression of CD45. The Treg subset was further identified as CD3+ CD4+ CD8− CD25+ Foxp3+. Numerical population values were calculated by applying subset frequencies to the initial cell count obtained following lymph node homogenization. Compensation controls were performed using single stained cellular suspensions and OneComp beads (eBioscience, San Diego, California) and fluorescence minus one staining controls were included to help set gating boundaries.
Treg suppression assay
The suppressive ability of Tregs was analyzed using an ex vivo Treg suppression assay as described by Kruisbeek et al. (2001) and Marshall et al. (2008) with some modifications. This assay evaluates the ability of naïve, conventional dLN-derived T cells (Tcons) to proliferate in the presence of varying numbers of Tregs isolated from acetone- or TDI-exposed mice. Mice were exposed to acetone (n = 7–11) or TDI (4%) (n = 4–5) as previously described and following sacrifice at 4 and 7 days postTDI (peak of the expansion) exposure the dLN and spleens were removed. Tregs (CD4+ CD25+) and Tcons (CD4+ CD25−) were isolated from the lymph nodes and CD4− accessory cells were isolated from naïve spleens using CD4 negative and CD25 positive selection-based magnetic separation kits (Stemcell, Vancouver, British of Columbia). Average Treg purity is as follows for 4 days: Acetone-70.65% of CD3+ CD4+ cells and 4% TDI-63.05% of CD3+ CD4+ cells and 7 days: Acetone-91.35% of CD3+ CD4+ cells and 4% TDI-82.8% of CD3+ CD4+ cells post 4% TDI exposure. Following isolation from naïve mouse dLNs, Tcons were labeled with 2 μM carboxyfluorescein succinimidyl ester (CFSE). Tcons and Tregs were cultured in a 96-well U-bottom plate with anti-CD3 (0.2 μg/ml; BD Biosciences) and accessory cells at a variety of Tcon:Treg ratios (1:1, 2:1, 4:1, and 8:1). Naïve CD4− splenocytes were treated with Mitomycin C (Sigma Aldrich, St Louis, Missouri) and utilized as accessory cells. Additional controls included stimulated Tcons only to assess baseline proliferation, Tregs only, accessory cells only, and Tcons only with no stimulation nor accessory cells. Cells from each treatment group were pooled and added to triplicate wells of the culture plate. Seventy-two hours following plating, cells were stained with anti-CD4 and Live/Dead Violet (Life Technologies, Carlsbad, California). Tcons were defined as CD4+ CFSE+ cells and suppression was measured based on changes in the frequency of dividing CFSE+ cells based on the dilution of CFSE. Tregs were analyzed for purity based on their expression of CD3, CD4, and Foxp3 as determined by flow cytometric analysis as previously described.
In vivo anti-CD25 antibody treatment
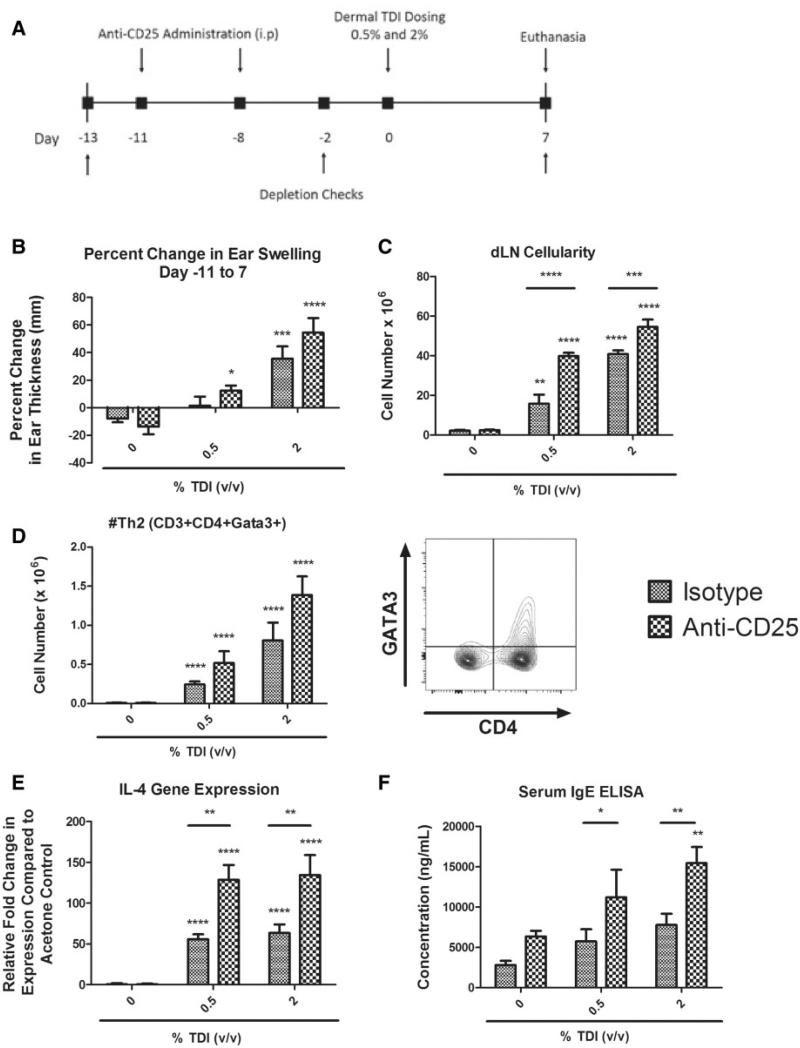
In order to deplete Tregs before and during TDI sensitization, InvivoMAb anti m CD25 (BioXCell, PC-61.5.3) was administered in vivo. The use of this antibody as a Treg depletion strategy is validated in vivo (Felonato et al., 2012; Setiady et al., 2010). InVivoMAb Rat IgG1 (Bioxcell, HRPN) was utilized as a negative, isotype control. Treatment groups for this study (n = 5 mice) are as follows: isotype/acetone, isotype/0.5% TDI, isotype/2% TDI, antiCD25/acetone, antiCD25/0.5% TDI, and antiCD25/2% TDI. 200 μg of the respective antibody in USP grade saline was administered intraperitoneally at days −11 and −8 during the depletion study (see Figure 6A for study timeline). Animal weights were recorded throughout the duration of the experiment to monitor potential toxicity. In order to confirm the effectiveness of the antibody, blood was collected from the lateral tail vein at days 2 and 7, and Tregs were measured by flow cytometry as previously described. Baseline blood Treg levels were assayed at day 12 to ensure equal pretreatment frequencies across all groups. Mice were exposed to a single dermal application of 0.5 or 2% TDI on day 0. The high dose of 2% TDI was selected in an effort to allow for a measurable increase in the sensitization response, as 4% TDI elicits a maximum sensitization response. Mice were euthanized 7 days following dermal chemical exposure. Specific measures of sensitization were evaluated, including examination of the Th2 population by flow cytometry (CD3+ CD4+ Gata3+), IL-4 dLN mRNA levels by RT-PCR, and total IgE levels in the serum. Total serum IgE was quantified following serum separation from whole blood (centrifugation) and analysis using a Mouse IgE Ready-SET-Go! Kit (Affymetrix eBioscience, San Diego, Califorrnia) according to the manufacturer's protocol. Absorbance was determined using a Spectramax Vmax plate reader (Molecular Devices, Sunnyvale, California) at 450 and 650 nm. Data analysis was performed using the IBM Softmax Pro 3.1 program (Molecular Devices, Sunnyvale, California) and the IgE concentrations for each sample were interpolated from a standard curve derived from multipoint analysis.
FIG. 6.
The severity of the sensitization response is intensified in the absence of Tregs during TDI sensitization. Tregs were depleted in mice prior to and during dermal TDI sensitization (A). Evaluation of dermal irritancy (B), dLN cellularity (C), dLN Th2 population (D), dLN IL-4 gene expression (E), and serum total IgE (F) were evaluated following a single exposure of 0.5 or 2% TDI with isotype control or anti-CD25. Bars represent mean (± SE) of 5 mice per group. Significance is indicated as follows: P ≤ .05 (*), P ≤ .01 (**), P ≤ .001 (***), and P ≤ .0001 (****). P values are represented by asterisks (comparison of acetone to TDI-exposed group from the same antibody treatment regimen) or horizontal bars with asterisks above (comparison of antibody and isotype-treated groups receiving identical chemical treatment).
Statistical analysis
Statistical analyses were generated using SAS/STAT software, version 9.3 (SAS Institute, Cary, North Carolina) and GraphPad Prism version 5.0 (San Diego, California). For irritancy and inflammatory gene expression analysis (Figure 1), a 1-way analysis of variance (ANOVA) was conducted. If the ANOVA showed significance at P < .05 or less, the Dunnett's Multiple Comparison Test was used to compare values from groups of mice treated with varying concentrations of TDI to the acetone control group. Figures 2–6 and Table 2 were analyzed by analysis of variance using PROC MIXED. In some cases, data were transformed using the natural log to meet the assumptions of the analysis. Significant interactions were explored utilizing the ‘slice’ option in PROC MIXED and pairwise differences were assessed using a Fishers Least Significant Difference Test. Supplemental data was analyzed by a Student t-test comparing groups as indicated in the figure legends. All differences were considered significant at P < .05; representative significance symbols varied by figure, as indicated in the figure legend.
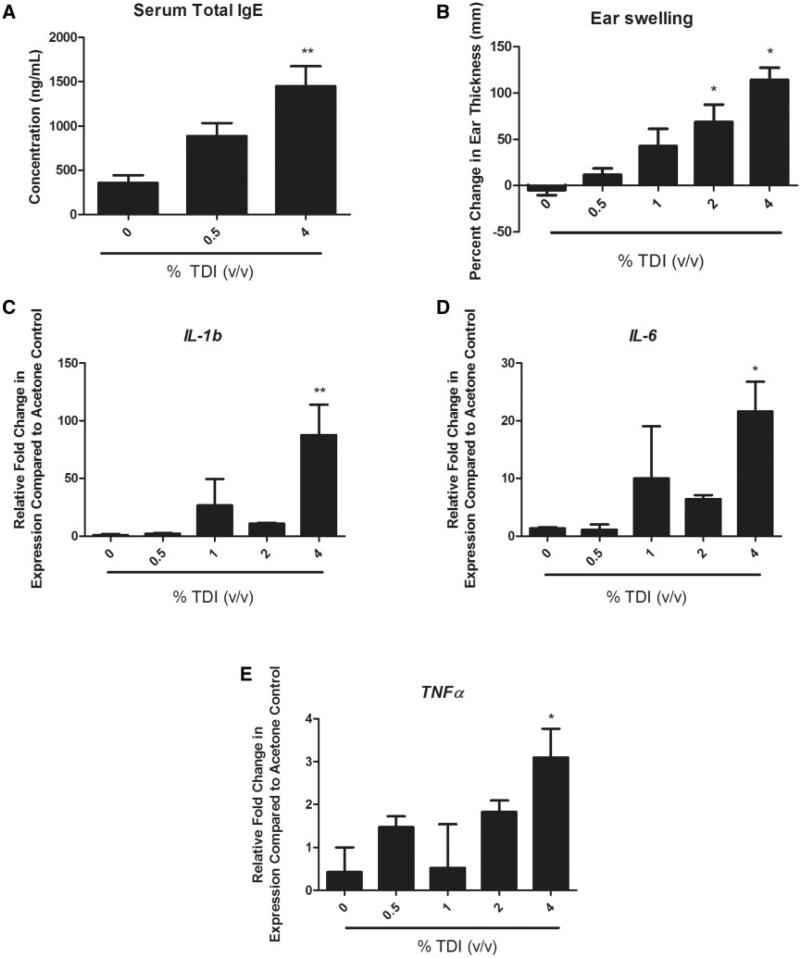
FIG. 1.
Confirmation of sensitization and evaluation of skin irritancy following dermal TDI exposure. ELISA analysis of total serum IgE levels 11 days following single TDI exposure at the indicated concentration (A). Percent change in ear thickness as determined 4 days following TDI exposure (B). Ear mRNA expression of the inflammatory cytokines il-1β (C), il-6 (D), and tnf-α (E) as determined 4 days following TDI exposure via RT-PCR. Bars represent mean (± SE) of 4–5 mice per group. Statistical significance is indicated by (*) at a P-value < .05 and (**) at a P-value < .01.
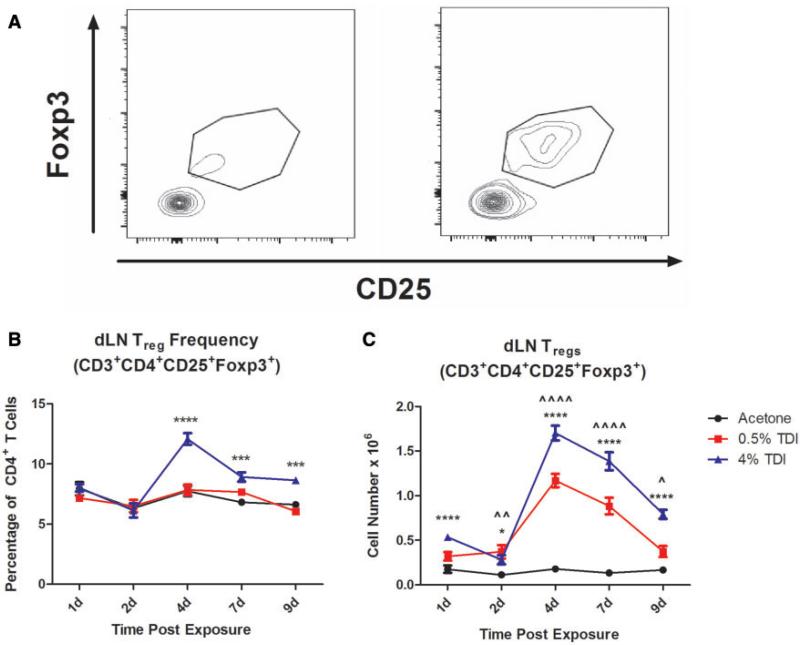
FIG. 2.
Treg subset expands during dermal TDI sensitization. Flow cytometric analysis of Tregs following dermal TDI sensitization (A) Tregs were first gated on their expression of CD3 and CD4, then were further identified by CD25 and Foxp3 expression at indicated time points. dLN Treg frequency (B) and number (C) were determined based on flow cytometry analysis and extrapolation of this data with total dLN cellularity. Graph symbols represent mean (± SE) of 5 mice per group. P-values are represented by (0.5% TDI) and asterisks (4% TDI) (P < .05). Significance is indicated as follows: P ≤ .05 (*), P ≤ .01 (**), P ≤ .001 (***), and P ≤ .0001 (****) for 4% TDI or P ≤ .05 (^), P ≤ .01 (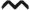 ), P ≤ .001 (^), and P ≤ .0001 (
), P ≤ .001 (^), and P ≤ .0001 (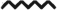 ) for 0.5% TDI. Dermal treatment groups are indicated by the following symbols: circle, acetone; square, 0.5% TDI; and triangle, 4% TDI.
) for 0.5% TDI. Dermal treatment groups are indicated by the following symbols: circle, acetone; square, 0.5% TDI; and triangle, 4% TDI.
TABLE 2.
dLN Migratory Effector Treg Population Expands During TDI Sensitization
| CCR6+ % (of Tregs) | 1 day | 2 day | 4 day | 7 day | 9 day |
|---|---|---|---|---|---|
| Acetone | 11.14 ± 0.7 | 12.72 ± 1.03 | 10.86 ± 0.55 | 18.22 ± 1.36 | 9.23 ± 0.8 |
| 0.5% TDI | 10.22 ± 0.68 | 17.7 ± 1.64** | 20.58 ± 0.84**** | 26.54 ± 0.68**** | 14.82 ± 0.99** |
| 4% TDI | 10.02 ± 0.92 | 10.2 ± 1.1 | 30.7 ± 1.75**** | 40.42 ± 1.87**** | 25.02 ± 1.98**** |
| CCR6+ #(× 105 cells) | |||||
| Acetone | 0.2 ± 0.05 | 0.14 ± 0.04 | 0.19 ± 0.02 | 0.24 ± 0.04 | 0.16 ± 0.03 |
| 0.5% TDI | 0.34 ± 0.06* | 0.61 ± 0.12**** | 2.41 ± 0.19**** | 2.35 ± 0.28**** | 0.57 ± 0.13**** |
| 4% TDI | 0.533 ± 0.05**** | 0.3 ± 0.07** | 5.25 ± 0.48**** | 5.59 ± 0.41**** | 1.99 ± 0.2**** |
| CD103+ % (of Tregs) | 1 day | 2 day | 4 day | 7 day | 9 day |
| Acetone | 22.86 ± 0.75 | 18.88 ± 4.24 | 20.82 ± 0.94 | 28.44 ± 2 | 21.18 ± 0.92 |
| 0.5% TDI | 18.38 ± 0.84 | 24.44 ± 2.99* | 35.88 ± 0.84**** | 38.8 ± 1.1*** | 32.72 ± 2.23**** |
| 4% TDI | 19.62 ± 1.25 | 16.5 ± 1.64 | 43.18 ± 1.78**** | 52.12 ± 1.36**** | 44.64 ± 1.45**** |
| CD103+ #(× 105 cells) | |||||
| Acetone | 0.41 ± 0.09 | 0.16 ± 0.03 | 0.37 ± 0.04 | 0.38 ± 0.07 | 0.36 ± 0.06 |
| 0.5% TDI | 0.59 ± 0.1 | 0.83 ± 0.15**** | 4.21 ± 0.33**** | 3.47 ± 0.45**** | 1.22 ± 0.23**** |
| 4% TDI | 1.04 ± 0.06**** | 0.48 ± 0.11**** | 7.4 ± 0.63**** | 7.23 ± 0.56**** | 3.53 ± 0.23**** |
| ICOS+ % (of Tregs) | 1 day | 2 day | 4 day | 7 day | 9 day |
| Acetone | 13.38 ± 0.36 | 15.92 ± 1.21 | 13.56 ± 0.41 | 18.7 ± 1.22 | 15.98 ± 1.24 |
| 0.5% TDI | 14.06 ± 1.03 | 36.42 ± 2.16**** | 47.8 ± 1.15**** | 38.38 ± 1.72**** | 33.36 ± 2**** |
| 4% TDI | 16.68 ± 1.45 | 33.18 ± 1.27**** | 62.64 ± 1.03**** | 55.02 ± 2.12**** | 45.54 ± 1.78**** |
| ICOS+ # (× 105 cells) | |||||
| Acetone | 0.23 ± 0.05 | 0.17 ± 0.04 | 0.24 ± 0.02 | 0.25 ± 0.04 | 0.28 ± 0.5 |
| 0.5% TDI | 0.46 ± 0.09** | 1.31 ± 0.27**** | 5.61 ± 0.45**** | 3.42 ± 0.45**** | 1.27 ± 0.26**** |
| 4% TDI | 0.88 ± 0.09**** | 0.94 ± 0.18**** | 10.68 ± 0.62**** | 7.62 ± 0.62**** | 3.62 ± 0.29**** |
Data represents mean frequency and number of each Treg population 1–9 days following chemical exposure. Significance is indicated as follows:
P ≤ .05
P ≤ .01
P ≤ .001
and P ≤ .0001 for each group compared with the acetone control value from the matching time point.
RESULTS
Examination of Sensitization and Skin Irritancy Potential of TDI
To confirm that a single dose exposure to TDI (0.5 and 4%) would sensitize animals, total serum IgE was evaluated following TDI exposure. Although not initially statistically significant, IgE levels appeared to increase in a dose-dependent manner, reaching significance following 4% exposure (Figure 1A). Because TDI is a known irritant (Daftarian et al., 2002), the selected doses of TDI (0.5–4% v/v) were assayed for dermal irritancy potential via ear swelling measurements and ear inflammatory cytokine mRNA production quantified via RT-PCR. Average ear swelling was significantly increased four days following 2 and 4% TDI exposure (Figure 1B); however, neither 0.5 nor 1% TDI exposure induced significant increases in ear swelling (Figure 1B). Inflammatory cytokine (IL-1β, IL-6, and TNF-α) mRNA levels were significantly increased in the ear four days following 4% TDI exposure (Figs. 1C–E). These data suggest that 2 and 4% TDI exposure induce a significant irritation response in the ear compared with 0.5 and 1% TDI exposure. Based on this data, 0.5 and 4% TDI were selected as the exposure concentrations in subsequent studies to represent sensitizing concentrations that encompassed both a low dose exhibiting a lack of irritation (0.5%) along with a high dose exhibiting significant irritation (4%).
dLN Treg Expression Kinetics Reveal an Expansion of This Population During TDI Sensitization
In order to profile the expression kinetics of the Treg subset during TDI sensitization we examined dLN cell populations at 1, 2, 4, 7, and 9 days postTDI exposure. Tregs were identified as CD3+ CD4+ CD25+ Foxp3+ cells by flow cytometry (Figure 2A). The frequency of Tregs was unchanged compared with control cells from acetone-treated mice during 0.5% TDI sensitization; however, Treg frequency increased significantly at 4, 7, and 9 days post 4% TDI exposure (Figure 2B). Tregs also significantly increased in number at all time points analyzed during 0.5 and 4% TDI sensitization (Figure 2C). The peak numbers of Tregs in the dLN appeared to occur at day 4 post TDI exposure for both concentrations (mean ± SEM; 1.17 × 106 cells ± 0.08 (0.5%) and 1.7 × 106 cells ± 0.08 (4%)) compared with the acetone control (0.18 × 106 cells ± 0.02). The Treg population remained elevated compared with the acetone control, but began to retract at days 7 (0.5% TDI- 0.88× 106±0.09, and 4% TDI- 1.39 × 106±0.1) and 9 (0.5% TDI- 0.37 × 106±0.06, and 4% TDI- 0.79 × 106±0.05) following TDI exposure relative to their peak at day 4.
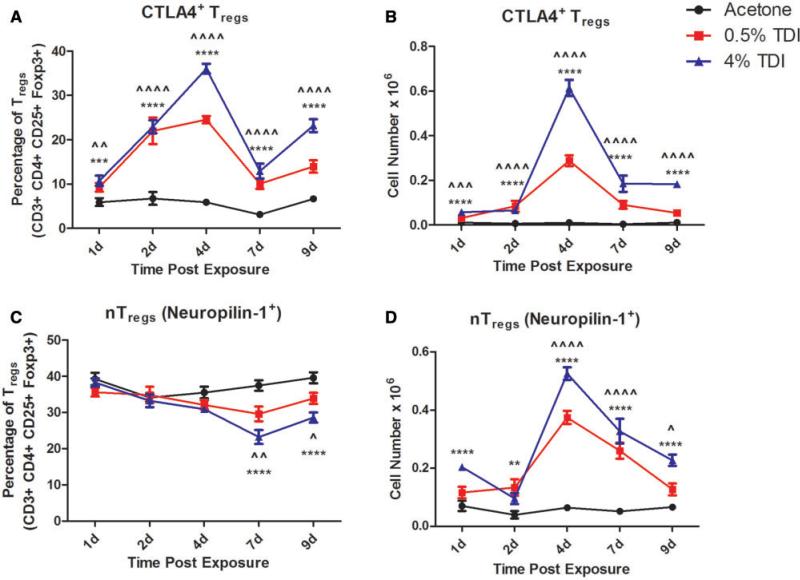
In order to better elucidate the origin of the Tregs involved in the TDI sensitization response, natural Tregs (nTregs) were identified based on their expression of neuropilin-1. Interestingly, the frequency of the nTreg subset as a percentage of total Tregs significantly decreased at 7 and 9 days during 0.5% and 4% TDI sensitization (Figure 3C). Although the frequency of this subset decreased in relation to the acetone control group, the numbers of nTregs in the dLN increased during 0.5 and 4% TDI sensitization (Figure 3D). This increase was significant for 4–9 days during 0.5% TDI sensitization and at all time points measured during 4% TDI sensitization. It is important to note that both the nTreg and the neuropilin-1neg Treg population (presumably induced Tregs [iTregs]) were represented as co-expressing populations examined using additional markers.dLN Treg subsets were further phenotyped by flow cytometry (Table 1). The phenotyping analysis revealed a number of Treg subpopulations that exhibited the potential to be functionally diverse in relation to their mechanism(s) of suppression (Table 1 and Supplementary Figure 1). An expansion in the frequency and number of the CTLA-4+Treg population was observed at all measured time points following both 0.5 and 4% TDI exposure (Figs. 3A and B). This population peaked in expression of both frequency (Figure 3A) and number (Figure 3B) at day 4 post TDI exposure. The emergence of the CTLA4+Treg population during TDI sensitization suggests the engagement of CTLA-4, a negative costimulatory molecule, as a potential suppressive mechanism during this response. As expected, presumably due to compensatory mechanisms during T cell activation, there was also an increase in CTLA4 expression in CD4+non-Tregs; however, at the peak of expression (4 days) the mean frequency of expression among all CD4+ cells was 0.54% ± 0.024 for acetone, 4.6% ± 0.24 for 0.5% TDI, and 7.85% ± 0.66 for 4% TDI groups. When compared with the Tregs expressing CTLA4 at the same time point, these numbers reveal a sizably smaller frequency of nonTregs expressing this marker, emphasizing the specificity of this marker to the Treg population during this response.
FIG. 3.
Expansion of CTLA4+ and nTreg populations during TDI sensitization. Flow cytometric analysis of dLN CTLA4+ Treg frequency (A) and number (B) and nTreg (Neuropilin-1+) frequency (C) and number (D) following TDI sensitization. Bars represent mean (± SE) of 5 mice per group. P values are represented by (0.5% TDI) and asterisks (4% TDI) (P < 0.05). Significance is indicated as follows: P ≤ .05 (*), P < .01 (**), P ≤ .001 (***), and P ≤ .0001 (****) for 4% TDI or P ≤ .05 (^), P ≤ .01 (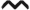 ), P ≤ .001 (
), P ≤ .001 (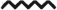 ), and P ≤ .0001 (
), and P ≤ .0001 ( ) for 0.5% TDI. Dermal treatment groups are indicated by the following symbols: circle, acetone; square, 0.5% TDI; and triangle, 4% TDI.
) for 0.5% TDI. Dermal treatment groups are indicated by the following symbols: circle, acetone; square, 0.5% TDI; and triangle, 4% TDI.
TABLE 1.
Treg Flow Cytometry Phenotyping Marker Guide
| Marker | Abbreviation | Surface or Intracellular Detection | Significance | T Cell Populations Expressing |
|---|---|---|---|---|
| Cluster of differentiation 3 | CD3 | S | Pan T cell marker | All T cells |
| Cluster of differentiation 4 | CD4 | S | CD4+ T cell marker | CD4+ T cells |
| Cluster of differentiation 25/IL2rα | CD25 | S | High affinity IL-2 receptor α, T and B cell growth factor (via IL-2 binding) (Fontenot et al., 2005; Lowenthal et al., 1985) | Tregs, some activated T cells (non-Tregs) |
| Forkhead box protein 3 | Foxp3 | IC | Master Treg transcription factor (Hori et al., 2003) | Tregs |
| Chemokine (CC-motif) receptor 6 | CCR6 | S | Lymphocyte chemoattractant CCL20 is its ligand (Schutyser et al., 2003) | Migratory Effector Tregs (Kleinewietfeld, et al., 2005; Yamazaki et al., 2008) and CD8+ T cells (Kondo et al., 2007) |
| Cluster of differentiation 103 | CD103 | S | Integrin involved in epithelial T cell migration and retention (Anz et al., 2011) | Migratory Effector Tregs (Matsushima and Takashima, 2010) and tissue resident CD8+ T cells (Mackay et al., 2013) |
| Cytotoxic T-Lymphocyte-Associated Protein 4 | CTLA4/CD152 | IC | Member of the CD28 family that is a potent inhibitor of T cell costimulation (Frauwirth and Thompson, 2002) | Tregs and activated T cells (non-Tregs) |
| Inducible T-cell costimulator | ICOS/CD278 | S | Member of the CD28 family that has costimulatory functionality during T cell activation (Hutloff et al., 1999) | Migratory Effector Tregs (Vocanson, et al., 2010) and activated T cells |
| Neuropilin-1 | Neuropilin-1 | S | Receptor for vascular endothelial growth factors and semaphorins (Gu et al., 2003) | nTregs (Weiss et al., 2012; Yadav et al., 2012), TFH cells (Renand et al., 2013), and CD8+ T cells (Jackson et al., 2014) |
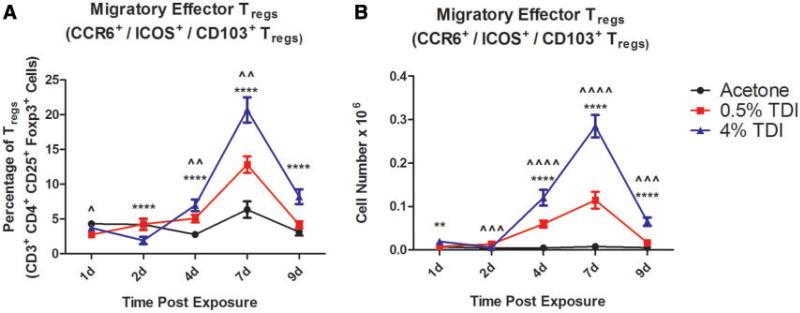
In addition to the CTLA4+Tregs and nTregs, cells expressing the homing molecules CD103, CCR6, and ICOS were analyzed during TDI sensitization. Treg expression of CD103, CCR6, and ICOS increased in both frequency and number throughout 0.5 and 4% TDI sensitization (Table 2). The CCR6+ population's expression frequency was highest at 7 days postTDI exposure, while the CD103+ and ICOS+ populations’ frequency peaked at 4 days postexposure. It is important to note that while there are significant changes in these subsets, they represent a small portion of the total CD4+ population, as their expression is presented on Tregs, which themselves constitute a minority of the total CD4+ population. Although there was robust Treg expression of CD103, CCR6, and ICOS as single markers, there was a significant population of dLN Tregs that co-expressed these molecules (Figs. 4A and B) with kinetics were similar to those exhibited by each single marker. This population likely represents a migratory subset of effector Tregs that have been activated during TDI sensitization. This co-expressing population expanded in the dLN in both number and percent during TDI sensitization and represented 6.34% ± 1.2 of Tregs following acetone exposure, 12.82% ± 1.2 of Tregs during 0.5% TDI sensitization and 20.7% ± 1.8 of Tregs during 4% TDI sensitization at 7 days postexposure (peak expression).
FIG. 4.
dLN migratory effector Treg population expands during TDI sensitization (co-expression). dLN CCR6+ CD103+ ICOS+ Treg frequency (A) and number (B) were determined based on flow cytometry analysis and extrapolation of this data with total dLN cellularity. Bars represent mean (± SE) of 5 mice per group. P values are represented by (0.5% TDI) and asterisks (4% TDI) (P < .05). Significance is indicated as follows: P ≤ .05 (*), P ≤ .01 (**), P ≤ .001 (***), and P ≤ .0001 (****) for 4% TDI or P ≤ .05 (^), P ≤ .01 (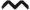 ), P ≤ .001 (^), and P ≤ .0001 (
), P ≤ .001 (^), and P ≤ .0001 (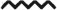 ) for 0.5% TDI. Dermal treatment groups are indicated by the following symbols: circle, acetone; square, 0.5% TDI; and triangle, 4% TDI.
) for 0.5% TDI. Dermal treatment groups are indicated by the following symbols: circle, acetone; square, 0.5% TDI; and triangle, 4% TDI.
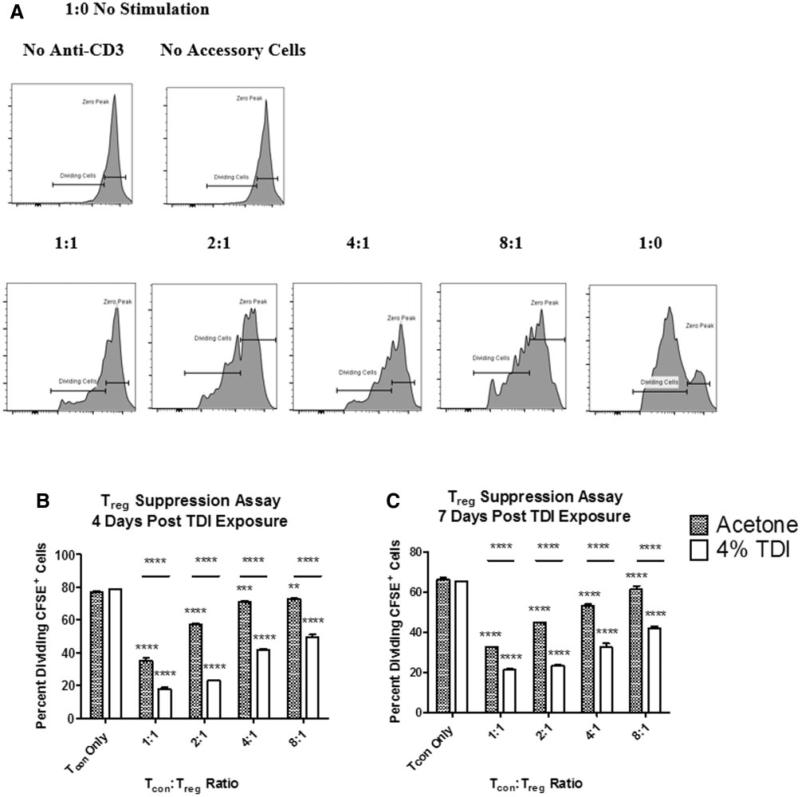
Tregs Have Potent Suppressive Ability During TDI Sensitization
Because the Treg population expanded during TDI sensitization, the functional role of Tregs was further examined by performing a CFSE-based Treg suppression assay with Tregs isolated from acetone or TDI-treated mice (Figure 5A). Tregs from acetone-treated mice were significantly suppressive at all Tcon:Treg ratios tested when isolated at 4 (Figure 5B) and 7 days (Figure 5C) postexposure. Acetone Treg-induced suppression was found to be equivalent to naïve-derived Treg-induced suppression (data not shown). Tregs from TDI-exposed mice exhibited increased suppressive ability compared with the acetone controls when isolated at both 4 (Figure 5B) and 7 days (Figure 5C) at all Tcon:Treg ratios tested. The heightened suppressive ability of Tregs was surprising, given the progression of sensitization at the selected concentrations of TDI. This data suggested a functional, suppressive role for Tregs in TDI sensitization.
FIG. 5.
Treg suppression assay reveals increased suppressive ability of Tregs during TDI sensitization. A CFSE-based Treg suppression assay was performed and percent dividing CFSE+ cells were quantified as illustrated in (A). The zero peak represents the cell population that retained all original CFSE stain. The percent dividing CFSE+ cells (Tcons) are represented in combination with Tregs from mice 4 (B) and 7 (C) days following TDI exposure at a variety of Tcon:Treg ratios. For (B) and (C) 3 plate replicates were utilized from groups of 4–11 mice, as described in the ‘Materials and Methods’ section. Significance is indicated as follows: P ≤ .05 (*), P ≤ .01 (**), P ≤ .001 (***), and P ≤ .0001 (****). P values are represented by asterisks (comparison of each treatment group to Tcon only from the same chemical treatment group) or horizontal bars with asterisks above (comparison of identical ratios between different chemical treatment groups).
Depletion of Tregs Before and During TDI Sensitization Augments the T cell-Mediated Allergic Response
In order to analyze the functional potential of Tregs in vivo during TDI sensitization, this subset was depleted by injecting mice with anti-CD25 antibody days 11 and 8 prior to TDI exposure (Figure 6A). The high dose of 2% TDI was selected in an effort to allow for a measurable increase in the sensitization response, as 4% TDI elicits a maximum sensitization response. The basal levels of Tregs in the blood of mice were determined to be comparable among all groups prior to dosing (Supplementary Figure 2A) and depletion was confirmed in the blood (Sup 2A) at days −2 and 7 and in the dLN (Supplementary Figure 2B) at day 7. Mice dosed with 2% TDI that received anti-CD25 lost significantly more body weight (grams; mean decrease 5.33% 6 3.9) than mice exposed to 2% TDI and isotype control antibody (mean increase 1.01% ± 4.3), suggesting enhanced toxicity following Treg depletion and high-dose TDI administration. Because local irritation is thought to influence allergic sensitization (Pauluhn, 2014) and TDI is a known irritant (Duprat et al., 1976), we analyzed the dermal irritation response at the site of TDI exposure by ear swelling measurements following Treg depletion. As expected, there was a dose-responsive increase (Linear trend test P < .01) in ear swelling following exposure to TDI for both the isotype control and anti-CD25 groups (Figure 6B). Although not statistically significant, ears from animals treated with anti-CD25 and either concentration of TDI exhibited increased swelling (12.4% ± 3.7 for 0.5%; 54.4% ± 10.7 for 2% TDI) compared with their isotype-treated counterparts (1.4% ± 6.6 for 0.5%; 35.6% ± 8.9 for 2% TDI). At day 7, dLN cellularity increased dose-responsively (Linear trend test P < .05) for both the isotype control and antiCD25-treated groups during TDI sensitization (Figure 6C). For both the 0.5 and 2% TDI-treated groups statistically significant increases in the dLN cellularity with anti-CD25 treatment compared with isotype were observed. Another effector CD4+T cell subset, Th2, which play an important role in allergic responses, was identified by GATA-3 expression and was found to dose-responsively expand in number during 0.5 and 2% TDI sensitization for both the isotype and anti-CD25-treated groups (Figure 6D; Linear trend test P < .01). When comparisons were made between the isotype control and anti-CD25 treated groups this population appeared to further expand during TDI sensitization, although these perceived trends were not statistically significant. Similar observations were made for the Th2 population's frequency (data not shown). IL-4 mRNA expression levels increased dose-responsively in the dLN following 0.5 and 2% TDI exposure (Linear trend test P < .05; isotype and anti-CD25 groups) and were significantly augmented in groups treated with anti-CD25 compared with isotype-treated controls (Figure 6E). Similarly, dose-responsive increases in total IgE levels in the blood appeared to occur at 7 days following exposure to TDI in mice treated with both isotype control and anti-CD25, although significance was only observed for the 2% TDI anti-CD25-treated group (Linear trend test P < .01; Figure 6F). Statistically significant increases in serum IgE levels were observed after anti-CD25 treatment following exposure to 0.5 and 2% TDI.
Other T cell-related dLN phenotyping was performed at day 7 including CD3, CD4, and CD8 expression, along with other effector CD4+Th1 and Th17 populations based on their expression of Tbet and Rorγt, respectively (Supplementary Figure 3). CD3+, CD3+ CD4+, CD3+ CD8+, Th1, and Th17 cells dose-responsively expanded in number during 0.5 and 2% TDI sensitization and antiCD25 depleted animals exhibited higher numbers of cells during TDI sensitization (Supplementary Figs. 3B, D, F, H, J). dLN CD3+ cellular frequency decreased in isotype and antiCD25-treated groups during 0.5 and 2% TDI sensitization compared with their respective acetone control (Supplementary Figure 3A). Interestingly, CD3+ cellular frequency was significantly increased in groups treated with anti-CD25 compared with isotype controls for acetone and 0.5% TDI, indicating that antiCD25 treatment did not affect general T cell frequencies in the dLN. CD4+ and CD8+T cell frequency (as a percentage of CD3+ cells) remained stable during TDI sensitization; however, the CD4+ frequency decreased following anti-CD25 treatment while the CD8+ frequency increased (Supplementary Figs.3C and E). The Th1 dLN population significantly expanded in frequency during TDI sensitization (Supplementary Figure 3C). This subset further expanded in mice treated with antiCD25 in frequency (2%) compared with the isotype control groups. The Th17 subset was also profiled and was not significantly altered in frequency during TDI sensitization in the isotype control treated groups; however, the frequency of Th17 cells was significantly increased above both the corresponding acetone and isotype-treated control groups during 2% TDI sensitization following anti-CD25 treatment (Supplementary Figure 3D).
DISCUSSION
Occupational exposure to sensitizing chemicals that are capable of inducing allergic disease is increasing globally and is an important public health concern. Although there continues to be progress made in the area of hazard identification, the limited knowledge of the immunologic mechanisms of sensitization induced by respiratory sensitizers such as TDI continues to complicate the development of these assays. Novel molecules and mechanisms involved in allergic disease need to be investigated in order to elucidate specific entities that can be utilized for the development of hazard identification assays for respiratory sensitizers. Due to the recognized role of Tregs in related allergic disease states, we chose to investigate the expression kinetics, phenotype, and functional capability of Tregs in a murine model of dermal TDI sensitization. To our knowledge, this is the first study that investigates the expression and functionality of Tregs in Th2-mediated chemical sensitization.
Treg involvement has been suggested in the prevention of the development of allergic disease in both mouse and human models of allergy, specifically impacting Th2-related responses (Robinson et al., 2004). Murine CD4+ CD25+ T cells can prevent the transition of naïve CD4+T cells to Th2 cells in vitro (Stassen et al., 2004). In a model of house dust mite antigen-induced airway inflammation decreased airway pathology and ex vivo-derived splenic IL-4 and IL-13 levels were observed following Treg adoptive transfer (Chen et al., 2003). Additionally, Tregs have been implicated in vivo-induced tolerance following inhaled ovalbumin exposure (Ostroukhova et al., 2004), highlighting the importance of these cells in the prevention of allergic disease. Supporting human studies have demonstrated the expansion of Tregs in the nasal mucosa following allergen immunotherapy and noted their association with the efficacy of treatment, suggesting a role for Tregs in the development of antigen-specific tolerance in allergic humans (Radulovic et al., 2008). In addition to their direct actions on Th2 cells, Tregs have also demonstrated the ability to suppress allergic disease by influencing granulocytes, antibody-producing B cells, and resident tissue cells (Palomares et al., 2010). In addition, Tregs have demonstrated involvement in maintaining tolerance and controlling the allergic response during the sensitization phase of both ovalbumin-induced allergic airway inflammation (Baru et al., 2010) and hapten-induced CD8+T cell-dependent contact hypersensitivity response (Christensen et al., 2015).
In an effort to explore the role of Tregs in TDI sensitization, this population was profiled following epicutaneous TDI exposure. Several basic Treg markers (CD3/CD4/CD25/Foxp3) were used to define the classical Treg population but it is important to note that some Treg subsets do not express CD25 and/or Foxp3 such as Tr1 (Passerini et al., 2011) and Th3 (Carrier et al., 2007) cells which typically exhibit TGF-β and IL-10-mediated suppression. Due to the use of CD25 as a functional tool in these studies, we have chosen to focus on classical Tregs. The basic Treg population encompasses numerous subpopulations including, but not limited to, nTregs, iTregs, migratory and homing Tregs, and IL-10 secreting Tregs. Beyond the nTregs and iTregs, these subsets are not mutually exclusive, so while phenotyping markers may be presented alone, many of these populations are co-expressers of a variety of markers. In general, the Treg populations (basic and specialized; Table 1) were identified to expand in both number and frequency, peaking around 4–7 days and beginning to retract around 7–9 days following TDI exposure. This retraction may be due to the early importance of this population in the dLN or may also be mediated by other compensatory or regulatory elements. Interestingly, the general Treg population's frequency did not significantly increase following 0.5% TDI exposure (Figure 2B), potentially implicating a role for the irritant response in both the induction of Tregs and as a precursor to the initiation of sensitization.
Further support for a role for Tregs in TDI sensitization was identified following in vivo administration of anti-CD25 treatment. In this Treg depletion study, augmentations in traditional Th2 allergic markers (dermal irritation, dLN cellularity, Th2 population, IL-4 mRNA expression, and serum total IgE production) were observed. Dose-responsive increases in dLN cellularity, IL4, Th2 cells, and IgE were also observed and were further augmented following antiCD25 treatment indicating increased sensitization in the absence of Tregs. dLN cellularity was measured as a gross marker of sensitization intensity, as this parameter is traditionally utilized in the standard sensitization assay known as the LLNA (Anderson et al., 2011). The dLN Th2 cellular subset was examined as the main effector T cell subset traditionally thought to be involved in allergic responses, with the allergic cytokine IL-4 being primarily produced by this subset, influencing the development and homeostasis of the allergic microenvironment (Brown, 2008). IgE expression indicates the development of an allergic humoral response. Although Treg depletion was identified to promote a Th2 response, the findings from the Treg suppression assay suggested that Tregs isolated from the TDI-sensitized animals were more suppressive compared with their acetone counterparts. These data suggest a significant role for Tregs in the regulation of chemical-induced allergy, implying that their presence may delay or reduce the intensity of sensitization.
Typically, the development of allergic disease is thought to involve an imbalance between Th1 and Th2 responses to allergens (Akdis et al., 2004), resulting in an ‘overzealous’ Th2 response. Although the Th2 response is an important mediator of TDI sensitization, it is important to note that TDI sensitization and resultant allergic disease also contain Th1 and Th17 components (Supplementary Figs. 3G–J) (Liu and Wisnewski, 2003). Tregs are capable of suppressing both Th1 (Xu et al., 2012) and Th17 (Jaffar et al., 2009)-mediated responses, indicating that they may be influencing these components of the TDI sensitization response. Selective suppression of the Th1 response during TDI sensitization could further enhance the development of Th2-mediated sensitization based on the Th1/Th2 imbalance hypothesis.
Although Tregs isolated from TDI sensitized mice were identified to be more suppressive than acetone-derived Tregs, this was reported based as a combined function for all populations of Tregs. This is important to note because TDI-induced Tregs were identified to be phenotypically heterogeneous. Although the kinetics of the specialized Treg populations generally mimic that of the basic Treg population several discrepancies were identified that may provide insight into the mechanisms driving this response. The CTLA4+Treg population was identified to increase in number and frequency earlier than the general Treg population with significant increases identified one day post exposure. The early expansion of this subset was intriguing, given the lack of any data implying a role for CTLA4-mediated suppression in chemical-induced allergy, although this population has been noted to play an immunosuppressive role in the sensitization phase of the ovalbumin-induced allergic response (Hellings et al., 2002). This population participates in contact-mediated suppression by binding to the B7 costimulatory complex expressed on APCs, thus inhibiting T cell activation in a highly suppressive manner (Tai et al., 2012). Based on the early expansion of this subset, it is possible that these cells are interacting with APCs in the early phase of the sensitization response. nTregs are thymus-derived cells whose counterparts are iTregs generated in the periphery; nTregs are identified by neuropilin-1 expression (Weiss et al., 2012; Yadav et al., 2012). Interestingly, the nTreg frequency significantly decreased between 7 and 9 days following TDI exposure compared with the acetone-treated population. This decrease may be attributed to the efflux of cells from the dLN into peripheral tissues and/or the influx and expansion of iTregs in the dLN. Because certain populations of Tregs are known to possess migratory capabilities, the Treg phenotyping panel included several markers associated with Treg migration and effector capabilities including CCR6, CD103, and ICOS. Tregs expressing any or all of these markers were defined as migratory effector Tregs due to the noted homing capabilities and the effector functions (Chang et al., 2012; Kleinewietfeld et al., 2005; Matsushima and Takashima, 2010; Vocanson et al., 2010; Yamazaki et al., 2008). The expansion of Tregs expressing CCR6, CD103, and/or ICOS may represent subsets that have acquired effector functions, such as IL-10 production, and possess migratory capabilities. Taken together, these findings suggest that Tregs likely utilize a variety of suppressive mechanisms as indicated by their phenotypic diversity. This could be a potential explanation for why TDI sensitized Tregs were identified as more suppressive than their acetone control counterparts.
These studies reveal an important role for Tregs in a murine model of TDI sensitization. Elucidation of the role of Tregs in this response will result in a better understanding of the immunologic mechanisms involved chemical sensitization. Due to the complexity of mechanisms involved in chemical allergy, the investigation of novel cellular subsets and mediators of allergic disease is imperative for the greater understanding of these conditions and the development of hazard identification strategies for respiratory chemical sensitizers. In conclusion, we have demonstrated that Tregs are a phenotypically heterogeneous population that expand, suppress, and play a part in controlling the allergic response during TDI sensitization.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Dr Paul Siegel for his interest in and support of this project. The findings and conclusion in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
FUNDING
This work was supported by intramural funds from the National Institute for Occupational Safety and Health.
Footnotes
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
REFERENCES
- Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergenspecific T regulatory 1 and T helper 2 cells. J. Exp. Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Beezhold K, Lukomska E, Richardson J, Long C, Anderson K, Franko J, Meade BJ, Beezhold DH. Expression kinetics of miRNA involved in dermal toluene 2,4-diisocyanate sensitization. J. Immunotoxicol. 2013;11:250–259. doi: 10.3109/1547691X.2013.835891. doi: 10.3109/1547691X.2013.835891. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Franko J, Jackson LG, Wells JR, Ham JE, Meade BJ. Irritancy and allergic responses induced by exposure to the indoor air chemical 4-oxopentanal. Toxicol. Sci. 2012;127:371–381. doi: 10.1093/toxsci/kfs102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Meade BJ. Potential health effects associated with dermal exposure to occupational chemicals. Environ. Health Insights. 2014;8:51–62. doi: 10.4137/EHI.S15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Siegel PD, Meade BJ. The LLNA: A brief review of recent advances and limitations. J. Allergy (Cairo) 2011;2011:424203. doi: 10.1155/2011/424203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Umbright C, Sellamuthu R, Fluharty K, Kashon M, Franko J, Jackson LG, Johnson VJ, Joseph P. Irritancy and allergic responses induced by topical application of ortho-phthalaldehyde. Toxicol. Sci. 2010;115:435–443. doi: 10.1093/toxsci/kfq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anz D, Mueller W, Golic M, Kunz WG, Rapp M, Koelzer VH, Ellermeier J, Ellwart JW, Schnurr M, Bourquin C, et al. CD103 is a hallmark of tumor-infiltrating regulatory T cells. Int. J. Cancer. 2011;129:2417–2426. doi: 10.1002/ijc.25902. [DOI] [PubMed] [Google Scholar]
- Baru AM, Hartl A, Lahl K, Krishnaswamy JK, Fehrenbach H, Yildirim AO, Garn H, Renz H, Behrens GM, Sparwasser T. Selective depletion of Foxp3+ Treg during sensitization phase aggravates experimental allergic airway inflammation. Eur. J. Immunol. 2010;40:2259–2266. doi: 10.1002/eji.200939972. [DOI] [PubMed] [Google Scholar]
- Bello D, Herrick CA, Smith TJ, Woskie SR, Streicher RP, Cullen MR, Liu Y, Redlich CA. Skin exposure to isocyanates: Reasons for concern. Environ. Health Perspect. 2007;115:328–335. doi: 10.1289/ehp.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA. IL-4 production by T cells: You need a little to get a lot. J. Immunol. 2008;181:2941–2942. doi: 10.4049/jimmunol.181.5.2941. [DOI] [PubMed] [Google Scholar]
- Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-β T cell-transgenic mice. J. Immunol. 2007;178:179–185. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- Chang LY, Lin YC, Kang CW, Hsu CY, Chu YY, Huang CT, Day YJ, Chen TC, Yeh CT, Lin CY. The indispensable role of CCR5 for in vivo suppressor function of tumor-derived CD103+ effector/memory regulatory T cells. J. Immunol. 2012;189:567–574. doi: 10.4049/jimmunol.1200266. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei K, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4(+)CD25(−) naive T cells to CD4(+)CD25(+) regulatory T cells by TGF-b induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AD, Skov S, Kvist PH, Haase C. Depletion of regulatory T cells in a hapten-induced inflammation model results in prolonged and increased inflammation driven by T cells. Clin. Exp. Immunol. 2015;179:485–499. doi: 10.1111/cei.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthay A. How do regulatory T cells work? Scand J. Immunol. 2009;70:326–336. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daftarian HS, Lushniak BD, Reh CM, Lewis DM. Evaluation of self-reported skin problems among workers exposed to toluene diisocyanate (TDI) at a foam manufacturing facility. J. Occup. Environ. Med. 2002;44:1197–1202. doi: 10.1097/00043764-200212000-00016. [DOI] [PubMed] [Google Scholar]
- Duprat P, Gradiski D, Marignac B. The irritant and allergenic action of two isocyanates: Toluene diisocyanate (TDI) and diphenylmethane diisocyanate (MDI). Eur. J. Toxicol. Environ. Hyg. 1976;9:43–53. [PubMed] [Google Scholar]
- Fabbri LM, Danieli D, Crescioli S, Bevilacqua P, Meli S, Saetta M, Mapp CE. Fatal asthma in a subject sensitized to toluene diisocyanate. Am. Rev. Respir. Dis. 1988;137:1494–1498. doi: 10.1164/ajrccm/137.6.1494. [DOI] [PubMed] [Google Scholar]
- Felonato M, Pina A, Araujo E. F d., Loures FV, Bazan SB, Feriotti C, Calich VLG. Anti-CD25 treatment depletes treg cells and decreases disease severity in susceptible and resistant mice infected with Paracoccidioides brasiliensis. PLoS One. 2012;7:e51071. doi: 10.1371/journal.pone.0051071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J. Clin. Invest. 2002;109:295–299. doi: 10.1172/JCI14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellings PW, Vandenberghe P, Kasran A, Coorevits L, Overbergh L, Mathieu C, Ceuppens JL. Blockade of CTLA-4 enhances allergic sensitization and eosinophilic airway inflammation in genetically predisposed mice. Eur. J. Immunol. 2002;32:585–594. doi: 10.1002/1521-4141(200202)32:2<585::AID-IMMU585>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Jackson SR, Berrien-Elliott M, Yuan J, Hsueh EC, Teague RM. Neuropilin-1 expression is induced on tolerant self-reactive CD8+ T cells but is dispensable for the tolerant phenotype. PLoS One. 2014;9:e110707. doi: 10.1371/journal.pone.0110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffar Z, Ferrini ME, Girtsman TA, Roberts K. Antigen-specific Treg regulate Th17-mediated lung neutrophilic inflammation, B cell recruitment and polymeric IgA and IgM levels in the airways. Eur. J. Immunol. 2009;39:3307–3314. doi: 10.1002/eji.200939498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber I, Travis MA, Martin SF, Dearman RJ. Immunoregulation of skin sensitization and regulatory T cells. Contact Dermatitis. 2012;67:179–183. doi: 10.1111/j.1600-0536.2012.02148.x. [DOI] [PubMed] [Google Scholar]
- Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rötzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25+ CD4+ T-cell subset. Blood. 2005;105:2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- Kondo T, Takata H, Takiguchi M. Functional expression of chemokine receptor CCR6 on human effector memory CD8+ T cells. Eur. J. Immunol. 2007;37:54–65. doi: 10.1002/eji.200636251. [DOI] [PubMed] [Google Scholar]
- Kruisbeek AM, Shevach E, Thornton AM. Current Protocols in Immunology. John Wiley & Sons, Inc; New York: 2001. Proliferative assays for T cell function. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wisnewski AV. Recent developments in diisocyanate asthma. Ann. Allergy, Asthma Immunol. 2003;90:35–41. doi: 10.1016/s1081-1206(10)61647-x. [DOI] [PubMed] [Google Scholar]
- Lowenthal JW, Zubler RH, Nabholz M, MacDonald HR. Similarities between interleukin-2 receptor number and affinity on activated B and T lymphocytes. Nature. 1985;315:669–672. doi: 10.1038/315669a0. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- Mapp CE. Agents, old and new, causing occupational asthma. Occup. Environ. Med. 2001;58:354–354. doi: 10.1136/oem.58.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NB, Vorachek WR, Steppan LB, Mourich DV, Kerkvliet NI. Functional characterization and gene expression analysis of CD4 +CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-pdioxin. J. Immunol. 2008;181:2382–2391. doi: 10.4049/jimmunol.181.4.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson JM, Johnson VJ, Luster MI. Immune mediators in a murine model for occupational asthma: Studies with toluene diisocyanate. Toxicol. Sci. 2005;84:99–109. doi: 10.1093/toxsci/kfi051. [DOI] [PubMed] [Google Scholar]
- Matsushima H, Takashima A. Bidirectional homing of Tregs between the skin and lymph nodes. J. Clin. Invest. 2010;120:653–656. doi: 10.1172/JCI42280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP. Report on carcinogens. 12th edn. P. H. S. U.S. Department of Health and Human Services (National Toxicology Program, Ed.); Research Triangle Park, NC.: 2011. p. 499. [Google Scholar]
- Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, Corcoran TE, Ray A. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J. Clin. Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of Treg in immune regulation of allergic diseases. Eur. J. Immunol. 2010;40:1232–1240. doi: 10.1002/eji.200940045. [DOI] [PubMed] [Google Scholar]
- Passerini L, Di Nunzio S, Gregori S, Gambineri E, Cecconi M, Seidel MG, Cazzola G, Perroni L, Tommasini A, Vignola S, et al. Functional type 1 regulatory T cells develop regardless of FOXP3 mutations in patients with IPEX syndrome. Eur. J. Immunol. 2011;41:1120–1131. doi: 10.1002/eji.201040909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauluhn J. Development of a respiratory sensitization/elicitation protocol of toluene diisocyanate (TDI) in Brown Norway rats to derive an elicitation-based occupational exposure level. Toxicology. 2014;319:10–22. doi: 10.1016/j.tox.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3- expressing CD4+ CD25+ cells in the nasal mucosa. J. Allergy Clin. Immunol. 2008;121:1467–1472. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Renand A, Milpied P, Rossignol J, Bruneau J, Lemonnier F, Dussiot M, Coulon S, Hermine O. Neuropilin-1 expression characterizes T follicular helper (Tfh) cells activated during B cell differentiation in human secondary lymphoid organs. PLoS One. 2013;8:e85589. doi: 10.1371/journal.pone.0085589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DS. Regulatory T cells and asthma. Clin. Exp. Allergy. 2009;39:1314–1323. doi: 10.1111/j.1365-2222.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- Robinson DS, Larché M, Durham SR. Tregs and allergic disease. J. Clin. Invest. 2004;114:1389–1397. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Setiady YY, Coccia JA, Park PU. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur. J. Immunol. 2010;40:780–786. doi: 10.1002/eji.200939613. [DOI] [PubMed] [Google Scholar]
- Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T-cell suppression – a diverse arsenal for a moving target. Immunology. 2008;124:13–22. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen M, Jonuleit H, Muller C, Klein M, Richter C, Bopp T, Schmitt S, Schmitt E. Differential regulatory capacity of CD25+ T regulatory cells and preactivated CD25+ T regulatory cells on development, functional activation, and proliferation of Th2 cells. J. Immunol. 2004;173:267–274. doi: 10.4049/jimmunol.173.1.267. [DOI] [PubMed] [Google Scholar]
- Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, et al. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood. 2012;119:5155–5163. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoirbeek JAJ, Tarkowski M, Ceuppens JL, Verbeken EK, Nemery B, Hoet PHM. Respiratory response to toluene diisocyanate depends on prior frequency and concentration of dermal sensitization in mice. Toxicol. Sci. 2004;80:310–321. doi: 10.1093/toxsci/kfh155. [DOI] [PubMed] [Google Scholar]
- Vocanson M, Rozieres A, Hennino A, Poyet G, Gaillard V, Renaudineau S, Achachi A, Benetiere J, Kaiserlian D, Dubois B, et al. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J. Allergy Clin. Immunol. 2010;126:280–289. doi: 10.1016/j.jaci.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 2012;209:1723–1742. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Lan Q, Chen M, Chen H, Zhu N, Zhou X, Wang J, Fan H, Yan CS, Kuang JL, et al. Adoptive transfer of induced-Treg cells effectively attenuates murine airway allergic inflammation. PLoS One. 2012;7:e40314. doi: 10.1371/journal.pone.0040314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 2012;209:1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, et al. CCR6 regulates the migration of inflamma-tory and regulatory T cells. J. Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.