Significance
Why has human life expectancy increased since 1850? A leading hypothesis proposes that limited exposure to childhood infections has reduced lifelong inflammation and enhanced survival, but tests of this hypothesis typically use all-cause mortality rates to estimate disease exposure. Meanwhile, links between early-life disease and reproduction have been neglected. We used data from preindustrial Finnish populations to show that early-life disease exposure was not associated with all-cause mortality, mortality from cardiovascular disease, stroke, and cancer, or reproductive success. Our study therefore does not support the prevailing contention that reduced exposure to early-life infections has increased life expectancy in modern populations.
Keywords: inflammation, stress, infection, life history, fitness
Abstract
A leading hypothesis proposes that increased human life span since 1850 has resulted from decreased exposure to childhood infections, which has reduced chronic inflammation and later-life mortality rates, particularly from cardiovascular disease, stroke, and cancer. Early-life cohort mortality rate often predicts later-life survival in humans, but such associations could arise from factors other than disease exposure. Additionally, the impact of early-life disease exposure on reproduction remains unknown, and thus previous work ignores a major component of fitness through which selection acts upon life-history strategy. We collected data from seven 18th- and 19th-century Finnish populations experiencing naturally varying mortality and fertility levels. We quantified early-life disease exposure as the detrended child mortality rate from infectious diseases during an individual’s first 5 y, controlling for important social factors. We found no support for an association between early-life disease exposure and all-cause mortality risk after age 15 or 50. We also found no link between early-life disease exposure and probability of death specifically from cardiovascular disease, stroke, or cancer. Independent of survival, there was no evidence to support associations between early-life disease exposure and any of several aspects of reproductive performance, including lifetime reproductive success and age at first birth, in either males or females. Our results do not support the prevailing assertion that exposure to infectious diseases in early life has long-lasting associations with later-life all-cause mortality risk or mortality putatively linked to chronic inflammation. Variation in adulthood conditions could therefore be the most likely source of recent increases in adult life span.
In industrialized nations, human life expectancy at birth has increased by around 3 mo/y between 1840 and 2002 (1). Although this is linked to improvements in child survival rates, there have been considerable increases in the life expectancy of adults: In the United Kingdom in 1843, the mean expected age at death of a 20-y-old was 60, whereas in 2011 it was 81 (2, 3), a 50% increase in remaining life span. Longer adult life expectancy has been accompanied by increased incidence of previously rare chronic health problems, and thus identifying the drivers of enhanced life span is an important question for global health. Declines in adult mortality rate since the 18th century in Europe were more closely linked to year of birth than to the year in which mortality was assessed, leading to the hypothesis that “the expectation of life was determined by the conditions which existed during the child’s earlier years” (4). The “cohort morbidity phenotype” hypothesis suggests that the link between declining birth year mortality rate and increasing adult survival is due to reduced exposure to infectious diseases in early life and reduced lifelong burden of chronic inflammation (5–8). Thus, individuals experiencing exposure to infectious disease in early life are expected to have higher adulthood mortality risk.
It is hypothesized that early-life exposure to infectious diseases could increase later-life mortality risk through a number of physiological mechanisms (6). Bacterial and viral infections elicit inflammatory immune responses, and chronic inflammation is associated with atherosclerosis, increased risk of stroke, cardiovascular disease (CVD), and mortality (9). Thus, early-life disease exposure is expected to be linked to adult mortality risk from these causes in particular (6). Support for this hypothesis comes from studies showing that exposure to high levels of early-life cohort mortality or infection is associated with middle-age mortality from CVD (10, 11); that exposure to infection is associated with chronically high levels of inflammation (12, 13); and that high levels of inflammation are associated with CVD (14). This physiological mechanism is underpinned by the fact that natural selection should favor the expression of robust immune responses at young ages, even at the cost of lower later-life survival (15).
Building on such knowledge, studies have tested the cohort morbidity phenotype hypothesis by showing that early-life infections are associated with chronic inflammation and impaired later health (16, 17). A drawback is that these studies are performed on modern populations with good health care and therefore cannot estimate associations between early-life disease exposure and natural mortality risk. A second approach is to use historical data on natural-mortality preindustrial populations and to link early-life cohort mortality (a proxy for early-life disease exposure) with later-life mortality (18–20). Studies of historical populations have, however, experienced at least one of several shortcomings. First, many studies are based on population-level statistics, meaning they cannot identify factors allowing some individuals to live long lives despite being born in high-mortality cohorts. Second, most studies only consider all-cause cohort mortality rate as a measure of disease exposure, which may be driven by nutrition and social factors as well as disease. Third, it is often unknown which types of deaths are associated with early stressors, obfuscating the mechanism through which early-life disease exposure is associated with later mortality.
The majority of studies of early-life disease exposure have focused solely on associations with later-life mortality, despite the fact that selection on life-history strategy should act through both survival and reproductive success. Life-history theory predicts that high mortality risk in early life should lead to earlier age at maturity (21), but associations between early-life disease exposure and between-individual variation in reproductive success have been neglected. A series of studies have shown that early-life trauma, such as sibling mortality, parental separation, disadvantageous birth location, and famine (22–25), is associated with earlier age at first reproduction and/or larger family size, suggesting adoption of a “fast” life history in high-stress, high-mortality environments. In contrast, childhood infections may have a nonlethal but damaging impact upon physiology, which may impede reproduction during adulthood (26). These could include infections after birth leading to stunted growth in childhood and eventually reduced adult size (27), or even infections of focal individuals’ mothers leading to reduced birth weight, which may lead to reduced birth weight of focal individuals’ offspring (28, 29). Nevertheless, it has been shown that the direction of the association between final adult height and fertility is variable across populations (30).
We used church register data collected from seven Finnish populations across the 18th and 19th centuries to determine the association between early-life exposure to infectious diseases and (i) later-life mortality risk, (ii) cause of death, and (iii) reproductive success. These populations had no access to modern contraception or health care (31), and as a result ∼40% of children died of disease before age 15. Having survived to age 15, mean life span in our sample was ∼56 y and individuals produced an average of ∼4 surviving offspring, with >99% of individuals born to parents aged <50. We used cause-of-death data recorded by churches to adopt a measure of disease exposure based on the local probability of child death from infectious diseases during the early years of an individual’s life. Crucially, we controlled for other salient variables reflecting early-life conditions and known to influence fitness in these populations (SI Appendix, Fig. S1 and Table S1), including social class at birth (32), twin status (33), interbirth interval (34), and season of birth (35).
Results
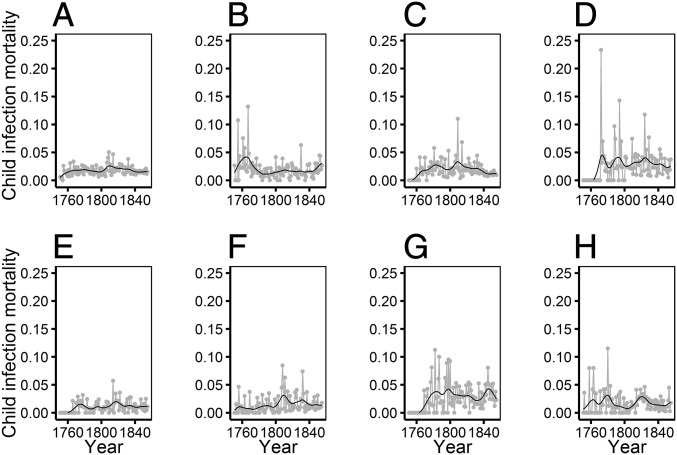
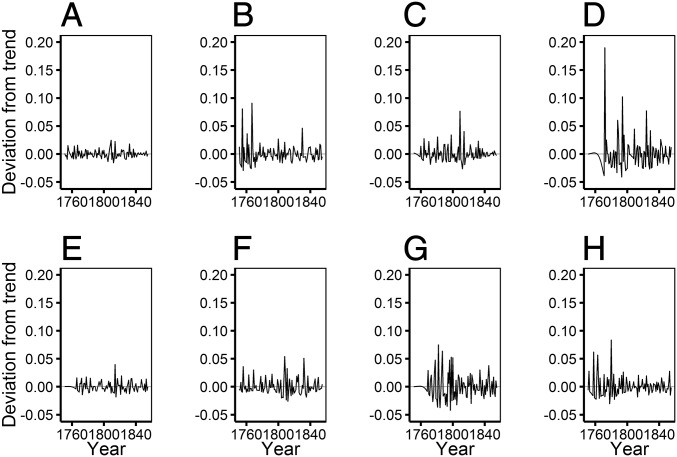
There was considerable between-year variation in the prevalence of deaths from infectious diseases in the early years of our study individuals’ lives. We used these data to calculate a measure of early-life exposure to infectious diseases for each of the first 5 y of life of each individual based on the proportion of children dying from infectious diseases in their parish in each year. We recorded the number of children aged 0–10 alive in each parish in each year, 1751–1855 (a total of 195,170 person-y). In 194,182 (>99.5%) cases, the child had a known cause of death or survived, whereas in the remaining 988 cases, the child died of an unknown cause; these 988 cases were not included in our calculations of early-life disease exposure (SI Appendix, Table S2). During these years, 4,780 child deaths of known cause were recorded, with 73% due to infectious diseases (SI Appendix, Table S3). We calculated annual parish-specific variation in child mortality rate from infectious diseases by dividing the number of child (aged 0–10) deaths in each parish due to infections in each year, 1751–1855, by the number of children alive in each parish in the same year. The child mortality rate from infectious diseases varied substantially across the study period in all seven parishes (Fig. 1). We detrended the child mortality rate from infectious diseases by applying a Hodrick–Prescott filter to data from each parish using the R package “mFilter”, with a filtering term of 100. This decomposed variation in child mortality rate from infectious diseases in each parish into a long-term smoothed “trend” component (Fig. 1) and the short-term deviation from the trend, or the “cycle” (Fig. 2). The cycle component for each year was multiplied by 100 to aid model convergence, and we refer to this measure herein as annual “disease exposure” (Fig. 2). Each individual was therefore defined by their potential early exposure to infections, rather than simply the level of all-cause cohort mortality (8, 18, 19), on a continuous quantitative scale, accounting for temporal changes in conditions and specific to the parish in which they were born. We designed analyses to determine whether our measure of disease exposure in each of the first 5 y of life was associated with later-life survival and reproductive success.
Fig. 1.
Changes in child mortality from infectious disease in each year, 1751–1855, and calculation of our measure of early-life disease exposure. Child (aged 0–10) mortality rate from infectious disease varied substantially across the early lives of our study individuals. Gray points and lines show the proportion of children alive in a given year who died of an infectious disease; black lines show the trend component of Hodrick–Prescott decomposition of the time series. Data and trends were calculated and are shown separately for (A) all parishes combined, (B) Hiittinen, (C) Ikaalinen, (D) Jaakkima, (E) Kustavi, (F) Pulkkila, (G) Rautu, and (H) Rymättylä.
Fig. 2.
Deviations from the trend in child mortality from infectious disease in the early years of our study individuals’ lives, represented as the cycle component of the Hodrick–Prescott decomposition. The parish-specific deviations from the trend, our measure of early-life disease exposure, varied substantially across cohorts in (A) all parishes combined, (B) Hiittinen, (C) Ikaalinen, (D) Jaakkima, (E) Kustavi, (F) Pulkkila, (G) Rautu, and (H) Rymättylä.
Mortality Risk.
Our mixed-effects Cox model analyses showed that greater disease exposure in each of the first 5 y of life was not associated with increased mortality risk in adulthood (n = 7,283). Controlling for a higher mortality risk among poor individuals and males and variation between parishes as well as other fixed and random effects (Table 1), disease exposure measured in each of the first 5 y of life was not significantly associated with hazard of mortality after age 15, nor was the association between early-life disease exposure and mortality risk dependent upon parish, social class, or sex (SI Appendix, Table S4). When we restricted our analyses to individuals who survived to at least the age of 50 (n = 3,822) and who were therefore most likely to experience stroke, CVD, and cancer, we again found no evidence for an association between early-life disease exposure and mortality risk and no indication of interactions with individual parish, social class, or sex (SI Appendix, Table S4).
Table 1.
Results from a mixed-effects Cox model of mortality risk after age 15
| Variable | Hazard (95% CI) | χ2 | df | P |
| Fixed effects | ||||
| Parish, Hiittinen | 1.00 (1.00–1.00) | 103.22 | 6 | <0.001 |
| Parish, Ikkalinen | 0.92 (0.82–1.02) | |||
| Parish, Jaakkima | 1.32 (1.12–1.56) | |||
| Parish, Kustavi | 1.15 (1.01–1.31) | |||
| Parish, Pulkkila | 1.43 (1.26–1.62) | |||
| Parish, Rautu | 1.61 (1.36–1.91) | |||
| Parish, Rymättylä | 1.11 (0.97–1.27) | |||
| Social, rich | 1.00 (1.00–1.00) | 65.01 | 1 | <0.001 |
| Social, poor | 1.36 (1.26–1.46) | |||
| Sex, male | 1.00 (1.00–1.00) | 55.51 | 1 | <0.001 |
| Sex, female | 0.79 (0.75–0.84) | |||
| IBI, 0 | 1.00 (1.00–1.00) | 4.38 | 3 | 0.223 |
| IBI, 1 | 0.97 (0.89–1.06) | |||
| IBI, 2 | 0.92 (0.85–1.00) | |||
| IBI, 3 | 0.98 (0.99–1.08) | |||
| Twin, 0 | 1.00 (1.00–1.00) | 2.01 | 1 | 0.156 |
| Twin, 1 | 0.89 (0.76–1.05) | |||
| Birth season, low | 1.00 (1.00–1.00) | 0.86 | 1 | 0.355 |
| Birth season, high | 1.03 (0.96–1.11) | |||
| Disease year 1 | 1.02 (0.99–1.04) | 1.89 | 1 | 0.169 |
| Random effects | Variance (SD) | |||
| Maternal ID | 0.11 (0.34) | |||
| Birth year | 0.01 (0.10) |
Hazards and 95% confidence intervals (CIs) are shown for a one-unit change in the explanatory variable. Disease year 1, disease exposure in the year after birth.
Cause of Death.
Mixed-effects Cox models showed that there was no significant association between early-life disease exposure and cause of death. We tested for an association between disease exposure in each of the first 5 y of life and death from CVD, stroke, and cancer, on which the cohort morbidity phenotype focuses (8). Individuals were scored as 1 (a “failure”) if they died of CVD, stroke, or cancer, and 0 if they died of other causes or were censored without dying (SI Appendix, Fig. S2 and Table S5). As above, we first performed the analysis on all individuals who survived to at least the age of 15. There was no statistical support for an association between early-life disease exposure in any of the first 5 y of life and death from CVD, stroke, or cancer (SI Appendix, Table S6). Although there was some evidence to support an interaction between early disease exposure and social class [disease year 4–social class (poor) hazard 0.79; 95% confidence interval 0.62–1.00; χ21 = 4.17; P = 0.0.041], suggesting that poor individuals with higher early-life disease exposure in the fourth year of life were less likely to later die of infection (SI Appendix, Table S7), this interaction was only weakly statistically supported, with an Akaike Information Criterion value relative to the base model (ΔAIC) of −2.02 (36). Nevertheless, it suggested a trend for a mildly protective effect of early disease exposure and later death risk from CVD, stroke, or cancer in poor individuals. We also repeated this analysis on individuals who survived to at least age 50, because the majority (263/346) of individuals affected by these death causes were aged over 50. Once again, there was no evidence that early-life disease exposure was associated with cause of death, nor any evidence for interactions between disease exposure and parish, social class, or sex (SI Appendix, Table S6).
Lifetime Reproductive Success.
We next assessed links between early-life disease exposure and later-life reproductive success in individuals who married and survived to at least age 50 and who had therefore completed their reproductive life spans with the opportunity to reproduce. We analyzed data from females and males separately, due to their differing social and physiological factors affecting reproductive schedules. First, we used linear mixed-effects models to show that there was no association between early-life disease exposure and Box–Cox–transformed lifetime reproductive success (LRS) in females (n = 1,512), defined as the number of offspring an individual produced who survived to age 15 (best-fitting single year; disease year 1 estimate −0.0202 ± 0.0112; χ21 = 3.25; P = 0.071; SI Appendix, Table S8). This model controlled for variation between parishes (χ26 = 68.60; P < 0.001), lower LRS in poor compared with rich individuals (estimate −0.24 ± 0.04; χ21 = 44.77; P < 0.001), and nonsignificant effects of birth interval, birth season, and twinning status. There was also no statistical support for interactions between early disease exposure and social class or parish (SI Appendix, Table S8). In males (n = 1,333), there was once again no support for an association between early-life disease exposure and LRS (best-fitting single year; disease year 4 estimate 0.0068 ± 0.0118; χ21 = 0.33; P = 0.563), nor any support for interactions with social class or parish (SI Appendix, Table S8).
We next determined whether there were any links between early-life disease exposure and other components of reproduction that constitute LRS, namely age at first reproduction, number of children born, or child survival rate. In neither sex, however, did we find any support for associations between early-life disease exposure and any of these measures of reproductive success, either as a main effect or in interactions with social class or parish (SI Appendix, Tables S9–S11).
Discussion
It has been hypothesized that recent increases in human life span have been brought about by reduced exposure to infectious diseases in early life and reduction of lifelong inflammation and associated chronic diseases such as CVD, stroke, and cancer (5–8). Tests of this hypothesis have used data from historical populations to support a link between cohort all-cause mortality rate and later-life survival, but have not assessed later-life survival in relation to measures of early-life disease exposure based on disease prevalence or determined how early disease exposure is linked to mortality risk specifically from CVD, stroke, and cancer. Studies of how early-life disease exposure may impact upon later-life reproductive success are also extremely rare. We used data from seven preindustrial Finnish populations and addressed these shortcomings by (i) quantifying variation in early-life disease exposure as the proportion of children alive in each of the first 5 y of an individual’s life who died of infectious diseases; (ii) assessing links between disease exposure and cause of death; and (iii) determining the association between early-life disease exposure and reproduction. Our results show that individuals experiencing higher early-life disease exposure do not experience higher all-cause mortality risk or risk of mortality from CVD, stroke, or cancer during adulthood. We also found no support for associations between early-life disease exposure and reproductive performance in either males or females. Our results do not support the link between early-life disease exposure and chronic inflammation-associated mortality risk, which forms the crux of the cohort morbidity phenotype hypothesis.
Our analyses showed that, controlling for individual parish, social class, sex, birth season, twinning status, birth interval, and temporal changes in disease exposure (Fig. 1), there was no statistical support for an association between early-life disease exposure and mortality risk after age 15 (Table 1 and SI Appendix, Table S4). Previous studies have found evidence for links between early-life cohort mortality rate and later survival (4, 5, 8), but without considering that such a measure of disease exposure could be confounded by spatial variation, social status, other intrinsic and extrinsic conditions as well as disease exposure, and improvements in living conditions and hygiene across time. Demographic studies have applied statistical techniques such as Hodrick–Prescott decomposition to trending environmental data and found generally weak associations between early and later cohort mortality (37–40), although some of these studies have still detected significant correlations (19, 41). By applying Hodrick–Prescott decomposition, we were able to account for declines in death rates from infection across time by using deviations from this trend as our measure of early-life disease exposure (Fig. 2). Unlike these studies, however, we use a measure of disease exposure explicitly based on prevalence of deaths from the infectious diseases most likely to affect infants (SI Appendix, Tables S2 and S3).
Early-life disease exposure may influence later-life health in a number of ways, and it is this complexity that may have prevented us from detecting significant associations. For example, early infections may have negative associations with later health through chronic physiological “scarring,” but may also have positive associations due to acquired immunity or selection of the most robust individuals who were able to survive the harsh early environment (42). There may also be changes in the factors that influence mortality in different parts of the life course, such that the relative importance of scarring and selection may vary with age (43) and the effects of early adversity may be short-lived, or be negligible in comparison with events that occur in adult life (39). A study using data from Sweden, collected 1813–1968 and calculating early-life disease risk based on neonatal mortality rates, showed that effects of exposure on mortality varied with age: Individuals born during times of higher neonatal mortality rates showed higher mortality initially but lower mortality in later life (44). These results suggest initial scarring effects of disease at younger ages, followed by a strengthening influence of acquired immunity and/or selection of robust individuals at later ages. Thus, rather than being unimportant for later-life survival, early-life infections may have both negative (through scarring) and positive (through acquired immunity and/or selection) associations with later survival, which may combine to culminate in no detectable overall effect.
The cohort morbidity phenotype (5–8) proposes a link between infection, chronic inflammation, and risk of CVD, stroke, and cancer (14). The exact prevalence of these diseases in our study population is unknown: Although 9.6% of deaths were due to CVD or stroke, a further 13.7% of deaths were due to “old age” and could have been related to chronic inflammation (SI Appendix, Table S5). Exposure to infection in early life has also been linked to respiratory disorders in later life (16, 17, 45), which is noteworthy given that 14.9% of deaths in our studied individuals were from tuberculosis (SI Appendix, Table S5). Focusing on deaths from CVD, stroke, and cancer, we did not find evidence that early-life disease exposure was associated with these causes of death, linked to chronic inflammation by the cohort morbidity phenotype (SI Appendix, Table S6). It should be noted that, with only 346/7,283 (5%) individuals scored as dying of CVD, stroke, or cancer, our statistical power to detect links between early disease exposure and cause of death may have been low. Meanwhile, studies of contemporary populations experiencing high exposure to infection and high levels of inflammatory markers rarely show signs of CVD despite their short life spans, suggesting that a low level of CVD, stroke, and cancer is simply the ancestral state and that high prevalence of these conditions may be a consequence of longer life spans in industrialized populations (46). Finally, it may be possible that adverse effects of chronic inflammation are only apparent in populations experiencing resource-rich or pathogen-poor environments, and that in our (relatively) resource-poor and pathogen-rich environments, heightened inflammatory responses are advantageous or neutral. Our study individuals were born before 1850, and thus it is possible that our results may not be generalizable to later cohorts, who experienced increased adult nutrition and health care. On the other hand, if chronic inflammation does not lead to CVD, stroke, and cancer in low-nutrition, high-pathogen environments, then the cohort morbidity phenotype would not offer an explanation for longer modern life spans being due to reduced or delayed instances of these disorders.
The majority of studies of early-life disease exposure have focused solely on associations with mortality, and our results are among the first to address links between early-life disease exposure and reproductive success. We found, however, that associations between early-life disease exposure and lifetime reproductive success, age at first reproduction, lifetime breeding success, and child survival rate were absent in both sexes. Life-history theory predicts that high mortality risk in early life should lead to earlier age at maturity (21), which should be associated with higher LRS, predictions that have been supported by population- (23, 47) and individual-level studies showing that adverse early-life conditions such as experience of mortality, deprived upbringing, experience of famine, or chronic childhood illness are associated with earlier age at first birth and higher fertility (22, 23, 25). In contrast, early-life stressors such as low food availability have been linked to later-life health problems (48) and, in our study population, reduced reproductive success (49, 50). These effects may be mediated through neonatal infections that stunt growth and diminish later reproductive success (26, 27) or maternal infections that reduce the birth weight of focal individuals (28, 29). In a historical German population, birth during a high-mortality period was associated with lower child survivorship in males and lower lifetime births in females (51). Thus, adverse conditions in early pre- or postnatal life may slow growth and development, affect reproductive physiology, and postpone first birth (52). As with survival, the conflicting negative (scarring) and positive (selection) effects of disease exposure may underpin our finding of no overall association between early-life disease exposure and later-life reproductive success. Alternatively, the lack of association in the present study could indicate that links detected in previous studies were due to factors other than disease. A recent study on data from 251 20th-century English women used path analysis to show that although social class was linked to age at first birth and illness in the first year of life was linked to adult height, there was no direct or indirect effect of childhood illness on age at first birth or LRS (53). Our results (with n > 1,000) are therefore unlikely to reflect lack of statistical power, and it may therefore simply be that effects of early-life infections on reproduction are weak and overshadowed by socioeconomic factors.
Unlike many previous studies testing for links between early-life disease exposure and later-life fitness, we (i) incorporated data on disease prevalence into our measure of early-life disease exposure; (ii) determined associations between disease exposure and cause of death; (iii) assessed associations between disease exposure and reproductive success; (iv) controlled for several important social factors; (v) detrended our measure of early-life disease exposure to account for temporal trends; and (vi) examined early-life effects not only around birth but up to age 5. An “ideal” study would link full health records from each individual documenting previous illness with lifetime follow-up on survival and reproduction. Our results should be interpreted with the acknowledgment that infection of any individual in our population at any time is unknown. Instead, our measure of disease exposure assumes that a higher child mortality rate from infection meant that, on average, individuals experiencing such conditions were more likely to be exposed to infections. In addition, we included the 22% of child deaths due to “unknown diseases” in the “infectious” category because deaths from infection were generally more common than those due to other causes, especially in children (SI Appendix, Tables S3 and S5), but this may have introduced noise into our calculation of disease exposure if not all such unknown diseases were indeed infectious. It therefore remains possible that inability to detect an association between early disease exposure and fitness is due to our way of calculating early disease exposure. Ability to accurately determine cause of death with poor medical knowledge is a general caveat of using historical data. These two caveats aside, a strength is that we have used parish-specific records rather than nationwide figures, thereby gaining the most geographically restricted estimate of disease exposure possible.
The results of our study strongly challenge the hypothesis that links between early- and later-life mortality rates are mediated through effects of early-life infectious diseases on chronic inflammation. Longitudinal study of contemporary populations will be essential for detecting the mechanisms underpinning such effects (53), for example for disentangling the role of early and later conditions in influencing mortality risk. With the increasing availability of national, individual-based health data, this endeavor will be achievable in a more explicit manner than shown here. Our study, however, uses data from 100 cohorts of a preindustrial population and controls for variation in social class and birth circumstances to provide a unique snapshot of how early-life conditions may have influenced human populations for much of our evolutionary history (6, 7). We conclude, in agreement with several other authors (37, 39, 40), that the influence of improved adult, rather than childhood, conditions is likely to have driven recent improvements in life expectancy.
Materials and Methods
Study Population and Data Collection.
We investigated associations between early-life disease exposure and later-life survival and reproductive success using longitudinal data collected from 18th- to 19th-century church records in seven populations, termed “parishes,” located across the southern half of Finland. Before the 1870s, these populations had high birth and death rates, primitive agricultural technology, and unreliable contraception and medical care (31) and were strictly monogamous. Individuals were divided into two social classes: “Rich” individuals included farm owners, merchants, and craftsmen, and “poor” individuals included crofters and laborers (32).
Causes of Death and Early-Life Disease Exposure.
We have collected data including birth, parentage, marriage, reproduction, and death for a total of 72,564 individuals in the parishes of Hiittinen, Ikaalinen, Jaakkima, Kustavi, Pulkkila, Rautu, and Rymättylä born 1702–2012. We restricted our analyses to 21,539 individuals born after 1750, because before this records were patchy, and born before 1851, ensuring that we only used data from individuals with natural mortality and fertility (31). We further restricted our analyses to individuals who survived to at least age 15 (15,237) and those of known maternal identity and social class (n = 7,327). This was done to ensure that we captured longer-term effects of disease exposure and to control for variation in survival and reproductive success between families and social classes (32). Finally, because birth order (34), twinning status (33), and birth season (35) are all linked to fitness in this population, we restricted our analyses to individuals for whom this information was recorded, leaving a final sample size of 7,283 individuals, born 1751–1850. None of our study individuals moved between parishes between birth and adulthood.
Based on previous studies examining links between early exposure to mortality and later-life health and fitness (19, 38–40), we aimed to estimate an individual’s experience of infectious diseases in their first 5 y of life, as illustrated by the prevalence of death from infectious diseases recorded by the church (54). Because children and adults are vulnerable to different diseases in this population (SI Appendix, Tables S3 and S5), we recorded the number of individuals aged 0–10 y who were alive in the parish in each year, 1751–1855, a total of 194,182 person-y (SI Appendix, Table S2), during which time 4,780 children died of a known cause, 73% of which were due to infectious diseases (SI Appendix, Table S3); 22% of deaths were from an unknown disease, which we included in the infectious category. We gained a parish-specific measure of disease exposure for each year by dividing the number of children dying of infectious diseases in a given year in a given parish by the number of children alive in the same parish in the same year (Fig. 1). Thus, our measure of early-life disease exposure is based on the child mortality rate from infectious diseases during an individual’s early life. We then detrended this measure using Hodrick–Prescott decomposition, and used the deviations from the trend (the cycle component) as our measure of disease exposure (Fig. 2). Early-life disease exposure was multiplied by 100 before use in analysis to aid model convergence.
Statistical Analysis.
We determined the association between disease exposure in each of the first 5 y of life and later-life fitness, controlling for other potentially confounding effects (SI Appendix, Table S1). All models described below were mixed-effects models with maternal identity and birth year as random effects, to account for between-family and -cohort variation in fitness. All models estimated the association between early-life disease exposure and fitness after controlling for the following fixed effects, which were retained in all models. Parish (seven levels), social class (rich or poor), and sex (male or female) were all included as categorical fixed effects. Because child survival is higher for those born in August, September, and October compared with other months (35), birth season was included as a two-level categorical variable (high or low survival). First-born males have higher prospects of wealth inheritance, marriage, and reproduction in this population, and individuals born with a longer intervening period after their previous sibling may have higher fitness (34). We incorporated both of these effects into a four-level categorical variable, interbirth interval (IBI; firstborn; born <2 y after sibling; born <3 y after sibling; born >3 y after sibling). Finally, all models accounted for the link between twin status at birth and later-life fitness (33) by including twin status at birth (singleton or twin) as a categorical fixed effect. All analyses were conducted in R version 3.2.3 (https://www.r-project.org/).
Mortality Risk.
We determined the association between early-life disease exposure and mortality risk after the age of 15 in 7,283 individuals using mixed-effects Cox models in the R package coxme. We constructed a “base” model containing the fixed and random effects described above (model 0) and then built a series of models testing the association between early-life disease exposure and mortality risk. We considered disease exposure in each of the first 5 y of an individual’s life as separate variables: year 0 (birth year), year 1, year 2, year 3, and year 4. We first tested for main effects of disease exposure in each of the first 5 y of life separately by (models 1–5) comparing the base model with separate models containing the disease exposure of each of the first 5 y, fitted as covariates, using likelihood ratio tests (LRTs), where the χ2-distributed test statistic is calculated as −2*(LogLikmodel1 − LogLikmodel2). We next (model 6) fitted all 5 disease-exposure years as covariates in the same model and compared model 6 with model 0 using an LRT. We determined which of models 1–5 fitted best by comparing their Akaike information criterion (AIC) values, where the lowest value provided the best fit (36). We did not encounter any situation where more than one of models 1–5 outperformed model 0. This allowed us to determine which disease-exposure year was most strongly associated with mortality risk. We took the best-fitting disease-exposure year forward into model 7, which tested for a nonlinear association between the best-fitting disease-exposure year and survival by comparing the model with a linear term of disease exposure to one with both linear and quadratic terms. Finally, we tested for interactions involving the best-fitting disease-exposure year by comparing the model with the main effect of the best-fitting disease-exposure year with models with interactions between disease exposure and parish (model 8), social class (model 9), and sex (model 10). We subsequently restricted the analyses to individuals who lived to at least age 50 (n = 3,822), because these individuals are most likely to fall victim to CVD, stroke, and cancer.
Cause of Death.
We next aimed to determine whether early-life disease exposure was associated with increased risk of death from stroke, CVD, or cancer versus other causes. Each individual was scored as dying from stroke, CVD, or cancer (1; 346 individuals), with the remainder (6,937 individuals) scored as 0. Cause-of-death analyses were performed using mixed-effects Cox models, following the same fixed- and random-effects structure and model selection procedure as described above. We then repeated this analysis on individuals who survived until at least the age of 50, because 263/346 (76%) of deaths from these causes occurred in individuals aged 50 and over.
Lifetime Reproductive Success.
We next determined the association between early-life disease exposure and later-life reproductive success by quantifying lifetime reproductive success as the number of children an individual produced who survived to age 15. From the original sample size of 7,283, we removed data from 1,200 individuals who were unmarried or whose marriage status was unknown; 488 individuals who had unknown LRS; 1,417 individuals whose reproductive success was unknown or whose children had unknown fates to age 15; and 1,333 individuals who failed to survive to the age of 50 and therefore complete their reproductive life span (49). We therefore focused on fertility differences within marriage to determine differences in reproductive capacity, rather than “mating success” (or marriage), which is influenced by a host of social and cultural factors that contribute to finding a partner. The final dataset for analysis of LRS contained 2,845 individuals, with 1,512 females and 1,333 males. The analyses were separated by sex due to the biological and social differences governing variation in reproductive success between the sexes in this population. We visually inspected diagnostic plots of several different modeling structures, including linear mixed-effects models (LMMs) of untransformed LRS; LMMs of log-transformed LRS; LMMs of Box–Cox–transformed LRS; and generalized linear mixed-effects models of untransformed LRS with Poisson, Poisson-lognormal, and negative binomial error structures. In both males and females, Box–Cox transformation provided the most favorable diagnostics, so LRS was modeled using LMMs of (LRS+1)0.5 in the R package lme4. The fixed- and random-effects structures of models 1–9 were the same as for the Cox models described above, although because we performed separate analysis for males and females, we did not fit model 10, which included the interaction between early-life disease exposure and sex.
Finally, we tested for associations between early-life disease exposure and the reproductive traits that contribute to LRS. We therefore performed analyses determining whether early-life disease exposure was associated with later age at first reproduction, reduced lifetime breeding success (number of children born), and/or child survival rate to age 15. These analyses are described in full in SI Appendix.
Supplementary Material
Acknowledgments
We thank Kimmo Pokkinen, Aino Siitonen, Timo Verho, Veli-Pekka Toropainen, Lasse Iso-Iivari, Sinikka Toijonen, Jarmo Piippo, and the Karelian Database for collection of the church record data and three reviewers for comments on previous versions that have greatly improved the manuscript. This research was funded by the Academy of Finland (V.L.) and Wellcome Trust (A.D.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data have been deposited in the Dryad Data Repository (www.datadryad.org), dx.doi.org/10.5061/dryad.966qt.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519820113/-/DCSupplemental.
References
- 1.Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296(5570):1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- 2.Kertzer DI, Laslett P. Aging in the Past: Demography, Society and Old Age. Univ of California Press; Berkeley, CA: 1995. [Google Scholar]
- 3.Roser M. 2016 Life expectancy. OurWorldInData.org. Available at ourworldindata.org/data/population-growth-vital-statistics/life-expectancy/. Accessed February 3, 2016.
- 4.Kermack WO, McKendrick AG, McKinlay PL. Death-rates in Great Britain and Sweden. Some general regularities and their significance. Lancet. 1934;223(5770):698–703. doi: 10.1093/ije/30.4.678. [DOI] [PubMed] [Google Scholar]
- 5.Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proc Natl Acad Sci USA. 2006;103(2):498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finch CE. Evolution of the human lifespan and diseases of aging: Roles of infection, inflammation, and nutrition. Proc Natl Acad Sci USA. 2010;107(Suppl 1):1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finch CE. Evolution of the human lifespan, past, present, and future: Phases in the evolution of human life expectancy in relation to the inflammatory load. Proc Am Philos Soc. 2012;156(1):9–44. [PubMed] [Google Scholar]
- 8.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305(5691):1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 9.Vasto S, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128(1):83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Mazumder B, Almond D, Park K, Crimmins EM, Finch CE. Lingering prenatal effects of the 1918 influenza pandemic on cardiovascular disease. J Dev Orig Health Dis. 2010;1(1):26–34. doi: 10.1017/S2040174409990031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myrskylä M, Mehta NK, Chang VW. Early life exposure to the 1918 influenza pandemic and old-age mortality by cause of death. Am J Public Health. 2013;103(7):e83–e90. doi: 10.2105/AJPH.2012.301060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurven M, Kaplan H, Winking J, Finch C, Crimmins EM. Aging and inflammation in two epidemiological worlds. J Gerontol A Biol Sci Med Sci. 2008;63(2):196–199. doi: 10.1093/gerona/63.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasunilashorn S, et al. Blood lipids, infection, and inflammatory markers in the Tsimane of Bolivia. Am J Hum Biol. 2010;22(6):731–740. doi: 10.1002/ajhb.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danesh J, et al. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. BMJ. 2000;321(7255):199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution (N Y) 1957;11:398–411. [Google Scholar]
- 16.Barker DJ, et al. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303(6804):671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Case A, Fertig A, Paxson C. The lasting impact of childhood health and circumstance. J Health Econ. 2005;24(2):365–389. doi: 10.1016/j.jhealeco.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Beltrán-Sáncheza H, Crimmins E, Finch C. Early cohort mortality predicts the rate of aging in the cohort: A historical analysis. J Dev Orig Health Dis. 2012;3(5):380–386. doi: 10.1017/S2040174412000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bengtsson T, Lindström M. Airborne infectious diseases during infancy and mortality in later life in southern Sweden, 1766–1894. Int J Epidemiol. 2003;32(2):286–294. doi: 10.1093/ije/dyg061. [DOI] [PubMed] [Google Scholar]
- 20.Bengtsson T, Lindström M. Childhood misery and disease in later life: The effects on mortality in old age of hazards experienced in early life, southern Sweden, 1760–1894. Popul Stud (Camb) 2000;54(3):263–277. doi: 10.1080/713779096. [DOI] [PubMed] [Google Scholar]
- 21.Stearns SC. The Evolution of Life Histories. Oxford Univ Press; Oxford: 1992. [Google Scholar]
- 22.Störmer C, Lummaa V. Increased mortality exposure within the family rather than individual mortality experiences triggers faster life-history strategies in historic human populations. PLoS One. 2014;9(1):e83633. doi: 10.1371/journal.pone.0083633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nettle D. Dying young and living fast: Variation in life history across English neighborhoods. Behav Ecol. 2010;21(2):387–395. [Google Scholar]
- 24.Nettle D, Coall DA, Dickins TE. Early-life conditions and age at first pregnancy in British women. Proc Biol Sci. 2011;278(1712):1721–1727. doi: 10.1098/rspb.2010.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Painter RC, et al. Increased reproductive success of women after prenatal undernutrition. Hum Reprod. 2008;23(11):2591–2595. doi: 10.1093/humrep/den274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan AD, Schroeder DG, Martorell R, Haas JD, Rivera J. Early childhood determinants of age at menarche in rural Guatemala. Am J Hum Biol. 1996;8(6):717–723. doi: 10.1002/(SICI)1520-6300(1996)8:6<717::AID-AJHB3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Beard AS, Blaser MJ. The ecology of height: The effect of microbial transmission on human height. Perspect Biol Med. 2002;45(4):475–498. doi: 10.1353/pbm.2002.0064. [DOI] [PubMed] [Google Scholar]
- 28.Nohr EA, Vaeth M, Rasmussen S, Ramlau-Hansen CH, Olsen J. Waiting time to pregnancy according to maternal birthweight and prepregnancy BMI. Hum Reprod. 2009;24(1):226–232. doi: 10.1093/humrep/den357. [DOI] [PubMed] [Google Scholar]
- 29.Ekholm K, Carstensen J, Finnström O, Sydsjö G. The probability of giving birth among women who were born preterm or with impaired fetal growth: A Swedish population-based registry study. Am J Epidemiol. 2005;161(8):725–733. doi: 10.1093/aje/kwi096. [DOI] [PubMed] [Google Scholar]
- 30.Sear R. Height and reproductive success: Is bigger always better? In: Frey U, Stoermer C, Willfuehr K, editors. Homo Novus—A Human Without Illusions. Springer; Berlin: 2010. pp. 127–143. [Google Scholar]
- 31.Korpelainen H. Human life histories and the demographic transition: A case study from Finland, 1870–1949. Am J Phys Anthropol. 2003;120(4):384–390. doi: 10.1002/ajpa.10191. [DOI] [PubMed] [Google Scholar]
- 32.Pettay JE, Helle S, Jokela J, Lummaa V. Natural selection on female life-history traits in relation to socio-economic class in pre-industrial human populations. PLoS One. 2007;2(7):e606. doi: 10.1371/journal.pone.0000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lummaa V, Jokela J, Haukioja E. Gender difference in benefits of twinning in pre-industrial humans: Boys did not pay. J Anim Ecol. 2001;70(5):739–746. [Google Scholar]
- 34.Faurie C, Russell AF, Lummaa V. Middleborns disadvantaged? Testing birth-order effects on fitness in pre-industrial Finns. PLoS One. 2009;4(5):e5680. doi: 10.1371/journal.pone.0005680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lummaa V, Lemmetyinen R, Haukioja E, Pikkola M. Seasonality of births in Homo sapiens in pre-industrial Finland: Maximisation of offspring survivorship? J Evol Biol. 1998;11(2):147–157. [Google Scholar]
- 36.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information Theoretic Approach. 2nd Ed Springer; New York: 2002. [Google Scholar]
- 37.Gagnon A, Mazan R. Does exposure to infectious diseases in infancy affect old-age mortality? Evidence from a pre-industrial population. Soc Sci Med. 2009;68(9):1609–1616. doi: 10.1016/j.socscimed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Myrskylä M. The effects of shocks in early life mortality on later life expectancy and mortality compression: A cohort analysis. Demogr Res. 2010;22:289–320. [Google Scholar]
- 39.Myrskylä M. The relative effects of shocks in early- and later-life conditions on mortality. Popul Dev Rev. 2010;36(4):803–829. doi: 10.1111/j.1728-4457.2010.00358.x. [DOI] [PubMed] [Google Scholar]
- 40.Bruckner TA, Catalano RA. Infant mortality and diminished entelechy in three European countries. Soc Sci Med. 2009;68(9):1617–1624. doi: 10.1016/j.socscimed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Bengtsson T, Broström G. Do conditions in early life affect old-age mortality directly and indirectly? Evidence from 19th-century rural Sweden. Soc Sci Med. 2009;68(9):1583–1590. doi: 10.1016/j.socscimed.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Preston SH, Hill ME, Drevenstedt GL. Childhood conditions that predict survival to advanced ages among African-Americans. Soc Sci Med. 1998;47(9):1231–1246. doi: 10.1016/s0277-9536(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 43.Myrskylä M, Gagnon A, Bengtsson T. Pathways to health and well-being. Soc Sci Med. 2014;119:175–179. doi: 10.1016/j.socscimed.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 44.Quaranta L. Early life effects across the life course: The impact of individually defined exogenous measures of disease exposure on mortality by sex in 19th- and 20th-century southern Sweden. Soc Sci Med. 2014;119:266–273. doi: 10.1016/j.socscimed.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Costa DL. Understanding the twentieth-century decline in chronic conditions among older men. Demography. 2000;37(1):53–72. [PubMed] [Google Scholar]
- 46.Gurven M, et al. Inflammation and infection do not promote arterial aging and cardiovascular disease risk factors among lean horticulturalists. PLoS One. 2009;4(8):e6590. doi: 10.1371/journal.pone.0006590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Placek CD, Quinlan RJ. Adolescent fertility and risky environments: A population-level perspective across the lifespan. Proc Biol Sci. 2012;279(1744):4003–8. doi: 10.1098/rspb.2012.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: An overview. Reprod Toxicol. 2005;20(3):345–352. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Rickard IJ, et al. Food availability at birth limited reproductive success in historical humans. Ecology. 2010;91(12):3515–3525. doi: 10.1890/10-0019.1. [DOI] [PubMed] [Google Scholar]
- 50.Hayward AD, Rickard IJ, Lummaa V. Influence of early-life nutrition on mortality and reproductive success during a subsequent famine in a preindustrial population. Proc Natl Acad Sci USA. 2013;110(34):13886–13891. doi: 10.1073/pnas.1301817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Störmer C. Sex differences in the consequences of early-life exposure to epidemiological stress—A life-history approach. Am J Hum Biol. 2011;23(2):201–208. doi: 10.1002/ajhb.21103. [DOI] [PubMed] [Google Scholar]
- 52.Ibáñez L, Potau N, Enriquez G, Marcos MV, de Zegher F. Hypergonadotrophinaemia with reduced uterine and ovarian size in women born small-for-gestational-age. Hum Reprod. 2003;18(8):1565–1569. doi: 10.1093/humrep/deg351. [DOI] [PubMed] [Google Scholar]
- 53.Sheppard P, Pearce MS, Sear R. How does childhood socioeconomic hardship affect reproductive strategy? Pathways of development. Am J Hum Biol. 2016;28(3):356–363. doi: 10.1002/ajhb.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helle S, Lummaa V, Jokela J. Accelerated immunosenescence in preindustrial twin mothers. Proc Natl Acad Sci USA. 2004;101(33):12391–12396. doi: 10.1073/pnas.0402215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.