Significance
Despite in vitro evidence, it remains unclear whether the rod visual pigment rhodopsin exists as dimers in vivo. With a unique mouse line that expresses the blue cone opsin transgenically in rods, we showed that, in the absence of chromophore, the transgenic cone opsin can mature and target to the outer segment if and only if rhodopsin is present to help it along (i.e., forming heterodimers in the endoplasmic reticulum). Furthermore, we have confirmed a key interaction domain for rhodopsin dimerization and show that, again in vivo, rhodopsin maturation and targeting to the outer segment becomes defective when dimerization is disrupted. This work provides in vivo evidence for rhodopsin existing as a dimer.
Keywords: rhodopsin, cone opsin, dimerization, protein trafficking
Abstract
It is a deeply engrained notion that the visual pigment rhodopsin signals light as a monomer, even though many G protein-coupled receptors are now known to exist and function as dimers. Nonetheless, recent studies (albeit all in vitro) have suggested that rhodopsin and its chromophore-free apoprotein, R-opsin, may indeed exist as a homodimer in rod disk membranes. Given the overwhelmingly strong historical context, the crucial remaining question, therefore, is whether pigment dimerization truly exists naturally and what function this dimerization may serve. We addressed this question in vivo with a unique mouse line (S-opsin+Lrat−/−) expressing, transgenically, short-wavelength–sensitive cone opsin (S-opsin) in rods and also lacking chromophore to exploit the fact that cone opsins, but not R-opsin, require chromophore for proper folding and trafficking to the photoreceptor’s outer segment. In R-opsin’s absence, S-opsin in these transgenic rods without chromophore was mislocalized; in R-opsin’s presence, however, S-opsin trafficked normally to the rod outer segment and produced functional S-pigment upon subsequent chromophore restoration. Introducing a competing R-opsin transmembrane helix H1 or helix H8 peptide, but not helix H4 or helix H5 peptide, into these transgenic rods caused mislocalization of R-opsin and S-opsin to the perinuclear endoplasmic reticulum. Importantly, a similar peptide-competition effect was observed even in WT rods. Our work provides convincing evidence for visual pigment dimerization in vivo under physiological conditions and for its role in pigment maturation and targeting. Our work raises new questions regarding a potential mechanistic role of dimerization in rhodopsin signaling.
Rhodopsin and cone pigments mediate scotopic and photopic vision, respectively. They consist of opsin, the apo-protein, and 11-cis-retinal, a vitamin A-based chromophore. Light absorption by 11-cis-retinal triggers a conformational change in opsin, which in turn initiates a G protein-coupled receptor (GPCR) signaling pathway to lead to vision. Indeed, rhodopsin signaling is a prominent prototypical GPCR pathway from which a huge quantity of mechanistic details about such signaling in general has emerged. All along, it is a dogma that rhodopsin exists and functions as a monomer (1–6). About a decade ago, evidence began to emerge that rhodopsin may exist as a dimer, based on atomic force microscopy and cross-linking experiments performed on rod outer-segment (ROS) disk membranes (7–9). However, this concept remains highly controversial because of the lack of in vivo evidence and also is puzzling because, unlike many GPCRs, monomeric rhodopsin is fully functional with respect to coupling to G protein (2, 4–6, 10) and to interactions with rhodopsin kinase and arrestin (11, 12). In vivo evidence, albeit of paramount importance, is also challenging, because rhodopsin always exists as a single isoform in rod photoreceptors, thus making homomeric, higher-order complexes difficult to distinguish from monomers. We addressed this question by taking advantage of the unique opportunity provided by a mouse line (S-opsin+Lrat−/−) that expresses transgenically the short wavelength-sensitive cone opsin (S-opsin) in rods and by exploiting the fact that cone opsins, but not the apoprotein of rhodopsin R-opsin, require chromophore for proper folding and trafficking to the photoreceptor’s outer segment (13–17). Our work shows that: (i) rhodopsin, and by extension cone pigments, natively mature in vivo in the endoplasmic reticulum (ER) as dimers and traffic as such to the outer segment; (ii) in S-opsin+Lrat−/− transgenic rods, R-opsin helps S-opsin fold and traffic by forming heterodimers with it; and (iii) the H1 and H8 helices are important for pigment dimerization.
Results
Correct Targeting of S-Opsin to the Outer Segment of S-opsin+Rho+/−Lrat−/− and S-opsin+Rho+/+Lrat−/− Rods in the Absence of 11-cis-Retinal.
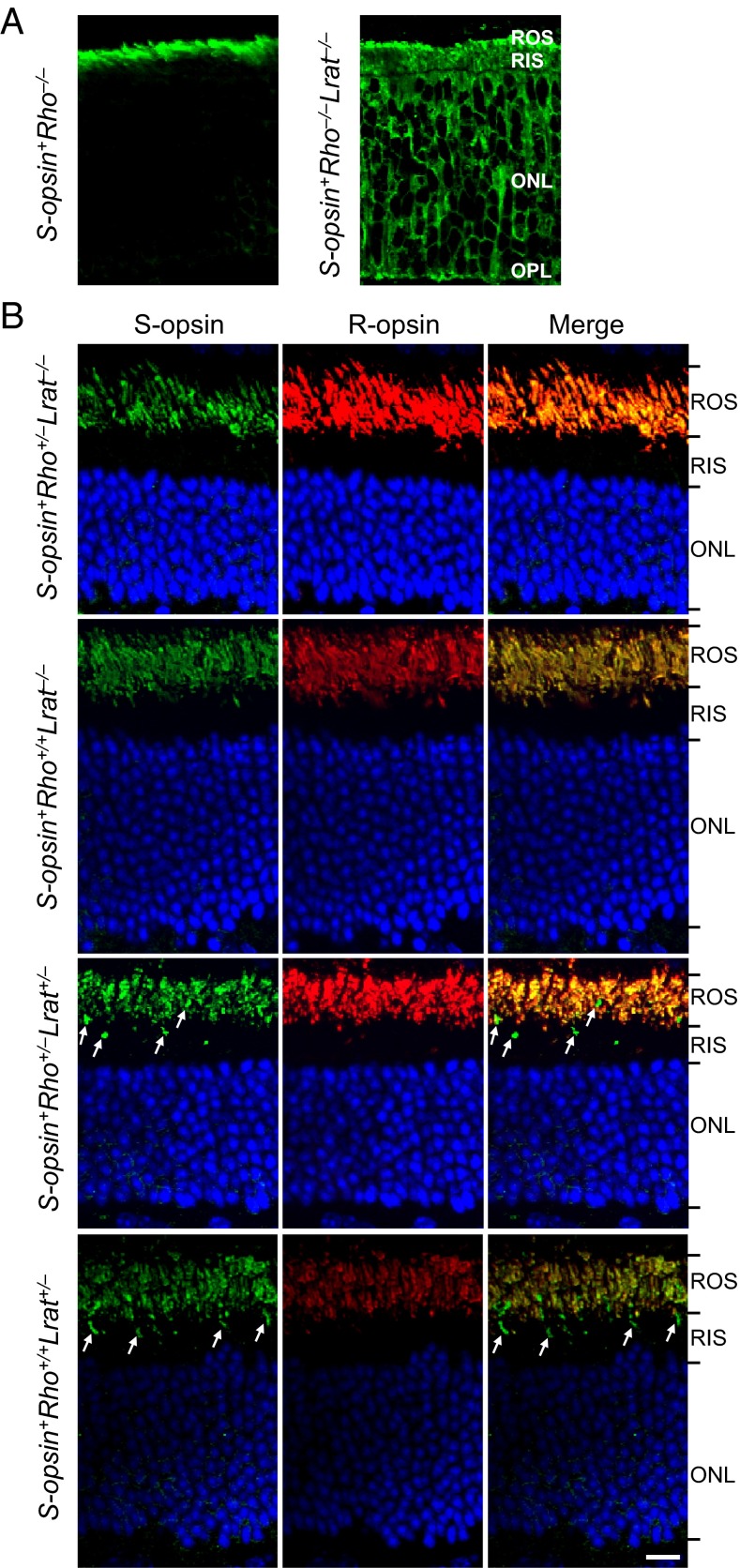
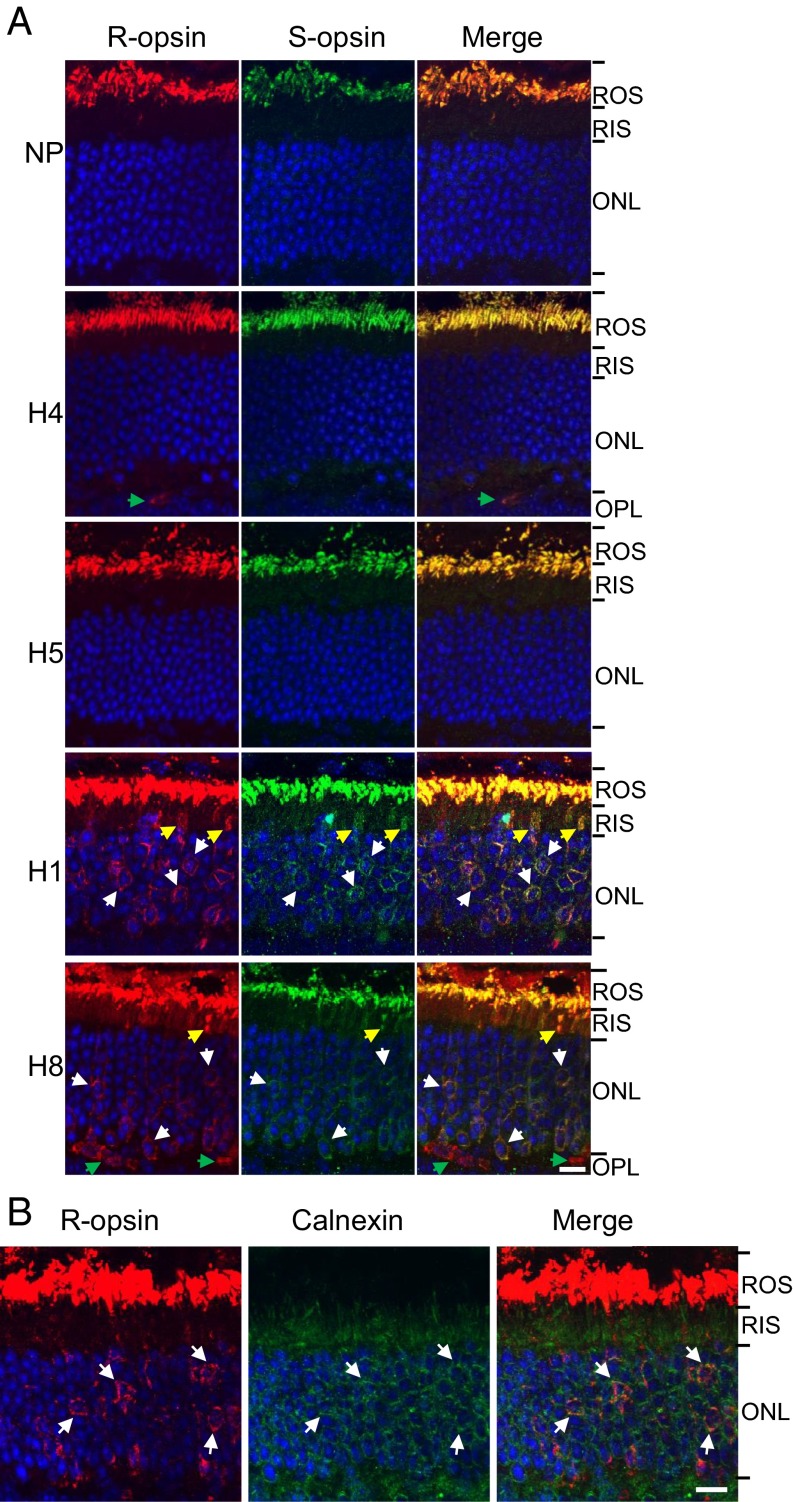
We generated various mouse lines by breeding S-opsin+ with Rho−/− and Lrat−/− mice (18–20). Lrat−/− mice lack lecithin-retinol acyltransferase, which is crucial for the regeneration of 11-cis-retinal in the retinal pigment epithelium (21). Previously, we and others found that transgenic cone pigment in rods and transgenic rhodopsin in cones, in an otherwise WT genetic background, signal much like the endogenous pigment in host cells except for a difference in the wavelength of peak absorbance (λmax) (18, 22, 23). In S-opsin+Rho−/−Lrat−/− rods, which have no endogenous R-opsin and no chromophore, immunolabeling with an S-opsin antibody (18) indicated S-opsin mislocalization to the inner segment, cell body, axon, and synaptic terminus in contrast to the correct rod outer segment (ROS) localization of S-opsin in S-opsin+Rho−/− rods (Fig. 1A). In the presence of R-opsin (i.e., in S-opsin+Rho+/−Lrat−/− or S-opsin+Rho+/+Lrat−/− rods), however, S-opsin trafficked properly to the ROS despite the absence of chromophore (Fig. 1B, Upper Two Rows), as it did when chromophore was present (S-opsin+Rho+/‒Lrat+/− and S-opsin+Rho+/+Lrat+/−) (Fig. 1B, Lower Two Rows; also see the viable cone outer segments indicated by white arrows when chromophore was present), presumably by heteromerizing with R-opsin. S-opsin and R-opsin heteromerization prevents rapid degeneration of S-opsin+Rho−/−Lrat−/− rods because of ER stress caused by misfolded and mistrafficked S-opsin [see Fig. S1 comparing the ROS, rod inner segments (RIS) and outer nuclear layer (ONL) in S-opsin+Rho−/−Lrat−/− and S-opsin+Rho+/−Lrat−/− mice at age 1 mo] (16). In the above experiments, we used S-opsin+Rho+/−Lrat−/− and S-opsin+Rho+/+Lrat−/− mice older than 1 mo in which most of the S-opsin–expressing cones had already died from the lack of chromophore, thus minimizing confounding signals from those cones (13, 15, 16). As a negative control for a generalized disruption of protein targeting to the ROS in the absence of chromophore and R-opsin, we also examined the localization of a rod cyclic nucleotide-gated cation channel subunit, CNGA1, a membrane protein mediating phototransduction in ROS. Even without R-opsin, and in either the absence (S-opsin+Rho−/−Lrat−/−) or presence (S-opsin+Rho−/−Lrat+/−) of chromophore, CNGA1 was targeted properly to the ROS (Fig. S2).
Fig. 1.
Trafficking of S-opsin in transgenic mouse rods of different genotypes. (A) S-opsin+Rho−/−Lrat−/− (i.e., lacking chromophore) and its control S-opsin+Rho−/− (i.e., containing chromophore). The age was postnatal day 14 (P14). (B) S-opsin+Rho+/−Lrat−/− and S-opsin+Rho+/+Lrat−/− (i.e., lacking chromophore) (two Upper rows) and their respective controls S-opsin+Rho+/−Lrat+/− and S-opsin+Rho+/+Lrat+/− (i.e., containing chromophore) (two Lower rows). The age was 2-mo-old. Retinal sections were stained with anti–S-opsin (green) in A and B and with anti–R-opsin (red) antibodies in B. Nuclei were stained with DAPI (blue) in B. S-opsin–positive cone photoreceptors (white arrows) are absent in S-opsin+Rho+/−Lrat−/− and S-opsin+Rho+/+Lrat−/− retinal sections lacking chromophore because of degeneration but are observable in S-opsin+Rho+/−Lrat+/− and S-opsin+Rho+/+Lrat+/− retinal sections containing chromophore. (Scale bar, 10 µm.)
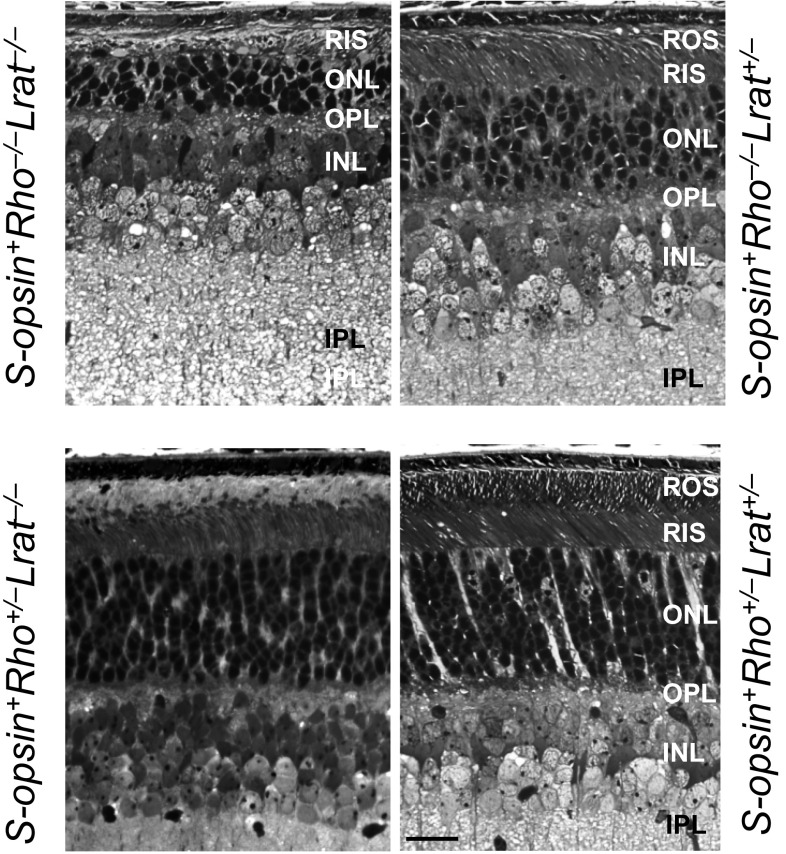
Fig. S1.
S-opsin and R-opsin dimerization prevents rapid rod degeneration. Retinal plastic sections (1 µm) of S-opsin+Rho−/−Lrat−/− and S-opsin+Rho−/−Lrat+/− mice (Upper) and S-opsin+Rho+/−Lrat−/− and S-opsin+Rho+/−Lrat+/− rods (Lower) at 1 mo were stained with Richardson’s stain. INL, inner nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer. (Scale bar, 20 µm.)
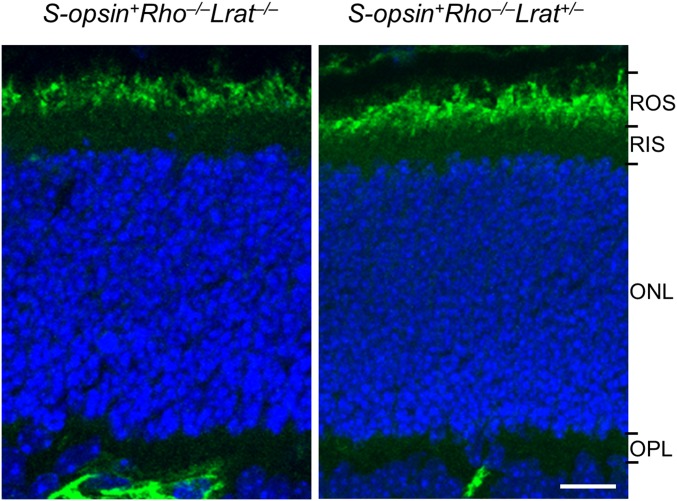
Fig. S2.
Immunolabeling of rod CNGA1 in P14 S-opsin+Rho−/−Lrat−/− and S-opsin+Rho−/−Lrat+/− mice. Mouse retinas were stained with a monoclonal antibody, 1D1, against rod CNGA1 subunit (green). Note the correct localization of CNGA1 in the ROS of S-opsin+Rho−/−Lrat−/− mice. Nuclei were stained with DAPI (blue). Green signals at or below the OPL were retinal blood vessels labeled by the Alexa 488-conjugated goat anti-mouse secondary antibody. (Scale bar, 10 µm.)
Dimerization of S-Opsin with R-Opsin in S-opsin+Rho+/−Lrat−/− Rods.
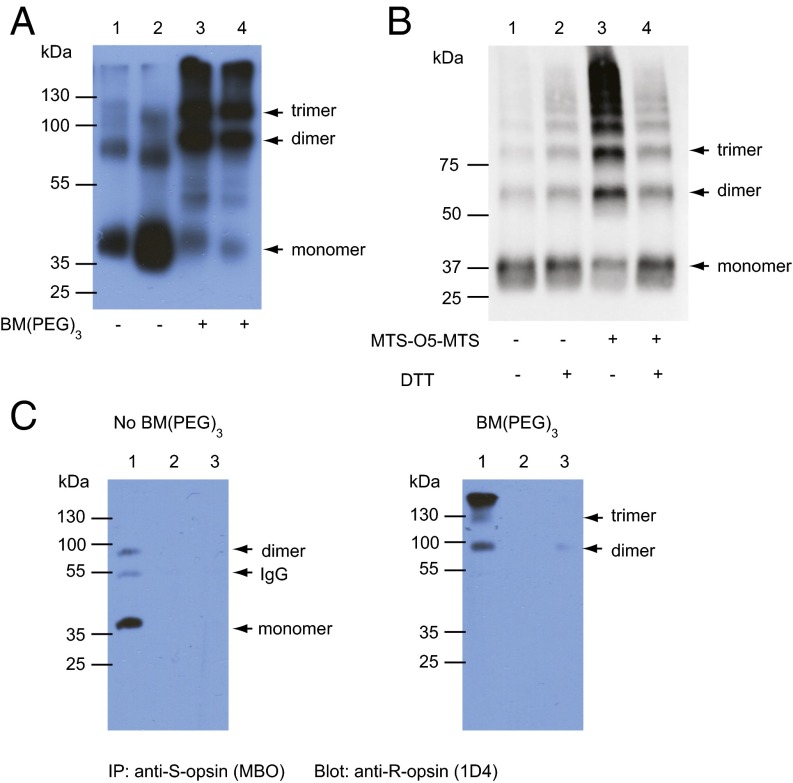
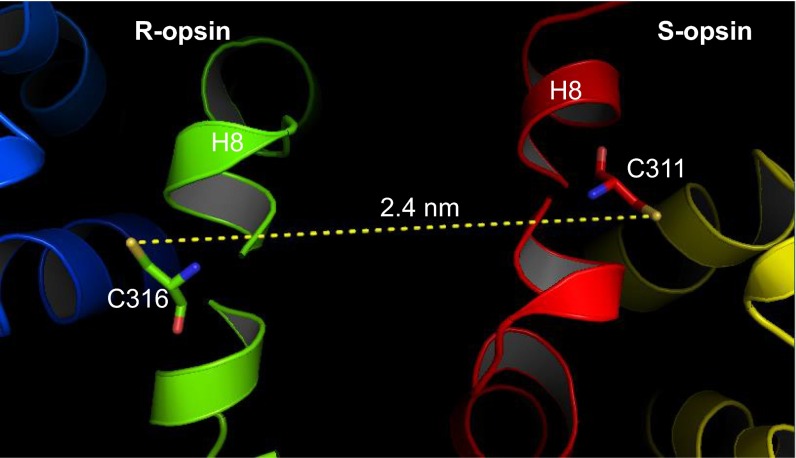
To probe for R/S-opsin heteromerization in transgenic rods, we performed cross-linking experiments on ROS membranes isolated from dark-adapted S-opsin+Rho+/−Lrat−/− mice using a noncleavable homobifunctional bis-maleimide reagent, BM(PEG)3 (24), followed by Western blot analysis with an anti–S-opsin (18) antibody. Without cross-linking, S-opsin was detected mainly as monomers in denaturing SDS/PAGE (Fig. 2A, lane 1). With cross-linking, we found S-opsin predominantly as dimers and oligomers, presumably mostly with R-opsin (Fig. 2A, lane 3). The reagent also cross-linked S-pigment in S-opsin+Rho−/−Lrat+/− rods (i.e., with chromophore but no R-opsin), in this case giving S-pigment homomers (Fig. 2A, lane 4). To confirm the specificity of cross-linking, we did cross-linking experiment using a cleavable methanethiosulfonate (MTS) cross-linker, MTS-O5-MTS (24). MTS-O5-MTS treatment shifted S-opsin from mainly monomers to dimers and oligomers (Fig. 2B, lane 3). DTT treatment, which cleaved the disulfide bond formed by MTS-O5-MTS, converted the higher-molecular-weight bands back to primarily monomers (Fig. 2B, lane 4), similar to samples without MTS-O5-MTS (Fig. 2B, lanes 1 and 2). This experiment suggests that the high-molecular-weight bands in the MTS-O5-MTS–treated samples were the result of cross-linking reactions and not nonspecific oligomerization. To confirm that the dimers in S-opsin+Rho+/−Lrat−/− ROS were indeed R/S-opsin heteromers, we performed immunoprecipitation on ROS membrane homogenate from these mice with the mouse blue opsin (MBO) antibody (18) followed by Western blot analysis with an anti–R-opsin antibody, 1D4 (25). Indeed, the anti–S-opsin antibody coimmunoprecipitated S-opsin and R-opsin in both non–cross-linked (Fig. 2C, Left, lane 1) and cross-linked ROS membrane fractions (Fig. 2C, Right, lane 1). The major bands were R-opsin monomers in the non–cross-linked samples but were R/S-opsin heterodimers and heterooligomers in the cross-linked samples, as is consistent with the results shown in Fig. 2A. As controls, no R-opsin was coimmunoprecipitated with S-opsin in the S-opsin+Rho−/−Lrat+/− sample (because R-opsin was absent) (Fig. 2C, lane 2 in both panels) or in the Rho+/−Lrat−/− sample (because S-opsin was absent) (Fig. 2C, lane 3 in both panels). Thus, S-opsin indeed heteromerized with R-opsin in S-opsin+Rho+/−Lrat−/− rods, underlying the former’s trafficking shown in Fig. 1. This property in turn suggests that R- and S-opsins must have sufficiently similar 3D topologies to allow R/S-opsin interactions (i.e., homologous to R/R-opsin interactions) and similar amino acid sequences for cross-linking. For example, both opsins have a key cysteine in H8 [C316 in R-opsin (24) and C311 in S-opsin] close enough to each other in a tail-to-tail dimer conformation for cross-linking by BM(PEG)3 and MTS-O5-MTS (Fig. 3 and Fig. S3). On the other hand, the presence of oligomers in the cross-linking experiments also may suggest higher-order complexes involving other cysteine pairs between the pigment proteins captured by the cross-linker (see ref. 24 and below).
Fig. 2.
Chemical cross-linking of cone and rod opsins in ROS membranes of different mouse lines followed by Western blot analysis. (A) Non–cross-linked (lanes 1 and 2) and cross-linked (lanes 3 and 4) S-opsin from ROS membranes of S-opsin+Rho+/−Lrat−/− mice (lanes 1 and 3) and S-opsin+Rho−/−Lrat+/− mice (lanes 2 and 4) following treatment with a noncleavable reagent, BM(PEG)3. The loading variability in different lanes was caused by the variable yield of the isolated ROS membrane fraction because the procedure was carried out in darkness. (B) Cross-linking of S-opsin in ROS membranes of S-opsin+Rho+/−Lrat−/− mice with a cleavable reagent, MTS-O5-MTS. Samples were analyzed in the absence (lanes 1 and 2) and presence (lanes 3 and 4) of cross-linker. The even-numbered lanes were treated with DTT before the gel was run. Arrows indicate the locations of monomers, dimers, and trimers. (C) Coimmunoprecipitation to detect dimeric interaction between S-opsin and R-opsin. Non–cross-linked and cross-linked ROS membranes from S-opsin+Rho+/−Lrat−/− (lane1), S-opsin+Rho−/−Lrat+/− (lane 2), and Rho+/−Lrat−/− (lane 3) mice were immunoprecipitated with an anti–S-opsin antibody, MBO, and were analyzed by immunoblotting with an anti–R-opsin antibody, 1D4. A very faint band in the cross-linked sample (Right, lane 3) is likely caused by some nonspecific interaction between the abundant R-opsin and protein A/G beads.
Fig. 3.
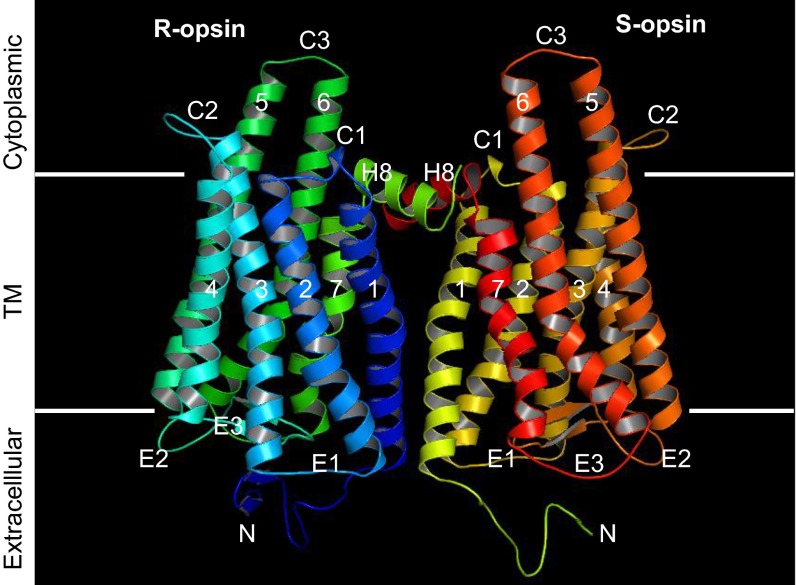
Structure of an R–S heterodimer via H1/H8 interface. The R–S heterodimer was modeled according to the crystal structure of the ligand-free opsin homodimer (PDB ID code 3CAP) (41) by homology modeling. The two monomers in the asymmetric unit are viewed from within the membrane. Seven transmembrane helices (1–7), extracellular loops (E1–E3), cytoplasmic loops (C1–C3), and a cytoplasmic helix (H8) are indicated. The figure was created with PyMOL (www.pymol.org/).
Fig. S3.
Predicted distance between Cys316 in rhodopsin and Cys311 in S-opsin in an R–S heterodimer. The distance of 2.4 nm is based on the R–S heterodimer model. In an R–R homodimer, the predicted C316–C316 distance is 2.1–2.9 nm (24). This cysteine pair was likely cross-linked by BM(PEG)3 or MTS-O5-MTS, whose extended S–S distance of 2.1 or 2.6 nm, respectively, is within the C316–C316 range.
Disruption by Helix Peptides of R- and S-Opsin Trafficking in S-opsin+Rho+/−Lrat−/− Rods.
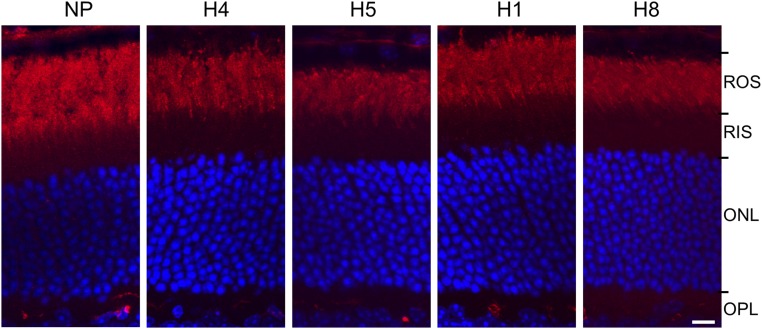
Two dimer interfaces between rhodopsin protomers have been suggested, with the first being formed by transmembrane helices 4 and 5 (i.e., H4/H5–H4/H5) (8, 9) and the second formed by transmembrane helix 1 and cytoplasmic helix 8 (i.e., H1/H8–H1/H8) (24). To determine whether the two interfaces were involved in the R/S-opsin interaction that we observed in vivo, we used peptides derived from these R-opsin domains to see whether they would disrupt, by competition (26–28), the R/S-opsin interaction and therefore trafficking. Each peptide was encapsulated in biodegradable polylactic acid-polyethylene oxide (PLA-PEO) nanoparticles (NPs) (29) for sustained delivery and was injected subretinally into S-opsin+Rho+/−Lrat−/− mouse eyes. Retinal sections were analyzed 6 d after injection. Both the H1 and H8 peptides caused mislocalization of R-opsin to the inner segment (yellow arrows in Fig. 4A) and the perinuclear ER region in the ONL (white arrows in Fig. 4A and white arrows indicating R-opsin colocalization with the ER marker Calnexin in Fig. 4B). S-opsin mislocalization was observed also (Fig. 4A). Injection of the carrier NPs alone (Fig. 4A) or of NPs encapsulating the rhodopsin H4 or H5 peptides (Fig. 4A) did not interfere with R-opsin and S-opsin trafficking.
Fig. 4.
Peptide-competition experiments in S-opsin+Rho+/−Lrat−/− rods. (A) Subretinal injection of NP-encapsulated R-opsin H1 and 8 peptides, but not R-opsin H4 and H5 peptides, caused mislocalization of both R-opsin and S-opsin in S-opsin+Rho+/−Lrat−/− rods. Mouse retinas were stained with anti–R-opsin (red) and anti–S-opsin (green) antibodies. White arrows indicate mislocalized R-opsin and S-opsin in the perinuclear region. Yellow arrows indicate mislocalized R-opsin and S-opsin in the inner segment. Green arrows indicate retinal blood vessels in the outer plexiform layer (OPL) labeled by the Cy3-conjugated goat anti-mouse secondary antibody. (B) Mislocalized R-opsin caused by subretinal injection of R-opsin H8 peptide colocalizes with the ER marker Calnexin in S-opsin+Rho+/−Lrat−/− rods. S-opsin+Rho+/−Lrat−/− mice were injected with NP-encapsulated R-opsin H8 peptide. Mouse retinas were stained with R-opsin (red) and Calnexin (green) antibodies. Nuclei were stained with DAPI (blue). White arrows indicate the colocalization of mislocalized R-opsin with Calnexin in the perinuclear region. (Scale bars, 10 µm.)
Functional S-Opsin After Trafficking to S-opsin+Rho+/−Lrat−/− ROS.
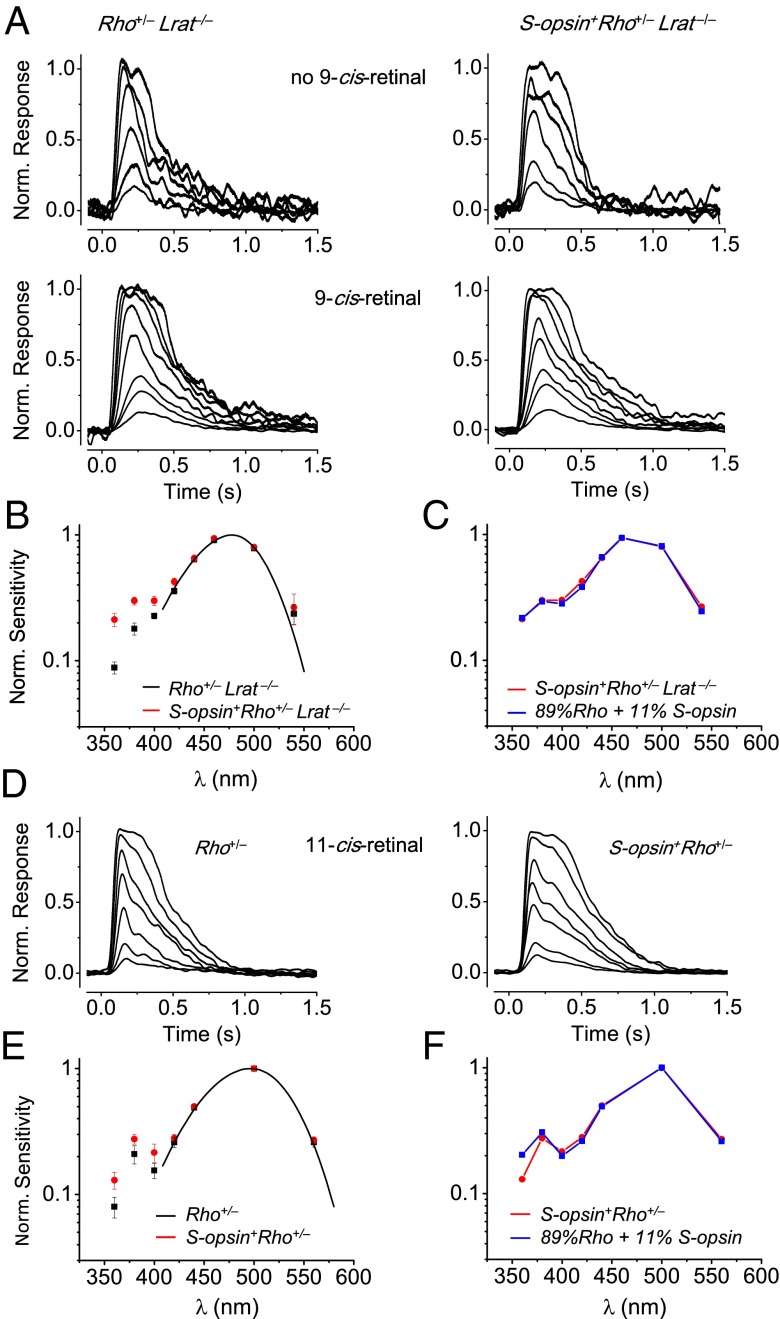
To explore whether the S-opsin helped by R-opsin to traffic to the ROS in the S-opsin+Rho+/−Lrat−/− retina was indeed functional, we perfused the isolated retina of this genotype in darkness with 9-cis-retinal [this chromophore was found to be naturally present in trace amounts in Lrat−/− retina (13), although far from sufficient for normal S-opsin trafficking (13, 19)] and made recordings from single rods with suction-pipette recording. The photosensitivity of both Rho+/−Lrat−/− and S-opsin+Rho+/−Lrat−/− rods after chromophore treatment increased by as much as ∼60-fold (Fig. 5A and legend), reflecting the regeneration of R-opsin into isorhodopsin (i.e., rhodopsin containing 9-cis-retinal). The dim flash–response kinetics also became slower after treatment (see legend of Fig. 5A and collected data in Table S1) because of the removal of the bleaching adaptation associated with chromophore-free R-opsin. Importantly, a comparison in the action spectra of 9-cis-retinal–treated Rho+/−Lrat−/− rods and S-opsin+Rho+/−Lrat−/− rods showed that the latter had extra photosensitivity in the UV region (red points in Fig. 5B), reflecting functional S-pigment (λmax = 365 nm with 9-cis-retinal). Fitting the action spectrum of S-opsin+Rho+/−Lrat−/− rods with a linear combination of the isorhodopsin data (black points in Fig. 5B) and the spectral template of 9-cis-S-cones, we arrived at an isorhodopsin/S-cone pigment ratio in the ROS of 89/11% (Fig. 5C). We made similar measurements on Rho+/− and S-opsin+Rho+/− rods, which have a normal 11-cis-retinal supply throughout life and therefore never have trafficking problems with their S-pigment, and arrived at a similar rhodopsin/S-pigment ratio in the ROS of 89/11% (Fig. 5 D–F and Table S1) (also see ref. 18). Thus, virtually all S-opsin in S-opsin+Rho+/−Lrat−/− rods trafficked successfully to the ROS through heteromerization with R-opsin; this result is not unreasonable, given the large excess of R-opsin available to S-opsin.
Fig. 5.
Flash responses and action spectra of Rho+/−Lrat−/− and S-opsin+Rho+/−Lrat−/− rods after perfusion with 9-cis-retinal (A–C), compared with those of Rho+/− and S-opsin+Rho+/− rods after perfusion with 11-cis-retinal (D–F). (A) Flash families from Rho+/−Lrat−/− and S-opsin+Rho+/−Lrat−/− rods before and after 9-cis-retinal treatment (four separate cells). A 12.1-ms flash at time 0 delivered 1,153, 2,772, 8,560, 15,400, 25,152, and 77,576 photons⋅μm−2 at 480 nm before 9-cis-retinal treatment and delivered 15.6, 35.7, 60.3, 105.4, 183.5, 250, 534, and 920 photons⋅μm−2 at 480 nm after 9-cis-retinal treatment. The saturated response amplitude was very small for rods of both lines before chromophore treatment (typically <2 pA) but increased substantially after treatment. (See Table S1 for collected data.) The flash sensitivity of both lines was also ∼60-fold higher after 9-cis-retinal treatment than before treatment, and the response integration time, tint, increased from ∼220 ms to 370 ms for the two cells. (B) The action spectra show S-opsin+Rho+/−Lrat−/− rods are more sensitive to UV than Rho+/−Lrat−/− rods. Shown are averaged, normalized data from 11 Rho+/−Lrat−/− rods and 13 S-opsin+Rho+/−Lrat−/− rods. The black curve is the spectral template for isorhodopsin with λmax at 477 nm. Incidentally, the rise in sensitivity of R pigment at λmax <400 nm does not belong to the main (α-) band of the pigment’s absorption spectrum and is not included in the fit by template (see also ref. 18), although it is included in the overall calculations in C. (C) The action spectrum of S-opsin+Rho+/−Lrat−/− rods (red points) was fit by a linear combination of isorhodopsin data in B (black points) and a spectral template for 9-cis-S-pigment with λmax at 365 nm, giving a ratio of 89:11% (blue). (D) Flash families from Rho+/− and S-opsin+Rho+/− rods after 11-cis-retinal treatment. Although both rods had 11-cis-retinal throughout life, we nonetheless pretreated them with 11-cis-retinal to remove any bare opsin (42), providing a better comparison with the data presented in A–C above. A 12.1-ms flash at time 0 delivered 4.5, 7.8, 16.7, 35.7, 60.2, 115, 204, and 472 photons⋅μm−2 at 500 nm in both cases. (E) The action spectrum of S-opsin+Rho+/− rods showed an increase in UV sensitivity compared with that of Rho+/− rods. Shown are averaged, normalized data from 6 Rho+/− rods and 12 S-opsin+Rho+/− rods. The black curve is spectral template for 11-cis-rhodopsin with λmax at 498 nm. Details are as in B. (F) The action spectrum of S-opsin+Rho+/− rods (red points) was fit by a linear combination of 11-cis-rhodopsin’s data in E (black points) and a spectral template for 11-cis-S-pigment with λmax at 360 nm, giving a ratio of 89:11% (blue). Error bars in B and E indicate SEM. See SI Methods for the procedure of spectral fitting.
Table S1.
Parameters of rod flash responses of transgenic mice expressing S-opsin in rods in various genetic backgrounds
| Response parameter | 9-cis-retinal | 11-cis-retinal | ||
| Rho+/−Lrat−/− | S-opsin+Rho+/−Lrat−/− | Rho+/− | S-opsin+Rho+/− | |
| Saturated-response amplitude, Rmax (pA) | 9.1 ± 0.6 | 7.5 ± 0.4 | 11.3 ± 0.4 | 11.2 ± 0.8 |
| Half-saturating flash intensity, σ, (photons⋅μm−2) | 187 ± 29 | 90 ± 10 | 25 ± 3 | 19 ± 3 |
| Single-photon response amplitude, a (pA) | 0.22 ± 0.03 | 0.20 ± 0.01 | 0.53 ± 0.07 | 0.42 ± 0.01 |
| Time-to-peak, tpeak (ms) | 222 ± 5 | 268 ± 6 | 185 ± 4 | 180 ± 2 |
| Integration time, tint (ms) | 280 ± 25 | 390 ± 7 | 265 ± 26 | 238 ± 20 |
| Recover time constant, τrec (ms) | 225 ± 43 | 230 ± 25 | 226 ± 19 | 200 ± 35 |
Values are mean ± SEM. Flash intensity–response relations such as those shown in Fig. 5 were fit with a normalized saturating-exponential function, R/Rmax = 1 – exp (- If/ρ), where R is the flash-response amplitude, Rmax is the saturating flash-response amplitude (same as dark current), If is the flash intensity, and ρ is a constant. The half-saturating flash intensity, σ, is given by σ = ρ ln 2. The single-photon response, a, is given by the dim-flash response amplitude (pA) divided by the product of flash intensity (photons⋅µm−2) and the ROS’s effective collecting area (in square micrometers), with the latter being 0.5 μm2 for Rho+/− and S-opsin+Rho+/− rods and 0.3 μm2 for Rho+/−Lrat−/− and S-opsin+Rho+/−Lrat−/− rods. The single-photon response of 9-cis-retinal–treated S-opsin+Rho+/−Lrat−/− and Rho+/−Lrat−/− rods was 2.1–2.4 times smaller than that of 11-cis-retinal–treated S-opsin+Rho+/− and control Rho+/− rods, as is consistent with the lower quantum efficiency (∼0.33) of isorhodopsin compared with that of rhodopsin (0.67) (51). For the single-photon response, n = 5, 13, 4, and 12 for Rho+/−Lrat−/−, S-opsin+Rho+/−Lrat−/−, Rho+/−, and S-opsin+Rho+/− rods, respectively. For all other measurements, n = 5, 5, 4, and 7 for the four genotypes, respectively. tpeak is the time lapse between the light flash and transient peak of the dim-flash response. tint is defined as = ∫ f(t)dt/fpeak, where f(t) is the waveform of the dim-flash response, and fpeak is the amplitude of f(t) at transient peak (52). τrec is the time constant of a single-exponential decay fit to the final decay of the dim-flash response.
Disruption by Helix Peptides of Rhodopsin Trafficking in WT Rods.
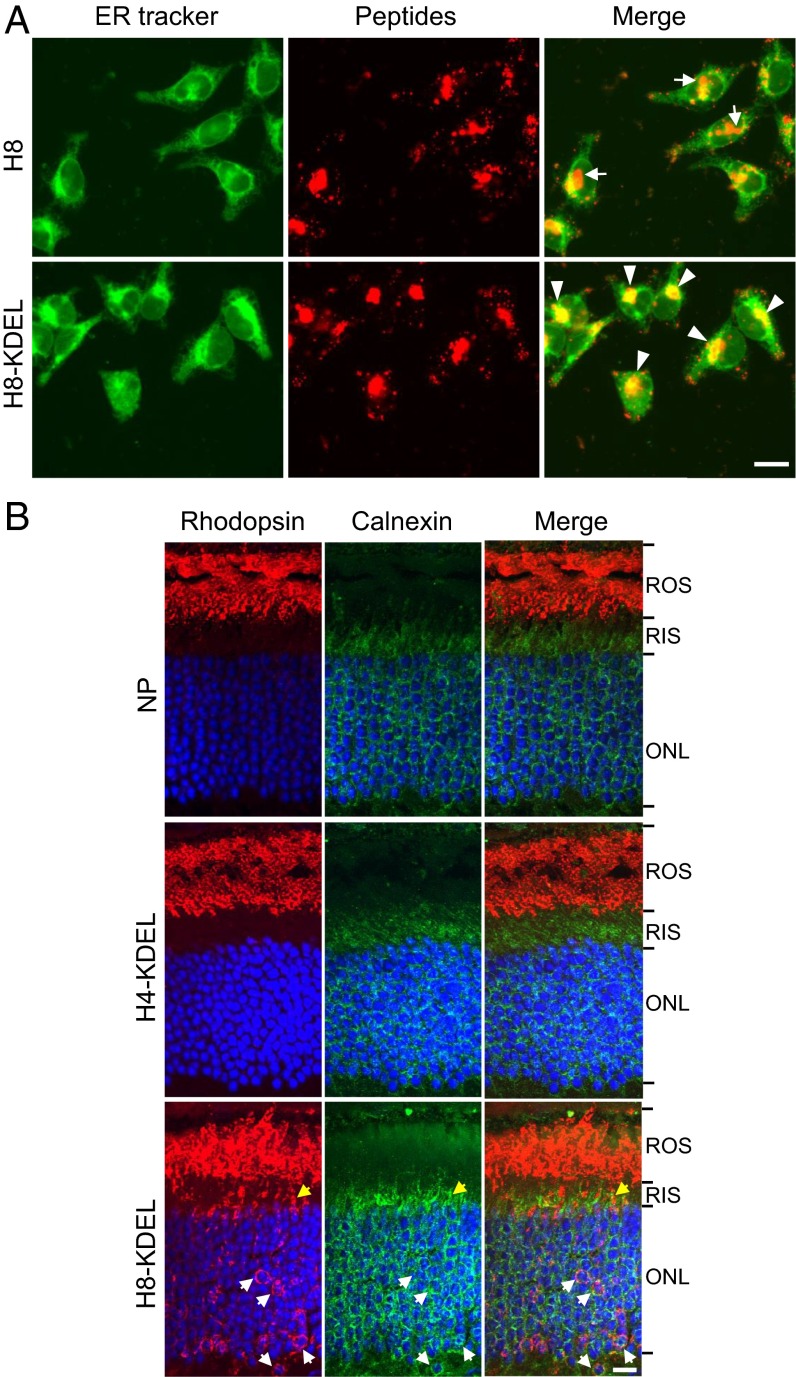
We tested whether subretinal delivery of NP-encapsulated H1 or H8 peptide could disrupt even the normal trafficking of rhodopsin in WT (i.e., Lrat+/+) rods. Neither peptide had an effect (Fig. S4). Possibly, homomeric R–R interaction is stronger than heteromeric R–S interaction and resists competition by the peptide. Accordingly, we tried to overcome this putative resistance by adding an ER-targeting signal, KDEL, at the C terminus of the competing peptide to increase its effective concentration in the ER. Indeed, in cell culture, the H8-KDEL peptide exhibited virtually complete colocalization with an ER marker (white arrowheads in Fig. 6A, Lower Right), whereas the H8 peptide only partially colocalized with the ER marker (white arrows indicating nonoverlapped peptide signals in Fig. 6A, Upper Right). The increased ER targeting efficiency of H8-KDEL is likely through the retrograde transport system from Golgi to ER via the KDEL receptors (30–32). In vivo, the H8-KDEL peptide caused mislocalization of rhodopsin to the inner segment and perinuclear ER region (yellow and white arrows, respectively, in Fig. 6B, Bottom Left), with the latter indicated by Calnexin labeling (Fig. 6B, Bottom Center). The same treatment with NP alone or with the H4-KDEL peptide had no effect (Fig. 6B, Top and Middle Rows). Thus, the H1/H8 domains rather than the H4/H5 domains may be the primary protomer–protomer interface in R/R-opsin and R/S-opsin dimerization during biosynthesis and targeting, although secondary interactions in the H4/H5 domains and even others are still possible in the ROS (33–36). This result is consistent with the cross-linking experiment involving BM(PEG)3 and MTS-O5-MTS, whose extended S–S distance of 2.1 and 2.6 nm, respectively, is within the predicted C316–C316 distance of 2.1–2.9 nm in the rhodopsin H1/H8 dimer (2.4 nm in the R/S-opsin heterodimer) (Fig. S3) (24).
Fig. S4.
Subretinal injection of R-opsin H1 and H8 peptides did not interfere with rhodopsin trafficking in WT mice. WT mice were injected with NP-encapsulated R-opsin H1, H4, H5, and H8 peptides or NPs alone. Mouse retinas were stained with rhodopsin (red) antibody 1D4. Nuclei were stained with DAPI (blue). (Scale bar, 10 µm.)
Fig. 6.
Peptide-competition experiments in WT rods. (A) Rhodamine-labeled H8-KDEL or H8 peptides (red) were delivered to HEK293 cells followed by live-cell imaging. The ER was labeled with ER-Tracker Green. White arrows indicate H8 peptides outside the ER. White arrowheads indicate the complete colocalization of H8-KDEL with ER-Tracker. (B) Subretinal injection of NP-encapsulated R-opsin H8-KDEL peptide but not R-opsin H4-KDEL peptide caused mislocalization of rhodopsin in WT rods. Mouse retinas were stained with anti–R-opsin (red) and anti-Calnexin (green) antibodies. White arrows indicate mislocalized R-opsin in the perinuclear ER region, which is colocalized with the ER marker Calnexin. Yellow arrows indicate mislocalized R-opsin in the inner segment. (Scale bars, 20 µm in A and 10 µm in B.)
Discussion
By exploiting transgenic S-opsin+Rho+/−Lrat−/− and S-opsin+Rho+/+Lrat−/− rods, we have demonstrated that R-opsin is able to facilitate the folding and proper trafficking of coexpressed S-opsin when the latter fails to do so by itself in the absence of chromophore. We conclude that heteromerization between R-opsin and S-opsin exists in the transgenic rod, a conclusion also supported by cross-linking, coimmunoprecipitation, and peptide-competition experiments. These experiments together provide in vivo evidence for visual pigment dimerization that begins during its biosynthesis at the ER. Importantly, we found the same specific competing-peptide effect on rhodopsin maturation and targeting in WT rods, thus extending the conclusion to the completely native and physiological situation. Two possibilities might account for H8-mediated rhodopsin mislocalization. One is that dimerization is required for rhodopsin trafficking. Thus, disruption of rhodopsin dimerization leads to trafficking defects. Alternatively, rhodopsin folding and dimerization are coupled during protein synthesis, as suggested by the rescue of the S-opsin folding defect in S-opsin+Rho+/−Lrat−/− mice. Disruption of rhodopsin dimerization may cause folding defects and subsequently affect trafficking. In both cases, our data suggest that dimerization involving homotypic H1 and H8 interactions is critical for correct visual pigment folding, maturation, and targeting. Recently, an in vitro study based on peptide competition suggests the involvement of two interfaces (i.e., H1/H2 and H4/H5) between the two rhodopsin protomers in the dimer (36). That work differs from our in vivo study in that it addresses the state of rhodopsin after the protein has reached the ROS, whereas our work addresses rhodopsin dimer formation in the ER. It is possible that rhodopsin first forms dimers in the ER via the H1/H8 interface (Fig. 3), followed by higher-order oligomerization involving multiple interfaces after reaching the ROS (perhaps promoted by the very high concentration of rhodopsin). Indeed, the possible existence of higher-order visual pigment oligomerization in the ROS has been suggested by experiments involving cross-linkers (this work and refs. 24 and 37) and cryoelectron tomography (38). Separately, visual pigment dimerization has interesting implications for mouse cones because they coexpress M and S pigments in the same cell, with an M/S pigment ratio dependent on cell location in the retina (39); thus, conceivably, M and S pigment homodimers as well as M/S pigment heterodimers may coexist in a given mouse cone. The important remaining question is whether a pigment dimer is stable during its lifetime or if mature dimers and monomers exist in equilibrium in the outer segment. If dimers are indeed stable, then it is possible that, besides being important for rhodopsin maturation and targeting, the dimer configuration may actually have a subtle, hitherto unknown mechanistic role in rhodopsin’s activation of transducin and possibly even its interactions with rhodopsin kinase and arrestin. These are new questions to ponder, even though rhodopsin monomers themselves are capable of these functions (2, 4–6, 10–12). Last, more than 100 mutations of rhodopsin are associated with the degenerative disease autosomal-dominant retinitis pigmentosa (ADRP), with WT and mutant rhodopsin being copresent in rods. Some of the pathology conceivably could involve defective dimerization of rhodopsin in the ER. Understanding this process might help in the development of future therapies for this class of ADRP.
Methods
Lrat−/−, S-opsin+, and Rho−/− mice were generated previously (18–20). All animal experiments were approved by the Institutional Animal Care and Use Committees at Baylor College of Medicine, the University of Utah, and the Johns Hopkins University School of Medicine and were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animal in Ophthalmic and Vision Research. Peptides corresponding to H1 (WQFSMLAAYMFLLIVLGFPINFLTLYVTV), H4 (HAIMGVVFTWIMALACAAPPLV), H5 (ESFVIYMFVVHFTIPMIVIFFCYGQLV), H8 (NKQFRNCMLTTL), H4-KDEL (HAIMGVVFTWIMALACAAPPLVKDEL), and H8-KDEL (NKQFRNCMLTTLKDEL) of mouse rhodopsin were synthesized and purified by Selleck Chemicals LLC. Rhodamine-labeled H8 or H8-KDEL via the N terminus was synthesized and purified by Peptide 2.0 Inc. NPs were fabricated as previously described (29). KDEL is a C-terminal signal for the localization in the ER of many soluble proteins residing in the ER lumen in eukaryotic cells (40). Chemical cross-linking was performed under dim red light as previously described (24). Immunohistochemistry, immunoprecipitation, Western blot, subretinal injection, and electrical recording were done with standard protocols. Detailed methods are described in SI Methods.
SI Methods
Animals.
WT (C57BL/6J) mice were purchased from Jackson Laboratory. P14 S-opsin+Rho−/−Lrat−/− mice were used because their rods degenerate rapidly after age 2 wk (16). We used S-opsin+Rho+/−Lrat−/− and S-opsin+Rho+/+Lrat−/− mice older than 1 mo because by that age most S-opsin–containing cones have died because of the lack of chromophore (13–16), thus minimizing confounding signals from cones when detecting S-opsin in rods. All animal experiments were approved by the Institutional Animal Care and Use Committees at Baylor College of Medicine, the University of Utah, and the Johns Hopkins University School of Medicine and were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animal in Ophthalmic and Vision Research. Mice were reared under cyclic light (12-h light/12-h dark).
Biochemical Cross-Linking.
Mouse outer-segment membranes were first prepared as follows. Retinas were removed from overnight dark-adapted mice under dim red light and were homogenized in 100 µL H buffer [29% (wt/vol) sucrose, 65 mM NaCl, 2 mM MgCl2, 10 mM Tris⋅HCl (pH 7.4), 1 mM DTT, 0.1 mM EDTA, and protease inhibitor mixture from Roche]. The retinal extract was spun at 1,789 × g at 4 °C, and the supernatant was removed. The pellet was resuspended in H buffer and spun again. Supernatants were combined and diluted 5× with dilution buffer [100 mM NaCl, 2 mM MgCl2, 10 mM Tris⋅HCl (pH 7.5), 0.1 mM DTT, 0.1 mM EDTA, and protease inhibitor mixture] and then were spun at 18,894 × g for 5 min at 4 °C. The pellet contained outer-segment membranes with rod opsin, cone opsins, and other membrane proteins. Chemical cross-linking was performed under dim red light as previously described (24) with minor modifications. The outer-segment membrane fraction was resuspended in 100 µL of buffer C [100 mM Na2HPO4 (pH 7.0), 150 mM NaCl] and 2 µL of protease inhibitor mixture. The noncleavable cross-linking reagent BM(PEG)3 or the cleavable cross-linking reagent MTS-O5-MTS (Toronto Research Chemicals) was solubilized in DMSO and added to a final concentration of 200 µM. The reaction was allowed to proceed for 24 h at room temperature for BM(PEG)3 or 15 min for MTS-O5-MTS before quenching with 10 mM l-cysteine or 20 mM N-ethylmaleimide, respectively, for 5 min. In controls without cross-linker, BM(PEG)3 or MTS-O5-MTS was omitted. To cleave MTS-O5-MTS cross-links, samples were treated with DTT at 100 mM for 60 min at room temperature.
Histology.
Mouse eyes were immersion-fixed from overnight to several weeks in a fixative containing 2.5% (wt/vol) glutaraldehyde/1% formaldehyde and were resin embedded as previously described (43). Samples were sectioned at 1 μm for Richardson’s staining.
Immunohistochemistry.
Immunohistochemistry was performed as previously described (23) with the following modifications. Fresh eyes were fixed for 2 h using 4% (wt/vol) paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. After further incubation overnight in 0.1 M phosphate buffer/30% (wt/vol) sucrose at 4 °C, the eyes were embedded in optimum cutting temperature (OCT) compound (Tissue-Tek) and were sectioned at 12-μm thickness. The sections were processed for immunostaining with the following primary antibodies: anti–S-opsin (MBO) (18), anti–M-opsin (AB5405; Millipore), anti–R-opsin (1D4) (25), anti-Calnexin (sc-11397; Santa Cruz Biotechnology), and anti-CNGA1 (1D1) (44). Primary antibodies were detected by Alexa 488 or Cy3-conjugated goat secondary antibodies. Specimens were imaged under identical scanning conditions with an Olympus Fluoview FV1000 or Zeiss LSM510 confocal microscope.
Immunoprecipitation and Western Blot.
Mouse retinas were homogenized in 100 µL lysis buffer [20 mM Tris⋅HCl (pH 7.5), 15 mM NaCl, 1% CHAPS, and protease inhibitor mixture]. The retinal extract was centrifuged at 16,200 × g for 10 min at 4 °C to remove cell debris. For immunoprecipitation, 20 µL of 50% (wt/vol) protein A/G beads were added to the supernatant and rotated for 2 h at 4 °C to preclear the samples. The samples were centrifuged at 16,200 × g for 20 s, and the agarose pellet was discarded. The precleared supernatant was mixed with ∼5 µg anti–S-opsin antibody and rotated overnight at 4 °C. Then 30 µL of 50% (wt/vol) protein A/G beads were added, and the tubes were rotated for 1.5 h. The immunoprecipitates were collected by centrifugation at 16,200 × g for 20 s, washed three times with lysis buffer, and solubilized in 50 µL of SDS/PAGE sample buffer. For Western blots, protein samples were separated by SDS/PAGE and transferred to PVDF membrane. To minimize protein aggregation, protein samples were kept at 4 °C after being dissolved in lysis buffer. Primary antibodies against R-opsin, M-opsin, and S-opsin were used. The primary antibodies were detected by incubation with goat anti-rabbit or anti-mouse secondary antibodies conjugated with HRP.
Subretinal Injection of Peptides.
Mice were anesthetized by intraperitoneal injection of ketamine/xylazine/acepromazine (70–100/10–20/1–3 mg/kg body weight). Pupils were dilated with 1% tropicamide (Bausch & Lomb), and 0.5% proparacaine (Bausch & Lomb) was applied to the eyes as a local anesthetic. The mouse was placed on a sterile pad under a stereomicroscope. A sclerotomy was made just posterior to the limbus using a 30.5-gauge needle. A 32-gauge blunt needle attached to a 5-μL syringe (Hamilton) was inserted into the subretinal space through the vitreous and retinal layers, and NPs with the peptide were delivered slowly. A total of 2.25 µg of peptide-conjugated NP (1.5 µL of 1.5 mg/mL) was delivered to one eye, and the same amount of control NP was injected into the other eye of the mouse. The needle was left in the injection site for several seconds after the NP-peptide delivery. A 0.5% erythromycin ophthalmic ointment (Bausch & Lomb) then was applied to the eyes. Mice were killed 6 d after injection, and their eyes were enucleated for immunohistochemistry. In transgenic S-opsin+Rho+/−Lrat−/− mice, H1- and H8-induced opsin mislocalization was widespread across more than one-third of the retina. In WT mice, H8-KDEL-induced opsin mislocalization was more localized, within 100–200 µm of the injection site, probably because of stronger homomeric R–R interaction in WT rods. We excluded the injection site in all analyses to avoid any injury effect.
Cell Culture.
HEK293 cells were grown in DMEM supplemented with 10% (vol/vol) FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin. Cells were seeded on an eight-well chambered coverglass (Eppendorf). Eighteen hours after seeding, cells were washed with HBSS and incubated for 30 min at 37o C in 1 µM endoplasmic reticulum dye ER-Tracker Green (Thermo Fisher Scientific). Next, cells were transfected with rhodamine-labeled H8-KDEL or H8 using Xfect protein transfection reagent (Clontech) following the manufacturer’s instructions. For vehicle control, DMSO was used. After 60 min, the transfection reagent was removed, and cells were washed in PBS and imaged live on a Leica DMi8 inverted microscope (Leica).
Electrical Recordings.
Mice reared in cyclic light were dark-adapted overnight and killed by CO2 asphyxiation. The eyes were enucleated under dim red light, and the retina was isolated, chopped into tiny pieces, and transferred to the recording chamber containing HCO3-Locke’s solution: 112.5 mM NaCl, 3.6 mM KCl, 2.4 mM MgCl2, 1.2 mM CaCl2, 10 mM Hepes (pH 7.4), 0.02 mM EGTA, 20 mM NaHCO3, 3 mM Na2-succinate, 0.5 mM Na-glutamate, 10 mM glucose. Membrane current was recorded with a suction pipette from an ROS projecting from a piece of retina as previously described (23). For chromophore treatment, the mouse retina was incubated with 35 μM 9-cis-retinal (Sigma) or 11-cis-retinal (US National Eye Institute) in 1% BSA-Ames solution for 1 h in complete darkness before chopping and recording. The chromophore solution was prepared following a published procedure (45). The signals were filtered at 20 Hz (eight-pole Bessel filter) and digitized at 10 kHz.
With 11-cis-retinal as chromophore, mouse rhodopsin and S-pigment have a λmax of 498 nm (ref. 46 and the data presented here) and 360 nm (47), respectively. For 9-cis-pigments, we used the equation: 9-cis λmax = 0.81 × (11-cis λmax) + 73 (48), giving 477 nm and 365 nm for isorhodopsin and S-pigment, respectively. The spectral template for 11-cis-pigments was adopted from Govardovskii et al. (49). The template for 9-cis-pigments was from Makino et al. (50) with some modifications for S-opsin (namely, the Gaussian component was removed because of the lack of parameters, but this removal only slightly affected the very long wavelengths in the spectrum for a UV-opsin with λmax at 365 nm). For fitting the 9-cis-S-opsin+Rho+/−Lrat−/− data in Fig. 5C (red points), we used a linear combination of the 9-cis-Rho+/−Lrat−/− data in Fig. 5B (black points) and the spectral template above (46) for 9-cis-S-pigment with λmax at 365 nm, with the best-fit weighting factors giving the rhodopsin/S-opsin ratio (also see the legend of Fig. 5). The same calculation was done for the corresponding 11-cis data in Fig. 5F.
Acknowledgments
We thank Jeannie Chen for the S-opsin+ mouse line and the S-opsin antibody; Wolfgang Baehr for the Lrat−/− mouse line; Janis Lem for the Rho−/− mouse line; Robert S. Molday for the 1D4 and 1D1 antibodies; Thomas Huber for providing information on cross-linkers; and Zheng Jiang, Xiaozhi Ren, Yanghui Sheng, Wendy W.-S. Yue, Katie Pennington, and Jacki Roberts for comments on the manuscript. T.Z. was supported by a Career Initiation Research Grant Award from Knights Templar; Y.F. was supported by NIH Grant EY022614, the E. Matilda Ziegler Foundation for the Blind, a Career Development Award from Research to Prevent Blindness (RPB), NIH Core Grant 2P30EY002520, and an unrestricted RPB grant to the Department of Ophthalmology at Baylor College of Medicine; and K.-W.Y. was supported by NIH Grant EY06837 and the António Champalimaud Vision Award from the Champalimaud Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609018113/-/DCSupplemental.
References
- 1.Chabre M, le Maire M. Monomeric G-protein-coupled receptor as a functional unit. Biochemistry. 2005;44(27):9395–9403. doi: 10.1021/bi050720o. [DOI] [PubMed] [Google Scholar]
- 2.Ernst OP, Gramse V, Kolbe M, Hofmann KP, Heck M. Monomeric G protein-coupled receptor rhodopsin in solution activates its G protein transducin at the diffusion limit. Proc Natl Acad Sci USA. 2007;104(26):10859–10864. doi: 10.1073/pnas.0701967104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chabre M, Cone R, Saibil H. Biophysics: Is rhodopsin dimeric in native retinal rods? Nature. 2003;426(6962):30–31, discussion 31. doi: 10.1038/426030b. [DOI] [PubMed] [Google Scholar]
- 4.Whorton MR, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci USA. 2007;104(18):7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whorton MR, et al. Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J Biol Chem. 2008;283(7):4387–4394. doi: 10.1074/jbc.M703346200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee S, Huber T, Sakmar TP. Rapid incorporation of functional rhodopsin into nanoscale apolipoprotein bound bilayer (NABB) particles. J Mol Biol. 2008;377(4):1067–1081. doi: 10.1016/j.jmb.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 7.Fotiadis D, et al. The G protein-coupled receptor rhodopsin in the native membrane. FEBS Lett. 2004;564(3):281–288. doi: 10.1016/S0014-5793(04)00194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fotiadis D, et al. Atomic-force microscopy: Rhodopsin dimers in native disc membranes. Nature. 2003;421(6919):127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, et al. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J Biol Chem. 2003;278(24):21655–21662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG. Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J Biol Chem. 2007;282(20):14875–14881. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- 11.Bayburt TH, et al. Monomeric rhodopsin is sufficient for normal rhodopsin kinase (GRK1) phosphorylation and arrestin-1 binding. J Biol Chem. 2011;286(2):1420–1428. doi: 10.1074/jbc.M110.151043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukamoto H, Sinha A, DeWitt M, Farrens DL. Monomeric rhodopsin is the minimal functional unit required for arrestin binding. J Mol Biol. 2010;399(3):501–511. doi: 10.1016/j.jmb.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan J, Rohrer B, Frederick JM, Baehr W, Crouch RK. Rpe65-/- and Lrat-/- mice: Comparable models of leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2008;49(6):2384–2389. doi: 10.1167/iovs.08-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Znoiko SL, et al. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65-/- mouse at early ages. Invest Ophthalmol Vis Sci. 2005;46(4):1473–1479. doi: 10.1167/iovs.04-0653. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, et al. Trafficking of membrane-associated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci. 2008;28(15):4008–4014. doi: 10.1523/JNEUROSCI.0317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, Zhang N, Baehr W, Fu Y. Cone opsin determines the time course of cone photoreceptor degeneration in Leber congenital amaurosis. Proc Natl Acad Sci USA. 2011;108(21):8879–8884. doi: 10.1073/pnas.1017127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Insinna C, et al. An S-opsin knock-in mouse (F81Y) reveals a role for the native ligand 11-cis-retinal in cone opsin biosynthesis. J Neurosci. 2012;32(23):8094–8104. doi: 10.1523/JNEUROSCI.0131-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi G, Yau KW, Chen J, Kefalov VJ. Signaling properties of a short-wave cone visual pigment and its role in phototransduction. J Neurosci. 2007;27(38):10084–10093. doi: 10.1523/JNEUROSCI.2211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batten ML, et al. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279(11):10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lem J, et al. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci USA. 1999;96(2):736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz A, et al. Molecular and biochemical characterization of lecithin retinol acyltransferase. J Biol Chem. 1999;274(6):3834–3841. doi: 10.1074/jbc.274.6.3834. [DOI] [PubMed] [Google Scholar]
- 22.Kefalov V, Fu Y, Marsh-Armstrong N, Yau KW. Role of visual pigment properties in rod and cone phototransduction. Nature. 2003;425(6957):526–531. doi: 10.1038/nature01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Y, Kefalov V, Luo DG, Xue T, Yau KW. Quantal noise from human red cone pigment. Nat Neurosci. 2008;11(5):565–571. doi: 10.1038/nn.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knepp AM, Periole X, Marrink SJ, Sakmar TP, Huber T. Rhodopsin forms a dimer with cytoplasmic helix 8 contacts in native membranes. Biochemistry. 2012;51(9):1819–1821. doi: 10.1021/bi3001598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacKenzie D, Arendt A, Hargrave P, McDowell JH, Molday RS. Localization of binding sites for carboxyl terminal specific anti-rhodopsin monoclonal antibodies using synthetic peptides. Biochemistry. 1984;23(26):6544–6549. doi: 10.1021/bi00321a041. [DOI] [PubMed] [Google Scholar]
- 26.Harikumar KG, et al. Glucagon-like peptide-1 receptor dimerization differentially regulates agonist signaling but does not affect small molecule allostery. Proc Natl Acad Sci USA. 2012;109(45):18607–18612. doi: 10.1073/pnas.1205227109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, He L, Combs CA, Roderiquez G, Norcross MA. Dimerization of CXCR4 in living malignant cells: Control of cell migration by a synthetic peptide that reduces homologous CXCR4 interactions. Mol Cancer Ther. 2006;5(10):2474–2483. doi: 10.1158/1535-7163.MCT-05-0261. [DOI] [PubMed] [Google Scholar]
- 28.Harikumar KG, et al. Transmembrane segment peptides can disrupt cholecystokinin receptor oligomerization without affecting receptor function. Biochemistry. 2006;45(49):14706–14716. doi: 10.1021/bi061107n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Csaky KG. Nanoparticle-integrin antagonist C16Y peptide treatment of choroidal neovascularization in rats. J Control Release. 2010;142(2):286–293. doi: 10.1016/j.jconrel.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 30.Sandvig K, van Deurs B. Transport of protein toxins into cells: Pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 2002;529(1):49–53. doi: 10.1016/s0014-5793(02)03182-4. [DOI] [PubMed] [Google Scholar]
- 31.Miesenböck G, Rothman JE. The capacity to retrieve escaped ER proteins extends to the trans-most cisterna of the Golgi stack. J Cell Biol. 1995;129(2):309–319. doi: 10.1083/jcb.129.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raykhel I, et al. A molecular specificity code for the three mammalian KDEL receptors. J Cell Biol. 2007;179(6):1193–1204. doi: 10.1083/jcb.200705180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards PC, et al. Crystals of native and modified bovine rhodopsins and their heavy atom derivatives. J Mol Biol. 2004;343(5):1439–1450. doi: 10.1016/j.jmb.2004.08.089. [DOI] [PubMed] [Google Scholar]
- 34.Salom D, et al. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc Natl Acad Sci USA. 2006;103(44):16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Periole X, Huber T, Marrink SJ, Sakmar TP. G protein-coupled receptors self-assemble in dynamics simulations of model bilayers. J Am Chem Soc. 2007;129(33):10126–10132. doi: 10.1021/ja0706246. [DOI] [PubMed] [Google Scholar]
- 36.Jastrzebska B, et al. Disruption of rhodopsin dimerization with synthetic peptides targeting an interaction interface. J Biol Chem. 2015;290(42):25728–25744. doi: 10.1074/jbc.M115.662684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jastrzebska B, et al. Functional characterization of rhodopsin monomers and dimers in detergents. J Biol Chem. 2004;279(52):54663–54675. doi: 10.1074/jbc.M408691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunkel M, et al. Higher-order architecture of rhodopsin in intact photoreceptors and its implication for phototransduction kinetics. Structure. 2015;23(4):628–638. doi: 10.1016/j.str.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Applebury ML, et al. The murine cone photoreceptor: A single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27(3):513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 40.Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 41.Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454(7201):183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 42.Kefalov VJ, et al. Breaking the covalent bond--a pigment property that contributes to desensitization in cones. Neuron. 2005;46(6):879–890. doi: 10.1016/j.neuron.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson JR, et al. A computational framework for ultrastructural mapping of neural circuitry. PLoS Biol. 2009;7(3):e1000074. doi: 10.1371/journal.pbio.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molday LL, Cook NJ, Kaupp UB, Molday RS. The cGMP-gated cation channel of bovine rod photoreceptor cells is associated with a 240-kDa protein exhibiting immunochemical cross-reactivity with spectrin. J Biol Chem. 1990;265(30):18690–18695. [PubMed] [Google Scholar]
- 45.Cornwall MC, Jones GJ, Kefalov VJ, Fain GL, Matthews HR. Electrophysiological methods for measurement of activation of phototransduction by bleached visual pigment in salamander photoreceptors. Methods Enzymol. 2000;316:224–252. doi: 10.1016/s0076-6879(00)16726-6. [DOI] [PubMed] [Google Scholar]
- 46.Yokoyama R, Yokoyama S. Comparative molecular biology of visual pigements. In: Stavenga DG, de Grip W, Pugh E, editors. Molecular Mechanisms in Visual Transduction. Elsevier; New York: 2000. pp. 257–296. [Google Scholar]
- 47.Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127(4):359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parry JW, Bowmaker JK. Visual pigment reconstitution in intact goldfish retina using synthetic retinaldehyde isomers. Vision Res. 2000;40(17):2241–2247. doi: 10.1016/s0042-6989(00)00101-2. [DOI] [PubMed] [Google Scholar]
- 49.Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. In search of the visual pigment template. Vis Neurosci. 2000;17(4):509–528. doi: 10.1017/s0952523800174036. [DOI] [PubMed] [Google Scholar]
- 50.Makino CL, Groesbeek M, Lugtenburg J, Baylor DA. Spectral tuning in salamander visual pigments studied with dihydroretinal chromophores. Biophys J. 1999;77(2):1024–1035. doi: 10.1016/S0006-3495(99)76953-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hubbard R, Kropf A. The action of light on rhodopsin. Proc Natl Acad Sci USA. 1958;44(2):130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo DG, Yau KW. Rod sensitivity of neonatal mouse and rat. J Gen Physiol. 2005;126(3):263–269. doi: 10.1085/jgp.200509342. [DOI] [PMC free article] [PubMed] [Google Scholar]