Malaria burdens have fallen dramatically this century, in large part because around a billion long-lasting insecticide-treated bed nets (LLINs) have been introduced into Africa (1, 2). Hanging over this success is a key question: What if the insecticides stop working? Pyrethroids are the only chemical class currently approved for bed net use and, predictably, pyrethroid resistance is emerging in Anopheles populations (1, 3). How much does this matter? Incredibly, we have little idea. The paper by Viana et al. (4) in PNAS explores one piece of the puzzle.
In agriculture, the impact of insecticide resistance is relatively straightforward to anticipate: insects eat crops and insects that survive otherwise effective insecticide exposure continue to eat crops and reduce yield. But in public health, the situation is more nuanced. What matters is not simply whether mosquitoes survive insecticide exposure but, rather, whether insecticide resistance enhances the ability of mosquitoes to acquire and transmit pathogens (vectorial capacity). For example, most mosquitoes do not survive the 10–14 d it typically takes malaria parasites to become infectious. Insecticides work by reducing the number of survivors still further. Whether insecticide resistance negates this reduction depends critically on the lifespan of resistant mosquitoes. If resistance is incomplete—so the mosquitoes nonetheless die younger after repeated insecticide exposure—or if resistance itself is a life-shortening trait because it is metabolically costly, the impact of resistance on disease transmission might be negligible (5).
Could it be that LLINs will continue to function in the face of increasing resistance? Although far from definitive, certain field data are consistent with this possibility. Genetic markers for known resistance alleles (such as knockdown resistance, KDR) and insecticidal bioassays (WHO standard cone and tube tests) point to the emergence of insecticide resistance in many malaria endemic areas, but as yet there is little evidence of widespread reduction in the efficacy of LLINS (6–8). A recent meta-analysis of laboratory and field studies evaluating the impact of resistance found that insecticide-treated nets were more effective than untreated nets, regardless of resistance (9). History is not much of a guide. The folklore that DDT resistance caused the failure of the 20th century malaria eradication program is poorly supported. Just 14 of 75 (19%) documented malaria resurgences in the 20th century were attributed at the time to insecticide resistance, and it is hard to know what to make of even those 14 reports; none were supported by quantitative analysis, and all 14 involved other factors, most commonly political failures (10).
Relevant laboratory data are remarkably sparse. The new paper by Viana et al. (4) shows just how needed such studies are. The WHO standard bioassay method for monitoring and evaluating insecticide resistance exposes young (3–5 d old) female mosquitoes to a single, discriminating dose of the relevant insecticide for a fixed time (11). Mortality is then assessed after 24 h to determine the frequency of resistance within mosquito populations; if a population suffers <90% mortality it is considered “resistant.” Viana et al. (4) took two different laboratory strains of Anopheles gambiae (the primary malaria vector in large parts of Africa) that are classified as “resistant” according to these criteria, and exposed them to an LLIN for 3 min following WHO protocols. One strain suffered 60–100% mortality after 24 h and the other suffered 3–61%. Equivalent mortality following exposure to a net without insecticide was <20%. Viana et al. then added further realism by repeating exposures at various intervals over subsequent days (this approach simulates the fact that mosquitoes require blood meals to produce eggs and are expected to attempt to feed every 2–4 d). This longer-term evaluation revealed two key results. First, the cumulative effect of even moderate mortality over subsequent exposures drives down survival of the mosquito cohorts over time. In other words, resistance is incomplete. Second, there appears to be delayed mortality following exposure. This additional mortality is a novel observation that is not captured in the standard 24-h mortality readout. From these data, Viana et al. estimate that LLINs would reduce the number of infectious bites delivered by their mosquito strains 3.3- and 7.8-fold, with the delayed mortality accounting for at least half of this reduction. Thus, despite these mosquito strains being classified as resistant [one of them highly so (12)], realistic exposure to LLINs across the lifetime of these mosquitoes is predicted to substantially reduce transmission potential.
The paper by Viana et al. (4) adds to a growing body of research challenging the link between widely used phenotypic measures of resistance and ultimate transmission. The standard WHO assays prescribe the use of 3- to 5-d-old mosquitoes, yet several studies observe that adult female Anopheles become more susceptible to insecticides with increasing age (13, 14). This rising susceptibility is important, as it is only the older mosquitoes that are responsible for transmitting malaria parasites, so if they remain susceptible, transmission can still be reduced (15). Furthermore, some recent studies report that when insecticide-resistant An. gambiae strains are exposed to insecticides, they are less likely to develop a malaria infection (16, 17). Other research shows sublethal exposure to LLINs changes mosquito feeding behavior and responsiveness to host feeding cues (18). Lower parasite burdens and changes in feeding behavior could contribute to reduced transmission, even in the absence of mortality. Such effects could all reduce the operational significance of resistance, yet are not captured in the standard resistance assays. Similar challenges exist for common genetic markers for resistance alleles, such as KDR. There is no convincing evidence that KDR alone produces operationally significant levels of pyrethroid resistance (19). Indeed a study from Bioko Island showed that Anopheles mosquitoes that were homozygous for KDR appeared less likely to be able to transmit malaria than susceptibles (20).
The emphasis of most insecticide-resistance research to date has been to detect resistance using simple diagnostic tests, characterize the mechanisms (e.g., target site mutations, or metabolic detoxification), and monitor the spread. These are sensible starting points but it is not clear that any of the current standard resistance measures (bioassays or molecular markers) tell us much more than the obvious: insecticides select for resistance. As the work of Viana et al. (4) and others demonstrates, there is an urgent need to characterize resistance phenotypes in terms of the properties that impact parasite transmission: vector longevity, competence, and behavior (5).
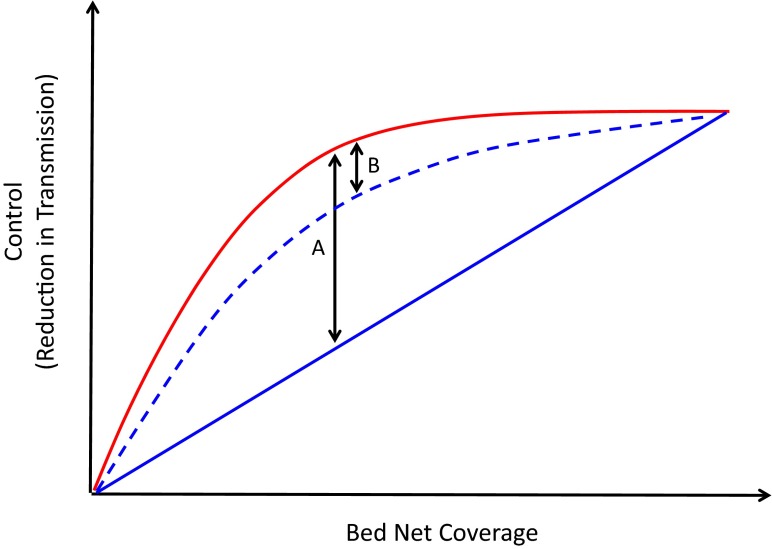
By how much might the emergence of pyrethroid-resistant mosquitoes undo the malaria control gains achieved by LLINs? One possible scenario is outlined in Fig. 1. LLINs impact malaria transmission in two ways. They physically protect users from
As the work of Viana et al. and others demonstrates, there is an urgent need to characterize resistance phenotypes in terms of the properties that impact parasite transmission: vector longevity, competence, and behavior.
mosquitoes and, by killing mosquitoes that contact the nets, they also protect those without nets. To a first approximation, it is largely this community protection that is vulnerable to insecticide resistance (because in a world of high-quality nets, with good campaigns to ensure they are replaced before they become overly damaged, untreated nets protect those who use them). This vulnerability puts bounds on the problems pyrethroid resistance could cause (Fig. 1). The impact will likely be greatest in areas where community protection is greatest (moderate-to-high but incomplete coverage). Just how great the problem could become depends critically on how the resistance phenotype impacts parasite transmission in those settings. Quite plausibly, the problem could be relatively minor (Fig. 1), which is perhaps why there is not yet evidence for wholesale failure of LLINS.
Fig. 1.
The possible impact of insecticide resistance on the efficacy of LLINs. As bed net coverage (proportion of the population using nets) increases, more people gain personal protection from malaria (solid blue line). Mosquitoes seeking those people are killed by insecticides on the nets, and so even people not sleeping under nets experience less exposure to mosquito bites, giving the combined population-wide protective effect of LLINs against a susceptible mosquito population shown in red. If insecticide resistance renders the insecticide completely ineffective, the community benefit is lost and LLINs provide physical protection only (an important assumption here is that the nets are intact and are used effectively). Thus, the difference between the red and blue lines represents the “potential effect size” of resistance. At intermediate levels of coverage we expect the largest effect size, as this is where the mass action effect of the insecticide provides the greatest relative contribution to control (indicated by arrow A). However, indirect impacts of insecticide exposure, such as delayed mortality, reductions in feeding, or pleiotropic effects of resistance, such as increased refractoriness to malaria parasites, could still contribute to reductions in transmission (dashed blue line). Such effects could result in a reduced “realized effect size” of resistance (arrow B).
On the other hand, this speculation could be wrong, and catastrophic LLIN failure might be just around the corner. Continued selection pressure could drive mosquitoes to become much more resistant than the two strains studied by Viana et al. (4). It could even enhance their capacity to transmit malaria (21, 22). We just don’t know. LLINs are one of the cheapest and most effective malaria control tools available. Whether resistance will render them ineffective should not be an open question. The entomological work required to characterize the vectorial capacity of resistant mosquitoes in relevant ecological settings is nontrivial and unglamorous. This work should, nonetheless, be considered one of the greatest priorities in contemporary malaria research.
Acknowledgments
This research was funded in part by NIH-NIAID (Grant R21AI113609).
Footnotes
The authors declare no conflict of interest.
See companion article on page 8975.
References
- 1.WHO . World Malaria Report 2015. WHO; Geneva: 2015. [Google Scholar]
- 2.Bhatt S, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranson H, et al. Pyrethroid resistance in African anopheline mosquitoes: What are the implications for malaria control? Trends Parasitol. 2011;27(2):91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Viana M, Hughes A, Matthiopoulos J, Ranson H, Ferguson HM. Delayed mortality effects cut the malaria transmission potential of insecticide-resistant mosquitoes. Proc Natl Acad Sci USA. 2016;113:8975–8980. doi: 10.1073/pnas.1603431113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivero A, Vézilier J, Weill M, Read AF, Gandon S. Insecticide control of vector-borne diseases: When is insecticide resistance a problem? PLoS Pathog. 2010;6(8):e1001000. doi: 10.1371/journal.ppat.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanda E, et al. Insecticide resistance and the future of malaria control in Zambia. PLoS One. 2011;6(9):e24336. doi: 10.1371/journal.pone.0024336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenwood B, Targett G, Chandramohan D, Logan J, Schellenberg D. Are insecticide treated bednets failing? Lancet Infect Dis. 2012;12(7):512; author reply 514. doi: 10.1016/S1473-3099(12)70105-6. [DOI] [PubMed] [Google Scholar]
- 8.Lindblade KA, et al. A cohort study of the effectiveness of insecticide-treated bed nets to prevent malaria in an area of moderate pyrethroid resistance, Malawi. Malar J. 2015;14:31. doi: 10.1186/s12936-015-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strode C, Donegan S, Garner P, Enayati AA, Hemingway J. The impact of pyrethroid resistance on the efficacy of insecticide-treated bed nets against African anopheline mosquitoes: Systematic review and meta-analysis. PLoS Med. 2014;11(3):e1001619. doi: 10.1371/journal.pmed.1001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JM, et al. Malaria resurgence: A systematic review and assessment of its causes. Malar J. 2012;11:122. doi: 10.1186/1475-2875-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO . Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes. WHO; Geneva: 2013. [Google Scholar]
- 12.Bagi J, et al. When a discriminating dose assay is not enough: Measuring the intensity of insecticide resistance in malaria vectors. Malar J. 2015;14:210. doi: 10.1186/s12936-015-0721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones CM, et al. Aging partially restores the efficacy of malaria vector control in insecticide-resistant populations of Anopheles gambiae s.l. from Burkina Faso. Malar J. 2012;11:24. doi: 10.1186/1475-2875-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glunt KD, Thomas MB, Read AF. The effects of age, exposure history and malaria infection on the susceptibility of Anopheles mosquitoes to low concentrations of pyrethroid. PLoS One. 2011;6(9):e24968. doi: 10.1371/journal.pone.0024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Read AF, Lynch PA, Thomas MB. How to make evolution-proof insecticides for malaria control. PLoS Biol. 2009;7(4):e1000058. doi: 10.1371/journal.pbio.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alout H, et al. Insecticide exposure impacts vector-parasite interactions in insecticide-resistant malaria vectors. Proc R Soc B-Biol Sci. 2014;281(1786):7. doi: 10.1098/rspb.2014.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kristan M, et al. Exposure to deltamethrin affects development of Plasmodium falciparum inside wild pyrethroid resistant Anopheles gambiae s.s. mosquitoes in Uganda. Parasit Vectors. 2016;9:100. doi: 10.1186/s13071-016-1384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegert PY, Walker E, Miller JR. Differential behavioral responses of Anopheles gambiae (Diptera: Culicidae) modulate mortality caused by pyrethroid-treated bednets. J Econ Entomol. 2009;102(6):2061–2071. doi: 10.1603/029.102.0607. [DOI] [PubMed] [Google Scholar]
- 19.Hemingway J. The role of vector control in stopping the transmission of malaria: Threats and opportunities. Philos Trans R Soc B-Biol Sci. 2014;369(1645):2013041. doi: 10.1098/rstb.2013.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemingway J, et al. Country-level operational implementation of the Global Plan for Insecticide Resistance Management. Proc Natl Acad Sci USA. 2013;110(23):9397–9402. doi: 10.1073/pnas.1307656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alout H, et al. Insecticide resistance alleles affect vector competence of Anopheles gambiae s.s. for Plasmodium falciparum field isolates. PLoS One. 2013;8(5):e63849. doi: 10.1371/journal.pone.0063849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ndiath MO, et al. Effects of the KDR resistance mutation on the susceptibility of wild Anopheles gambiae populations to Plasmodium falciparum: a hindrance for vector control. Malar J. 2014;13:340. doi: 10.1186/1475-2875-13-340. [DOI] [PMC free article] [PubMed] [Google Scholar]