Significance
Earth is currently the only planet known to harbor complex life. Understanding whether terrestrial biotic complexity is a unique phenomenon or can be expected to be widespread in the universe depends on a mechanistic understanding of the factors that led to the emergence of complex life on Earth. Here, we use geochemical constraints and quantitative models to suggest that marine environments may have been unfavorable for the emergence and large-scale proliferation of motile multicellular life for most of Earth’s history. Further, we argue that a holistic evaluation of environmental variability, organismal life history, and spatial ecological dynamics is essential for a full accounting of the factors that have allowed for the emergence of biological complexity on Earth.
Keywords: oxygen, animals, evolution, Proterozoic
Abstract
The emergence and expansion of complex eukaryotic life on Earth is linked at a basic level to the secular evolution of surface oxygen levels. However, the role that planetary redox evolution has played in controlling the timing of metazoan (animal) emergence and diversification, if any, has been intensely debated. Discussion has gravitated toward threshold levels of environmental free oxygen (O2) necessary for early evolving animals to survive under controlled conditions. However, defining such thresholds in practice is not straightforward, and environmental O2 levels can potentially constrain animal life in ways distinct from threshold O2 tolerance. Herein, we quantitatively explore one aspect of the evolutionary coupling between animal life and Earth’s oxygen cycle—the influence of spatial and temporal variability in surface ocean O2 levels on the ecology of early metazoan organisms. Through the application of a series of quantitative biogeochemical models, we find that large spatiotemporal variations in surface ocean O2 levels and pervasive benthic anoxia are expected in a world with much lower atmospheric pO2 than at present, resulting in severe ecological constraints and a challenging evolutionary landscape for early metazoan life. We argue that these effects, when considered in the light of synergistic interactions with other environmental parameters and variable O2 demand throughout an organism’s life history, would have resulted in long-term evolutionary and ecological inhibition of animal life on Earth for much of Middle Proterozoic time (∼1.8–0.8 billion years ago).
A long-standing and pervasive view is that there have been intimate mechanistic links between the evolution of complex life on Earth—in other words, the emergence and ecological expansion of eukaryotic cells and their aggregation into multicellular organisms—and the secular evolution of ocean−atmosphere oxygen levels (1). Molecular oxygen (O2) is by far the most energetic of the abundant terminal oxidants used in biological metabolism (e.g., ref. 2). When this energetic capacity is harnessed by mitochondria in eukaryotic cells, the energy flux supported by a given genome size increases by a factor of ∼8,000 (3), potentially paving the way for increased complexity at the cellular level (but see ref. 4). Oxygen is also a crucial component of enzymatic pathways leading to the synthesis of regulatory membrane lipids (5) and structural proteins (6) in eukaryotic organisms and provides a powerful shield against solar UV radiation at Earth’s surface through stratospheric ozone production. Furthermore, O2 is the only respiratory electron acceptor that can meet the metabolic demands required for attaining the large sizes and active lifestyles characteristic of metazoan life (e.g., ref. 7). There is thus ample support for the view that Earth’s oxygen cycle provided a crucial evolutionary and ecological constraint on the road to increased biotic complexity, both at the cellular level and on the road to motile multicellular life.
However, it is important to distinguish between the O2 levels required for: (i) the emergence and ecological expansion of complex (multicellular) eukaryotes, including red/brown algae, land plants, fungi, and animals; (ii) the initial origin of the metazoan (animal) last common ancestor (LCA); (iii) the diversification of basal (e.g., Ctenophora, Cnidaria, Porifera) and more derived (e.g., Bilateria) metazoan clades; and (iv) emergence of the larger body sizes and more complex ecological interactions (such as predation and burrowing) likely to leave robust signals in the fossil record (8). The requisite environmental O2 levels for each of these biotic milestones may vary substantially, and some may depend strongly on environmental variability, whereas others may be more directly linked to the environment achieving a discrete “threshold” or range of oxygen levels. Our focus here is on reconstructing an ecological context for the emergence and expansion of early animal life during the Middle to Late Proterozoic [∼1.8–0.6 billion years ago (Ga)] in the context of evolving Earth surface O2 levels.
Most work on the coevolution of metazoan life and surface oxygen levels can be characterized as either biological (e.g., attempting to constrain threshold environmental O2 levels for different organisms), or geochemical (e.g., attempting to constrain environmental O2 levels before, during, and after the emergence of metazoan life). This discussion is typically framed in terms of a relatively sharp dichotomy—e.g., either environmental oxygen levels have had little to do with the timing of the rise of animal life (e.g., refs. 9 and 10) or oxygenation of Earth’s surface has been of primary importance (e.g., ref. 11). Both approaches have emphasized the relationship (or lack thereof) between metazoan evolution and the secular oxygenation of Earth’s ocean−atmosphere system, and both provide a critical backdrop for efforts to understand the importance of environmental factors in early animal evolution.
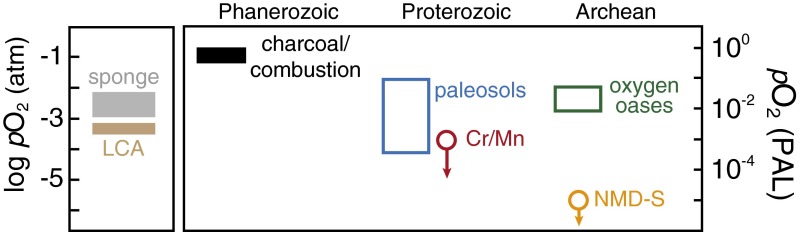
Experimental work with the modern demosponge Halichondria panacea suggests an empirical O2 threshold of ∼0.5–4.0% of the present atmospheric level (PAL; corresponding to an equilibrium concentration of ∼1–10 µmol⋅kg−1 for air-saturated water at 25 °C and a salinity of 35‰) for basal metazoan life (12) and perhaps lower for smaller sponge species. Theoretical calculations for the LCA of bilaterian life indicate an even lower threshold range of ∼0.14–0.36% PAL (ref. 13, Fig. 1). In addition, all major eukaryotic lineages contain genes and enzymes allowing for facultatively anaerobic energy metabolism (14), which indicates that either (i) anaerobic pathways of ATP synthesis in primitively aerobic eukaryotes were obtained through interdomain and intradomain lateral gene transfer (15, 16), or (ii) the earliest eukaryotic organisms contained mitochondria that were facultatively anaerobic (17). In any case, it is possible that early animals were able to respire at very low environmental O2 levels and possibly even survive periodic episodes of local anoxia.
Fig. 1.
Biological thresholds and geochemical constraints on Earth surface O2 levels. (Left) Estimated minimum metabolic O2 requirements of sponges (gray) (12) and the LCA of bilaterian metazoans (brown) (13). (Right) Estimated environmental O2 levels based on the plant fossil and charcoal record during the Phanerozoic (37); fossilized soil profiles during the Proterozoic (blue), recalculated following ref. 38 according to pCO2 values in ref. 39; chromium (Cr) and manganese (Mn) systematics of marine chemical sediments and ancient soil profiles (red) (11); non-mass-dependent sulfur (S) isotope fractionation in sedimentary rocks (orange) (40); and 0D and 3D Earth system models for Archean oxygen oasis systems (green) (20−22). Downward arrows represent upper bounds on atmospheric O2 levels, and the range for oxygen oases refers to the estimated atmospheric pO2 value at gas exchange equilibrium.
Geochemical estimates of atmospheric pO2 levels during the Proterozoic before the emergence and ecological expansion of metazoan life span orders of magnitude, from less than 0.1% PAL (11) to ∼10% PAL (18, 19). Estimates thus span levels well below what active aerobic metazoans would have been able to tolerate on long timescales to values well above those that should be required for animal respiration (Fig. 1). An additional complication is that, after the evolution of oxygenic photosynthesis, disequilibrium between surface marine and atmospheric oxygen levels would have been common, as it is in the modern oceans. A range of biogeochemical models applied to simulate this disequilibrium indicate that bacterial oxygen production in the surface ocean can sustain local dissolved O2 concentrations of ∼1–10 µmol⋅kg−1 at steady state (20–22), even at exceptionally low atmospheric O2 levels (Fig. 1). Thus, local biological O2 production in some regions of the surface ocean may have allowed for O2 levels above the respiratory requirements of basal metazoan organisms regardless of background atmospheric pO2 (10).
In principle, one could compare a geochemical estimate of atmospheric pO2 to an estimate of the dissolved O2 level required for respiration in a particular metazoan organism and use this to infer whether there would have been any respiratory “metabolic barrier” to animal emergence and ecological expansion during a given period in Earth’s history (e.g., refs. 11 and 23). However, this basic approach has a number of drawbacks. First, it neglects spatial heterogeneity in oceanic O2 levels—for example, decoupling between atmospheric pO2 levels and oxygen availability in benthic habitats or the existence of surface ocean regions with relatively high dissolved O2 as a result of local O2 production. Second, a system with an average steady-state O2 level above that required for a given metabolism could nonetheless be inhospitable overall from an evolutionary perspective if O2 levels oscillate significantly on timescales that are unfavorable for the long-term persistence of robust populations (e.g., refs. 24 and 25). Finally, the comparative threshold approach ignores variable metabolic O2 demand during an organism’s life history, the ecology of the organism, and the potential synergistic interactions between O2 and other key environmental variables, such as temperature and pH.
Here we explore a series of quantitative biogeochemical models with the goal of providing a new framework for understanding the constraints posed by a relatively low oxygen Earth system on early animal ecology and evolution. First, we use a 3D Earth system model to assess spatial variability in surface ocean O2 and surface−benthic decoupling at a range of atmospheric pO2 values relevant for the Late Proterozoic Earth [∼800–600 million years ago (Ma)]. We then use a regional time-dependent box model of the surface ocean to evaluate the potential for temporal variability in O2 levels within productive regions of the surface ocean. Finally, we build on these perspectives to propose a simple conceptual model that frames the links between Earth’s oxygen cycle and the early evolution of animal life as a question of habitat viability, highlighting the stability of environmental O2 levels (e.g., ref. 25) against the backdrop of evolving baseline pO2 (11, 26), rather than the attainment of threshold O2 levels for a single life history stage (10, 11, 23). We suggest that this view provides a more realistic and powerful framework for understanding the links between environmental stress and the early evolution of complex metazoan life on Earth. Finally, we argue that spatiotemporal O2 variability during Middle Proterozoic time would have resulted in a challenging evolutionary and ecological landscape for early animal life, even at relatively high background atmospheric pO2.
Oceanic Oxygen Landscapes
We evaluate steady-state spatial heterogeneity in surface ocean and benthic O2 levels by using a 3D Earth system model of intermediate complexity (EMIC) configured for a low pO2 world (see ref. 22). The model ocean is integrated to steady state at a range of imposed atmospheric pO2 values, and the resulting distribution of dissolved oxygen concentrations at the surface and ocean interior is evaluated. We explore a range of atmospheric pO2 levels over two orders of magnitude (0.1–10% PAL), meant to be broadly inclusive of estimates for the Mid- and Late-Proterozoic Earth system (Fig. 1), and we focus our analysis on the spatial arrangement and concentration distributions of both surface and benthic grid cells at steady state.
Although we use an effectively modern continental distribution, we do not mean to suggest that large-scale ocean circulation during the Proterozoic was necessarily similar to that characteristic of modern Earth. However, we consider it likely that the basic range of large-scale “circulatory settings”—low-latitude to midlatitude equatorial upwelling due to Hadley cell convergence, high-latitude deep convection, eastern boundary upwelling—is well represented by the zonal and meridional circulation patterns of modern Earth. This assumption should be particularly true with respect to nutrient recharge to the surface ocean (of primary importance for our simulations here). Although more accurately diagnosing patterns of large-scale ocean circulation under different continental configurations and explicitly linking these to the fossil record of early metazoan life is a crucially important topic for future research, we consider it unlikely that changes in large-scale ocean circulation would dramatically alter our primary conclusions.
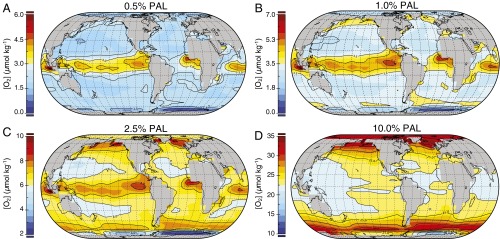
Consistent with previous work (20–22), we find that broad regions of the ocean can be characterized by dissolved O2 levels significantly above ambient gas exchange equilibrium at steady state as a result of localized biological O2 release (Fig. 2). At atmospheric pO2 levels below ∼2.5% PAL, the distribution of surface ocean O2 is controlled principally by the biosphere, with the highest dissolved O2 levels located primarily in areas of high productivity (and O2 release) along eastern boundaries and along regions of equatorial divergence (Fig. 2 A and B). However, a critical transition occurs at pO2 levels above ∼2.5% PAL, at which point surface O2 distributions are controlled by the atmosphere rather than local O2 release in the surface ocean. In this regime, high O2 levels are more commonly observed in colder, high-latitude surface waters where aqueous O2 solubility is relatively high (Fig. 2D).
Fig. 2.
Results from the EMIC [Grid Enabled Integrated Earth System Model [GENIE]). Steady-state dissolved oxygen concentrations in the surface layer of the ocean after 20-thousand-year spinup for atmospheric pO2 values of (A) 0.5%, (B) 1.0%, (C) 2.5%, and (D) 10.0% of the PAL. Note the differing scales for [O2] for each background pO2 level.
The precise atmospheric pO2 threshold at which this transition occurs will ultimately depend on a number of factors, but the presence of this transition and its functional relationship with atmospheric pO2 is unlikely to be dramatically different from that observed here (Supporting Information and Fig. S1). At pO2 levels above this transition point, most of the surface ocean will be near gas exchange equilibrium with atmospheric pO2 (Fig. 2D). Below this transitional atmospheric pO2, surface ocean dissolved O2 levels are expected to be extremely variable in time and space and may often have been well below levels that would be expected to challenge aerobic metazoan organisms (Fig. 2 A and B), despite locally elevated values in productive regions of the surface ocean.
Fig. S1.
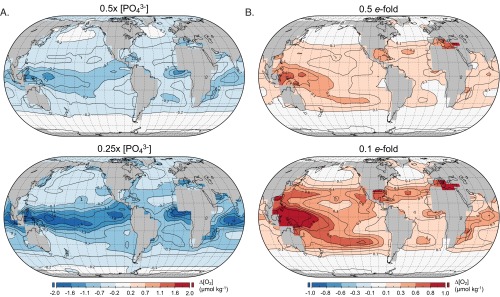
Sensitivity simulations for steady-state surface ocean O2 distributions. All panels show oxygen concentration anomalies (∆[O2], in micromoles per kilogram) for a given experiment relative to the baseline case presented in Fig. 1 (e.g., [O2]experiment – [O2]baseline, such that negative values represent lower oxygen levels than the baseline case and positive values represent higher levels). (A) Simulations with 0.5× and 0.25× the modern marine nutrient inventory. (B) Simulations with 0.5× and 0.1× the modern organic carbon remineralization length scale.
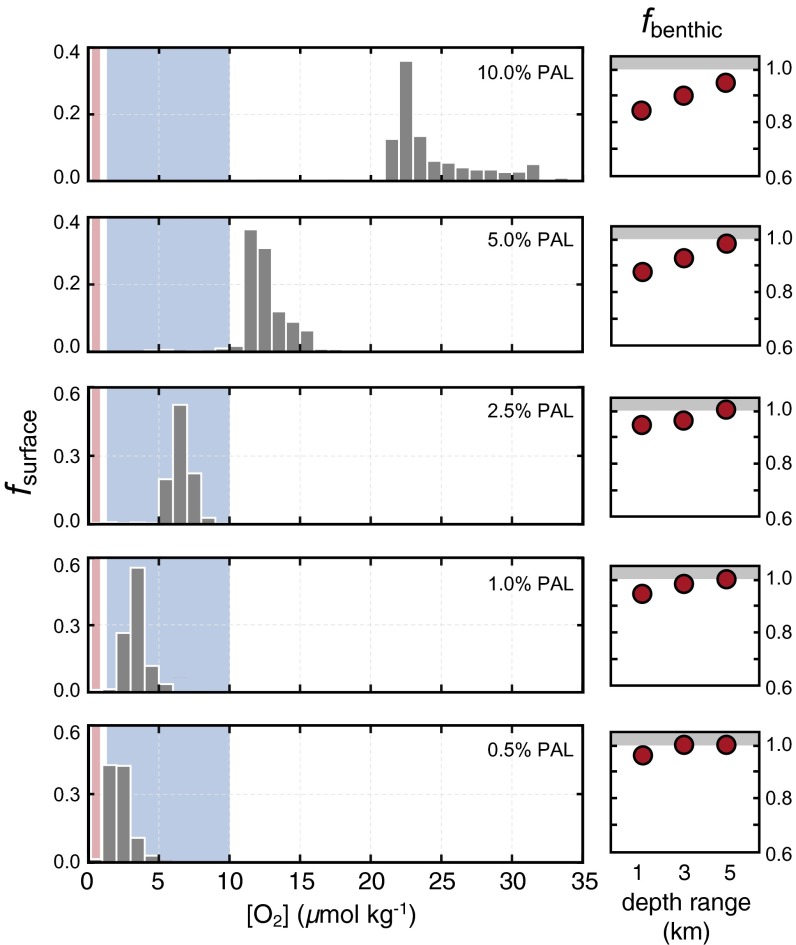
Perhaps more importantly, we observe strong decoupling between atmospheric pO2, surface ocean O2 distributions, and O2 availability in the benthic realm. On an ocean scale, benthic grid cells are almost entirely devoid of O2 at steady state at pO2 levels below ∼2–3% PAL, and, even above this level, the seafloor is dominated by anoxic conditions in our model (Fig. 3). The pervasiveness of benthic anoxia in shallow settings may also have been exacerbated by nutrient limitation of biological oxygen production in the surface ocean during much of Proterozoic time (refs. 27 and 28, Supporting Information). The upper ocean grid size in our model does not fully resolve the shallowest of continental margin settings (29), and it will be important to leverage high-resolution models of the upper ocean (e.g., ref. 30) in future research. Nevertheless, our results imply the potential for a severe O2-based ecological bottleneck for the large-scale dispersal and expansion of basal metazoan organisms with a benthic life stage (e.g., ref. 31), despite background atmospheric pO2 above the levels currently considered to be required for respiration (Fig. 2). We emphasize that this decoupling is a robust feature and arises as a natural consequence of the ocean’s biological pump—for example, any changes to the nutrient status of the ocean interior that favor greater local dissolved O2 accumulation in the surface ocean will tend to exacerbate O2 deficiency in the benthic realm.
Fig. 3.
(Left) Relative frequency distributions for dissolved oxygen concentration in surface ocean grid cells (fsurface) and (Right) the fraction of benthic grid cells that are anoxic (fbenthic) for a range of atmospheric pO2 levels. Relative fraction of benthic grid cells that are anoxic are calculated for three different cumulative depth ranges, the upper 1 km, upper 3 km, and upper 5 km of the ocean. Shaded blue boxes denote estimated ranges for basal metazoan organisms derived from laboratory experiments (12), and red boxes show lower limits for bilaterian organisms estimated from theoretical calculations (13).
In summary, our 3D model results suggest a complex interplay between atmospheric pO2, surface ocean dissolved O2 distributions, and O2 availability in the benthic marine realm over the range of atmospheric pO2 values envisioned for the Late Proterozoic Earth system. Surface ocean dissolved O2 levels would have been spatially variable and, in many cases, well above those predicted based on ambient atmospheric pO2. At the same time, O2 availability in benthic habitats would have been largely insulated from surface ocean−atmosphere O2 dynamics, making dispersal and long-term survival of metazoans with a benthic life stage extremely difficult—even at background atmospheric pO2 levels well above those conventionally assumed to be challenging for basal metazoan life.
Temporal Variability in Surface O2 Levels
Although the Earth system model results provide insight into the long-term mean state of the ocean’s oxygen landscape, we can also diagnose temporal variability in surface O2 levels using a simpler regional model (21) that tracks dissolved O2 concentrations in a generalized coastal surface ocean environment as governed by physical transport, gas exchange, and biogeochemical processes. This approach has a number of advantages for our purposes. First, it allows us to apply dynamic wind stress on diurnal and seasonal timescales such that air−sea gas exchange can be periodically forced. Second, it allows us to explicitly explore the role of externally sourced deep ocean reductants (e.g., Fe2+) on regionally large O2 production fluxes.
Briefly, our model assumes that a fraction of primary production in surface waters escapes respiration and becomes exported beneath the photic zone. This exported carbon is offset by a stoichiometric production of O2. Oxygen that accumulates in the surface ocean is transported by upwelling circulation and equilibrates with the atmosphere through air−sea gas exchange on a timescale governed by temperature, salinity, and surface wind speed (21). The upwelling circulation also injects ferrous iron (Fe2+) into surface waters, which can react with O2 produced by photosynthesis and thus represents a potential sink term for O2. Ferrous iron concentrations were kept constant in the simulations explored here. It is likely that pulsed benthic and/or hydrothermal sources of Fe2+ would have resulted in temporally variable bottom water ferrous Fe concentrations, leading to periodic anoxia. Given poor empirical constraints on local and global variability in Fe2+ fluxes, we do not explicitly model these processes here, rendering our conclusions conservative.
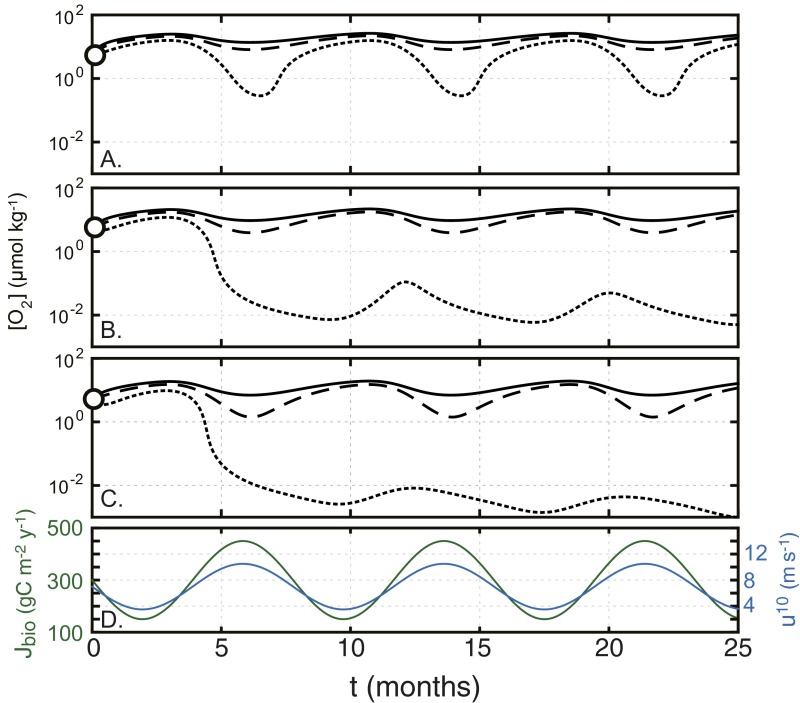
Seasonal (monthly) oscillations in biological carbon fluxes and air−sea gas exchange result in oscillatory changes in dissolved [O2] that are, in some cases, very large (Supporting Information and Fig. S2). To diagnose the potential biological consequences of this temporal variability, we evaluate the minimum surface O2 level attained during a seasonal cycle at a range of atmospheric pO2 values similar to those explored above and assuming a range of deep water dissolved [Fe2+] levels (Fig. 4). Relatively modest [Fe2+] levels well below some recent predictions for ferruginous Proterozoic oceans (32) can significantly depress seasonal minima and maxima in dissolved [O2], even at very high rates of biological productivity (Fig. 4B). Indeed, under most conditions, the estimated tolerance range for basal metazoan organisms (12) is only safely exceeded throughout the year at pO2 on the order of ∼5–10% PAL.
Fig. S2.
Representative results for seasonal [O2] oscillation in the time-dependent model. (A−C) Dissolved [O2] in the surface ocean box is shown as a function of time according to the time-dependent forcing in organic carbon export (green) and surface wind speed (blue) shown in D. Results are shown for atmospheric pO2 values of (A) 2.5%, (B) 1.0%, and (C) 0.5% of the PAL. In each panel, curves correspond to assumed deep ocean [Fe2+] values of 50 µmol⋅kg−1 (solid), 150 µmol⋅kg−1 (dashed), and 250 µmol⋅kg−1 (dotted).
Fig. 4.
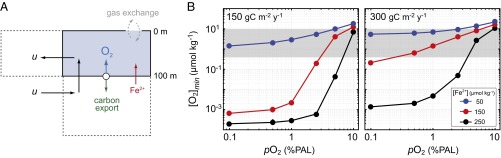
Results of the time-dependent biogeochemical model. (A) A schematic depiction of the main processes represented by the model. (B) Minimum seasonal dissolved oxygen concentrations in the surface ocean as a function of background atmospheric pO2 value for two different baseline carbon export rates (Left and Right), and at deep [Fe2+] levels of 50 µmol⋅kg−1 (blue), 150 µmol⋅kg−1 (red), and 250 µmol⋅kg−1 (black). Gray shaded box encompasses the biological limits denoted in Fig. 1.
In summary, seasonal variability in biological O2 production, carbon export, and surface winds would have resulted in significant temporal variability in surface O2 within productive regions of the surface ocean and in shallow continental margin settings. In addition, even modest amounts of Fe2+ relative to some predictions for the Proterozoic ocean interior (18, 32) would have been sufficient to depress seasonal minima in dissolved O2 to levels that would be expected to have inhibitory metabolic effects on emerging metazoan organisms for much of Proterozoic time. Such temporal variability in redox structure, particularly in light of the spatial complexity of the long-term mean state oxygen landscape discussed above, would have provided an additional environmental constraint on the early evolution of animal life.
Conclusions and Future Directions
Taken together, our results imply a long-term mean surface ocean oxygen landscape during the Proterozoic that would have been characterized by significant spatial variability (Fig. 2). Superimposed on this long-term mean state were surface ocean dissolved O2 distributions that would have varied significantly on seasonal timescales (Fig. 4), all against a backdrop of consistently low benthic O2 levels (Fig. 3). Although it should be stressed that many key biological innovations must have preceded the Late Proterozoic emergence of basal metazoan clades (33), we suggest that early multicellular organisms would have had to contend with a “patchy” and evolutionarily restrictive oxygen landscape for much of Proterozoic time, despite background local O2 levels that may, in some cases, have been sufficient to fuel their metabolic needs. Although our discussion here focuses on metazoans, if periodic anoxia was common throughout shallow oceans during the Neoproterozoic, all eukaryotic life may have been oxygen-stressed on evolutionary timescales. This relationship may, in part, explain the early rise but protracted diversification of eukaryotes (34).
More broadly, we stress that geochemical reconstructions of background atmospheric pO2 (11, 26) and experimental/theoretical reconstructions of resting O2 demand in basal animal organisms (12, 13), although critical, only provide part of the picture. Proterozoic marine environments would have been characterized by O2 levels that departed strongly from background mean pO2, and the oxygen demand and marine habitat of different life history stages could present physiological bottlenecks that are currently poorly known for basal metazoan organisms. Indeed, microfossil evidence for metazoan life stages adapted for large-scale dispersal across inhospitable environments may represent a paleontological signal for such ecosystem oxygen stress during the Late Proterozoic (24). In this light, our results strongly suggest that the distribution of O2 within the ocean−atmosphere system played a significant role in structuring evolutionary innovation and ecological success among early animals.
Finally, we hypothesize that the interplay between long-term changes in base-level pO2 and some degree of spatiotemporal oxygen variability may promote rather than inhibit biological and ecological innovation. For example, theory predicts that environmental fluctuation can often reinforce evolutionary transitions in individuality (35), such as multicellularity. A critical combination of baseline pO2 and spatiotemporal variability may thus have ratcheted the evolution of more advanced forms of multicellularity in some eukaryotic lineages. In addition, variability in the marine oxygen landscape against the backdrop of increasing baseline O2 during the Late Proterozoic (36) may have acted as a creative evolutionary force, ultimately favoring rapid phylogenetic diversification and ecological expansion among early animal lineages. Future work pinpointing when and how this transition occurred will be essential for fully understanding how changes in surface O2 levels have affected the tempo and mode of early metazoan evolution.
Spatial Heterogeneity in Steady-State O2 Levels
To explore spatial variations in surface ocean dissolved oxygen levels, we perform simulations using the GENIE framework, an EMIC (e.g., ref. 41). GENIE considers a frictional geostrophic 3D ocean circulation coupled to a marine biosphere that specifies nutrient-limited export production, aerobic respiration, denitrification, sulfate reduction, and methanogenesis. The marine biogeochemistry module also includes a range of oxidative processes, including aerobic methanotrophy, nitrification, and sulfide oxidation (see ref. 29). The ocean is represented as a 36 × 36 equal-area horizontal grid with 16 depth layers, and is coupled to a 2D energy/moisture balance model, a sea ice model, and a 2D atmospheric model for calculating sea−air exchange fluxes and atmospheric chemistry (42). The model is well calibrated for simulations of the Cenozoic Earth system (29, 43) and has also been used successfully to interrogate older intervals of Earth’s history, including the Cretaceous (41), the end-Permian (45), and the Archean/Proterozoic (22).
Our default simulations assume many effectively modern boundary conditions, including climate (e.g., pCO2 and pCH4), continental configuration (e.g., large-scale ocean circulation), marine nutrient inventory, and organic carbon remineralization length scale in the water column (e.g., the “penetration depth” of organic carbon exported from the photic zone). However, it is clear that all of these parameters have changed dramatically throughout Earth’s history. It is thus important to establish broadly the sensitivity of any model result to plausible changes in these parameters through time. We focus below on the sensitivity of our results to ocean circulation, nutrient inventory, and biological carbon fluxes through the water column, while noting that our assumption of roughly modern preindustrial greenhouse gas inventories is conservative for our purposes in that the relatively cold preindustrial climate state should on its own act to increase O2 solubility in the ocean (e.g., ref. 46).
Quantitative reconstructions of marine nutrient inventory that are currently available suggest that long-term oceanic fertility was, if anything, less than that of modern Earth for most of Earth’s history (47−50). The effects of this are illustrated in a series of sensitivity simulations in which the initial oceanic phosphorus inventory is reduced to 0.5× and 0.25× the modern value (Fig. S1A). Intuitively, decreasing the marine nutrient inventory leads to decreased local production and accumulation of O2 in surface marine environments. For reasonable decreases in nutrient inventory as envisioned for much of the Precambrian (50), surface ocean oxygen levels for a given set of identical boundary conditions are lower by up to ∼2 µmol⋅kg−1. Thus, as is argued here, all other things being equal, the marine nutrient inventory would need to be significantly elevated relative to that of modern Earth to effectively counter the magnitude of variability in oxygen concentrations implied by our time-dependent simulations. In addition, it is possible (if not likely) that nutrient availability would have exacerbated the spatial heterogeneity in surface oxygen levels discussed here.
It has long been suggested that reduced sinking speeds of marine aggregates during much of the Precambrian would have led to shallower organic carbon penetration into the ocean interior (see ref. 9 for a recent review). However, this conceptual model was originally based on the notion of enhanced vertical transport due to zooplankton fecal pellets (51), which have been shown to be a relatively small component of vertical carbon transport, even in modern surface ocean ecosystems (52). In addition, zooplankton activity is very effective at promoting particle deaggregation as particles sink through the water column (e.g., ref. 53). In other words, zooplankton may, in fact, reduce overall sinking speeds of marine aggregates while contributing only minor direct fluxes due to fecal pellet production. Perhaps more importantly, this framework has yet to rigorously incorporate the clear importance of picoplankton in vertical carbon fluxes within modern marine ecosystems (54, 55). Our overall understanding of evolving biological pump strength is thus rather poorly developed at present, and it is far from clear that Precambrian surface ecosystems would result in markedly reduced sinking speeds of organic aggregates through the water column.
Nevertheless, if shallower remineralization of sinking organic matter were pervasive during Precambrian time, this could result in more efficient nutrient recycling and increased input fluxes of nutrients through the main thermocline. Such a mechanism could, to some degree, counteract the effects of a smaller overall oceanic nutrient inventory. Indeed, simulations with shallower organic carbon remineralization result in relative increases in local surface oxygen levels for a given set of parameters (Fig. S1B). However, rather extreme decreases in organic carbon penetration would be required to counteract the scale of seasonal variability observed in our time-dependent simulations—for example, simulations in which the remineralization length scale for organic carbon in the model is decreased by an order of magnitude result in rather modest local increases in O2, generally less than ∼1 µmol⋅kg−1. This is well below the temporal variability we observe in our regional model and could easily be counteracted by modest reductions in nutrient inventory.
It is difficult to accurately diagnose the effects of changing oceanic circulation for the vast majority of Earth’s history, simply because accurate continental reconstructions are not available until the latest Proterozoic (56). We do not mean to suggest that large-scale ocean circulation during the Proterozoic was necessarily similar to that characteristic of modern Earth. However, it is likely that the basic range of large-scale “circulatory settings”—low-latitude to midlatitude equatorial upwelling due to Hadley cell convergence, high-latitude deep convection, eastern boundary upwelling—is well represented by the zonal and meridional circulation patterns of modern Earth. This should be particularly true with respect to nutrient recharge to the surface ocean (of primary importance for our simulations here), although large-scale patterns of ocean ventilation should change dramatically with varying continental configuration. In short, we consider it difficult to imagine an open ocean system unusually favorable for local O2 accumulation that is not represented in some region of the modern Earth system. Although more accurately diagnosing patterns of large-scale ocean circulation and how they have responded to shifts in continental configuration through time is a crucially important topic, and should, in general, be a major focus of future quantitative modeling efforts, we consider it unlikely that changes in continental configuration would dramatically alter our primary conclusions here.
Temporal Heterogeneity in Surface Ocean O2 Levels
We attempt to diagnose temporal heterogeneity in surface oxygen levels by using a simple regional model of a coastal upwelling system (Fig. 2A), modified from ref. 21, that tracks dissolved oxygen in the surface ocean as governed locally by physical transport, biological productivity, gas exchange, and biogeochemical processes. Briefly, concentrations of dissolved O2 in the surface ocean are governed by sources minus sinks due to physical transport (∆mix), gas exchange (Jgas), and biogeochemical processes (Jbio),
where
In the above expressions, wu represents the upwelling rate (meters per day), brackets denote concentrations in either surface or deep waters (the latter of which are specified as boundary conditions for the active surface layer), kO2 is the gas exchange coefficient for O2 (governed by surface wind speed and corrected Schmidt number at a given temperature), KH is the Henry’s Law constant for O2 at a given seawater temperature and salinity, and ri values represent stoichiometric factors for a given reaction. Physical transport and gas exchange are driven by surface winds as described in ref. 21. The model has been modified to allow for time-dependent forcing of physical boundary conditions and deepwater chemistry, most notably by including an explicit expression for the kinetics of Fe2+ oxidation. The rate constant of this process (kox) depends on seawater temperature and ionic strength according to ref. 57, with [OH−] derived based on an assumed pH (taken to be constant at 8.3). We neglect CH4 in the present analysis, for simplicity, but note that this is conservative for our purposes here.
We assume a given deepwater [Fe2+] value and allow the system to equilibrate from an initial assumed surface [O2] value (taken here to be 5 µmol⋅kg−1, but the exact value is irrelevant) while subjecting the model to periodic forcing in surface wind speed (and thus upwelling rate) and export production (net rates of O2 production by photosynthesis). We explore two timescales of periodic forcing. In the first case, oscillations have a period of 24 h, meant to represent diurnal fluctuations in, for example, photosynthetic activity. In the second case, oscillations have a period of ∼120 d, chosen to represent roughly seasonal variability in upwelling, nutrient recharge, and biological productivity (ignoring subtle differences in the length of the Precambrian day and year). The latter timescale is examined in detail here.
Acknowledgments
This research was supported by a National Science Foundation Sedimentary Geology and Paleobiology grant and a grant from the NASA Astrobiology Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1521544113/-/DCSupplemental.
References
- 1.Nursall JR. Oxygen as a prerequisite to the origin of the Metazoa. Nature. 1959;183(4669):1170–1172. [Google Scholar]
- 2.Froelich PN, et al. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: Suboxic diagenesis. Geochim Cosmochim Acta. 1979;43(7):1075–1090. [Google Scholar]
- 3.Lane N, Martin W. The energetics of genome complexity. Nature. 2010;467(7318):929–934. doi: 10.1038/nature09486. [DOI] [PubMed] [Google Scholar]
- 4.Booth A, Doolittle WF. Reply to Lane and Martin: Being and becoming eukaryotes. Proc Natl Acad Sci USA. 2015;112(35):E4824. doi: 10.1073/pnas.1513285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Summons RE, Bradley AS, Jahnke LL, Waldbauer JR. Steroids, triterpenoids and molecular oxygen. Philos Trans R Soc Lond B Biol Sci. 2006;361(1470):951–968. doi: 10.1098/rstb.2006.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Towe KM. Oxygen-collagen priority and the early metazoan fossil record. Proc Natl Acad Sci USA. 1970;65(4):781–788. doi: 10.1073/pnas.65.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catling DC, Glein CR, Zahnle KJ, McKay CP. Why O2 is required by complex life on habitable planets and the concept of planetary “oxygenation time”. Astrobiology. 2005;5(3):415–438. doi: 10.1089/ast.2005.5.415. [DOI] [PubMed] [Google Scholar]
- 8.Sperling EA, Knoll AH. The ecological physiology of Earth’s second oxygen revolution. Annu Rev Earth Planet Sci. 2015;46:215–235. [Google Scholar]
- 9.Butterfield NJ. Oxygen, animals and oceanic ventilation: An alternative view. Geobiology. 2009;7(1):1–7. doi: 10.1111/j.1472-4669.2009.00188.x. [DOI] [PubMed] [Google Scholar]
- 10.Mills DB, Canfield DE. Oxygen and animal evolution: Did a rise of atmospheric oxygen “trigger” the origin of animals? BioEssays. 2014;36(12):1145–1155. doi: 10.1002/bies.201400101. [DOI] [PubMed] [Google Scholar]
- 11.Planavsky NJ, et al. Earth history. Low mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science. 2014;346(6209):635–638. doi: 10.1126/science.1258410. [DOI] [PubMed] [Google Scholar]
- 12.Mills DB, et al. Oxygen requirements of the earliest animals. Proc Natl Acad Sci USA. 2014;111(11):4168–4172. doi: 10.1073/pnas.1400547111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperling EA, Halverson GP, Knoll AH, Macdonald FA, Johnston DT. A basin redox transect at the dawn of animal life. Earth Planet Sci Lett. 2013;371-372:143–155. [Google Scholar]
- 14.Müller M, et al. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol Mol Biol Rev. 2012;76(2):444–495. doi: 10.1128/MMBR.05024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hug LA, Stechmann A, Roger AJ. Phylogenetic distributions and histories of proteins involved in anaerobic pyruvate metabolism in eukaryotes. Mol Biol Evol. 2010;27(2):311–324. doi: 10.1093/molbev/msp237. [DOI] [PubMed] [Google Scholar]
- 16.Hampl V, Stairs CW, Roger AJ. The tangled past of eukaryotic enzymes involved in anaerobic metabolism. Mob Genet Elements. 2011;1(1):71–74. doi: 10.4161/mge.1.1.15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mentel M, Röttger M, Leys S, Tielens AGM, Martin WF. Of early animals, anaerobic mitochondria, and a modern sponge. BioEssays. 2014;36(10):924–932. doi: 10.1002/bies.201400060. [DOI] [PubMed] [Google Scholar]
- 18.Canfield DE. The early history of atmospheric oxygen: Homage to Robert M. Garrels. Annu Rev Earth Planet Sci. 2005;33:1–36. [Google Scholar]
- 19.Lenton TM, Boyle RA, Poulton SW, Shields-Zhou GA, Butterfield NJ. Co-evolution of eukaryotes and ocean oxygenation in the Neoproterozoic era. Nat Geosci. 2014;7(4):257–265. [Google Scholar]
- 20.Kasting JF. Box models for the evolution of atmospheric oxygen: An update. Global Planet Change. 1991;97(1-2):125–131. [PubMed] [Google Scholar]
- 21.Reinhard CT, Lalonde S, Lyons TW. Oxidative sulfide dissolution on the early Earth. Chem Geol. 2013;362:44–55. [Google Scholar]
- 22.Olson SL, Kump LR, Kasting JF. Quantifying the areal extent and dissolved oxygen concentrations of Archean oxygen oases. Chem Geol. 2013;362:35–43. [Google Scholar]
- 23.Zhang S, et al. Sufficient oxygen for animal respiration 1,400 million years ago. Proc Natl Acad Sci USA. 2016;113(7):1731–1736. doi: 10.1073/pnas.1523449113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen PA, Knoll AH, Kodner RB. Large spinose microfossils in Ediacaran rocks as resting stages of early animals. Proc Natl Acad Sci USA. 2009;106(16):6519–6524. doi: 10.1073/pnas.0902322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston DT, et al. Late Ediacaran redox stability and metazoan evolution. Earth Planet Sci Lett. 2012;335-336:25–35. [Google Scholar]
- 26.Cole DB, et al. A shale-hosted Cr isotope record of low atmospheric oxygen during the Proterozoic. Geology. June 2016;44(7):555–558. [Google Scholar]
- 27.Reinhard CT, et al. Proterozoic ocean redox and biogeochemical stasis. Proc Natl Acad Sci USA. 2013;110(14):5357–5362. doi: 10.1073/pnas.1208622110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones C, Nomosatryo S, Crowe SA, Bjerrum CJ, Canfield DE. Iron oxides, divalent cations, silica, and the early earth phosphorus crisis. Geology. 2015;43(2):135–138. [Google Scholar]
- 29.Ridgwell A, et al. Marine geochemical data assimilation in an efficient Earth System Model of global biogeochemical cycling. Biogeosciences. 2007;4(1):87–104. [Google Scholar]
- 30.Shchepetkin AF, McWilliams JC. The regional oceanic modeling system (ROMS): A split-explicit, free-surface, topography-following-coordinate oceanic model. Ocean Model. 2005;9(4):347–404. [Google Scholar]
- 31.Nielsen C. Six major steps in animal evolution: Are we derived sponge larvae? Evol Dev. 2008;10(2):241–257. doi: 10.1111/j.1525-142X.2008.00231.x. [DOI] [PubMed] [Google Scholar]
- 32.Derry LA. Causes and consequences of mid-Proterozoic anoxia. Geophys Res Lett. 2015;42(20):8538–8546. [Google Scholar]
- 33.Tweedt SM, Erwin DH. 2015. Origin of metazoan developmental toolkits and their expression in the fossil record. Evolutionary Transitions to Multicellular Life, Advances in Marine Genomics, eds Ruiz-Trillo I, Nedelcu AM (Springer, New York), Vol 2, pp 47–77.
- 34.Knoll AH. Paleobiological perspectives on early eukaryotic evolution. Cold Spring Harb Perspect Biol. 2014;6(1):a016121. doi: 10.1101/cshperspect.a016121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyle RA, Lenton TM. Fluctuation in the physical environment as a mechanism for reinforcing evolutionary transitions. J Theor Biol. 2006;242(4):832–843. doi: 10.1016/j.jtbi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Sperling EA, et al. Statistical analysis of iron geochemical data suggests limited late Proterozoic oxygenation. Nature. 2015;523(7561):451–454. doi: 10.1038/nature14589. [DOI] [PubMed] [Google Scholar]
- 37.Glasspool IJ, Scott AC. Phanerozoic concentrations of atmospheric oxygen reconstructed from sedimentary charcoal. Nat Geosci. 2010;3(9):627–630. [Google Scholar]
- 38.Zbinden EA, Holland HD, Feakes CR. The Sturgeon Falls paleosol and the composition of the atmosphere 1.1 Ga BP. Precambrian Res. 1988;42:141–163. doi: 10.1016/0301-9268(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 39.Sheldon ND. Precambrian paleosols and atmospheric CO2 levels. Precambrian Res. 2006;147:148–155. [Google Scholar]
- 40.Pavlov AA, Kasting JF. Mass-independent fractionation of sulfur isotopes in Archean sediments: Strong evidence for an anoxic Archean atmosphere. Astrobiology. 2002;2(1):27–41. doi: 10.1089/153110702753621321. [DOI] [PubMed] [Google Scholar]
- 41.Claussen M, et al. Earth system models of intermediate complexity: closing the gap in the spectrum of climate system models. Clim Dyn. 2002;18:579–586. [Google Scholar]
- 42.Edwards NR, Marsh R. Uncertainties due to transport-parameter sensitivity in an efficient 3-D ocean-climate model. Clim Dyn. 2005;24:415–433. [Google Scholar]
- 43.Cui Y, et al. Slow release of fossil carbon during the Palaeocene-Eocene Thermal Maximum. Nat Geosci. 2011;4:481–485. [Google Scholar]
- 44.Monteiro FM, Pancost RD, Ridgwell A, Donnadieu Y. Nutrients as the dominant control on the spread of anoxia and euxinia across the Cenomanian-Turonian oceanic anoxic event (OAE2): Model-data comparison. Paleoceanography. 2012;27:PA4209. [Google Scholar]
- 45.Meyer KM, Kump LR. Oceanic euxinia in Earth history: Causes and consequences. Annu Rev Earth Planet Sci. 2008;36:251–288. [Google Scholar]
- 46.Keeling RF, Körtzinger A, Gruber N. Ocean deoxygenation in a warming world. Ann Rev Mar Sci. 2010;2:199–229. doi: 10.1146/annurev.marine.010908.163855. [DOI] [PubMed] [Google Scholar]
- 47.Bjerrum CJ, Canfield DE. Ocean productivity before about 1.9 Ga ago limited by phosphorus adsorption onto iron oxides. Nature. 2002;417:159–162. doi: 10.1038/417159a. [DOI] [PubMed] [Google Scholar]
- 48.Godfrey LV, Falkowski PG. The cycling and redox state of nitrogen in the Archaean ocean. Nat Geosci. 2009;2(10):725–729. [Google Scholar]
- 49.Reinhard CT, et al. Proterozoic ocean redox and biogeochemical stasis. Proc Natl Acad Sci USA. 2013;110:5357–5362. doi: 10.1073/pnas.1208622110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones C, Nomosatryo S, Crowe SA, Bjerrum CJ, Canfield DE. Iron oxides, divalent cations, silica, and the early earth phosphorus crisis. Geology. 2015;43:135–138. [Google Scholar]
- 51.Logan GA, Hayes JM, Hieshima GB, Summons RE. Terminal Proterozoic reorganization of biogeochemical cycles. Nature. 1995;376:53–56. doi: 10.1038/376053a0. [DOI] [PubMed] [Google Scholar]
- 52.Turner JT. Zooplankton fecal pellets, marine snow and sinking phytoplaknton blooms. Aquat Microb Ecol. 2002;27:57–102. [Google Scholar]
- 53.Dilling L, Alldredge AL. Fragmentation of marine snow by swimming macrozooplankton: A new process impacting carbon cycling in the sea. Deep-Sea Research I. 2000;47:1227–1245. [Google Scholar]
- 54.Richardson TL, Jackson GA. Small phytoplankton and carbon export from the surface ocean. Science. 2007;315:838–840. doi: 10.1126/science.1133471. [DOI] [PubMed] [Google Scholar]
- 55.Close HG, et al. Export of submicron particulate organic matter to mesopelagic depth in an oligotrophic gyre. Proc Natl Acad Sci USA. 2013;110:12545–12570. doi: 10.1073/pnas.1217514110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Evans DAD. Reconstructing pre-Pangean supercontinents. GSA Bulletin. 2013;125:1735–1751. [Google Scholar]
- 57.Millero FJ, Sotolongo S, Izaguirre M. The oxidation kinetics of Fe(II) in seawater. Geochimica et Cosmochimica Acta. 1987;51:793–801. [Google Scholar]