Significance
Throughout the life of female mammals, only a small number of viable oocytes are produced. The mechanisms underlying the creation and selection of competent oocytes remain unclear. Here, we propose a novel approach for elucidating these unsolved questions, involving the use of an in vitro system established in the present study, which can fully reproduce mammalian oogenesis from mouse fetal primordial germ cells. Reconstitution of the entire oogenesis process has not been previously accomplished. Our system will assist in understanding the mechanisms of oogenesis and also create a new gamete resource in mammals.
Keywords: oogenesis, primordial germ cells, follicle formation, oocytes, in vitro
Abstract
Reconstituting gametogenesis in vitro is a key goal for reproductive biology and regenerative medicine. Successful in vitro reconstitution of primordial germ cells and spermatogenesis has recently had a significant effect in the field. However, recapitulation of oogenesis in vitro remains unachieved. Here we demonstrate the first reconstitution, to our knowledge, of the entire process of mammalian oogenesis in vitro from primordial germ cells, using an estrogen-receptor antagonist that promotes normal follicle formation, which in turn is crucial for supporting oocyte growth. The fundamental events in oogenesis (i.e., meiosis, oocyte growth, and genomic imprinting) were reproduced in the culture system. The most rigorous evidence of the recapitulation of oogenesis was the birth of fertile offspring, with a maximum of seven pups obtained from a cultured gonad. Moreover, cryopreserved gonads yielded functional oocytes and offspring in this culture system. Thus, our in vitro system will enable both innovative approaches for a deeper understanding of oogenesis and a new avenue to create and preserve female germ cells.
Oocytes contain fundamental materials for perpetuating a species; that is, the maternal genome and ooplasm filled with maternal factors that are essential for totipotency, including mitochondria, which are transmitted to the next generation. Therefore, the process of oogenesis is of wide interest in reproductive biology and regenerative medicine. However, mechanisms underlying the process are not fully elucidated. An approach to comprehensively resolve the mechanisms of oogenesis is to reconstitute the entire process of oogenesis in vitro (1, 2).
In mice, primordial germ cells (PGCs) mitotically divide until 13.5 d postcoitum (dpc) in the fetal gonads, after which they immediately enter meiosis. Passage through prophase of the first meiotic division and preparation for primordial follicle formation, including the breakdown of oocyte cysts, occurs during the remainder of the prenatal period. Shortly after birth, a cohort of primordial follicles enters the growth phase, and after 3 wk, oocyte growth culminates in the acquisition of competencies to resume meiosis, complete maternal imprinting, undergo fertilization, and support full-term development. Applications of in vitro systems to study the events involved in oogenesis have been widely used, although none has been successful in reconstituting the entire process. To date, most successful reconstitutions of oogenesis (but not from the onset) with proven fertility have used neonatal oocytes, which were already in the prophase of the first meiosis and were ready to be assembled into primordial follicles (3–6).
Thus far, the ectopic oogenesis from PGCs to fertile mature oocytes has been achieved only by means of grafting PGCs into other mice to facilitate key steps (7, 8). Even PGC-like cells, originally produced from mouse embryonic stem and induced pluripotent stem cells, can develop into functional oocytes after reaggregation with gonadal somatic cells and grafting beneath the bursa (9, 10). However, many previous studies attempting in vitro oogenesis without the help of grafting have documented the occurrence of aberrant follicular formation and insufficient growth and ability of the oocytes after in vitro growth (IVG) (1, 2, 11). Thus, the crucial step remaining to create a connected “chain” of in vitro oogenesis is to eliminate the gap between what can be achieved using grafting and what has become possible by culture (i.e., IVG of oocytes from the primordial follicle stage) (3–5).
Three events need to be achieved to successfully reconstitute oogenesis in vitro: the initial phase of meiosis, follicular assembly, and appropriate conditions to support sufficient oocyte growth and complete maturation. Elucidating the mechanisms of oogenesis is important, and if the above three requirements are fulfilled, then in vitro oogenesis can enable mass generation of oocytes.
In this study, we reconstituted the entire process of oogenesis across meiosis and complete maturation in vitro. Meiosis, follicular formation, oocyte growth, and maternal imprinting, all of which are required for the development of a functional oocyte, were found to be accomplished without apparent abnormality.
Results
Meiotic Entry and Follicular Assembly in Vitro.
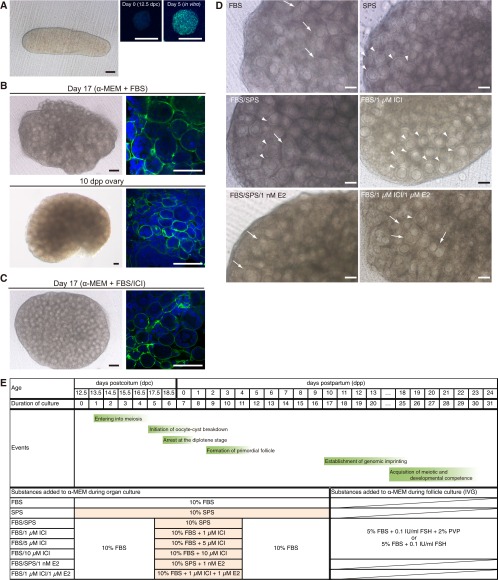
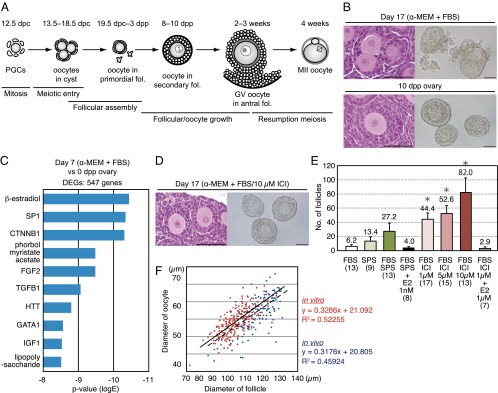
In our initial experiments, female gonads obtained at 12.5 dpc were cultured on Transwell-COL membranes, using the α-MEM supplemented with 10% (vol/vol) FBS, as previously described (3, 11). On the basis of the meiotic marker synaptonemal complex protein 3 (SYCP3) (12), entry into meiosis occurred during the first 5 d of culture, irrespective of the absence of the mesonephros (Fig. S1A), which contrasts with previous data that suggested the involvement of mesonephroi in the entry into meiosis (13). On day 17 of culture, corresponding to an age of 10 d in vivo (Fig. 1A), the ovary contained many growing oocytes; however, most were contained in hypoplastic follicles with abnormal and unclear external layers (Fig. S1 B and D). The oocytes in these follicles were easily denuded during follicle isolation attempts and were no longer capable of growth in culture, thereby indicating severe defects in follicular assembly. Despite the presence of >100 growing oocytes per ovary, only four to six secondary follicles were successfully isolated (Fig. 1 B and E), which is consistent with previous studies (1, 11). An analysis of laminin-immunostained images (14) of the follicle basement membrane indicated that the hypoplastic follicles had incomplete laminin envelopes, unlike normal in vivo follicles, which develop a complete envelope (Fig. S1B). Some neighboring in vitro follicles shared the same theca or granulosa cell layer, and multioocyte follicles were occasionally detected (Fig. 1B, Fig. S1B, and Movie S1).
Fig. S1.
Refinement of culture condition for follicular assembly. (A) Gonad derived from a mouse embryo at 12.5 dpc (Left). (Black scale bar, 100 μm; white scale bars, 20 μm.) Immunostaining for SCP3 (green) in female germ cells derived from an ovary on day 0 (Center) and day 5 of culture without the mesonephros (Right). Nuclei were stained with 4′,6-diamidino-2-pheylindole (DAPI; blue). (B) Ovary cultured for 17 d (α-MEM + FBS) and the ovary at 10 dpp (control). Shown are bright-field images (Left) and laminin immunostaining (green) and DAPI staining (blue) of the ovary (Right). (Black scale bars, 100 μm; white scale bars, 50 μm.) (C) Ovary cultured for 17 d (α-MEM + FBS/10 μM ICI). (Black scale bars, 100 μm; white scale bars, 50 μm.) (D) Ovary cultured in the medium indicated. The parts where the border of follicles was not clear and multioocytes were contained in a follicle were indicated by white arrows. The parts where each follicle was clearly visible with a single oocyte were indicated by white arrowheads. Note that the addition of ICI drastically improved the formation of follicles with single oocytes. In contrast, the addition of estradiol disturbed such follicular formation. (Scale bars, 100 μm.) (E) Oogenetic events and examined conditions for the culture. α-MEM was used as the basal medium. The effects of supplementation of FBS, SPS, estradiol (E2), ICI, and PVP were examined. On day 17 of culture, secondary follicles were isolated from the in vitro-derived ovaries and subjected to IVG. On day 20 of culture, follicles were treated with collagenase and subjected to further IVG.
Fig. 1.
Refinement of follicular (fol) assembly in cultured gonads. (A) Schematic representation of oogenesis and crucial events that require reconstitution in vitro. (B) Histological section (Left) and isolated follicles (Right) from the ovary cultured for 17 d in α-MEM + FBS or the 10-dpp ovary. (Scale bars, 50 µm.) (C) Predicted upstream transcriptional regulators of 547 DEGs in ovaries cultured with α-MEM + FBS. (D) Histological section (Left) and isolated follicles (Right) from the ovary cultured for 17 d with α-MEM + FBS/10 µM ICI. (Scale bars, 50 µm.) (E) Mean number of secondary follicles successfully isolated from each ovary under various culture conditions. Significant differences (*P < 1.0E−3) in the number of isolated secondary follicles were determined by the Bonferroni multiple-comparison correction. Error bars indicate SDs. Numbers in parentheses indicate the number of ovaries examined. (F) A scatter plot showing the ratio of oocyte/follicle diameters from ovaries cultured with α-MEM + FBS/10 µM ICI (red) and those of in vivo origin (blue). Regression lines of in vitro-derived (solid) and in vivo-derived (dashed) ovaries are shown with the slope, intercept, and correlation coefficient. The slopes of the regressions were not significantly different.
Cause of Hypoplastic Follicle Formation in Vitro.
We hypothesized that this abnormally structured secondary follicle has its root in the early stage of primordial follicle assembly; if so, this could be caused by a mechanism that involves estrogen and governs the oocyte cyst breakdown and primordial follicular formation (15, 16).
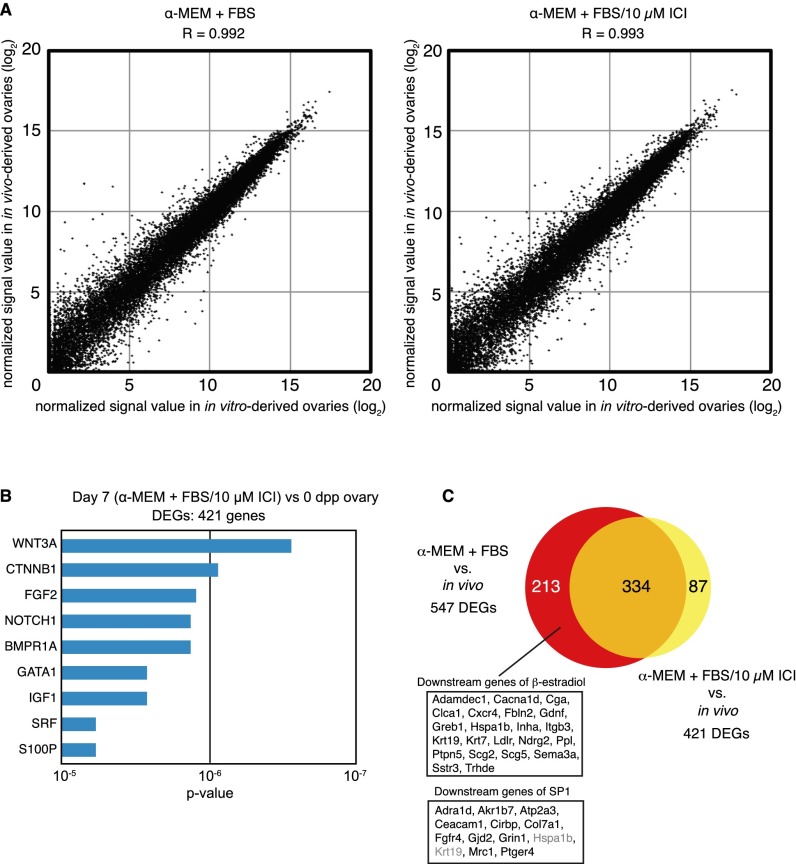
To explore the cause of an abnormal follicular state and test this hypothesis, RNA sequencing (RNA-seq) analysis was performed using ovaries cultured in α-MEM + FBS on day 7 and ovaries from mice at 0 d postpartum (dpp) (Fig. S1E). In the RNA-seq analysis, the global gene expression pattern that was observed for in vitro-derived ovaries was highly correlated (R = 0.992) (Fig. S2A) with that of neonatal ovaries, indicating that developmental progression in culture was similar to development in vivo. However, 547 genes were differentially expressed between the in vitro-cultured ovaries and neonatal ovaries (normalized signal value, >5; P < 0.05; greater than threefold change) (Table S1). Ingenuity pathway analysis clearly demonstrated that β-estradiol, trans-acting transcription factor 1 (SP1), and β-catenin (CTNNB1), which bind to estrogen receptors and regulate gene expression (17), constituted the top three predicted upstream regulators of the 547 genes (Fig. 1C and Table S2). These results indicated that an elevated estrogen signaling level, probably triggered by some ligands in FBS, caused hypoplastic follicle formation in vitro.
Fig. S2.
Correlation of RNA-seq analysis for in vitro and in vivo-derived ovaries. (A) Correlation of all genes identified by RNA-seq that were expressed in in vitro-derived ovaries on day 7 cultured with α-MEM + FBS (Left) or α-MEM + FBS/10 μM ICI (Right) and in ovaries of 0-d-old mice. (B) Ingenuity pathway analysis of the 421 genes differentially expressed in the cultured ovaries (α-MEM + FBS/10 μM ICI). (C) Venn diagram showing a comparison of DEGs in each culture condition indicated. Among 213 genes specifically listed as DEGs in the cultured ovaries (α-MEM + FBS), 22 and 13 genes of β-estradiol– and SP1-downstream targets, respectively, were identified; these could be responsible for abnormal follicle formation. Genes in gray in the SP1 downstream targets are also β-estradiol downstream targets.
Overcoming Hypoplastic Follicle Formation in Vitro.
The potential effects of FBS-derived estrogen in the culture medium were minimized as follows (Fig. S1E): A serum protein substitute (SPS; 44 mg/mL normal human serum albumin and 6 mg/mL α- and β-globulins in PBS) was used instead of FBS (α-MEM + SPS); α-MEM + SPS was used only from day 5 to day 11, when oocyte cyst breakdown and primordial follicle assembly occurs (6) (α-MEM + FBS/SPS); and an estrogen-receptor antagonist (ICI 182,780; ICI) was added from day 5 to day 11 (α-MEM + FBS/ICI). These test conditions significantly improved the efficiency of the isolation of single secondary follicles, each of which encapsulated a single primary oocyte (Fig. 1D and Fig. S1 C and D). In particular, the addition of ICI resulted in a more than sevenfold increase in the number of single secondary follicles compared with the original conditions (Fig. 1E) (P < 1 × 10−3). Furthermore, ICI treatment promoted the formation of a complete laminin envelope by individual follicles (Fig. S1C). Although oocytes in the secondary follicles that were cultured in vitro were smaller than those growing in vivo, the ratios of oocyte/follicle diameters were comparable (Fig. 1F), suggesting the follicles assembled normally in vitro. On the basis of an RNA-seq analysis of ovaries that were cultured for 7 d in α-MEM + FBS/10 µM ICI, 421 genes were differentially expressed between ovaries cultured with α-MEM + FBS/ICI and neonatal ovaries (Table S3), although the global pattern of gene expression was highly correlated with that of neonatal ovaries (Fig. S2A). Ingenuity pathway analysis demonstrated that β-estradiol and SP1 were not on the top 10 list of predicted upstream regulators of the 421 genes (Fig. S2B and Table S4). Potential downstream target genes responsible for hypoplastic follicle formation were identified among 213 genes whose transcript levels differed specifically in α-MEM + FBS-cultured ovaries (Fig. S2C). Finally, supplementing the culture medium with estradiol impaired follicle formation; therefore, the efficiency of secondary follicle isolation was reduced (Fig. 1E and Fig. S1D). Collectively, this series of analyses clearly demonstrated that the appropriate regulation of estrogen binding to its receptors is crucial for initial in vitro folliculogenesis.
Oocyte and Follicular Growth in Vitro.
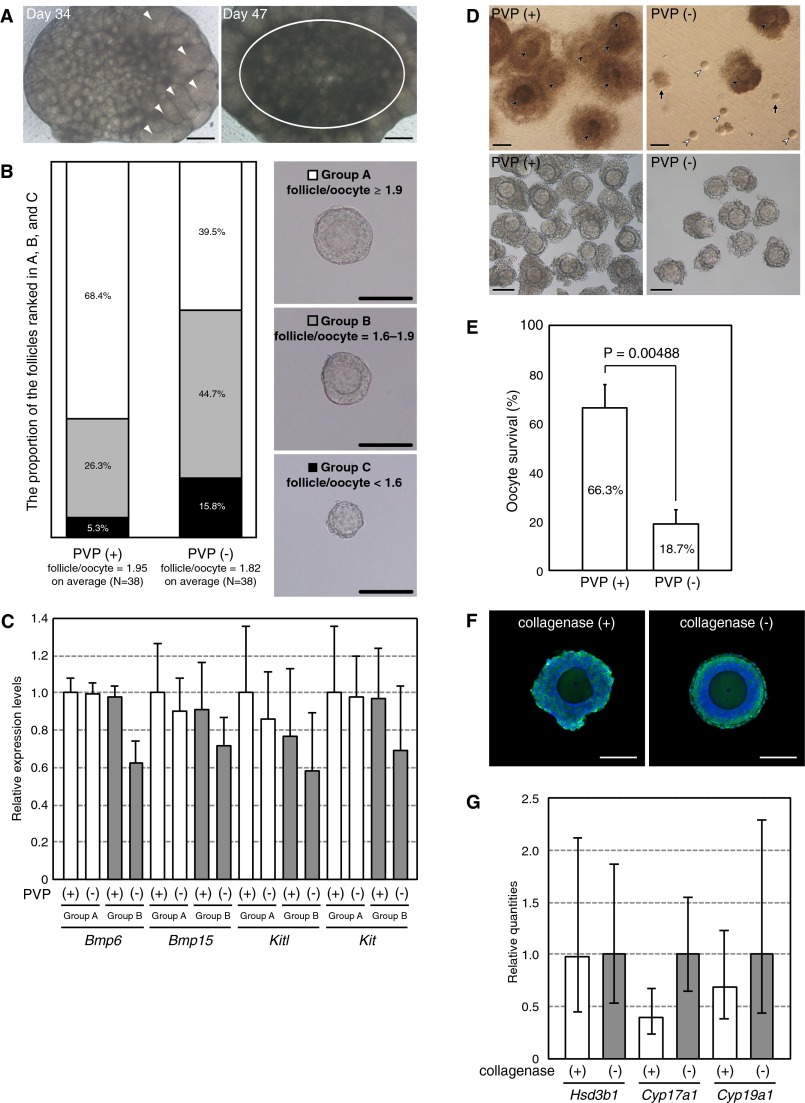
Secondary follicles were isolated on day 17 of culture because a prolonged culture of whole ovaries eventually results in large-scale follicular degeneration (Fig. S3A). The isolated follicles were cultured in α-MEM supplemented with 5% (vol/vol) FBS, 2% (wt/vol) polyvinylpyrrolidone (PVP; 360 kDa) (18), and 0.1 IU/mL follicle-stimulating hormone for IVG. The PVP-supplemented medium was used because of its effect on maintaining the integrity of oocyte–granulosa cell complexes in a long-term culture, which was found in our previous study in cattle (19). We demonstrated here that without PVP supplementation, even in the early phase of IVG (day 20), the ratio of follicle/oocyte diameters was significantly smaller than that cultured in medium supplemented with 2% (wt/vol) PVP (1.82 vs. 1.95; P = 0.0049) (Fig. S3B). Moreover, the mRNA expression levels of born morphogenetic protein 6 (Bmp6), Bmp15, kit ligand (Kitl), and kit oncogene (Kit), which are involved in follicle growth (20), were slightly reduced in follicles cultured in the PVP-free medium (Fig. S3C). After a full term of IVG, a significantly larger proportion of oocyte–granulosa cell complexes were recovered from the medium with PVP than from the medium without PVP supplementation (Fig. S3 D and E) (with PVP: 344/519; without PVP: 98/524; P = 0.00488).
Fig. S3.
Optimization of in vitro follicle growth. (A) An ovary cultured for 34 d (Left) and 47 d (Right). Prolonged culture of a whole ovary resulted in limited follicle growth (only a few follicles formed antrums; arrowhead) and large-scale follicular degeneration (circle). (Scale bars, 200 μm.) (B) Effect of PVP during follicle culture. On day 20 of culture, follicles were classified into groups A, B, and C, depending on the ratio of the follicle/oocyte diameter, which was in the range of ≥1.9 (Upper), 1.6–1.9 (Middle), and <1.6 (Lower), respectively. The proportion of the follicles ranked in groups A, B, and C is indicated with white, gray, and black boxes, respectively. The ratio of the follicle/oocyte diameter was much greater in follicles cultured with PVP [average ratio; PVP (+) vs. PVP (−); 1.95 vs. 1.82; P = 0.0049]. Before IVG, no morphological differences were found in the examined follicles [average ratio; PVP (+) vs. PVP (−); 1.78 vs. 1.78; P = 0.9243]. (Scale bars, 100 μm.) (C) Relative expression levels of genes involved in follicle growth on day 20 of culture. Expression levels were measured by qRT-PCR, using cDNA derived from follicles cultured with or without PVP. White and gray bars indicate groups A and B, respectively. Tbp was used as an internal control. Error bars indicate the SD (n = 3). (D) Morphology of follicles (Upper) and COCs (Lower) developed in medium with (Left) or without PVP (Right) on day 33 of culture. Closed arrowheads indicate the oocytes within follicle; open arrowheads represent denuded oocytes. The arrows indicate the remnants of follicles left on the insert membrane. [Scale bars, 200 μm (Upper) and 100 μm (Lower).] (E) Comparison of the viability of oocytes after the 33-d culture period. Oocytes with firmly attached granulosa (cumulus) cells over at least half of their surface and showing no signs of degeneration were considered as surviving. Data from three independent experiments are shown as means ± SD. The total numbers of follicles included after the collagenase treatment were 524 and 519 in medium with or without PVP, respectively. The Student’s t test was used for the comparison between two groups after arcsine transformation of the percentage data. (F) Evidence for the growth of steroidogenic cells during follicle culture on day 26 of culture. Immunostaining of HSD3B in the follicles with (Left) or without (Right) collagenase treatment. (Scale bars, 100 μm.) (G) Relative quantities (RQs) of genes involved in steroidogenesis on day 26 of culture. RQ was measured by qRT-PCR and ΔΔ Ct method, using cDNAs derived from the follicles treated with (white bars) or without (gray bars) collagenase. Tbp was used as an internal control. Error bars indicate the RQ max and RQ min (n = 4).
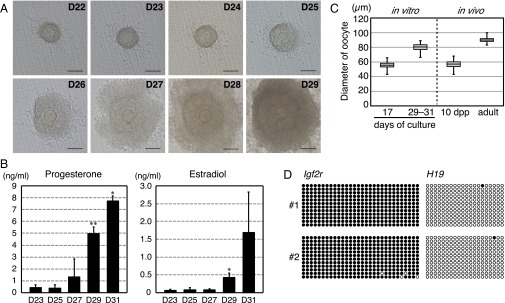
Previous reports have described two possible IVG culture methods, wherein the intact follicle structure (i.e., oocytes, granulosa cells, the basal lamina, and thecal layers) is maintained or the oocyte–granulosa cell complex is directly exposed to the medium. The latter method requires collagenase treatment for digestion of the outer layer of follicles. In preliminary experiments in which IVG was conducted without collagenase treatment, 83% of isolated follicles survived (116/140). However, only a few oocytes reached metaphase during the second meiosis [MII; 26/116; MII oocytes/cumulus cells-oocyte complexes (COCs)], supposedly because of the insufficient growth of oocytes/follicles, none of which were normally fertilized. Therefore, we treated the follicles with 0.1% collagenase for at least 15 min and largely removed the thecal layers by pipetting on day 20 of culture. During IVG culture with collagenase treatment, granulosa cell proliferation, oocytes, and clearly outlined germinal vesicles (GV) with a characteristic nucleolus were observed in most of the follicular structures (Fig. 2A and Table 1). Mural granulosa cells often formed dorm-like structures. Immunostaining of hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase (HSD3B), which is essential for progesterone production (21, 22), showed the presence of a population of steroidogenic cells on day 26 of culture (Fig. S3F). Continuous increases in the progesterone and estradiol concentrations in the medium suggested persistent steroidogenic activity in the follicles (Fig. 2B). Steroidogenic markers, such as Hsd3b1, cytochrome P450, family 17, subfamily a, polypeptide 1 (Cyp17a1) and Cyp19a1 mRNA, were also expressed in the follicles on day 26 of culture (Fig. S3G). On days 29–33, COCs were collected from approximately half of the cultured follicles (Table 1). During the IVG culture, the oocytes increased in size to a mean diameter of 80.0 µm (n = 85) (Fig. 2C). Because the average diameter before IVG was 54.4 µm (n = 203) (Figs. 1F and 2C), the oocyte volume increased by 3.18-fold. A control experiment showed that the average diameter of fully grown oocytes collected from superovulated mice was 89.9 µm (n = 74) (Fig. 2C), suggesting that the volume increased by 3.98-fold in vivo compared with oocytes in secondary follicles of 10-dpp ovaries, whose average diameter was 56.6 µm (n = 175). Although the size and growth rate of oocytes were slightly attenuated in the in vitro culture, oocyte-specific methylation imprints, which are necessary for mammalian ontogeny (23), were nevertheless established in the oocytes grown in vitro (Fig. 2D).
Fig. 2.
In vitro growth of oocytes and follicles. (A) A representative follicle cultured on a Transwell-COL membrane on days 22–29 of culture. (Scale bars, 100 μm.) (B) Concentration of progesterone (Left) and estradiol (Right) in the medium. Steroid hormones were measured by an enzyme-linked immunoassay during follicular culture. Significant increases in the steroid concentration in the medium were determined by t tests. Asterisks indicate a significant increase in the steroid concentration compared with that measured 2 d before (*P < 0.05, **P < 0.01). Error bars indicate SD (n = 4). (C) Oocyte growth in IVG culture. The box plot shows the diameters of oocytes on the days that IVG started (day 17) and ended (day 29–33), the diameters of oocytes in secondary follicles at 10 dpp, and the diameter of mature oocytes derived from adult mice. (D) DNA methylation imprints in the in vitro-derived GV oocytes at the Igf2r and H19 loci, which are methylated specifically in oogenesis and spermatogenesis, respectively. “#1” and “#2” represent two independent samples. Black and white circles indicate methylated and nonmethylated cytosines at CpG sites in the analyzed imprinted regions, respectively.
Table 1.
Developmental ability of oocytes differentiated from PGCs after growth, maturation, and fertilization in vitro
| Condition for organ culture | No. of cultured gonads | No. of cultured follicles | No. of survived follicles | No. of collected COCs | No. of oocytes matured into MII | No. of eggs fertilized normally | No of embryos developed to two-cell | No. of embryos developed to pup | No. of pups from a gonad |
| FBS/SPS | 9 | 216 | 96 (44%) | 60 (63%) | 46 (77%) | 18 (39%) | 15 (83%) | 6 (40%) | 0.7 |
| FBS/1 µM ICI | 16 | 641 | 470 (73%) | 340 (72%) | 321 (94%) | 168 (52%) | 155 (92%) | 31 (20%) | 1.6 |
| FBS/5 µM ICI | 6 | 312 | 223 (71%) | 178 (80%) | 168 (94%) | 94 (56%) | 91 (97%) | 20 (22%) | 3.3 |
| FBS/10 µM ICI | 6 | 505 | 337 (67%) | 281 (83%) | 256 (91%) | 148 (58%) | 138 (93%) | 19 (14%) | 3.2 |
| Vitrification | 4 | 87 | 57 (66%) | 39 (68%) | 37 (95%) | 19 (51%) | 15 (79%) | 2 (13%) | 0.5 |
| GV (control) | 19 | 155 | 139 (90%) | 70 (45%) | 67 (96%) | 40 (60%) | 2.1 |
Evidence for Recapitulation of Oogenesis in Vitro.
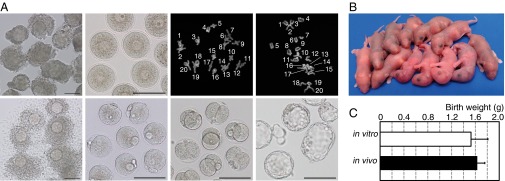
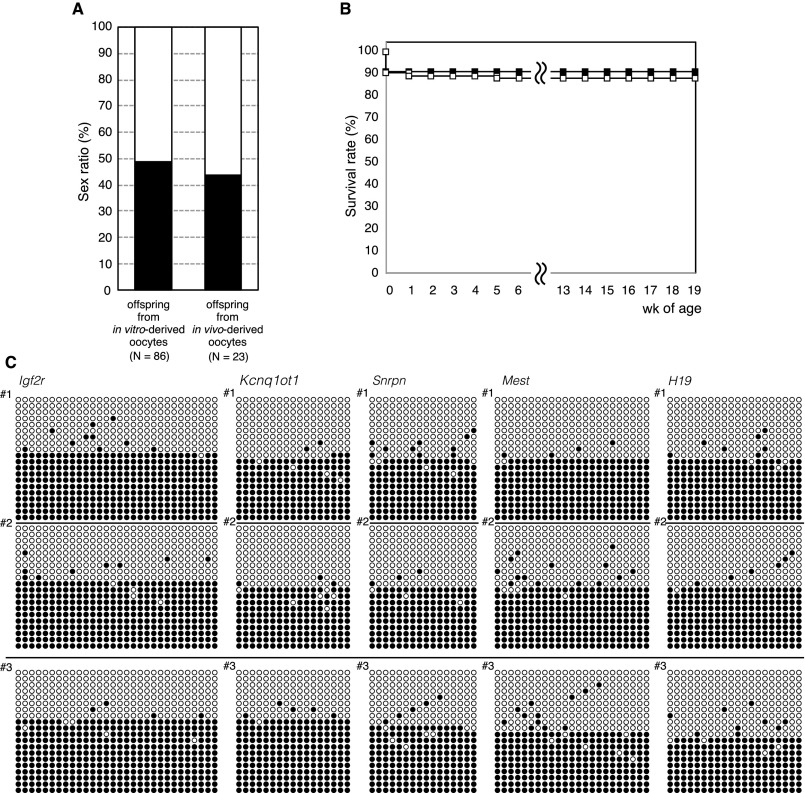
The most rigorous evidence for recapitulation of oogenesis in vitro originates from the demonstration of the reproductive ability of oocytes. To evaluate this, the oocytes grown in this system were subjected to in vitro maturation, followed by in vitro fertilization. The collected COCs were induced to resume meiosis, using gonadotropins and epidermal growth factor (24). The majority of oocytes released the first polar body after 17 h and became MII oocytes, as confirmed by a karyotype analysis (Fig. 3A). Approximately half (39–58%) of the MII oocytes were normally fertilized (Fig. 3A and Table 1), and 83–97% of the eggs developed to the two-cell stage on the next day and were transferred into 0.5-dpc pseudopregnant ICR albino mice (Fig. 3A). These transplantation experiments resulted in the birth of healthy pups with comparable body weights to those derived from oocytes in vivo (Fig. 3 B and C and Table 1). All the pups had pigmented eyes, and hence were considered to have clearly originated from in vitro-derived oocytes. The frequency of development from two-cell-stage embryos to pups was 14–40% (Table 1). A maximum of seven pups was obtained from a single gonad (Table S5), which is comparable with the number seen by natural delivery in mice. On average, 0.7–3.3 pups were obtained from each gonad, and they showed normal appearance and fertility (Fig. S4).
Fig. 3.
Developmental competence of oocytes produced from PGCs in vitro. (A) Maturation, resumption of meiosis, fertilization, and embryonic development in vitro. Shown are COCs obtained from follicles on day 29 after the beginning of organ culture (Upper Left); denuded fully grown oocytes at the GV stage after follicular culture (Upper Middle Left); the karyotype of in vitro-derived oocytes at the first (Upper Middle Right) and second (Upper Right) meiotic stage with the number of chiasmata and 20 pairs of homologous chromosomes, respectively; cumulus cell-enclosed in vitro-derived oocytes at the MII stage (Lower Left); normally fertilized eggs with two pronuclei and the second polar body (Lower Middle Left), indicating the completion of meiosis after fertilization; and two-cell (Lower Middle Right) and blastocyst-stage (Lower Middle Right) embryos. (Scale bars, 100 µm.) (B) Pups from in vitro-derived oocytes. (C) Birth weights of the offspring. White and black bars indicate the mean body weights of offspring (1.53 ± 0.282 g vs. 1.63 ± 0.128 g) from in vitro-derived oocytes (n = 68) and in vivo-derived oocytes (n = 23), respectively. Error bars indicate SD. No significant difference in body weights between the groups was observed (t test).
Fig. S4.
Evaluation of pups from in vitro-derived oocytes. (A) Sex ratio of offspring. Black and white bars indicate the frequency of male and female offspring, respectively. The χ2 test was used for statistical analysis. (B) Proportion of surviving offspring from in vitro-derived (white squares, n = 86) and in vivo-derived (black squares, n = 23) oocytes. Offspring were weaned at around 4 wk of age. Few offspring were lost from 1 wk of age onward. The oldest offspring were 85 wk old on December 21, 2015. Offspring grew well and exhibited a healthy appearance. Offspring from in vivo-derived oocytes were produced from fully grown GV oocytes of adult mice by maturation, in vitro fertilization, and embryo transfer as a control. (C) DNA methylation imprints in offspring from in vitro-derived oocytes (#1 and #2, two individuals) and in vivo-derived oocyte (#3) loci indicated. Black and white circles indicate methylated and nonmethylated cytosines at CpG sites in the analyzed imprinted regions, respectively.
Cryopreservation of Ovary: Approach to Applicable System.
Cryopreservation of ovarian tissue is an essential technology for preserving animal fertility and gamete resources. Accordingly, the use of our in vitro system to produce functional oocytes from cryopreserved tissues is an important criterion to evaluate the application of the culture system. We dissected each of the fetal gonads into two or three sections, after which they were cryopreserved by vitrification (25). After placing the gonads in liquid nitrogen for at least 3 d, the gonads were rewarmed and cultured under the same conditions (α-MEM + FBS/1 µM ICI) that were used for fresh gonads. Although a decreased number of follicles formed in the thawed gonads, apparently normal offspring were nevertheless derived from the resultant oocytes (Table 1). These results demonstrated the feasibility of cryopreserving gonads and subsequent functional oocyte production that are expected to serve as an alternative source of oocytes in the future.
Discussion
Here, we demonstrated the first completely connected chain of in vitro oogenesis that permits full differentiation of PGCs into mature mouse oocytes. Breaks in the developmental “chain” have been bypassed previously through in vivo conditions by grafting PGCs beneath the renal capsules (7, 8) or the ovarian bursa (9). We used a different kind of bypass, involving whole cytoplasm prepared from in vivo-grown oocytes, which was combined with genomic materials from in vitro-grown oocytes cultured from PGCs, using the nuclear transfer technique (11, 26). All such bypasses demand additional animals and complex procedures. More important, the existence of a period during which oocytes are not observable and controllable diminishes the potential of the experimental scheme involving oocyte culture as the model of oogenesis. Thus, the use of our in vitro system without breaking the “chain” can add convenience in conducting experiments and open up new possibilities, such as enabling observation and manipulation throughout the course of oogenesis.
A crucial factor underlying the successful establishment of our in vitro system stemmed from an observation of hypoplastic follicle formation in the ovaries cultured in α-MEM + FBS (Fig. 1B). The addition of FBS caused a delay or failure of oocyte cyst breakdown in the ovary, as suggested by the typical occurrence of multioocyte follicle-like structures. Recently, follicular assembly in newborn mouse ovaries was shown to be correlated with the loss of maternally, fetal, and/or ovary-derived estrogen (27, 28). A markedly reduced concentration of estrogen and/or progesterone is essential to promote oocyte cyst breakdown and follicle formation in mice and rats (15, 29). This mechanism was apparently dominant in the case of ovaries cultured in the present study, despite the absence of maternal and fetal estrogen after 5 d in culture. FBS used in the medium is most likely the primary candidate of the source of estrogen. This assumption is consistent with the finding that no apparent inhibition of follicle assembly was observed when SPS was used in place of FBS. Moreover, the positive effect of the use of SPS disappeared on the addition of estradiol (Fig. 1 and Fig. S1). However, further study is necessary to determine whether the cultured fetal ovary itself can generate estrogen; for example, in response to the absence of maternally derived estrogen. In fact, Fortune and Eppig have shown that neonatal ovaries can produce small amounts of estrogen (30).
It is of special importance that ovaries cultured in α-MEM + FBS showed differential expression of genes regulated by estrogen (Fig. 1C and Table S1). Estrogen receptors comprise estrogen receptor 1 (alpha) (ESR1), estrogen receptor 2 (beta) (ESR2), and G protein-coupled estrogen receptor 1 (GPER1) (17). It is well known that ESRs are activated by estrogen binding to consensus DNA sequences and that they regulate the expression of various genes (17). ICI, an antagonist of ESR1 and ESR2, caused dramatic changes of differentially expressed genes (DEGs), which include genes responsible for hypoplastic follicle formation (Fig. S2). These previous findings and our current results strongly suggest the involvement of induced ectopic activation of estrogen receptors in hypoplastic follicle formation after the addition of FBS. Therefore, it is a reasonable conclusion that the cyst breakdown problem was avoided by the addition of ICI.
There are differences among mammalian species regarding the course of follicle formation. For example, the number of primordial follicles reaches a maximum value in the ovaries of 141- to 210-d-old bovine fetuses, when the secretion of estradiol from the ovaries is almost undetectable (31). In contrast, estradiol promotes primordial follicle formation in nonhuman primates (baboons) and hamsters (32, 33), but not by an excess of estradiol (33). The reason for such differences among species should be clarified to make our in vitro system applicable in practice to animals other than mice.
Normal follicle formation is important for oocyte production in vitro as well as in vivo (34, 35). However, an additional and perhaps more dominant reason existed in the present study: normal robust follicles that developed after ICI treatment showed the maximum number of isolated secondary follicles from cultured ovaries. A more than sevenfold increase in the efficiency of follicle recovery after ICI exposure demonstrated a tremendous effect on the design and scale of experiments for the latter part of oogenesis in vitro.
Although a key to success in the present study was the ability to generate an increased yield of intact follicles until day 17, collagenase treatment of the isolated follicles also played an important role in acquiring oocyte competency. The developmental competence of oocytes was elicited by the collagenase treatment, which disrupts integral follicle structure and may promote oocyte development by supporting the direct transfer of materials to the exposed oocyte–granulosa cell complexes. In contrast, potentially because of the removal of a proportion of theca cells by collagenase, there was a delay in the marked acceleration of steroidogenesis until day 25 of culture (Fig. 2B).
Another modification of the culture medium was the supplementation of PVP, which increased the recovery rate of bovine oocyte-granulosa cell complexes after 2 wk of IVG (18, 19). In addition to its remarkable effect on maintaining the oocyte–granulosa cell complexes in an integrated form and attached to the insert membrane (Fig. S3D), PVP may positively affect the expression of genes involved in granulosa cell proliferation, such as Bmp6, Bmp15, Kit, and Kitl (Fig. S3C). It is also of interest that BMP6, a paracrine and autocrine factor expressed in granulosa cells and oocytes, can modulate steroidogenesis and follicle development (20).
Our in vitro system will be useful as a model for visualizing and manipulating PGCs/oocytes throughout oogenesis and promoting the development of functionally mature oocytes. In a recent study by Pfender et al. (36), IVG of mouse oocytes from secondary follicles was combined with a live-cell imaging, using RNA interference screening to identify genes essential for meiosis. Our in vitro oocyte production system expands the applications of such approaches to as early as the PGC stage and is especially effective for elucidating mechanisms regulating the early stage of meiosis. The same system is also applicable to PGC-like cells derived from embryonic stem and induced pluripotent stem cells (9, 10). Our system will assist in bridging the current technical gaps in the entire in vitro oocyte production process from stem cells.
Spermatogenesis has been recently reconstituted in an organ culture of neonatal testicular tissue (37). Thus, complete reconstitution of gametogenesis is now possible in both males and females.
Materials and Methods
Expanded methods are available in SI Materials and Methods. All the animals were purchased from CLEA Japan. BDF1 mouse fetuses were collected from C57BL/6N female mice crossed with DBA/2J male mice at 12.5 dpc and were used for the culture experiments. The animals were maintained in accordance with the guidelines of the Science Council of Japan, and all experiments were approved by the Institutional Animal Care and Use Committee of the Tokyo University of Agriculture.
Female fetal gonads without mesonephros were cultured in Transwell-COL membranes (Corning) for 17 d. α-MEM (Gibco, Thermo Fisher Scientific) supplemented with 1.5 mM 2-O-α-d glucopyranosyl-l-ascorbic acid (Tokyo Chemical Industry), 10 units/mL penicillin, and 10 µg/mL streptomycin (Sigma-Aldrich) was used as a basal medium (referred to here simply as α-MEM). FBS (Gibco, Thermo Fisher Scientific), SPS (SAGE In-Vitro Fertilization), β-estradiol (Santa Cruz Biotechnology), and the estrogen receptor antagonist ICI 182,780 (Tocris Bioscience) were added at the indicated concentrations for each experiment. Gonads were cultured for 17 d at 37 °C under 5% CO2 and 95% air. Approximately half of the medium in each well was replaced with fresh medium every other day (3, 11, 15, 38).
To optimize the organ culture conditions, the following conditions were evaluated (Fig. S1E): culture for the complete 17-d period in α-MEM supplemented with 10% (vol/vol) FBS (α-MEM + FBS), culture for the complete 17-d period in α-MEM supplemented with 10% (vol/vol) SPS (α-MEM + SPS), culture in α-MEM + FBS with a shift to α-MEM + SPS from day 5 to day 11 (α-MEM + FBS/SPS), and culture in α-MEM + FBS with the addition of 1, 5, or 10 µM ICI from day 5 to day 11 (α-MEM + FBS/1, 5, or 10 µM ICI).
After 17 d of organ culture, the secondary follicles were isolated from the ovaries, using a Tungsten needle in L15 medium (Sigma-Aldrich). Follicles were further cultured in α-MEM supplemented with 2% (wt/vol) PVP (Sigma-Aldrich), 5% (vol/vol) FBS, and 0.1 IU/mL FSH (FOLLISTIM Injection 50; MSD). Follicles were cultured on a Millicell membrane (Merck Millipore) in a 35-mm culture dish (Falcon, Corning) for 12–16 d.
On day 20 of culture, follicles were treated with 0.1% collagenase type I (Worthington Biochemicals) in L15 medium for 15 min at 37 °C. Then, the theca layer of the follicles was removed by pipetting in part. Follicles were cultured on Transwell-COL or Millicell membranes for another 9–13 d at 37 °C in medium under 5% CO2 and 95% air. Approximately half the medium in each well was replaced with fresh medium every other day (26).
SI Materials and Methods
Animals.
All animals were purchased from CLEA Japan, Inc. BDF1 mouse fetuses (coat color loci; aaBbCCDd) were collected from C57BL/6N female mice (coat color loci; aaBBCCDD) crossed with DBA/2J male mice (coat color loci; aabbCCdd) at 12.5 dpc and were used for culture experiments. Juvenile and adult female BDF1 mice (C57BL/6N × DBA/2J hybrid) were used for control experiments, and male BDF1 mice were used as sperm donors. To produce pseudopregnant mice, outbred albino female ICR mice (coat color locus; cc) were mated with vasectomized male ICR mice. The animals were maintained in accordance with the guidelines of the Science Council of Japan, and all experiments were approved by the Institutional Animal Care and Use Committee of the Tokyo University of Agriculture.
Organ Culture.
Female fetal gonads without mesonephros were cultured in Transwell-COL membranes (3.0-µm pore size, 24-mm diameter; Corning, Inc.) for 17 d by the gas–liquid interphase method (3). Each membrane was transferred to a single well of a 6-well culture plate, and 2.2 mL of medium was added. α-MEM (Gibco, Thermo Fisher Scientific Inc.) supplemented with 1.5 mM 2-O-α-d glucopyranosyl-l-ascorbic acid (Tokyo Chemical Industry), 10 units/mL penicillin, and 10 µg/mL streptomycin (Sigma-Aldrich) was used as a basal medium (referred to here simply as α-MEM). FBS (Gibco, Thermo Fisher Scientific Inc.), SPS (SAGE In-Vitro Fertilization), β-estradiol (Santa Cruz Biotechnology), and the estrogen receptor antagonist, 7α, 17β-[9-([4,4,5,5,5-pentafluoropentyl]sulfinyl)nonyl]estra-1,3,5(10)-triene-3,17-diol; ICI 182,780 (ICI; Tocris Bioscience) were added at the indicated concentrations for each experiment. Gonads were cultured for 17 d at 37 °C under 5% CO2 and 95% air. Approximately half of the medium in each well was replaced with fresh medium every other day (3, 11, 15, 38).
To optimize the organ-culture conditions, the following conditions were evaluated (Fig. S1E): culture for the complete 17-d period in α-MEM supplemented with 10% (vol/vol) FBS (α-MEM + FBS), culture for the complete 17-d period in α-MEM supplemented with 10% (vol/vol) SPS (α-MEM + SPS); culture in α-MEM + FBS with a shift to α-MEM + SPS from day 5 to day 11 (α-MEM + FBS/SPS), and culture in α-MEM + FBS with the addition of 1, 5, or 10 µM ICI from day 5 to day 11 (α-MEM + FBS/1 µM ICI, α-MEM + FBS/5 µM ICI, or α-MEM + FBS/10 µM ICI, respectively). To examine the inhibitory effect of estrogen on follicle formation, organ culture was performed under the α-MEM + FBS/SPS condition, but with 1 nM β-estradiol added from day 5 to day 11 (α-MEM + FBS/SPS/1 nM E2), and under the α-MEM + FBS/1 µM ICI condition, but with 1 µM β-estradiol added from day 5 to day 11 (α-MEM + FBS/1 µM ICI/1 µM E2).
Follicular Culture.
After 17 d of organ culture, the secondary follicles were isolated from the ovaries, using a fine Tungsten needle in L15 medium (Sigma-Aldrich). Follicles were further cultured in α-MEM supplemented with 2% (wt/vol) PVP (360 kDa, Sigma-Aldrich), 5% (vol/vol) FBS, and 0.1 IU/mL FSH (FOLLISTIM Injection 50; MSD). Follicles were cultured on a Millicell membrane (0.4-µm pore size, 27-mm diameter; Merck Millipore) in a 35-mm culture dish (Falcon, Corning).
On day 20 of culture, follicles were treated with 0.1% (wt/vol) collagenase type I (Worthington Biochemicals) in L15 medium for 15 min at 37 °C. Then, the theca layer of the follicles was removed by pipetting with a pulled fine-glass capillary. Fifty to 60 follicles were cultured on Transwell-COL or Millicell membranes for another 9–13 d at 37 °C in medium under 5% CO2 and 95% air. The inside and outside of the membrane insert were filled with 1 mL and 2 mL medium, respectively. Approximately half the medium in each well was replaced with fresh medium every other day (19, 26).
In Vitro Maturation, in Vitro Fertilization, and Embryo Transfer.
COCs collected from in vitro-derived follicles were cultured with α-MEM containing 5% (vol/vol) FBS, 0.1 IU/mL FSH, 1.2 IU/mL CG (Gonatropin, ASKA Pharmaceutical), and 4 ng/mL epidermal growth factor (Gibco, Thermo Fisher Scientific Inc.) (24). Control COCs were collected from adult female mice 44 h after injection with equine CG (Serotropin; ASKA Pharmaceutical). The obtained in vivo-derived COCs were processed using the same methods described for the in vitro-derived COCs. After 17 h of culture, the oocytes with expanded cumulus cells were fertilized in TYH medium (LSI Medience Corporation) with epididymal sperm. Normally fertilized eggs with 2 pronuclei were cultured in KSOM medium. Embryos developed to the 2-cell stage were transferred into the oviducts of pseudopregnant females at 0.5 dpc. Offspring were delivered by caesarean section at 19.5 dpc and were raised by foster mothers. The offspring were weaned at ∼4 wk of age.
Histological Analysis.
Ovaries were cultured for 17 d in α-MEM + FBS or α-MEM + FBS/10 µM ICI, and ovaries derived from 10-d-old mice were processed for H&E staining. The ovaries were fixed for 4 h at room temperature in 1% paraformaldehyde (PFA)/0.1% glutaraldehyde in 0.05 M phosphate buffer. For H&E staining, the ovaries were embedded in paraffin blocks after a routine protocol, and 4-µm-thick serial sections were prepared. After H&E staining, sections were observed under an IX71 microscope (Olympus).
Immunostaining.
For laminin immunofluorescence staining, ovaries were cultured for 17 d in α-MEM + FBS or α-MEM + FBS/10 µM ICI, and ovaries derived from 10-d-old mice were cut into four to eight pieces. The ovaries were fixed for 4 h at room temperature in 1% PFA/0.1% glutaraldehyde in 0.05 M phosphate buffer and incubated for 3 d at 4 °C with an anti-laminin rabbit polyclonal antibody (Abcam) at a dilution of 1:100. Then the samples were incubated for 2 d at 4 °C with an Alexa Fluor 488-conjugated goat anti-rabbit IgG at a dilution of 1:500 (Molecular Probes, Thermo Fisher Scientific Inc.).
To evaluate entry into meiosis in vitro, ovaries cultured for 5 d were processed for SYCP3 expression analysis. The ovaries were incubated at 37 °C for 30 min in a hypotonic buffer solution containing 17 mM trisodium citrate dehydrate and then suspended in 100 mM sucrose solution on the surface of a glass slide. The cell suspension was spread on the glass slide and fixed with 1% PFA containing 0.15% Triton X-100. After air drying, the spreads were incubated overnight at 4 °C with a primary anti-SYCP3 rabbit polyclonal antibody (Abcam; 1:100 dilution), and then at room temperature for 1 h with secondary Alexa Fluor 488-conjugated goat anti-rabbit IgG. Ovaries derived from 12.5-dpc fetuses were also used for SYCP3 staining.
To detect steroidogenic markers, secondary follicles isolated from ovaries cultured for 17 d with α-MEM + FBS/ICI were further cultured in α-MEM supplemented with 2% (wt/vol) PVP, 5% (vol/vol) FBS, and 0.1 IU/mL FSH. The follicles were treated with or without collagenase on day 20, and each follicle was fixed on day 26 of culture in 0.05 M phosphate buffer containing 1% PFA/0.1% glutaraldehyde. Then, the follicles were incubated for 1 d at 4 °C with an anti-HSD3B rabbit polyclonal antibody at a dilution of 1:500 (21). Next, the samples were incubated for 1 d at 4 °C with an Alexa Fluor 488-conjugated goat anti-rabbit IgG at a dilution of 1:500 (Molecular Probes, Thermo Fisher Scientific Inc.).
All samples were counterstained with DAPI (Molecular Probes, Thermo Fisher Scientific Inc.) or Hoechst 33342 (Dojin Molecular Technologies, Inc.), after which they were observed with a Zeiss LSM 710 confocal microscope (Carl Zeiss).
Hormone Assays.
To assess steroidogenesis during follicular culture, estradiol and progesterone levels in the medium were measured in duplicate, using an enzyme immunoassay kit (Cayman), according to the manufacturer’s instructions.
Karyotype Analysis.
Fully grown oocytes in first and second meiosis were incubated in 0.6% sodium citrate for 5 min at room temperature and then spread on glass slides with Carnoy’s solution. Chromosomes were stained with DAPI, and the karyotype was analyzed using a Zeiss LSM 710 confocal microscope.
Vitrification and Warming.
Vitrification and warming were conducted following previously reported methods (25), with minor modifications. Gonads collected from female fetuses at 12.5 dpc were dissected into two to three pieces. Gonadal tissues were immersed in 2 mL solution 1, which consisted of L15 medium containing 4 mg/mL BSA (Sigma-Aldrich), 10% (vol/vol) ethylene glycol (Wako), and 10% (vol/vol) DMSO (Sigma-Aldrich). Twenty minutes later, gonadal tissues were transferred to 2 mL solution 2, consisting of L15 medium containing 4 mg/mL BSA, 17% (vol/vol) ethylene glycol, 17% (vol/vol) DMSO, and 0.75 M sucrose (Wako). Three minutes later, gonadal tissues were placed into cryotubes (Iwaki, Asahi Glass Co.) with 3 µL solution 2 and preserved in liquid nitrogen. To warm the vitrified gonadal tissues, 1 mL of 0.5 M sucrose solution was added to the cryotube and then mixed by pipetting. The gonadal tissues were then washed sequentially with 0.25 M, 0.125 M, and 0 M sucrose solution every 5 min. Warmed gonadal tissues were cultured in α-MEM + FBS/1 µM ICI, and isolated follicles were cultured as described earlier.
qRT-PCR Experiments.
To evaluate the effect of PVP supplementation on IVG, follicles isolated from ovaries cultured for 17 d with α-MEM + FBS/10 µM ICI were further cultured in medium supplemented with or without 2% (wt/vol) PVP for IVG. On day 20 after the beginning of organ culture, follicles were classified into three groups, depending on the ratio of the follicle-to-oocyte diameter: group A had a ratio of ≥1.9, group B had a ratio of 1.9–1.6, and group C had a ratio of <1.6. Total RNA was extracted using the RNeasy Micro Kit (Qiagen) from a single follicle in group A and from four follicles in group B. RNA from each sample was reverse-transcribed, using an Invitrogen SuperScript III Reverse Transcriptase (Life Technologies). The cDNAs were studied by quantitative PCR (qPCR), using SYBR Green (Life Technologies) in a QuantStudio 3 Real-Time PCR System (Applied Biosystems). All copy numbers were normalized to an internal control gene, TATA box binding protein (Tbp).
Primers with the following sequences were used in this study: Tbp (NM_013684.3, 206 bp): 5′-ATC CCA AGC GAT TTG C-3′ and 5′-GCT CCC CAC CAT GTT C-3′; Bmp6 (NM_007556.3, 163 bp): 5′-TGG GAT GGC AGG ACT GGA T-3′ and 5′-CAG CAT GGT TTG GGG ACG TA-3′; Bmp15 (NM_009757, 168 bp): 5′-CTC CCA GAG GTT CCT GGC AT-3′ and 5′-GCT TGG TCC GGC ATT TAG GA-3′; Kitl (NM_013598.2, 112 bp): 5′-GCG GGA ATC CTG TGA CTG AT-3′ and 5′-CTA GGC AAA ACA TCC ATC CCG-3′; Kit (NM_001122733.1, 161 bp): 5′-GCC TGC CGA AAT GTA TGA CG-3′ and 5′-GGT TCT CTG GGT TGG GGT TG-3′.
To detect the expression of steroidogenic genes, secondary follicles isolated from ovaries cultured for 17 d in α-MEM + FBS/ICI were further cultured in α-MEM supplemented with 2% (wt/vol) PVP, 5% (vol/vol) FBS, and 0.1 IU/mL FSH. The follicles were treated with or without collagenase on day 20, and each follicle was subjected to RNA extraction on day 26 of culture. Total RNA was extracted using the RNeasy Micro Kit (Qiagen) from 10 follicles. RNA from each sample was reverse-transcribed using Invitrogen SuperScript III Reverse Transcriptase (Life Technologies). The cDNAs were studied by qPCR, using TaqMan probes (Life Technologies) in a QuantStudio 3 Real-Time PCR System (Applied Biosystems). Quantification of transcripts was analyzed by the comparative Delta Delta Ct method, and the expression was normalized to an internal control gene, Tbp. The IDs of the TaqMan Probe used in this study are as follows: Tbp (Mm01277042_m1), Cyp17a1 (Mm00484040_m1), Cyp19a1 (Mm00484049_m1), and Hsd3b1 (Mm01261921_mH).
RNA-Seq.
Three ovaries were subjected to RNA-seq analysis for each experimental group. Ninety nanograms total RNA was extracted from each ovary cultured for 7 d in the presence of either of 0 or 10 µM ICI, and from a newborn ovary at 0 dpp. cDNA libraries were prepared using the TruSeq RNA Sample Preparation Kit v2 (Illumina), following the manufacturer’s instructions. Each sample, with a unique bar code, was pooled in a lane, and clusters were generated on a cBot to obtain short 50-bp single reads in a HiSeq 2500 sequencer (Illumina). Image analysis, base calling, and mapping were performed using CASAVA software ver.1.8.3 (Illumina) and the reference mouse genome, GRCm/mm10. Reads aligned to the mouse genome (University of California, Santa Cruz) were normalized using the DESeq method with Strand NGS software version 2.1 (Strand Genomics, Inc.), and the counts of normalized reads were log2-transformed to obtain normalized signal values for statistical analyses. Genes with normalized signal values greater than 5 were considered stably expressed. To identify DEGs, stably expressed genes were filtered according to statistical significance (P < 0.05) and greater than threefold changes. Moderated t tests were used to determine significant differences. Multiple testing corrections were carried, using the Benjamin Hochberg FDR method. Predictive causal analysis was performed, using Ingenuity Pathway Analysis software (Ingenuity).
DNA Methylation Analysis at Imprinted Loci.
DNA was isolated from in vitro-derived oocytes at the GV stage, and pups were developed from the in vitro-derived oocytes. DNA was then treated with sodium bisulfite (Qiagen). Bisulfite-treated DNA was subjected to PCR (23).
Primers with the following sequences were used in this study: insulin-like growth factor 2 receptor (Igf2r) (L06446.1): first PCR (549 bp), 5′-CAC TTT TAA ACT TAC CTC TCT TAC-3′ and 5′-TAG AGG ATT TTA GTA TAA TTT TAA-3′; second PCR (490 bp), 5′-GAG GTT AAG GGT GAA AAG TTG TAT-3′ and 5′-CAC TTT TAA ACT TAC CTC TCT TAC-3′; H19 (AF049091.1): first PCR (440 bp), 5′-TTT GGG TAG TTT TTT TAG TT-3′ and 5′-TCC TAA TCT CTA ATC TCA AC -3′; second PCR (368 bp), 5′-TTT GGG TAG TTT TTT TAG TT-3′ and 5′-AAC CCC AAC CTC TAC TTT TA-3′; KCNQ1 overlapping transcript 1 (Kcnq1ot1) (AP001295.1, 337 bp), 5′-TAA GGT GAG TGG TTT AGG AT-3′ and 5′-CCA CTA TAA ACC CAC ACA TA-3′; small nuclear ribonucleoprotein N (Snrpn) (AF081460.1, 420 bp), 5′-AAT TTG TGT GAT GTT TGT AAT TAT TTG G-3′ and 5′-ATA AAA TAC ACT TTC ACT ACT AAA ATC C-3′; mesoderm specific transcript (Mest) (AF017994.1, 563 bp), 5′-TTT TAG ATT TTG AGG GTT TTA GGT TG-3′ and 5′-AAT CCC TTA AAA ATC ATC TTT CAC AC-3′.
PCR products were cloned into the pGEM-T Easy vector (Promega) and sequenced on an ABI PRISM 3100 system (Applied Biosystems, Thermo Fisher Scientific). At least 20 plasmid DNA clones in each sample were sequenced at each imprinted locus, using QUMA software (39).
Supplementary Material
Acknowledgments
We thank Prof. Satoru Kobayashi (University of Tsukuba), Prof. Takehiko Ogawa (Yokohama City University), Ms. Naoko Mochida, Dr. Akiko Hasegawa, and Prof. Hiroaki Shibahara (Hyogo College of Medicine) for their comments; Prof. Ken-ichirou Morohashi (Kyushu University) for giving us the HSD3B antibody; and Dr. Kazuya Kobayashi (Hirosaki University) for preparing the histologic sections of the ovaries. This work was supported by Grants-in-Aid for Scientific Research 26450449 (to Y.O.), 25114008 (to Y.O. and Y.H.), and 25114006 (to K.H.); and by a Ministry of Education, Culture, Sports, Science, and Technology (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities (S1311017).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603817113/-/DCSupplemental.
References
- 1.Shen W, et al. In vitro development of mouse fetal germ cells into mature oocytes. Reproduction. 2007;134(2):223–231. doi: 10.1530/REP-06-0378. [DOI] [PubMed] [Google Scholar]
- 2.Zhang ZP, et al. Growth of mouse oocytes to maturity from premeiotic germ cells in vitro. PLoS One. 2012;7(7):e41771. doi: 10.1371/journal.pone.0041771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54(1):197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68(5):1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 5.Morohaku K, Hirao Y, Obata Y. Developmental competence of oocytes grown in vitro: Has it peaked already? J Reprod Dev. 2016;62(1):1–5. doi: 10.1262/jrd.2015-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234(2):339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 7.Shen W, et al. Live offspring produced by mouse oocytes derived from premeiotic fetal germ cells. Biol Reprod. 2006;75(4):615–623. doi: 10.1095/biolreprod.106.051482. [DOI] [PubMed] [Google Scholar]
- 8.Matoba S, Ogura A. Generation of functional oocytes and spermatids from fetal primordial germ cells after ectopic transplantation in adult mice. Biol Reprod. 2011;84(4):631–638. doi: 10.1095/biolreprod.110.087122. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi K, et al. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338(6109):971–975. doi: 10.1126/science.1226889. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146(4):519–532. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 11.Obata Y, Kono T, Hatada I. Gene silencing: Maturation of mouse fetal germ cells in vitro. Nature. 2002;418(6897):497. doi: 10.1038/418497a. [DOI] [PubMed] [Google Scholar]
- 12.Lammers JH, et al. The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol Cell Biol. 1994;14(2):1137–1146. doi: 10.1128/mcb.14.2.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowles J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312(5773):596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 14.Berkholtz CB, Lai BE, Woodruff TK, Shea LD. Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol. 2006;126(5):583–592. doi: 10.1007/s00418-006-0194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Breen K, Pepling ME. Estrogen can signal through multiple pathways to regulate oocyte cyst breakdown and primordial follicle assembly in the neonatal mouse ovary. J Endocrinol. 2009;202(3):407–417. doi: 10.1677/JOE-09-0109. [DOI] [PubMed] [Google Scholar]
- 16.Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: Inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod. 2006;74(1):161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- 17.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19(8):1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirao Y. Isolation of ovarian components essential for growth and development of mammalian oocytes in vitro. J Reprod Dev. 2012;58(2):167–174. doi: 10.1262/jrd.2011-052. [DOI] [PubMed] [Google Scholar]
- 19.Hirao Y, et al. Production of fertile offspring from oocytes grown in vitro by nuclear transfer in cattle. Biol Reprod. 2013;89(3):57. doi: 10.1095/biolreprod.113.109439. [DOI] [PubMed] [Google Scholar]
- 20.Otsuka F. Multifunctional bone morphogenetic protein system in endocrinology. Acta Med Okayama. 2013;67(2):75–86. doi: 10.18926/AMO/49665. [DOI] [PubMed] [Google Scholar]
- 21.Miyabayashi K, et al. Heterogeneity of ovarian theca and interstitial gland cells in mice. PLoS One. 2015;10(6):e0128352. doi: 10.1371/journal.pone.0128352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morohashi K, Baba T, Tanaka M. Steroid hormones and the development of reproductive organs. Sex Dev. 2013;7(1-3):61–79. doi: 10.1159/000342272. [DOI] [PubMed] [Google Scholar]
- 23.Obata Y, et al. Epigenetically immature oocytes lead to loss of imprinting during embryogenesis. J Reprod Dev. 2011;57(3):327–334. doi: 10.1262/jrd.10-145a. [DOI] [PubMed] [Google Scholar]
- 24.Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996;11(12):2656–2666. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Catt S, Pangestu M, Temple-Smith P. Successful in vitro culture of pre-antral follicles derived from vitrified murine ovarian tissue: Oocyte maturation, fertilization, and live births. Reproduction. 2011;141(2):183–191. doi: 10.1530/REP-10-0383. [DOI] [PubMed] [Google Scholar]
- 26.Obata Y, Maeda Y, Hatada I, Kono T. Long-term effects of in vitro growth of mouse oocytes on their maturation and development. J Reprod Dev. 2007;53(6):1183–1190. doi: 10.1262/jrd.19079. [DOI] [PubMed] [Google Scholar]
- 27.Lei L, Jin S, Mayo KE, Woodruff TK. The interactions between the stimulatory effect of follicle-stimulating hormone and the inhibitory effect of estrogen on mouse primordial folliculogenesis. Biol Reprod. 2010;82(1):13–22. doi: 10.1095/biolreprod.109.077404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutta S, Mark-Kappeler CJ, Hoyer PB, Pepling ME. The steroid hormone environment during primordial follicle formation in perinatal mouse ovaries. Biol Reprod. 2014;91(3):68. doi: 10.1095/biolreprod.114.119214. [DOI] [PubMed] [Google Scholar]
- 29.Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: Endocrine model of follicle assembly. Endocrinology. 2003;144(8):3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- 30.Fortune JE, Eppig JJ. Effects of gonadotropins on steroid secretion by infantile and juvenile mouse ovaries in vitro. Endocrinology. 1979;105(3):760–768. doi: 10.1210/endo-105-3-760. [DOI] [PubMed] [Google Scholar]
- 31.Yang MY, Fortune JE. The capacity of primordial follicles in fetal bovine ovaries to initiate growth in vitro develops during mid-gestation and is associated with meiotic arrest of oocytes. Biol Reprod. 2008;78(6):1153–1161. doi: 10.1095/biolreprod.107.066688. [DOI] [PubMed] [Google Scholar]
- 32.Wandji SA, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod. 1997;12(9):1993–2001. doi: 10.1093/humrep/12.9.1993. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Roy SK. Development of primordial follicles in the hamster: Role of estradiol-17beta. Endocrinology. 2007;148(4):1707–1716. doi: 10.1210/en.2006-1193. [DOI] [PubMed] [Google Scholar]
- 34.Heller DT, Cahill DM, Schultz RM. Biochemical studies of mammalian oogenesis: Metabolic cooperativity between granulosa cells and growing mouse oocytes. Dev Biol. 1981;84(2):455–464. doi: 10.1016/0012-1606(81)90415-2. [DOI] [PubMed] [Google Scholar]
- 35.Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol. 2000;226(2):167–179. doi: 10.1006/dbio.2000.9863. [DOI] [PubMed] [Google Scholar]
- 36.Pfender S, et al. Live imaging RNAi screen reveals genes essential for meiosis in mammalian oocytes. Nature. 2015;524(7564):239–242. doi: 10.1038/nature14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato T, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471(7339):504–507. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- 38.Mochida N, et al. Live births from isolated primary/early secondary follicles following a multistep culture without organ culture in mice. Reproduction. 2013;146(1):37–47. doi: 10.1530/REP-13-0020. [DOI] [PubMed] [Google Scholar]
- 39.Kumaki Y, Oda M, Okano M. QUMA: Quantification tool for methylation analysis. Nucleic Acids Res. 2008;36(Web Server issue):W170–W175. doi: 10.1093/nar/gkn294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.