Significance
Breeding crops with more biomass produced per drop of water transpired is a key challenge in the context of climate change. However, the tight coupling between transpiration and carbon assimilation during the day makes it challenging to decrease water loss without altering photosynthesis and reducing crop yield. We tested whether reducing transpiration at night when photosynthesis is inactive could substantially reduce water loss without altering growth—a hypothesis that, to our knowledge, has never been genetically addressed in any species. By studying a whole progeny in grapevine, a major crop for drought-prone areas, we identified genomic regions where selection could be operated to reduce transpiration at night and maintain growth. This opens new horizons for breeding crops with higher water-use efficiency.
Keywords: night transpiration, transpiration efficiency, growth, stomata, QTL
Abstract
Increasing water scarcity challenges crop sustainability in many regions. As a consequence, the enhancement of transpiration efficiency (TE)—that is, the biomass produced per unit of water transpired—has become crucial in breeding programs. This could be achieved by reducing plant transpiration through a better closure of the stomatal pores at the leaf surface. However, this strategy generally also lowers growth, as stomatal opening is necessary for the capture of atmospheric CO2 that feeds daytime photosynthesis. Here, we considered the reduction in transpiration rate at night (En) as a possible strategy to limit water use without altering growth. For this purpose, we carried out a genetic analysis for En and TE in grapevine, a major crop in drought-prone areas. Using recently developed phenotyping facilities, potted plants of a cross between Syrah and Grenache cultivars were screened for 2 y under well-watered and moderate soil water deficit scenarios. High genetic variability was found for En under both scenarios and was primarily associated with residual diffusion through the stomata. Five quantitative trait loci (QTLs) were detected that underlay genetic variability in En. Interestingly, four of them colocalized with QTLs for TE. Moreover, genotypes with favorable alleles on these common QTLs exhibited reduced En without altered growth. These results demonstrate the interest of breeding grapevine for lower water loss at night and pave the way to breeding other crops with this underexploited trait for higher TE.
Understanding how plants make efficient use of water has become a priority in the context of climate change and reduced water availability (1, 2). Plants inevitably lose water vapor by transpiration while capturing atmospheric CO2 for photosynthesis through stomatal pores at the leaf surface. Photosynthesis and transpiration covary with the aperture and density of stomatal pores depending on genotypes and environmental conditions (3, 4). In addition, photosynthesis and transpiration rates are codetermined by the leaf area, which is involved in light capture and is subjected to evaporation. Thus, plant productivity is positively coupled with transpirational water losses through stomatal characteristics and shoot development. This coupling has prompted plant scientists to define transpiration efficiency (TE) as the amount of biomass produced per unit of water used through transpiration (5). Because TE shows significant variations across species and varieties, it has been proposed as a relevant target in breeding programs for areas with restricted water availability (2).
Breeders have substantially improved TE by selecting plants with more efficient photosynthesis or higher allocation of photosynthates to harvested organs (6). Another way to improve TE is to decrease the amount of water lost by transpiration. However, the tight coupling between transpiration and photosynthesis during the day in C3 and C4 species makes it challenging to decrease water loss without reducing crop yield: genotypes that save the most water often show the lowest photosynthesis rate and yield (7). This drawback may be circumvented by selecting alleles or manipulating genes that uncouple transpiration from photosynthesis. Such a selection has been facilitated by the widespread use of δ13C, a proxy for daytime TE based on the carbon isotope discrimination that occurs during photosynthesis (8). This strategy has been successfully implemented in both model plants and crops to detect genomic regions associated with the control of TE, which are being exploited in breeding programs (9, 10), or serve as starting points to investigate the genetic determinism and ecological significance of the variation in TE (11–13).
An alternative yet unexplored strategy to improve TE (14) could be to select plants with a reduced rate of water loss at night (En), when photosynthesis is not operating due to the absence of light. Although plants actively close their stomata in the dark, stomatal closure is largely incomplete and nighttime transpiration can be substantial, accounting for up to 30% of the plant’s daytime water loss (15). Furthermore, variation in En has been identified across (15–17) and within (15, 18–20) plant species, but little is known about the underlying genetic determinisms. In the model plant Arabidopsis thaliana, natural variation in nighttime transpiration was detected (21, 22) and found to contribute significantly to total transpiration (23). However, genotypes with reduced En also exhibited lower photosynthesis rate and growth (21), hindering further developments in breeding programs. More recently, A. thaliana mutants were isolated, showing impaired nighttime transpiration and intact growth (24). This suggests that genomic regions may be identified with beneficial alleles, allowing plants to save water at night without altering growth. However, no study so far has attempted to jointly analyze the genetic determinisms of En, TE, and growth at the whole genome scale of a plant species.
Here we show that TE can be bred by reducing En independently of daytime stomatal behavior in grapevine (Vitis vinifera L.), a cultivated perennial species of high economic importance for drought-prone areas. Genetic variations in TE (25) and En (20, 26, 27) have been independently reported in V. vinifera, but the genetic basis of a possible link between these two traits has never been examined. In our study, we jointly examine TE and En in a mapping population obtained from the cross between two widespread cultivars, Syrah and Grenache (S×G), previously described as exhibiting different daytime water use (28, 29). We highlight that part of the genetic variation in TE is tightly linked to the variation in En. Incomplete stomatal closure and, to a lesser extent, water loss through the cuticle account for a large part of the genetic variability in En. Several genomic loci underlying the variation in En are identified under well-watered (WW) conditions and soil water deficit (WD). Based on genetic information on these loci, offspring genotypes could be selected with favorable alleles that enhance TE by reducing En without altering plant growth.
Results and Discussion
Nocturnal Transpiration in Grapevine: Substantial Losses Under Tight Genetic Control.
We first characterized the variability of nighttime water loss and its response to soil WD in a pseudo-F1 population obtained from a cross between the two grapevine cultivars Syrah and Grenache. Potted plants (186 offspring plus the parents) were grown in a phenotyping platform in a greenhouse under both WW and WD conditions. Transpiration rates were determined in a chamber under stabilized climatic conditions. Experiments were repeated over 2 successive years and gave similar mean values with similar genetic variability, although with slight differences between years for most of the traits (SI Appendix, Table S1). Genotypic values reported hereafter for all traits were estimated as Best Linear Unbiased Predictions (BLUPs) from mixed models using replicates from either 2012, 2013, or both years.
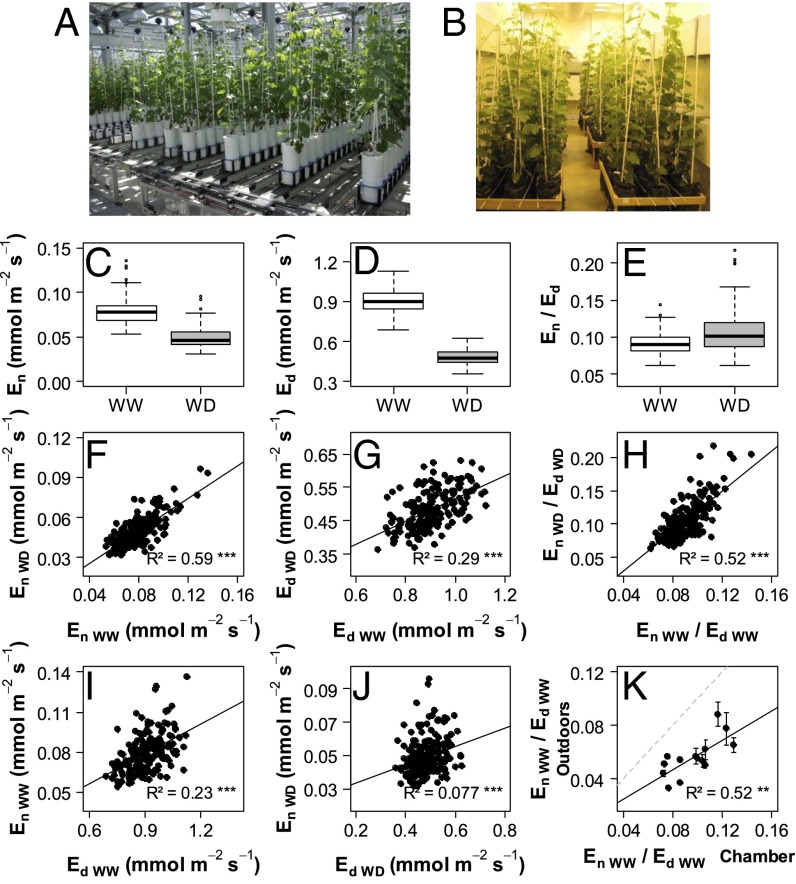
Substantial, nocturnal rates of water loss (En) were recorded with a highly significant genotypic effect regardless of the year and watering scenario (SI Appendix, Table S1), resulting in broad-sense heritability up to 0.86 (SI Appendix, Table S2). Mean genotypic En values recorded under WW conditions for Grenache and Syrah (0.070 and 0.091 mmol·m−2·s−1, respectively) were consistent with previous reports (20). The offspring exhibited much higher contrasts, with En ranging from 0.054 to 0.136 mmol·m−2·s−1 (Fig. 1C), similar to ranges reported in several species (15). Comparatively, diurnal rates of water loss (Ed) ranged from 0.69 to 1.13 mmol·m−2·s−1 (Fig. 1D). WD decreased mean En of the population by 37% compared with WW (Fig. 1C), whereas it decreased Ed by 50% (Fig. 1D), resulting in a stronger contribution of En to daily water loss under WD. Interestingly, genotype ranking for En was mostly conserved between WW and WD conditions, as indicated by the tight correlation between the genotypic values of En under these two conditions (Fig. 1F). The correlation was looser for Ed (Fig. 1G). This suggests that the genetic determinism of En is less subjected to environmental interaction than Ed, making En a simpler target for breeding. The En/Ed ratio, which gives the extent of nighttime compared with daytime transpiration, was ruled by a significant effect of the genotype (SI Appendix, Table S1) and high heritability (up to 0.87). En accounted for 6–14% of Ed under WW conditions, whereas it reached up to 23% of Ed under WD conditions (Fig. 1E). The ranking of genotypes for En/Ed was largely conserved between both watering scenarios (Fig. 1H). Most importantly, the genotypic values of En and Ed only loosely correlated, notably under WD (Fig. 1 I and J). Deviation from this correlation suggests that genotypes with low En but high Ed could be exploited to substantially reduce water loss at night without lowering gas exchange in the daytime.
Fig. 1.
Genetic variability in transpiration rates measured in the nighttime (En) and daytime (Ed) on potted plants of an S×G population. Offspring and parents (188 genotypes) were grown and subjected to either WW or WD conditions on a greenhouse phenotyping platform (A) during two experiments in 2012 and 2013. Plants were transferred to a controlled environment chamber (B) to determine En and Ed. (C–E) Boxplots of the genotypic values (BLUPs for the whole dataset merging 2012 and 2013) for En, Ed, and En/Ed under WW and WD conditions. (F–H) Comparisons of genotypic values between WW and WD scenarios. (I and J) Correlation between daytime and nighttime genotypic values of transpiration rates under WW (I) and WD (J) conditions. (K) Comparison of genotypic values of En/Ed between controlled (x axis) and outdoor (y axis) conditions for a subset of 14 genotypes. Pearson’s determination coefficients (R2) are indicated with their significance level as follows: **P < 0.01, ***P < 0.001. Regression lines are represented in black and bisecting lines in dotted gray. Means and SD of genotypic values are presented in SI Appendix, Table S1 together with effects of genotype, water scenario, and year.
To test whether results obtained in controlled environment were conserved in outdoor conditions, a subset of 14 genotypes with contrasting transpiration rates were grown outdoors. Potted plants were regularly weighed over a 24-h period on a clear summer night followed by a bright sunny day, typical of the Mediterranean climate. Overall, genotype ranks for the contribution of night relative to daytime water losses (En/Ed) were mostly conserved regardless of conditions (Fig. 1K). Compared with controlled conditions, outdoor daytime evaporative demand and light were particularly high (SI Appendix, Table S3), resulting in a general decrease in En/Ed (Fig. 1K). Nevertheless, En/Ed values still reached up to 10% for extreme genotypes (Fig. 1K).
Genetic Variability in Nighttime Transpiration Mostly Originates from Incomplete Stomatal Closure in the Dark.
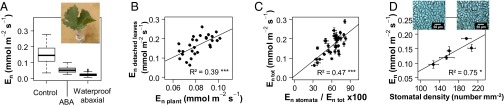
The fact that a significant transpiration rate was found at night even under WD (Fig. 1C) raises questions about the origin of this nocturnal water loss. Although recent studies have shown that stomata remain partially open in the dark (30, 31), including in grapevine (32), a slightly permeable cuticle might also be responsible for these observations. To decipher the respective contributions of stomata and cuticle to the genetic variability of En, a subset of 28 genotypes with contrasting En were selected from the S×G population. Detached leaves from potted plants cultivated outdoors were fed with solutions of artificial sap, and En was determined once stabilized under controlled atmospheric conditions in darkness (control in Fig. 2A). A significant effect of the genotype on En was detected (P < 0.001), and genotypic values measured on detached leaves correlated with values obtained on whole plants (Fig. 2B). Higher values for detached leaves than for whole plants were consistent with a higher stomatal density (168 ± 31 stomata mm−2) that is typical of plants grown outdoors rather than under greenhouse conditions (108 ± 25 stomata mm−2). The detached leaves were then transferred to a solution supplemented with abscisic acid (ABA), a drought hormone that induces stomatal closure. The ABA treatment significantly reduced En by 70% on average compared with control (Fig. 2A), indicating that stomata had remained substantially open under dark conditions before ABA addition. Stomatal contribution to En was calculated as the percentage variation in En induced by ABA, assuming that feeding detached leaves with ABA at supraphysiological concentration induced maximal stomatal closure. Stomatal contribution largely varied across genotypes (P < 0.001), ranging from 30% to 90%, and strongly correlated with En (Fig. 2C). This indicates that variability in the stomatal contribution to water loss at night, as estimated with the ABA assay, accounted for an important part of the genetic variability of En. Genotypes with higher En also displayed higher stomatal density in a subset of five genotypes (Fig. 2D). This suggests that stomatal density also contributed to the genetic variability of En observed in our study, contrasting with a previous report (33).
Fig. 2.
Genetic variability in nighttime transpiration (En) measured on detached leaves subjected to different treatments for a subset of genotypes selected in the S×G population. (A) Detached leaves fed with control, artificial sap; effect of 128 mmol m−3 (+) ABA added to the solution (boxplots for 28 genotypes) and effect of waterproofing of the abaxial side (boxplot for 15 genotypes). The Inset picture shows a representative leaf in solution. (B) Comparison for 28 genotypes between En measured on detached leaves fed with control, artificial sap (mean for n = 5 leaves per genotype, y axis) and genotypic En values measured on WW whole plants (2013 experiment, x axis). (C) Comparison between total En measured on detached leaves fed with artificial sap (En tot) and estimate of stomatal contribution to En calculated as the percentage reduction in En induced by ABA (En stomata) relative to En tot for 28 genotypes. (D) Correlation between En measured on detached leaves fed with artificial sap and stomatal density for five genotypes; pictures show imprints of the abaxial leaf surface for two genotypes with low (Left) or high (Right) stomatal density. (Scale bar, 60 µm.) In C and D, mean ± SE for five leaves per genotype.
Cuticular Water Losses: Tight Genetic Control for a Small Fraction of Nighttime Transpiration.
A small but consistent rate of water loss was still recorded on detached leaves after the ABA treatment (Fig. 2A). This remaining loss may be due to the inability of stomata to fully close (34) or to a slight permeability of the cuticle on the leaf epidermis. Taking advantage of the hypostomatous feature of grapevine—that is, the absence of stomata on the adaxial side of the leaves—we quantified water loss through the adaxial cuticle by waterproofing the abaxial side of detached leaves with petroleum jelly. As an average for all genotypes tested, the resulting, residual rate of water loss accounted for 15% of total En observed for control leaves (Fig. 2A). It approached half of En observed upon ABA treatment, suggesting that water loss through the abaxial cuticle was close to that of the adaxial cuticle. Assuming that water loss through the cuticle was similar on both leaf sides (i.e., negligible part played by water vapor leaks through ABA-treated stomata), we inferred that total cuticular water loss from both sides accounted for 30% of En, the remaining 70% being due to incomplete stomatal closure in the dark, in agreement with previous estimates in grapevine (27, 35). Moreover, we found a significant effect of the genotype on cuticular water loss (P < 0.001). This suggests that cuticular water loss, although low compared with stomatal transpiration under WW conditions, may represent a relevant breeding target, with a likely higher contribution under WD when stomata close more fully.
Nighttime Transpiration, Daytime Transpiration, and Growth Only Partly Share Their Genetic Determinisms.
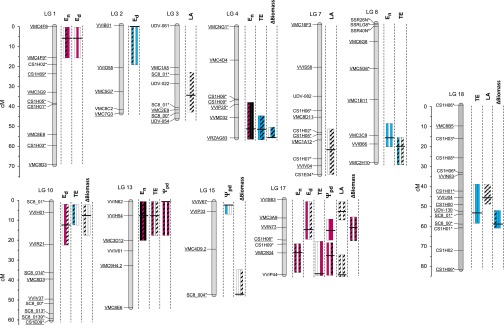
The genetic determinism of En was examined in whole plants by searching for underlying QTLs. Detection was performed on BLUPs calculated for individual years (2012 or 2013) and for the 2-y dataset on the consensus S×G map (36). Significant QTLs for En were detected on five linkage groups (LGs) for 2012 and/or 2013 and WW and/or WD (Fig. 3), with a higher confidence level under WD (SI Appendix, Table S5). Contributions of alleles to individual QTLs were mainly due to additive effects with very few dominance effects between alleles (SI Appendix, Table S5). Each of these QTLs individually accounted for 8–24% of the total variance (SI Appendix, Table S5) and for up to 36% altogether (for WD in 2012). Three genomic regions of particular interest, located on LGs 1, 4, and 13, contained stable QTLs for En in 2012 and 2013 under WD. The identification of stable QTLs where alleles had mostly additive effects is a promising, primary step toward marker-assisted selection on En.
Fig. 3.
Localization on the S×G linkage map of the most important QTLs detected for transpiration rates during the nighttime (En) and daytime (Ed), transpiration efficiency (TE), predawn water potential (Ψpd), shoot growth (ΔBiomass), and leaf area (LA). Each QTL is represented by three bars, either filled when significant in 2012 (left), 2013 (middle), and 2012+2013 (right) or left empty if not, and colored in blue or red when detected under WW or WD conditions or filled with black when detected under both conditions or else hatched when detected with the multiscenario dataset (WW+WD). Central mark in the bars indicates the position L where maximum logarithm of odds (LOD) score was obtained, and bar length represents the confidence region for the QTL (where LOD score exceeded maximum LOD – 1). When several QTLs were detected for the same trait with different positions L but with overlapping confidence regions, only one bar was figured with L corresponding to the highest LOD score; when the length of their confidence regions differed, the shorter one was figured. Fully informative markers (segregating in four allelic classes) are underlined. The longest marker names have been truncated and suffixed * (29). Complete description of the QTLs is provided in SI Appendix, Table S5.
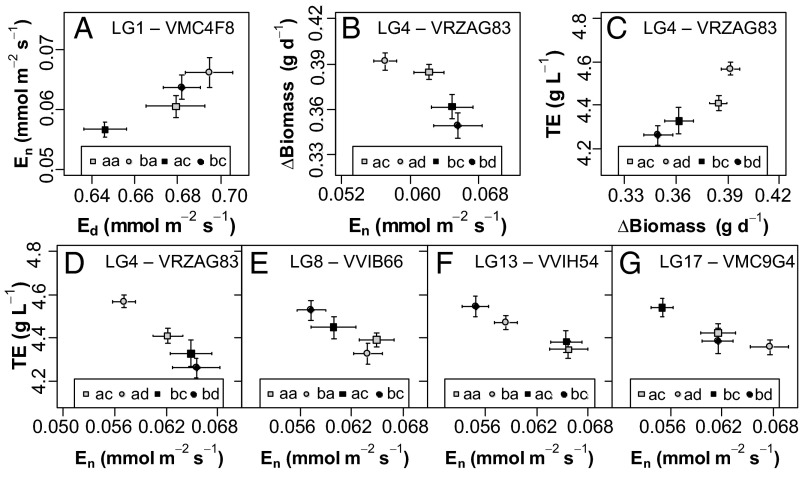
We then examined whether the genetic variations of En and Ed could be uncoupled from each other, as suggested by the weak correlations between the two traits (Fig. 1 I and J). Five QTLs were found on LGs 1, 2, 10, and 17 as determining genetic variation in Ed (Fig. 3 and SI Appendix, Table S5). Although Ed was much larger than En, the associated QTLs were less stable across years and watering scenarios, probably due to stronger differences in climatic conditions during the day between the 2 y of experiment (29). Importantly, most of the QTLs detected for Ed did not colocalize with QTLs for En. The only exception was a colocalization on LG 1 (Fig. 3), where allelic variation had parallel effects on both traits (Fig. 4A and SI Appendix, Fig. S1 A and B). The four other QTLs that were exclusively detected for En suggested they could be related to specific regulators of stomatal closure in darkness, such as those recently classified as OPEN ALL NIGHT LONG (24). Thus, choosing favorable alleles on QTLs for En that do not colocalize with QTLs for Ed should allow a reduction in nighttime water loss without altering daytime gas exchange.
Fig. 4.
Relationships between allelic values for transpiration rates during the nighttime (En) and daytime (Ed), growth (ΔBiomass), and transpiration efficiency (TE) at the main QTL colocalizations. QTLs are identified in plots by the name and LG number of the nearest marker. Pairs of letters in the legends indicate the different allelic combinations on markers associated to each QTL, with different letters when alleles differed and the first and second letters corresponding to alleles, respectively, inherited from Syrah and Grenache parents. (A) Biplot of allelic values for En vs. Ed at the VMC4F8 marker on LG 1. (B) Biplot of allelic values for ΔBiomass vs. En at the VRZAG83 marker on LG 4. (C) Biplot of allelic values for TE vs. ΔBiomass at the VRZAG83 marker on LG 4. (D–G) Biplots of allelic values for TE vs. En at the VRZAG83 marker on LG 4 (D), at the VVIB66 marker on LG 8 (E), at the VVIH54 marker on LG 13 (F), and at the VMC9G4 marker on LG 17 (G). Means and SEs of allelic values are calculated as BLUPs from the whole dataset. Separate analyses for each water scenario are detailed in SI Appendix, Figs. S1, S2, and S5.
Finally, we assessed whether genetic variations in Ed and En were associated to variations in growth. Growth rate (ΔBiomass) was estimated for the whole offspring in the greenhouse over periods of 10–15 d with stabilized soil water content by analyzing sequences of images taken in the phenotyping platform. ΔBiomass was ruled by a highly significant effect of the genotype, and heritability reached up to 0.71. Five QTLs were detected on LGs 4, 10, 15, 17, and 18 (Fig. 3), altogether accounting for up to 30% of the variance. Stable localizations between years were found on two LGs (4 and 18; SI Appendix, Table S5). Two QTL colocalizations were found for ΔBiomass and Ed with parallel allelic effects (SI Appendix, Fig. S2), reflecting the positive relationship between transpiration and photosynthesis rates in the daytime. As expected from this relationship, genotypes with reduced transpiration rates also exhibited reduced growth, especially under WD (SI Appendix, Table S4). By contrast, the genotypic values of En and ΔBiomass did not correlate under WW (SI Appendix, Table S4). Furthermore, a negative correlation was observed under WD (SI Appendix, Table S4): genotypes with reduced En tended to maintain higher growth rates. Accordingly, a common QTL for En and ΔBiomass was detected on LG 4 (Fig. 3) with opposite allelic effects on each trait (Fig. 4B and SI Appendix, Fig. S1 C and D). The relationship between reduced transpiration at night and enhanced growth may be due to an improved restoration of the plant water status during the night with low En, favoring cell expansion before the onset of transpiration at dawn (37). High transpiration rate at night, by contrast, prevents overnight equilibration between plant and soil water potentials (38, 39). In line with this interpretation, a significant, negative correlation was observed between the genotypic values of En and predawn leaf water potential (Ψpd; SI Appendix, Table S4). Moreover, two colocalizations between QTLs for En and Ψpd were detected (on LG 13 and 17) with opposite allelic effects (SI Appendix, Fig. S3). Reduced transpiration at night may also favor plant growth by limiting plant vulnerability to embolism in the daytime (20). Our results support that reduced En does not necessarily decrease productivity and can even result in higher growth.
Shared Genetic Determinisms for Nighttime Transpiration and TE.
The genetic analysis of the S×G population suggested that selection could be effectively operated on En independently of Ed and without causing detrimental effects on plant growth or even having a positive influence under WD. Such a breeding strategy would be of agronomical relevance if lower En significantly improved TE. We addressed this possibility by determining TE at the whole shoot level as the ratio of ΔBiomass to the amount of water lost over a period of 10–15 d. A highly significant genotypic effect on TE was found (SI Appendix, Table S1), with TE ranging from 5.0 to 6.7 g·L−1 under WW and from 1.8 to 3.2 g·L−1 under WD (SI Appendix, Fig. S4A). A negative correlation was found between TE and En under both watering scenarios, supporting our initial hypothesis that water saving at night could substantially contribute to an efficient use of this resource (SI Appendix, Fig. S4 B and C). However, individual genotypes largely deviated from this general trend, which prompted us to dissect the genetic links between En and TE.
Six QTLs for TE were detected on six LGs (Fig. 3), altogether accounting for up to 33% of the variance (for WW in 2012). The most significant QTL, on LG 4, remained stable over the 2 y and accounted for more than 18% of the variance (SI Appendix, Table S5). Four QTLs for TE colocalized with QTLs for En on LGs 4, 8, 13, and 17 (Fig. 3), with opposite allelic effects (Fig. 4 D–G). Allelic variation at the QTL detected on LG 4 combined an increase in TE not only with a reduction in En (Fig. 4D) but also with an enhancement of growth (Fig. 4C). The three remaining loci (on LGs 8, 13, and 17) hosted genetic variation with specific impacts on En without significant effect on Ed or growth (Figs. 3 and 4 E–G). All these QTLs therefore arise as preferential targets to breed for higher TE. Only one QTL of TE was common to Ed and growth (on LG 10), suggesting a dominating role of En in determining the genetic variability of TE in the S×G population. A lot of genetic analyses of TE that are based on gas exchange in the daytime or δ13C may therefore miss important components that are unrelated to daytime physiology (40, 41). In our study, this drawback was circumvented by using an integrated estimation of TE over several day–night cycles to reveal the effect of nighttime transpiration therein.
That nocturnal transpiration might be associated to a less efficient use of water raises the question of why plants evolved with substantial water losses at night. First, night transpiration may lower leaf temperature by evaporative cooling, thereby decreasing carbon losses through dark respiration (42). Night water fluxes may otherwise have beneficial roles in nutrient transport (43) and O2 supply to the xylem parenchyma (16, 44). Incomplete stomatal closure at night may also accelerate photosynthesis resumption at sunrise (45), but this has not always been observed (46). All these putative benefits were probably masked by stronger physiological influences in our study, as we did not observe any general, positive relationship between night transpiration and plant growth.
Lowering Nighttime Transpiration as a Breeding Strategy to Anticipate Climate Change.
The genetic uncoupling between day and night water losses allowed us to identify QTLs for nighttime transpiration with an associated impact on TE in grapevine. One of these QTLs where alleles also favor plant growth arose as a promising target for marker-assisted selection. Implementing such a breeding strategy becomes all the more relevant in light of climate change projections (1).
First, the intensity and duration of drought episodes are likely to increase in Mediterranean regions. Our study highlights that soil WD further loosens the relationship between day and night transpiration (Fig. 1 I and J) and increases the confidence level on the QTLs identified (Fig. 3 and SI Appendix, Table S5). The level of water stress we imposed was deliberately moderate, to capture the physiological events triggered at incipient stages of soil drying. Such stages correspond to extended periods of major relevance for water saving in Mediterranean vineyards (47). Investigating more stressful conditions would certainly have led to the detection of alternative QTL localizations but most likely associated with avoidance or survival strategies, which are less relevant in an agricultural perspective (48). Gradual change in the physiological processes mobilized by plants to cope with progressive soil drying could be illustrated by the QTLs of En that were detected exclusively under WD but not under WW conditions (e.g., on LG 17). Such QTLs could be related to a substantial influence of the cuticle on nocturnal transpiration under WD, whereas it could be masked by the predominant contribution of stomata under WW conditions (Fig. 2). These QTLs could also be related to differences in stomatal density, which is affected by water stress and ABA (49) and modulates TE (50). Further studies are required to dissect these previously unidentified QTLs of En and their interaction with the severity of water stress.
Second, evaporative demand and thus transpiration are expected to increase faster in the nighttime than in the daytime due to multiple impacts of climate change. An increase in temperature that drives higher vapor pressure deficit appears to proceed faster at night with global warming (1). Consequences on transpiration rate are expected to be attenuated preferentially in the daytime due to a forecasted increase in atmospheric water vapor concentration (51). Additionally, the projected elevation in atmospheric CO2 concentration is expected to reduce stomatal aperture and transpiration more strongly in the presence of light than in darkness (52). Overall, climate projections therefore intimate an enhancement of transpiration at night relative to the day, making nocturnal control of transpiration a relevant target to breed crops for enhanced TE.
In conclusion, this study supports that crops can be bred for reduced transpiration at night with a substantial gain in TE. Field experiments that address the control of nighttime transpiration in relation to climate change projections will contribute to the design of a more sustainable agriculture.
Materials and Methods
The S×G pseudo-F1 population was obtained from the reciprocal cross between Syrah and Grenache V. vinifera cultivars. In 2012 and 2013, six and five clones, respectively, of each genotype (all offspring and the two parents) were studied as replicates in randomized blocks into the PHENOARCH high-throughput phenotyping platform (53) in a greenhouse. Watering scenarios were imposed by maintaining soil water content in pots at target values using watering stations. Total water loss by transpiration over a period of 10–15 d, when soil water content had stabilized, was calculated as the sum of daily water losses recorded for each potted plant by the weighing terminals and corrected for soil evaporation. Individual plant fresh weights were calculated daily using images taken in the platform and processed for conversion into biomass. Growth rates over 10–15 d periods were then calculated as increases in dry biomass per unit time (ΔBiomass) from processed images of plants. Whole plant TE was determined as the ratio between biomass increase and the total amount of transpired water over the same period. En, Ed, and predawn water potential (Ψpd) were measured on a specific day when the plants were taken off the platform and placed into a controlled chamber to ensure stable and repeatable climatic conditions. The weight loss of each plant in a bagged pot to prevent soil evaporation was calculated over periods of darkness (ca. 12 h) followed by at least 6 h of constant light. Results were related to the duration of the measurement period and to the leaf area obtained by image analysis, yielding mean specific transpiration rates overnight (En) and daytime (Ed) periods. For each trait, QTL detection was performed on BLUPs with MapQTL 4.0 software (54) using the consensus map, which combined segregation information from the two parents. Further experiments were performed on subsets of genotypes selected for their contrasting En, Ed, or allelic composition at the main QTLs identified. More details on all experiments, analyses, and any associated references are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank P. Péchier, P. Hamard, and the technical staff from the team “Diversité, Adaptation et Amélioration de la Vigne” for technical assistance, and L. Cabrera-Bosquet and Antonin Grau for great help with platform management. This work was supported by funding from the project Long Term Impacts and Adaptations to Climate Change in Viticulture and Oenology (LACCAVE) of the French National Institute for Agricultural Research (INRA) and by Grant ANR-09-GENM-024-002 from the French National Research Agency. A.C.-L. received a PhD grant from the French government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600826113/-/DCSupplemental.
References
- 1.Intergovernmental Panel on Climate Change . In: Climate Change 2013: The Physical Science Basis. Stocker TF, et al., editors. Cambridge Univ Press; New York: 2013. [Google Scholar]
- 2.Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. Breeding for high water-use efficiency. J Exp Bot. 2004;55(407):2447–2460. doi: 10.1093/jxb/erh277. [DOI] [PubMed] [Google Scholar]
- 3.Lawson T, Blatt MR. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014;164(4):1556–1570. doi: 10.1104/pp.114.237107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424(6951):901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- 5.Bacon MA. What Is Water-Use Efficiency? Water Use Efficiency in Plant Biology. Blackwell; Oxford: 2004. pp. 27–41. [Google Scholar]
- 6.Furbank RT, Quick WP, Sirault XRR. Improving photosynthesis and yield potential in cereal crops by targeted genetic manipulation: Prospects, progress and challenges. Field Crops Res. 2015;182:19–29. [Google Scholar]
- 7.Blum A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009;112(2–3):119–123. [Google Scholar]
- 8.Farquhar G, Oleary M, Berry J. On the relationship between carbon isotope discrimination and the inter-cellular carbon-dioxide concentration in leaves. Aust J Plant Physiol. 1982;9(2):121–137. [Google Scholar]
- 9.Rebetzke GJ, Condon AG, Richards RA, Farquhar GD. Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Sci. 2002;42(3):739–745. [Google Scholar]
- 10.Viger M, Rodriguez-Acosta M, Rae AM, Morison JIL, Taylor G. Toward improved drought tolerance in bioenergy crops: QTL for carbon isotope composition and stomatal conductance in Populus. Food Energy Secur. 2013;2(3):220–236. [Google Scholar]
- 11.Masle J, Gilmore SR, Farquhar GD. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature. 2005;436(7052):866–870. doi: 10.1038/nature03835. [DOI] [PubMed] [Google Scholar]
- 12.Juenger TE, et al. Identification and characterization of QTL underlying whole-plant physiology in Arabidopsis thaliana: δ13C, stomatal conductance and transpiration efficiency. Plant Cell Environ. 2005;28(6):697–708. [Google Scholar]
- 13.Campitelli BE, Des Marais DL, Juenger TE. Ecological interactions and the fitness effect of water-use efficiency: Competition and drought alter the impact of natural MPK12 alleles in Arabidopsis. Ecol Lett. 2016;19(4):424–434. doi: 10.1111/ele.12575. [DOI] [PubMed] [Google Scholar]
- 14.Yoo CY, Pence HE, Hasegawa PM, Mickelbart MV. Regulation of transpiration to improve crop water use. Crit Rev Plant Sci. 2009;28(6):410–431. [Google Scholar]
- 15.Caird MA, Richards JH, Donovan LA. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol. 2007;143(1):4–10. doi: 10.1104/pp.106.092940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marks CO, Lechowicz MJ. The ecological and functional correlates of nocturnal transpiration. Tree Physiol. 2007;27(4):577–584. doi: 10.1093/treephys/27.4.577. [DOI] [PubMed] [Google Scholar]
- 17.Ogle K, et al. Differential daytime and night-time stomatal behavior in plants from North American deserts. New Phytol. 2012;194(2):464–476. doi: 10.1111/j.1469-8137.2012.04068.x. [DOI] [PubMed] [Google Scholar]
- 18.Schoppach R, Claverie E, Sadok W. Genotype-dependent influence of night-time vapour pressure deficit on night-time transpiration and daytime gas exchange in wheat. Funct Plant Biol. 2014;41(9):963–971. doi: 10.1071/FP14067. [DOI] [PubMed] [Google Scholar]
- 19.Phillips NG, Lewis JD, Logan BA, Tissue DT. Inter- and intra-specific variation in nocturnal water transport in Eucalyptus. Tree Physiol. 2010;30(5):586–596. doi: 10.1093/treephys/tpq009. [DOI] [PubMed] [Google Scholar]
- 20.Rogiers SY, Greer DH, Hutton RJ, Landsberg JJ. Does night-time transpiration contribute to anisohydric behaviour in a Vitis vinifera cultivar? J Exp Bot. 2009;60(13):3751–3763. doi: 10.1093/jxb/erp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christman MA, et al. Genetic variation in Arabidopsis thaliana for night-time leaf conductance. Plant Cell Environ. 2008;31(8):1170–1178. doi: 10.1111/j.1365-3040.2008.01833.x. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher RS, et al. Development of a next-generation NIL library in Arabidopsis thaliana for dissecting complex traits. BMC Genomics. 2013;14(1):655. doi: 10.1186/1471-2164-14-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reuning GA, Bauerle WL, Mullen JL, McKay JK. Combining quantitative trait loci analysis with physiological models to predict genotype-specific transpiration rates. Plant Cell Environ. 2015;38(4):710–717. doi: 10.1111/pce.12429. [DOI] [PubMed] [Google Scholar]
- 24.Costa JM, et al. Open All Night Long: The dark side of stomatal control. Plant Physiol. 2015;167(2):289–294. doi: 10.1104/pp.114.253369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomás M, et al. Variability of water use efficiency in grapevines. Environ Exp Bot. 2014;103:148–157. [Google Scholar]
- 26.Fuentes S, De Bei R, Tyerman S. Night-time plant water loss: The unseen process for local and global water footprint and water balance estimations in grapevines. Wine Vitic J. 2013;28(4):50–52. [Google Scholar]
- 27.Escalona JM, et al. Responses of leaf night transpiration to drought stress in Vitis vinifera L. Agric Water Manage. 2013;118:50–58. [Google Scholar]
- 28.Schultz HR. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 2003;26(8):1393–1405. [Google Scholar]
- 29.Coupel-Ledru A, et al. Genetic variation in a grapevine progeny (Vitis vinifera L. cvs Grenache×Syrah) reveals inconsistencies between maintenance of daytime leaf water potential and response of transpiration rate under drought. J Exp Bot. 2014;65(21):6205–6218. doi: 10.1093/jxb/eru228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mott KA, Peak D. Stomatal responses to humidity and temperature in darkness. Plant Cell Environ. 2010;33(7):1084–1090. doi: 10.1111/j.1365-3040.2010.02129.x. [DOI] [PubMed] [Google Scholar]
- 31.Howard AR, Donovan LA. Helianthus nighttime conductance and transpiration respond to soil water but not nutrient availability. Plant Physiol. 2007;143(1):145–155. doi: 10.1104/pp.106.089383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogiers SY, et al. Stomatal response of an anisohydric grapevine cultivar to evaporative demand, available soil moisture and abscisic acid. Tree Physiol. 2012;32(3):249–261. doi: 10.1093/treephys/tpr131. [DOI] [PubMed] [Google Scholar]
- 33.Rogiers SY, Clarke SJ. Nocturnal and daytime stomatal conductance respond to root-zone temperature in ‘Shiraz’ grapevines. Ann Bot (Lond) 2013;111(3):433–444. doi: 10.1093/aob/mcs298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santamaria JM, Davies WJ, Atkinson CJ. Stomata of micropropagated Delphinium plants respond to ABA, CO2, light and water potential, but fail to close fully. J Exp Bot. 1993;44(1):99–107. [Google Scholar]
- 35.Boyer JS, Wong SC, Farquhar GD. CO2 and water vapor exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiol. 1997;114(1):185–191. doi: 10.1104/pp.114.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fournier-Level A, et al. Quantitative genetic bases of anthocyanin variation in grape (Vitis vinifera L. ssp. sativa) berry: A quantitative trait locus to quantitative trait nucleotide integrated study. Genetics. 2009;183(3):1127–1139. doi: 10.1534/genetics.109.103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caldeira CF, Jeanguenin L, Chaumont F, Tardieu F. Circadian rhythms of hydraulic conductance and growth are enhanced by drought and improve plant performance. Nat Commun. 2014;5:5365. doi: 10.1038/ncomms6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donovan L, Linton M, Richards J. Predawn plant water potential does not necessarily equilibrate with soil water potential under well-watered conditions. Oecologia. 2001;129(3):328–335. doi: 10.1007/s004420100738. [DOI] [PubMed] [Google Scholar]
- 39.Kavanagh KL, Pangle R, Schotzko AD. Nocturnal transpiration causing disequilibrium between soil and stem predawn water potential in mixed conifer forests of Idaho. Tree Physiol. 2007;27(4):621–629. doi: 10.1093/treephys/27.4.621. [DOI] [PubMed] [Google Scholar]
- 40.Tambussi EA, Bort J, Araus JL. Water use efficiency in C3 cereals under Mediterranean conditions: A review of physiological aspects. Ann Appl Biol. 2007;150(3):307–321. [Google Scholar]
- 41.Medrano H, et al. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 2015;3(3):220–228. [Google Scholar]
- 42.Peraudeau S, et al. Effect of carbohydrates and night temperature on night respiration in rice. J Exp Bot. 2015;66(13):3931–3944. doi: 10.1093/jxb/erv193. [DOI] [PubMed] [Google Scholar]
- 43.Zeppel MJB, Lewis JD, Phillips NG, Tissue DT. Consequences of nocturnal water loss: A synthesis of regulating factors and implications for capacitance, embolism and use in models. Tree Physiol. 2014;34(10):1047–1055. doi: 10.1093/treephys/tpu089. [DOI] [PubMed] [Google Scholar]
- 44.Gansert D. Xylem sap flow as a major pathway for oxygen supply to the sapwood of birch (Betula pubescens Ehr.) Plant Cell Environ. 2003;26(11):1803–1814. [Google Scholar]
- 45.Resco de Dios V, Loik ME, Smith R, Aspinwall MJ, Tissue DT. Genetic variation in circadian regulation of nocturnal stomatal conductance enhances carbon assimilation and growth. Plant Cell Environ. 2016;39(1):3–11. doi: 10.1111/pce.12598. [DOI] [PubMed] [Google Scholar]
- 46.Auchincloss L, Easlon HM, Levine D, Donovan L, Richards JH. Pre-dawn stomatal opening does not substantially enhance early-morning photosynthesis in Helianthus annuus. Plant Cell Environ. 2014;37(6):1364–1370. doi: 10.1111/pce.12241. [DOI] [PubMed] [Google Scholar]
- 47.Lebon E, Pellegrino A, Louarn G, Lecoeur J. Branch development controls leaf area dynamics in grapevine (Vitis vinifera) growing in drying soil. Ann Bot (Lond) 2006;98(1):175–185. doi: 10.1093/aob/mcl085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skirycz A, et al. Survival and growth of Arabidopsis plants given limited water are not equal. Nat Biotechnol. 2011;29(3):212–214. doi: 10.1038/nbt.1800. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka Y, Nose T, Jikumaru Y, Kamiya Y. ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. Plant J. 2013;74(3):448–457. doi: 10.1111/tpj.12136. [DOI] [PubMed] [Google Scholar]
- 50.Franks PJW, W Doheny-Adams T, Britton-Harper ZJ, Gray JE. Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol. 2015;207(1):188–195. doi: 10.1111/nph.13347. [DOI] [PubMed] [Google Scholar]
- 51.Robinson PJ. Temporal trends in United States dew point temperatures. International Journal of Climatology. 2000;20(9):985–1002. [Google Scholar]
- 52.Messinger SM, Buckley TN, Mott KA. Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiol. 2006;140(2):771–778. doi: 10.1104/pp.105.073676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cabrera-Bosquet L, et al. High throughput estimation of incident light, light interception and radiation-use efficiency of thousands of plants in a phenotyping platform. New Phytologist. June 3, 2016 doi: 10.1111/nph.14027. [DOI] [PubMed] [Google Scholar]
- 54.Van Ooijen JW, Maliepaard C. MapQTL Version 4.0: Software for the Calculation of QTL Positions on Genetic Maps. CPRO-DLO; Wageningen, The Netherlands: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.