Significance
Actin is a prominent component of the cytoskeleton in the cytoplasm of eukaryotic cells. Its presence in the nucleus was once considered controversial. However, it is now widely accepted that nuclear actin (N-actin) is a constitutive subunit of several chromatin-remodeling complexes. Yet, the specific structure of N-actin and how its polymerization is regulated remain unresolved. Our study presents the first crystal structure of the N-actin complex to our knowledge and illustrates the salient features that distinguish N-actin from its cytoplasmic counterpart. These features prevent the self-polymerization of N-actin and its regulation by many known actin-binding proteins. Our findings provide a rational basis for the functions of N-actin, laying the groundwork for future studies in this field.
Keywords: nuclear actin, Arp4, chromatin remodeling, crystal structure
Abstract
Actin polymerizes and forms filamentous structures (F-actin) in the cytoplasm of eukaryotic cells. It also exists in the nucleus and regulates various nucleic acid transactions, particularly through its incorporation into multiple chromatin-remodeling complexes. However, the specific structure of actin and the mechanisms that regulate its polymeric nature inside the nucleus remain unknown. Here, we report the crystal structure of nuclear actin (N-actin) complexed with actin-related protein 4 (Arp4) and the helicase-SANT–associated (HSA) domain of the chromatin remodeler Swr1. The inner face and barbed end of N-actin are sequestered by interactions with Arp4 and the HSA domain, respectively, which prevents N-actin from polymerization and binding to many actin regulators. The two major domains of N-actin are more twisted than those of globular actin (G-actin), and its nucleotide-binding pocket is occluded, freeing N-actin from binding to and regulation by ATP. These findings revealed the salient structural features of N-actin that distinguish it from its cytoplasmic counterpart and provide a rational basis for its functions and regulation inside the nucleus.
Actin self-assembles into a two-stranded filamentous structure (F-actin) in the cytoplasm, where it acts as a key component of the eukaryotic cytoskeleton. The polymerization of actin is regulated by the bound nucleotide and several actin-binding proteins (ABPs) (1). The binding of ATP promotes actin polymerization, whereas ADP-bound actin is susceptible to depolymerization. Numerous ABPs, including cofilin, profilin, and thymosin-β4, have been identified, and they regulate the dynamics of actin in the cytoplasm. The actin-related proteins (ARPs) are homologs of actin. Two of these, Arp2 and Arp3, form a tight complex (the Arp2/3 complex) and bind to the side of a mother filament to promote the formation of a branched F-actin structure (2).
Actin, several ARPs, and many ABPs are also found in the nucleus. The presence of actin in the nucleus was once considered controversial (3, 4). The most convincing evidence for the presence of N-actin in the nucleus comes from studies of chromatin regulatory complexes (5–8). Actin and Arp4 form a conjugated pair and are assembled into several chromatin-remodeling complexes, including the Swr1, Ino80, and BAF complexes, and the acetyltransferase NuA4 complex (9, 10). In addition to N-actin and Arp4, the Swr1 complex also contains Arp6, and the Ino80 complex contains Arp5 and Arp8. These chromatin-remodeling complexes are ATP-driven molecular machines that slide, remove, and reconstruct nucleosomes, thus regulating gene transcription, DNA repair, homologous recombination, and many other nucleic acid transactions (9). It is known that the Swr1 complex is responsible for reconstruction of the nucleosome through replacement of canonical H2A-H2B dimers with H2A.Z-H2B dimers, whereas the Ino80 complex performs diverse functions, including transcription activation and DNA repair (9). Deregulation of the chromatin-remodeling processes is associated with various human diseases, particularly cancers (11–13).
Although N-actin and Arp4 are widely conserved from yeast to humans, their specific structures and functions within the chromatin regulatory complexes remain largely unknown. N-actin and Arp4 are incorporated into different chromatin regulatory complexes through a common motif, the HSA domain (10). In yeast, the HSA domains of Snf2 and Sth1 bind to the Arp7/Apr9 pair, whereas those of Swr1, Ino80, and Eaf1 (a subunit of the NuA4 complex) specifically recognize the N-actin/Arp4 pair. Arp4 directly interacts with G-actin and inhibits its polymerization in solution (14). In yeast, Arp4 is suggested to interact with histone and target the NuA4 complex to the site of DNA damage (15, 16). BAF53/Actl6 (an Arp4 homolog in mammalian cells) regulates the recruitment of the BAF complex to the promoters of specific genes, controlling neural development, epidermal differentiation, and hematopoietic stem cell function (17–20).
Less is known about N-actin. The current understanding of N-actin is mostly related to the prior acknowledge of its well-studied counterpart in the cytoplasm (3, 21–23). Early studies suggested that N-actin within the BAF complex interacts with actin filaments, cofilin, and profilin (5, 24). By contrast, a recent study showed that N-actin within the Ino80 complex is in a monomeric state and does not bind profilin (25). Instead, N-actin might play a role in chromatin substrate handling. How N-actin is regulated by various nuclear ABPs is unclear.
In this study, we determined the structure of N-actin in complex with Arp4 and the HSA domain of Swr1 at a resolution of 2.8 Å. The structure illustrates the salient features of N-actin within the chromatin-remodeling complex, which distinguish it from cytoplasmic actin and provide the structural foundation for understanding its functions and regulation inside the nucleus.
Results and Discussion
Overall Structure of the Actin–Arp4–HSAswr1 Complex.
We report the crystal structure of actin of the budding yeast Saccharomyces cerevisiae in complex with Arp4 and the HSA domain of the chromatin remodeler Swr1. Swr1 has an N-terminal HSA domain, which recognizes N-actin and Arp4, and a C-terminal ATPase core domain, which performs the key enzymatic reaction. The actin–Arp4–HSASwr1 complex was reconstituted in vitro with actin and Arp4 expressed individually in insect cells and the HSA domain of Swr1 expressed in Escherichia coli (Fig. S1). The final model was refined to 2.8 Å, with Rwork/Rfeee = 0.23/0.28 (Table 1).
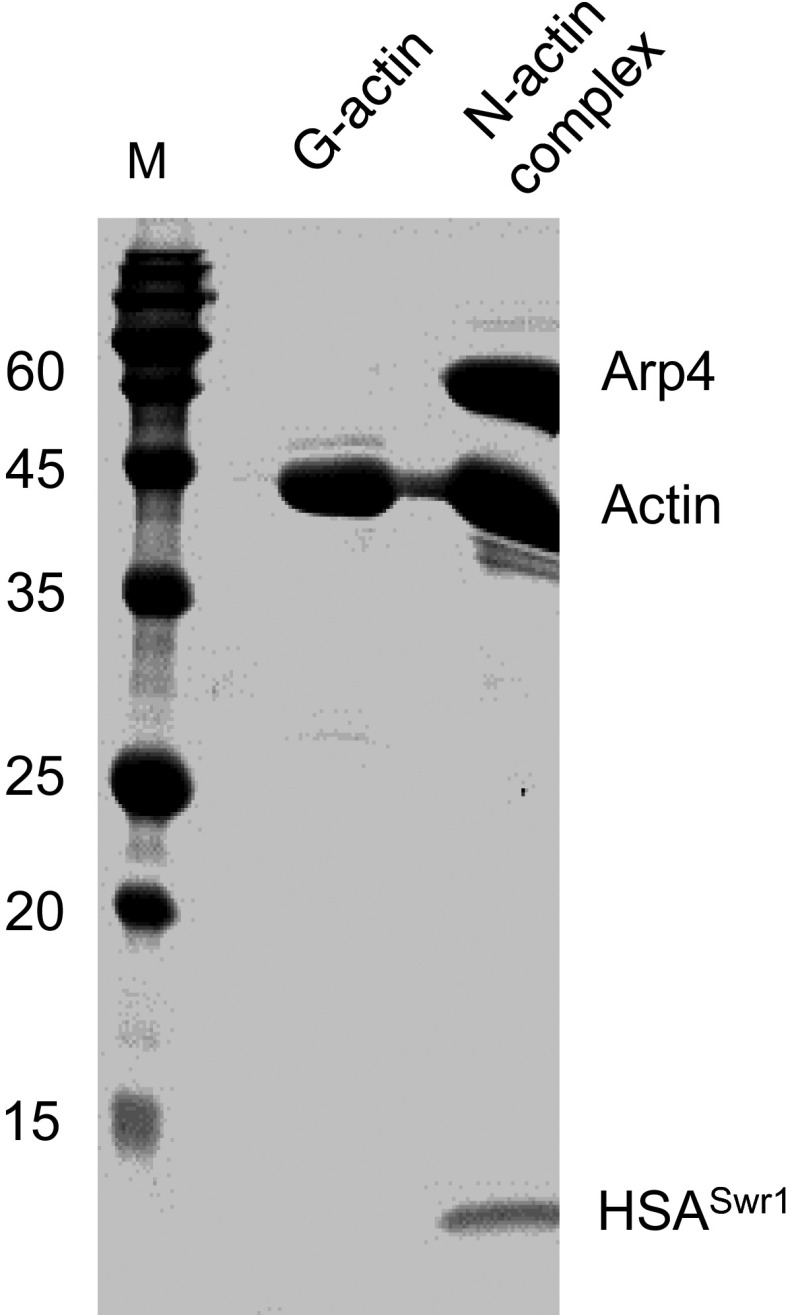
Fig. S1.
SDS/PAGE analysis of G-actin and the N-actin complex. G-actin and the N-actin complex were purified close to homogeneity through multiple steps of chromatography.
Table 1.
Data collection and refinement statistics
| Data collection | |
| Space group | P21212 |
| Cell dimensions | |
| a, b, c, Å | 110.260, 202.220, 87.010 |
| α,β,γ, ° | 90.00, 90.00, 90.00 |
| Resolution, Å | 50–2.8 (2.87–2.8)* |
| Rsym or Rmerge | 0.115 (0.972) |
| I/σI | 15.4 (1.15) |
| Completeness, % | 99.7 (99.2) |
| Redundancy | 9.4 (3.9) |
| CC1/2 | 0.585 |
| Refinement | |
| Resolution, Å | 48.4–2.80 |
| No. reflections | 48,531 |
| Rwork/Rfree | 0.2317/0.2844 |
| No. of atoms | |
| Protein | 12,600 |
| Ligand/ion | 66 |
| Water | 0 |
| B factors | |
| Protein | 108 |
| Ligand/ion | 92 |
| Water | / |
| rms deviations | |
| Bond lengths, Å | 0.007 |
| Bond angles, ° | 0.708 |
Values in parentheses are for the highest-resolution shell.
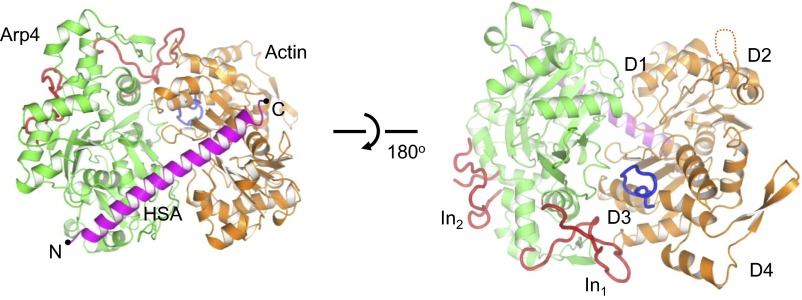
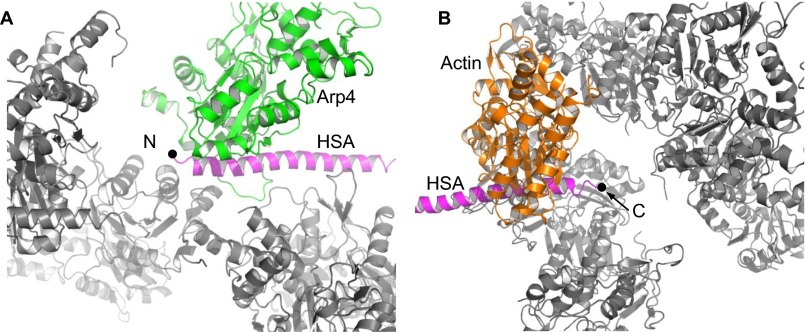
The HSA domain functions as the key organizer in the assembly of the ternary complex. It forms a long helix, with its N and C termini binding to Arp4 and N-actin, respectively (Fig. 1). As suggested (26), the barbed end of N-actin interacts with the amphipathic HSA domain through its hydrophobic cleft. The DNase-I–binding loop in N-actin subdomain D2, which is suggested to be involved in chromatin binding (25), is disordered. The inner face of N-actin, including the “hydrophobic plug,” makes multiple contacts with Arp4 (Fig. 1 and Fig. S2). The barbed end of actin is responsible for its longitudinal interactions within the actin filament, whereas the inner face of actin is responsible for its lateral interactions and is buried inside two-stranded F-actin (27–29). These key elements for F-actin assembly are sequestered by the interactions with Arp4 and the HSA domain, which provides the structural basis for preventing N-actin from polymerization. This structure is also consistent with an earlier study, which showed that Arp4 reduces the polymerization rate of G-actin and cannot bind to F-actin (14).
Fig. 1.
Two different views of the overall structure of the actin–Arp4–HSA complex. Actin, orange; Arp4, green; HSA domain, magenta; the “hydrophobic plug” of actin, blue; the two insertions (In1 and In2) of Arp4, red. The N and C termini of the HSA domain are labeled with black dots. The four subdomains of actin are labeled D1, D2, D3, and D4.
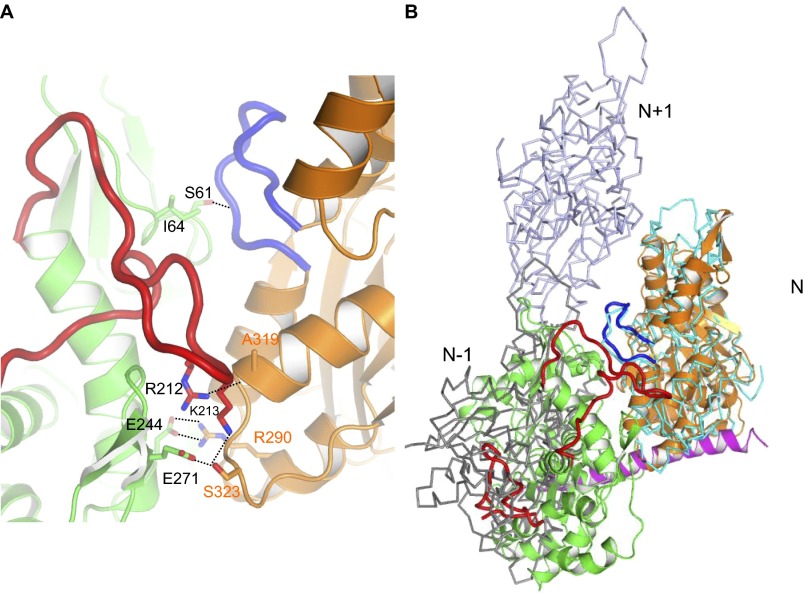
Fig. S2.
Interactions with Arp4 prevent N-actin from polymerization. (A) Arp4 mainly interacts with N-actin at subdomain D3. N-actin and Arp4 are colored as in Fig. 1. Glu244 of Arp4 forms two salt bridge interactions with Arg290 of actin. Arg212 and Lys213 of the In1 of Arp4 form H bonds with Ala319 and Ser323 of actin, respectively. Ile64 and Ser61 of Arp4 pack against the main chain of the “hydrophobic plug” of actin. (B) Supposition of the actin–Arp4 complex with a F-actin model (PDB ID code 3MFP) (29). The actin protomers along the F-actin model are labeled N-1, N, and N+1, and colored gray, cyan, and light blue, respectively. The alignment was done on actin N. Arp4 clashes with the actin protomers located at the adjacent positions (N-1 or N+1) across the two-stranded filament, which provides a mechanism for preventing N-actin from polymerization.
Interactions Between the HSA Domain and actin/Arp4.
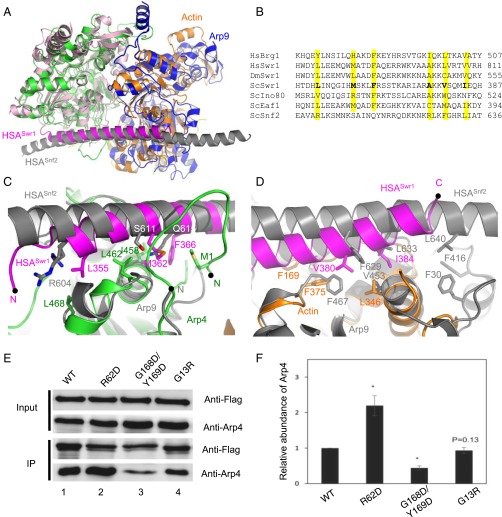
The structures of the yeast-specific ARPs, Arp7 and Arp9, when complexed with the HSA domain of the chromatin remodeler Snf2, have been reported (26). To compare these two HSA domain-containing complexes, we aligned the structures of Arp4 and Arp7 (Fig. 2A). This structural superposition leads to more accurate sequence alignments of the HSA domains (Fig. 2B), which mostly agree with the earlier result, except in the alignment of Snf2 and Ino80 (10). Consistent with a previous suggestion (26), the actin/Apr4 pair is organized in the same way as the Arp7/Arp9 pair, with Arp4 and actin located at the positions of Arp7 and Arp9, respectively (Fig. 2A). The sequence conservation of actin and the ARPs and the overall structure of the complex suggest that the actin/Arp4 and Arp7/9 modules have evolved from a common ancestor and have similar functions in the cell. Consistent with this notion, both the Arp7/9 and actin/Arp4 modules were shown to modulate chromatin remodeling activities in vivo and in vitro (10, 25, 30).
Fig. 2.
Interactions between the HSA domain and actin/Arp4. (A) Superposition of the actin/Arp4 module with the Arp7/9 module. Arp7, pink; Arp9, blue; HSA domain of Snf2, gray. Arp4 and Arp7 were structurally aligned. (B) Multiple sequence alignments of the HSA domain. The residues involved in binding to Arp4 and actin are shown in bold (ScSwr1). The hydrophobic residues implicated in binding to N-actin and ARPs are highlighted in yellow. Dm, Drosophila melanogaster; Hs: Homo sapiens; Sc, S. cerevisiae. (C) Comparison of the HSASwr1–Arp4 interaction with the HSASnf2–Arp7 interaction. The structures of Arp4 and Arp7 are aligned. Three key hydrophobic residues of HSASwr1 (L355, M362, and F366) are labeled, corresponding to R604, S611, and Q615 of HSASnf2, respectively. (D) Comparison of the interactions around the C termini of the HSA domains. The structures of N-actin and Arp9 are aligned. The C terminus of HSASwr1 binds to the barbed end of N-actin and contacts multiple hydrophobic residues, including F169 (corresponding to Y169 in human β-actin), F375, and L346. Two key residues of HSASwr1 (V380 and I384) are labeled. Similar hydrophobic interactions were also observed at the HSASnf2–Arp9 interface. (E) The coimmunoprecipitation of endogenous Arp4 with Flag-tagged actin in HEK293 cells. (F) Quantification of the normalized amounts of Arp4 coimmunoprecipitated with WT actin and three mutant actins. Error bars show the SEM of at least five independent experiments. *P < 0.01.
The HSA domain of Snf2 binds to Arp7 and Arp9, whereas the HSA domain of Swr1 binds to Arp4 and actin (10). Our structure reveals the specific recognition between different HSA helices and the N-actin/ARP pairs. The HSASnf2 helix is longer than the HSASwr1 helix, with the N-terminal end binding to the next molecule in the crystal lattice (26). In the structure of the N-actin complex, both the N and C termini of the HSASwr1 helix are free of crystal packing interactions (Fig. S3). The N terminus of the HSA domain of Swr1, which involves three bulky residues (Leu355, Met362, and Phe366), binds the hydrophobic cleft and the N-terminal loop of Arp4, covering a large surface area of ∼1,200 Å2 (Fig. 2C). By contrast, the HSA domain of Snf2 lacks two of the important hydrophobic residues (Met362 and Phe366) at the corresponding positions, and forms less-extensive contacts with Arp7, covering a smaller surface area of ∼600 Å2. The C terminus of the HSA domain of Swr1, which involves three hydrophobic residues (Ala377, Val380, and Ile384), interacts with the hydrophobic cleft of N-actin (Fig. 2D). The structural superposition indicated that the longer C terminus of the HSASnf2 helix mediates additional interactions with Arp9 that are not found at the HSASwr1–actin interface (Fig. 2D). In particular, Leu640 of HSASnf2 makes hydrophobic contacts with Phe30 and Phe416 of Arp9. These results suggest that the N-actin/Arp4 and Arp7/Arp9 pairs recognize the unique compositions of the target HSA helices.
Fig. S3.
Crystal packing environments of the N and C termini of the HSASwr1 helix. (A) Crystal packing environment of the N-terminal end of the HSASwr1 helix. Arp4 and the HSA helix within one asymmetry unit are colored as in Fig. 1A, and the nearby molecules in the crystal lattice were colored gray. (B) Crystal packing environment of the C-terminal end of the HSASwr1 domain. Both the N- and C-terminal ends of the HSASwr1 domain are free of crystal packing interactions.
The HSA domains of Ino80 and Eaf1 also recognize the actin/Arp4 pair in yeast. Sequence alignments showed that residues with similar hydrophobic properties are found at most of the equivalent positions of the HSA domains of Eaf1 and Ino80 (Fig. 2B). These results suggest that the actin/Arp4/HSA module in the NuA4 and Ino80 complexes assembles in a way similar to the structure reported here.
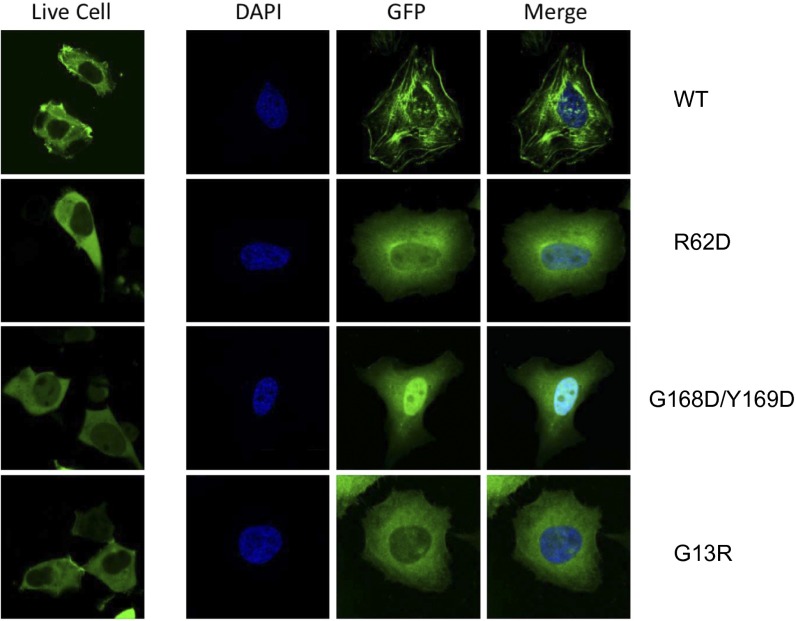
In human cells, N-actin and Arp4 bind to the HSA domains of the Swr1 homolog P400 and the Snf2 homolog Brg1 (10). Our structure suggests that they interact in a similar way. To test this model, we transfected human embryonic kidney (HEK) 293 cells with plasmids encoding Flag-tagged wild-type (WT) and mutant human β-actin, immunoprecipitated the Flag-tagged actin from the nuclear extract, and probed the coimmunoprecipitate for endogenous Arp4. As expected, we coimmunoprecipitated endogenous Arp4 with WT actin, but at significantly lower levels when actin was mutated in the hydrophobic cleft (G168D/F169D) (Fig. 2 E and F) (29). By contrast, mutation R62D at the pointed end of actin, which prevents its polymerization but does not perturb its interaction with the HSA domain, enhanced the coimmunoprecipitation of Arp4. This enhancement is probably attributable to the nuclear enrichment of the mutant, as reported (31) (Fig. S4).
Fig. S4.
Cellular localization of the WT and three mutant β-actins. Live cell images were obtained with EGFP-actin by using HEK293 cells (left column). Fixed cell images were obtained by using HeLa cells with antibody against the EGFP tag of actin, and nuclei were stained with DAPI (right three columns). WT actin formed filaments and puncta in both cell types, and distributed predominantly in the cytoplasm. In sharp contrast to the WT actin, all three mutants lost the ability to polymerize and showed diffused distribution, as indicated by the absence of filamentous structure/punctum. The G168D/Y169D mutant actin showed strong accumulation inside the nucleus in HeLa cells, which may be due to the perturbation of the nucleus-exporting pathway.
Structural Features of N-Actin.
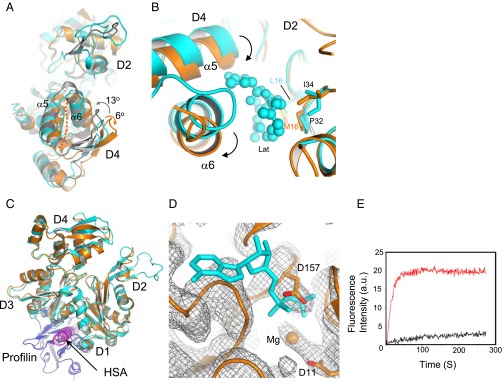
The two major domains of cytoplasmic G-actin (D1/D2 and D3/D4) are twisted, which become flat upon actin polymerization (27, 29). Interactions with Arp4 and the HSA domain twist the two major domains of N-actin by a further ∼6°, with the N terminus of the α6 helix (Thr202–Cys217) and the preceding loop becoming disordered (Fig. 3A). Consequently, the pointed end of N-actin is reshaped. The more-twisted major domains of N-actin inhibit the binding of another actin molecule to the pointed end, which provides an additional mechanism preventing actin from polymerization.
Fig. 3.
Structural features of N-actin complexed with Arp4. (A) Superposition of N-actin (orange), G-actin [cyan; Protein Data Bank (PDB) ID code 1YAG], and an actin protomer in F-actin (gray, PDB ID code 3MFP). The structural alignments were constructed on the major domain D1–D2. Upon polymerization, the two major domains of G-actin show a counterclockwise rotation of ∼13° and become flat in F-actin, whereas it is additionally twisted by a clockwise rotation of ∼6° in the N-actin complex. (B) Conformational changes in the major-domain cleft of N-actin around the latrunculin-binding pocket. N-actin was structurally aligned with G-actin (PDB ID code 1ESV). Latrunculin (Lat), in a ball-and-stick model, is surrounded by hydrophobic contacts from L16, P32, and I34 in D2 subdomain of G-actin. The arrows indicated the movement of α5 and α6. (C) Superposition of the structures of N-actin and G-actin bound to profilin (blue; PDB ID code 2BTF). The HSA helix bound to the hydrophobic cleft of N-actin clashes with profilin. (D) Superposition of the structure of N-actin with the electron density map (contour level σ = 1) of the nucleotide-binding pocket. The ADP bound by G-actin (PDB ID code 1YAG) is indicated with a stick model. No electron density at the position of ADP was found in the crystal structure of the N-actin complex. (E) Time-course of the nucleotide exchange by G-actin (red curve) and the N-actin complex (black curve). Conditions: 3 μM G-actin or N-actin complex was mixed with 50 μM ε-ATP in buffer (2 mM Tris⋅HCl, pH 7.5 and 50 mM KCl).
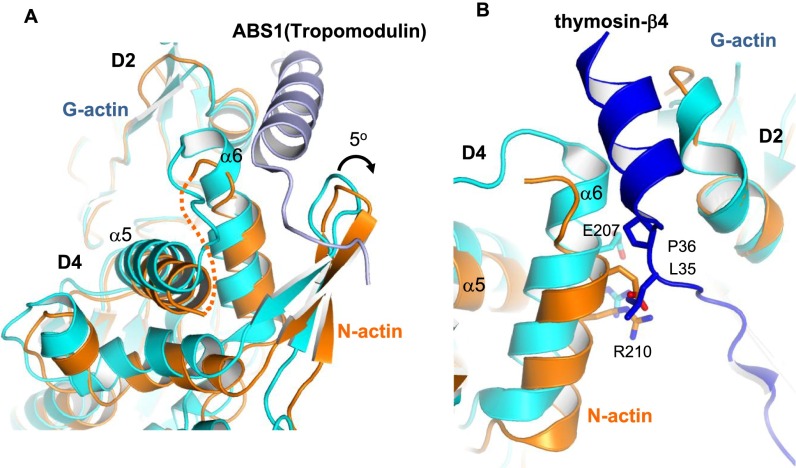
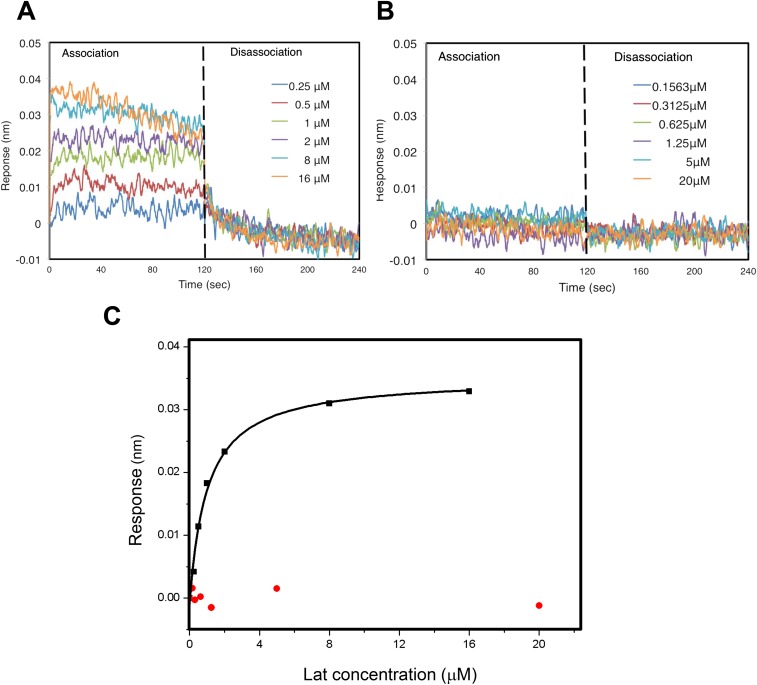
Several pointed end-binding proteins, including tropomodulin and thymosin-β4, bind to actin across the major-domain cleft and interact with α6 (32, 33). The conformational changes in N-actin hinder these interactions (Fig. S5). This cleft also binds several toxins, including latrunculin and phalloidin, which modulate the activity of actin in the cytoplasm. In G-actin, latrunculin is tightly bound to a pocket formed by α5 and α6 of the D4 subdomain and multiple hydrophobic residues in the D2 subdomain (34, 35). In N-actin, the twisting of the pointed end disrupts the latrunculin-binding pocket, with α5 moving into the binding pocket and α6 withdrawing from it (Fig. 3B). This structure suggests that the N-actin complex disfavors the binding of the drug. To confirm this inference, we compared the binding of latrunculin to the N-actin complex and G-actin by using biolayer interferometry (BLI). The data showed that latrunculin binds to G-actin, with a disassociation constant (Kd) ∼1 μM, consistent with an earlier measurement (36) (Fig. S6). By contrast, the N-actin complex did not interact with latrunculin, and no binding was observed even in the presence of 20 μM of the drug. These results are consistent with our model that the reshaped pointed end of N-actin disfavors the binding of latrunculin. Our findings differ from the previous suggestion that latrunculin targets N-actin within the BAF complex (5). This difference might be due to how the complexes were purified. Alternatively, the drug might bind to subunits of the BAF complex other than N-actin.
Fig. S5.
The twisted pointed end of N-actin disfavors the binding of tropomodulin and thymosin-β4. (A) Superposition of N-actin (orange) with G-actin (cyan) in complex with tropomodulin (light blue, PDB ID code 4PKG) (32). The actin-binding site 1 (ABS1) of tropomodulin binds across the major-domain cleft of G-actin, connecting subdomain D2 and the α6 helix of subdomain D4. In N-actin, the major-domain cleft is twisted ∼5° relative to the tropomodulin-bound G-actin, and the α6 helix is partially melted. These structural changes would disfavor the binding of tropomodulin at the pointed end of N-actin. (B) Superposition of N-actin with G-actin in complex with thymosin-β4 (blue, PDB ID code 1T44) (33). The C-terminal helix of thymosin-β4 binds across the major-domain cleft between subdomain D2 and D4 in G-actin. Because of the twisting of the pointed end of N-actin, Leu35 and Pro36 of thymosin-β4 clash with multiple sites of the α6 helix, which would inhibit the binding of thymosin-β4 to N-actin.
Fig. S6.
Binding of latrunculin to G-actin and the N-actin complex analyzed by BLI. (A) Binding of latrunculin to G-actin monitored by the super streptavidin biosensor using BLI. G-actin at a ∼2 μM concentration was labeled with biotin and bound to streptavidin-coated sensors. After washing away loosely bound materials, association was initiated by dipping the G-actin conjugated sensors in the buffer containing latrunculin at different concentrations and was monitored at real time for 120 s. Dissociation was initiated by dipping the same sensors into buffers without latrunculin and was monitored similarly. Individual graphs with indicated concentration of latrunculin were grouped together and showed. (B) Interaction of latrunculin with the N-actin complex monitored as in A. No association or dissociation of latrunculin was detected in the presence of the N-actin complex. (C) BLI analysis of the binding of latrunculin to G-actin (black) or the N-actin complex (red). Fitting the binding curve of latrunculin to G-actin yielded the Kd ∼ 1 μM, whereas no binding to the N-actin complex was observed.
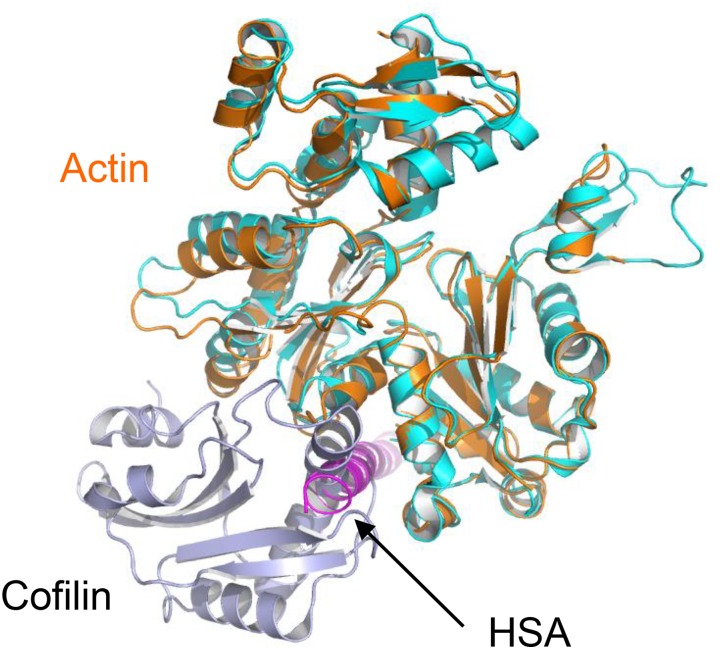
The barbed end of actin is the key element mediating its interactions with many ABPs, including profilin and cofilin, which are considered to regulate the dynamics of actin in the nucleus (5, 23). The superposition of actin in the complex with profilin or cofilin leads to severe clashes between the HSA domain and the bound ABPs (Fig. 3C and Fig. S7) (37, 38). The crystal structure of N-actin suggests that, within the chromatin-remodeling complex, it cannot interact with barbed end-binding proteins. To test this notion directly, we performed pull-down assays in vitro. Whereas GST-tagged profilin bound strongly to G-actin, it failed to interact with the N-actin complex (Fig. S8). These results are consistent with a recent report showing that N-actin within the Ino80 complex does not bind profilin (25), but differ from an earlier observation that the BAF complex binds to profilin (5). The reason for this difference is unknown. Our findings suggest the association between the BAF complex and profilin might be achieved through subunits other than N-actin or Arp4.
Fig. S7.
Structural superposition of N-actin and cytoplasmic G-actin bound to cofilin (blue, PDB ID code 3DAW; ref. 38). Interactions with the HSA domain are incompatible with the binding of cofilin to N-actin.
Fig. S8.
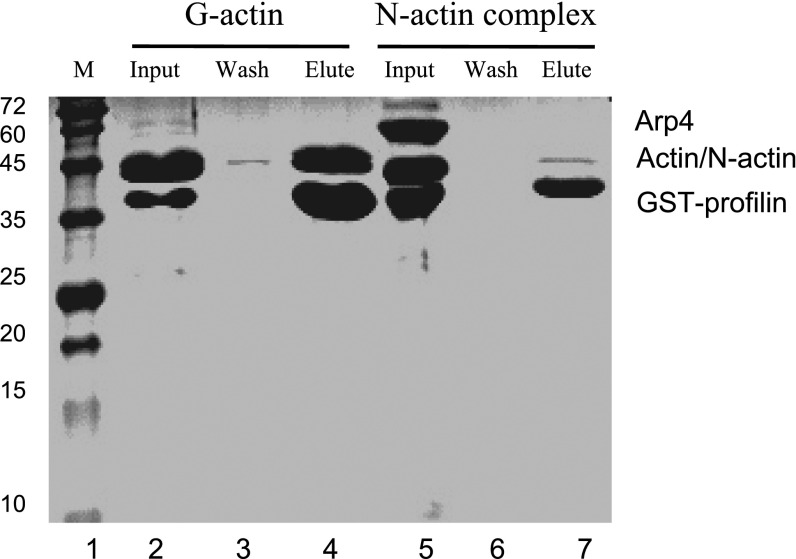
Interactions between profilin and the G-actin/N-actin complex. Five micromolar GST-tagged profilin was immobilized GST-resin and mixed with 10 μM G-actin or the N-actin complex. After washing, the bound materials were eluted and analyzed with SDS/PAGE. The HSA domain polypeptide (<10 kDa) ran out of the gel. G-actin was notably bound to profilin (lane 4), whereas little N-actin was retained by profilin (lane 7). The faint actin band in lane 7 suggested profilin competed with Arp4/HSA in binding to N-actin and led to partial disassembly of the N-actin complex, as indicated by the absence of Arp4 in the elution fraction.
Thus, once bound to Arp4 and HSASwr1, N-actin has several structural features, which distinguish it from cytoplasmic actin and inhibit its interaction with many ABPs. The N-actin molecule within other chromatin remodeling complexes should have similar features given the strong homology of the complex components.
N-Actin Within Remodeling Complexes Is in the Nucleotide-Free apo State.
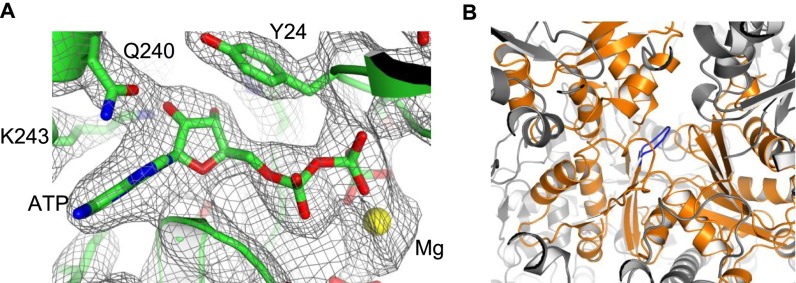
Critically, the nucleotide-binding pocket of N-actin is blocked. The movement of the loop (Ser155–Thr160) in subdomain D3 causes the side chain of Asp157 to insert into the nucleotide-binding pocket, thus preventing the binding of any nucleotide at this site (Fig. 3D). Therefore, N-actin complexed with Arp4 and the HSA domain is in a nucleotide-free apo state. However, Arp4 tightly binds ATP within the same complex (Fig. S9A). These results suggest that the lack of a nucleotide within N-actin is not due to nucleotide loss during sample preparation or a crystallographic artifact (Fig. S9B). Instead, it is an intrinsic property of N-actin once it is assembled into the chromatin-remodeling complex.
Fig. S9.
Structural analysis of the ATP-binding pocket of Arp4 and N-actin. (A) Superposition of the structure of the N-actin ternary complex with the electron density map at the nucleotide-binding pocket of Arp4. ATP and its contacting residues are shown as sticks, and Mg as a yellow sphere. The contour level of the map is set with σ = 1. (B) Crystal-packing environment of the ATP-binding pocket of N-actin. The Ser155-Thr160 loop, which is incompatible with the binding of nucleotide to N-actin, is colored blue, and the surrounding molecules in the crystals were colored gray. The Ser155-Thr160 loop is buried inside the major domain cleft and free of crystal packing contact.
The loss of the nucleotide from N-actin is surprising because it has long been known that actin in the nucleotide-free, apo state denatures rapidly (39, 40). To confirm the structural model, we performed ATP-exchange assays and examined the binding of ATP to N-actin. Consistent with previous results, the exchange of fluorescent ε-ATP with the ATP bound within G-actin increased the fluorescence intensity (41) (Fig. 3E). By contrast, no increase in fluorescence intensity was detected in the presence of the N-actin complex. These results support the idea that N-actin within the ternary complex does not bind ATP. These data also suggest that ATP bound by Arp4 is not readily released, consistent with an earlier study that showed ATP is intimately associated with Arp4 (14). To test the requirement of ATP binding for the actin–Arp4 interaction in cells, we introduced a G13R mutant human β-actin into HEK293 cells. The G13R mutation disrupts the ATP-binding pocket and reduces the stability of the protein (31). As expected, the mutant did not polymerize in cells (Fig. S4). However, it maintained its ability to interact with Arp4 (Fig. 2 E and F), consistent with the idea that nucleotide binding is not required by N-actin to associate with Arp4.
The absence of nucleotide in N-actin is intriguing. No nucleotide was found in the ARP subunits in the crystal structure of the Arp7–Arp9–HSASnf2–Rtt102 complex (26). A recent biochemical analysis showed that one ATP is bound to a single site within the Arp7–Arp9–HSASth1–Rtt102 complex, presumably by the Arp7 subunit. This result is consistent with our observation that ATP is tightly bound by Arp4, whereas the nucleotide-binding pocket of N-actin remains empty. The similar nucleotide-binding mode of the actin/Arp4 and Arp7/9 pairs (Arp4 and Arp7 are in the ATP-bound state, whereas N-actin and Arp9 are in the apo state) further strengthens their conservation. It has been suggested that N-actin is regulated by the bound nucleotide (22, 25). However, the structure suggests that N-actin within the chromatin-remodeling complex does not bind any nucleotide, freeing it from regulation by ATP hydrolysis. Because Arp4 does not show detectable ATPase activity (42), these results suggest that ATP hydrolysis by the actin/ARP module may play a less important role in controlling the activity of the chromatin-remodeling complexes than what was previously proposed (22, 25). Instead, we speculate that ATP depletion and the nuclear accumulation of actin under stress conditions might act as signals to up-regulate the activity of the chromatin-remodeling complexes (43).
Implications for the Functions of N-Actin.
Our structure shows that N-actin binds to the C-terminal end of the HSA domain, which is connected to the ATPase core domain of the remodeler. This structure suggests that N-actin may interact with the proximal catalytic core. This notion is consistent with a low-resolution map of the Swr1 holocomplex, in which the actin/Arp4 pair is close to the catalytic module (44). Alternatively, its close proximity to the catalytic core may allow N-actin to bind to the chromatin substrate, as suggested (25). Because the resolution of the map is limited, the precise interactions are unknown. More work is required to determine the interactions and specific functions of N-actin.
In summary, N-actin within the chromatin-remodeling complex has several important structural features, including a more twisted pointed end and a blocked barbed end. Additionally, it is not regulated by ATP hydrolysis. These unique features of N-actin distinguish it from its cytoplasmic counterpart and lay the groundwork for future studies of its functions inside the nucleus.
Materials and Methods
Protein Expression, Purification, Crystallization, and Structure Determination.
For a detailed description of protein purification and related structure determination, see SI Materials and Methods. Briefly, S. cerevisiae Swr1 protein (amino acids 340−410) was expressed in E. coli. The full-length sequence of yeast actin and Arp4 were expressed in insect cells. Crystals were grown at 18 °C with the hanging-drop vapor diffusion method. Diffraction data were collected at beamline BL17U1 at the Shanghai Synchrotron Radiation Facility. The structure was solved by molecular replacement, and the final model was refined to 2.8 Å, with Rwork/Rfeee = 0.25/0.29.
Cellular Localization and Coimmunoprecipitation.
HeLa cells and HEK293 cells were transferred with various actin constructs as described in SI Materials and Methods. The EGFP signal (HEK293 cells) was visualized directly 48 h after transfection. The HeLa cells were fixed with 4% (wt/vol) paraformaldehyde 48 h after transfection. A primary mouse anti-GFP monoclonal antibody and a goat anti-mouse IgG polyclonal secondary antibody conjugated to Alexa Fluor 488 were used for immunofluorescence detection.
HEK293 cells transfected with various actin constructs were harvested 48 h after transfection and lysed in hypotonic buffer. The nuclear extract was resuspended and then incubated with anti-Flag beads (M2; Sigma). After washing, samples were eluted with SDS loading buffer and analyzed with Western blotting by using antibodies specific for the Flag tag (Cell Signaling) and Arp4 (ACLT6A; Abcam). At least five independent experiments were performed, and representative blots are shown.
Nucleotide Exchange Assays, GST Pull-Down Assays, and BLI.
For a detailed description of the assays, see SI Materials and Methods. The fluorescent signal from 1,N6-ethenoadenosine 5′-triphosphate (ε-ATP; Jena Bioscience) bound to actin was used to measure the rate of exchange of the actin nucleotide (45). Briefly, ε-ATP (50 μM) was added to the solution of 3 μM G-actin or the N-actin complex. The reaction was monitored with a QuantaMaster Luminescence QM 3 PH Fluorometer.
GST-tagged profilin (5 µM) was mixed with 10 µM G-actin or the N-actin complex and then incubated with GST resin. The resin was washed three times and then eluted. The samples were analyzed with SDS/PAGE and stained with Coomassie blue.
An Octet RED BLI instrument (FortéBio) was used to examine the binding of latrunculin to G-actin or the N-actin complex. We exposed the biosensors to different concentrations of latrunculin in the association step and then washed the biosensors with buffer in the dissociation step. The BLI data were fitted with the Octet Data analysis software package and graphed with the Origin 8.0 software.
SI Materials and Methods
Protein Expression and Purification.
The sequence encoding the S. cerevisiae Swr1 protein (amino acids 340−410) with a C-terminal His tag was subcloned into the pMal-p2 expression vector. The full-length sequence of yeast actin with an N-terminal GGGS linker was subcloned into the pFastBac–HT1a vector, and the full-length Arp4 sequence was subcloned into a homemade pAV5a vector (46).
His-tagged actin and Arp4 were separately overexpressed in sf21 cells and harvested 72 h after transfection. The cells were lysed in G buffer (5 mM Tris⋅HCl, 0.5 mM CaCl2, 0.5 mM ATP, pH 7.5) and in 20 mM Tris⋅HCl, 200 mM NaCl (pH 8.0), respectively. Maltose-binding protein-tagged Swr1 (340−410)–6× His was overexpressed in E. coli BL21(DE3)RIL cells and induced with 0.5 mM isopropyl-β-d-thiogalactoside (IPTG) at 18 °C overnight. The cells were lysed in the buffer used for Arp4 (20 mM Tris⋅HCl, 200 mM NaCl, pH 8.0). Actin, Swr1, and Arp4 were purified with Ni+ affinity chromatography and assembled into the trimeric complex after they were mixed in a molar ratio of 1:4:1 and incubated overnight on ice in the presence of 1% Nonidet P-40, 200 mM NaCl, 20 mM Tris⋅HCl, 1 mM MgCl2, 1 mM ADP, 1 mM DTT, and 250 mM imidazole (pH 8.0). Unbound Arp4 and actin were removed with an amylose affinity column, and the fusion tags were removed by treatment with tobacco etch virus (TEV) protease on the column overnight. The trimeric complex was further purified with Q chromatography to remove various subcomplexes, and finally brought to homogeneity with size-exclusion chromatography in 200 mM NaCl, 10 mM Tris⋅HCl, 1 mM MgCl2, 1 mM ADP, and 5 mM DTT (pH 7.5), concentrated to approximately 20 mg⋅ml−1, and stored at −80 °C. G-actin was expressed and purified similarly. After Ni+ affinity chromatography, the fusion tag of G-actin was removed by treatment with TEV protease, subjected to Q chromatography, and finally dialyzed and stored in G buffer.
The coding of sequence of profilin was amplified from the genomic DNA of S. cerevisiae, and ligated into the expression vector pGEX-6P1 by using the restriction enzymes BamHI and XhoI. The protein was overexpressed in E. coli BL21(DE3) cells and induced with 0.5 mM IPTG at 18 °C overnight. The cells were lysed in buffer (20 mM Tris⋅HCl, 200 mM NaCl, pH 8.0) by using a high-pressure nano homogenizer (ATS) at 4 °C. After centrifugation at 18,000 rpm (Beckman; Rotor JA20) for 1 h, the supernatant was separated and loaded onto a gravity column with GST beads. After the column was washed, the protein was eluted with 25 mM glutathione (pH 8.0) in lysis buffer and further purified with gel filtration chromatography (Superdex 200; GE Healthcare) with protein buffer (10 mM Hepes, 0.2 mM CaCl2, 0.2 mM ATP, 1 mM DTT, pH 7.9).
Crystallization, Data Processing, and Structural Resolution.
Crystals were grown at 18 °C with the hanging-drop vapor diffusion method from a solution of 12–16% (wt/vol) polyethylene glycol (PEG) 3350, 0.052 M citric acid, and 0.048 M Bis-Tris propane (pH 4.8). The crystals were cryoprotected with 16% (wt/vol) PEG 3350, 100 mM NaCl, 0.5 mM MgCl2, 52 mM citric acid, 48 mM Bis-Tris propane, and 20% (wt/vol) glycerol (pH 4.8). The diffraction data were collected at beamline BL17U1 at the Shanghai Synchrotron Radiation Facility.
The data were processed with XDS (47). The structure was solved by molecular replacement using yeast actin (PDB ID code 1YAG) and Arp4 (PDB ID code 3QB0) as the search models. The model was completed manually in Coot and refined with Phenix. The final model was refined to 2.8 Å, with Rwork/Rfeee = 0.25/0.29.
Cellular Localization of EGFP–Actin.
The coding sequences of human WT and mutant actins (R62D, G168D/Y169D, and G13R) were subcloned into the vector pEGFP-C1, and used to transfect HEK293 and HeLa cells with Lipofectamine 3000 (Invitrogen). Sixty thousand HEK293 cells were plated in each chamber of a borosilicate coverglass (Lab-Tek) and incubated at 37 °C under 5% CO2. The EGFP signal was visualized directly 48 h after transfection by using an inverted confocal microscope (Zeiss LSM 780) with a 63×/1.4 N.A. oil immersion lens.
The HeLa cells (6–8 × 104) were fixed with 4% paraformaldehyde 48 h after transfection. A primary mouse anti-GFP monoclonal antibody (Roche; 1:200) was used to target EGFP. Goat anti-mouse IgG polyclonal secondary antibody conjugated to Alexa Fluor 488 (diluted 1:500; Roche) was used for immunofluorescence detection of EGFP. DNA was stained with 1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes). The prepared samples were observed with an inverted confocal microscope (Zeiss LSM 780) with a 40×/1.4 N.A. oil immersion lens. Images were illuminated with an Argon/2 laser (488 nm) and recorded by gallium arsenide phosphide high-quantum-efficiency detectors using Zen 2011 software (Zeiss).
Coimmunoprecipitation.
HEK293 cells were transfected with 4 µg of DNA by using Lipofectamine 2000 (Invitrogen), as described by the manufacturer. The cells were harvested 48 h after transfection and lysed in hypotonic buffer A [10 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5% Nonidet P-40, 1× protease inhibitor mixture (Biotool.com), 20 mM Hepes, pH 7.9]. After centrifugation at 17,000 × g, the nuclear extract was resuspended in 0.3 mL of buffer B (400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1× protease inhibitor mixture, 10 mM Hepes, pH 7.9), and incubated on ice for 30 min with intermittent vortexing. The supernatant was then incubated with anti-Flag beads (M2, Sigma) on ice. After the beads were washed with buffer B, they were eluted with SDS loading buffer and analyzed with Western blotting by using antibodies specific for the Flag tag (Cell Signaling) and Arp4 (ACLT6A; Abcam). The blots were exposed for 5 min and recorded with an ImageQuant LAS 4000 biomolecular imager (GE Healthcare). The band intensities were quantified with the Quantity One software. The band intensities of Arp4 were calculated relative to those of actin for each lane and normalized to the value for WT actin. At least five independent experiments were performed, and representative blots are shown.
Nucleotide Exchange Assay.
The fluorescent signal from 1,N6-ethenoadenosine 5′-triphosphate (ε-ATP; Jena Bioscience) bound to actin was used to measure the rate of exchange of the actin nucleotide (45). Briefly, 3 μM G-actin or the N-actin complex was dialyzed in G buffer lacking ATP and incubated with Tris⋅KCl buffer (2 mM Tris⋅HCl, pH 7.5, 50 mM KCl). ε-ATP (50 μM) was then added to the solution. The reaction was monitored with a QuantaMaster Luminescence QM 3 PH Fluorometer for at least 300 s with an excitation wavelength of 350 nm and an emission wavelength of 410 nm.
GST Pull-Down Assay.
GST-tagged profilin (5 µM) was mixed with 10 µM G-actin or the N-actin complex in 200 µL of binding buffer (10 mM Hepes, 50 mM NaCl, 0.2 mM CaCl2, 0.2 mM ATP, 1 mM DTT, pH 7.9) and then incubated with 20 µL of GST resin on a rotating platform at 4 °C for 30 min. The resin was washed three times with 500 µL of binding buffer and eluted with 50 µL of binding buffer containing 25 mM glutathione (pH 8.0). The samples were analyzed with SDS/PAGE and stained with Coomassie blue.
BLI.
An Octet RED instrument (FortéBio) was used to examine the binding of latrunculin to G-actin or the N-actin complex. Biotinylated actin or the N-actin complex at a concentration of ∼2 μM in buffer (10 mM Hepes, 0.2 mM CaCl2, 0.2 mM ATP, 1 mM DTT, pH 7.9) was incubated for 300 s with Super Streptavidin Biosensors (FortéBio) to attach to them. The biosensors were then washed with L-buffer (1% DMSO, 10 mM Hepes, 0.2 mM CaCl2, 0.2 mM ATP, 1 mM DTT, pH 7.9) for 120 s to remove excess protein. Biocytin was used to quench the loaded and empty biosensors. To determine the binding of latrunculin to G-actin or the N-actin complex, we exposed the biosensors to different concentrations of latrunculin (0.2–10 μM) in L buffer for 120 s in the association step, and then washed the biosensors with L buffer for another 120 s in the dissociation step. The BLI data were fitted with the Octet Data analysis software package and graphed with the Origin 8.0 software.
Acknowledgments
We thank Shilong Fan at the center of structure biology (Tsinghua University) and the staff at beamline BL17U of Shanghai Synchrotron Radiation Facility for help with diffraction data collection and the Tsinghua University Branch of China National Center for Protein Sciences Beijing for providing facility support. This work was supported by Chinese Key Research Plan-Protein Sciences Grant 2014CB910100, National Natural Science Foundation of China Grant 31270762, and the “Junior One Thousand Talents” program (to Z.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 5I9E).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602818113/-/DCSupplemental.
References
- 1.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326(5957):1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkmann N, et al. Structure of Arp2/3 complex in its activated state and in actin filament branch junctions. Science. 2001;293(5539):2456–2459. doi: 10.1126/science.1063025. [DOI] [PubMed] [Google Scholar]
- 3.Olave IA, Reck-Peterson SL, Crabtree GR. Nuclear actin and actin-related proteins in chromatin remodeling. Annu Rev Biochem. 2002;71:755–781. doi: 10.1146/annurev.biochem.71.110601.135507. [DOI] [PubMed] [Google Scholar]
- 4.Pederson T, Aebi U. Actin in the nucleus: What form and what for? J Struct Biol. 2002;140(1-3):3–9. doi: 10.1016/s1047-8477(02)00528-2. [DOI] [PubMed] [Google Scholar]
- 5.Zhao K, et al. Rapid and phosphoinositol-dependent binding of the SWI/SNF-like BAF complex to chromatin after T lymphocyte receptor signaling. Cell. 1998;95(5):625–636. doi: 10.1016/s0092-8674(00)81633-5. [DOI] [PubMed] [Google Scholar]
- 6.Galarneau L, et al. Multiple links between the NuA4 histone acetyltransferase complex and epigenetic control of transcription. Mol Cell. 2000;5(6):927–937. doi: 10.1016/s1097-2765(00)80258-0. [DOI] [PubMed] [Google Scholar]
- 7.Shen X, Mizuguchi G, Hamiche A, Wu C. A chromatin remodelling complex involved in transcription and DNA processing. Nature. 2000;406(6795):541–544. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- 8.Ikura T, et al. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell. 2000;102(4):463–473. doi: 10.1016/s0092-8674(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 9.Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009;78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- 10.Szerlong H, et al. The HSA domain binds nuclear actin-related proteins to regulate chromatin-remodeling ATPases. Nat Struct Mol Biol. 2008;15(5):469–476. doi: 10.1038/nsmb.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosho T, Miyake N, Carey JC. Coffin-Siris syndrome and related disorders involving components of the BAF (mSWI/SNF) complex: Historical review and recent advances using next generation sequencing. Am J Med Genet C Semin Med Genet. 2014;166C(3):241–251. doi: 10.1002/ajmg.c.31415. [DOI] [PubMed] [Google Scholar]
- 12.Kadoch C, Crabtree GR. Mammalian SWI/SNF chromatin remodeling complexes and cancer: Mechanistic insights gained from human genomics. Sci Adv. 2015;1(5):e1500447. doi: 10.1126/sciadv.1500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11(7):481–492. doi: 10.1038/nrc3068. [DOI] [PubMed] [Google Scholar]
- 14.Fenn S, et al. Structural biochemistry of nuclear actin-related proteins 4 and 8 reveals their interaction with actin. EMBO J. 2011;30(11):2153–2166. doi: 10.1038/emboj.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bird AW, et al. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature. 2002;419(6905):411–415. doi: 10.1038/nature01035. [DOI] [PubMed] [Google Scholar]
- 16.Downs JA, et al. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol Cell. 2004;16(6):979–990. doi: 10.1016/j.molcel.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Yoo AS, Staahl BT, Chen L, Crabtree GR. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460(7255):642–646. doi: 10.1038/nature08139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lessard J, et al. An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron. 2007;55(2):201–215. doi: 10.1016/j.neuron.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu JI, et al. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron. 2007;56(1):94–108. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Krasteva V, et al. The BAF53a subunit of SWI/SNF-like BAF complexes is essential for hemopoietic stem cell function. Blood. 2012;120(24):4720–4732. doi: 10.1182/blood-2012-04-427047. [DOI] [PubMed] [Google Scholar]
- 21.Kapoor P, Shen X. Mechanisms of nuclear actin in chromatin-remodeling complexes. Trends Cell Biol. 2014;24(4):238–246. doi: 10.1016/j.tcb.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Percipalle P, Visa N. Molecular functions of nuclear actin in transcription. J Cell Biol. 2006;172(7):967–971. doi: 10.1083/jcb.200512083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blessing CA, Ugrinova GT, Goodson HV. Actin and ARPs: Action in the nucleus. Trends Cell Biol. 2004;14(8):435–442. doi: 10.1016/j.tcb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Rando OJ, Zhao K, Janmey P, Crabtree GR. Phosphatidylinositol-dependent actin filament binding by the SWI/SNF-like BAF chromatin remodeling complex. Proc Natl Acad Sci USA. 2002;99(5):2824–2829. doi: 10.1073/pnas.032662899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapoor P, Chen M, Winkler DD, Luger K, Shen X. Evidence for monomeric actin function in INO80 chromatin remodeling. Nat Struct Mol Biol. 2013;20(4):426–432. doi: 10.1038/nsmb.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schubert HL, et al. Structure of an actin-related subcomplex of the SWI/SNF chromatin remodeler. Proc Natl Acad Sci USA. 2013;110(9):3345–3350. doi: 10.1073/pnas.1215379110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oda T, Iwasa M, Aihara T, Maéda Y, Narita A. The nature of the globular- to fibrous-actin transition. Nature. 2009;457(7228):441–445. doi: 10.1038/nature07685. [DOI] [PubMed] [Google Scholar]
- 28.Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347(6288):44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- 29.Fujii T, Iwane AH, Yanagida T, Namba K. Direct visualization of secondary structures of F-actin by electron cryomicroscopy. Nature. 2010;467(7316):724–728. doi: 10.1038/nature09372. [DOI] [PubMed] [Google Scholar]
- 30.Szerlong H, Saha A, Cairns BR. The nuclear actin-related proteins Arp7 and Arp9: A dimeric module that cooperates with architectural proteins for chromatin remodeling. EMBO J. 2003;22(12):3175–3187. doi: 10.1093/emboj/cdg296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Posern G, Sotiropoulos A, Treisman R. Mutant actins demonstrate a role for unpolymerized actin in control of transcription by serum response factor. Mol Biol Cell. 2002;13(12):4167–4178. doi: 10.1091/mbc.02-05-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao JN, Madasu Y, Dominguez R. Mechanism of actin filament pointed-end capping by tropomodulin. Science. 2014;345(6195):463–467. doi: 10.1126/science.1256159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irobi E, et al. Structural basis of actin sequestration by thymosin-beta4: Implications for WH2 proteins. EMBO J. 2004;23(18):3599–3608. doi: 10.1038/sj.emboj.7600372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat Cell Biol. 2000;2(6):376–378. doi: 10.1038/35014075. [DOI] [PubMed] [Google Scholar]
- 35.Belmont LD, Patterson GM, Drubin DG. New actin mutants allow further characterization of the nucleotide binding cleft and drug binding sites. J Cell Sci. 1999;112(Pt 9):1325–1336. doi: 10.1242/jcs.112.9.1325. [DOI] [PubMed] [Google Scholar]
- 36.Yarmola EG, Somasundaram T, Boring TA, Spector I, Bubb MR. Actin-latrunculin A structure and function. Differential modulation of actin-binding protein function by latrunculin A. J Biol Chem. 2000;275(36):28120–28127. doi: 10.1074/jbc.M004253200. [DOI] [PubMed] [Google Scholar]
- 37.Schutt CE, Myslik JC, Rozycki MD, Goonesekere NC, Lindberg U. The structure of crystalline profilin-beta-actin. Nature. 1993;365(6449):810–816. doi: 10.1038/365810a0. [DOI] [PubMed] [Google Scholar]
- 38.Paavilainen VO, Oksanen E, Goldman A, Lappalainen P. Structure of the actin-depolymerizing factor homology domain in complex with actin. J Cell Biol. 2008;182(1):51–59. doi: 10.1083/jcb.200803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asakura S. The interaction between G-actin and ATP. Arch Biochem Biophys. 1961;92:140–149. doi: 10.1016/0003-9861(61)90228-4. [DOI] [PubMed] [Google Scholar]
- 40.Kasai M, Nakano E, Oosawa F. Polymerization of actin free from nucleotides and divalent cations. Biochim Biophys Acta. 1965;94:494–503. doi: 10.1016/0926-6585(65)90058-0. [DOI] [PubMed] [Google Scholar]
- 41.Pollard TD, Goldberg I, Schwarz WH. Nucleotide exchange, structure, and mechanical properties of filaments assembled from ATP-actin and ADP-actin. J Biol Chem. 1992;267(28):20339–20345. [PubMed] [Google Scholar]
- 42.Gerhold CB, et al. Structure of Actin-related protein 8 and its contribution to nucleosome binding. Nucleic Acids Res. 2012;40(21):11036–11046. doi: 10.1093/nar/gks842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pendleton A, Pope B, Weeds A, Koffer A. Latrunculin B or ATP depletion induces cofilin-dependent translocation of actin into nuclei of mast cells. J Biol Chem. 2003;278(16):14394–14400. doi: 10.1074/jbc.M206393200. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen VQ, et al. Molecular architecture of the ATP-dependent chromatin-remodeling complex SWR1. Cell. 2013;154(6):1220–1231. doi: 10.1016/j.cell.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldschmidt-Clermont PJ, et al. The control of actin nucleotide exchange by thymosin beta 4 and profilin. A potential regulatory mechanism for actin polymerization in cells. Mol Biol Cell. 1992;3(9):1015–1024. doi: 10.1091/mbc.3.9.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Z, et al. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468(7323):533–538. doi: 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kabsch W. Xds. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]