Abstract
Benzodiazepines have been widely used for their anxiolytic actions. However, the contribution of GABAA receptor subtypes to anxiolysis is still controversial. Studies with mutant mice harboring diazepam-insensitive α-subunits α1, α2, α3, or α5 have revealed that α2-containing GABAA receptors (α2-GABAARs) are required for diazepam-induced anxiolysis, with no evidence for an involvement of any other α-subunit, whereas TP003, described as a selective modulator of α3-containing GABAA receptors, was shown to be anxiolytic. Here, we describe a novel, systematic approach to evaluate the role of positive allosteric modulation of each of the four diazepam-sensitive α-subtypes in anxiety-related behavioral paradigms. By combining H to R point mutations in three out of the four diazepam-sensitive α-subunits in mice with a 129X1/SvJ background, diazepam becomes a subtype-specific modulator of the remaining non-mutated α-subtype. Modulation of α5-GABAARs, but not of α2-GABAARs, increased the time in the light side of the light–dark box as well as open-arm exploration in the elevated plus maze. In contrast, modulation of α3-GABAARs decreased open-arm exploration, whereas modulation of α2-GABAARs increased time in the center in the open-field test. Modulation of any single α-subtype had no effect on stress-induced hyperthermia. Our results provide evidence that modulation of α5-GABAARs elicits anxiolytic-like actions, whereas our data do not provide evidence for an anxiolytic-like action of α3-GABAARs. Thus, α5-GABAARs may be suitable targets for novel anxiolytic drugs.
Introduction
Benzodiazepines–pharmacological agents whose primary mechanism of action is the positive allosteric modulation of GABAA receptors—have been a major pharmacotherapeutic tool to manage pathological anxiety in the clinic for decades (Shader and Greenblatt, 1993). Although highly effective in reducing anxiety symptoms, the long-term use of benzodiazepines has been fraught with an unfavorable side-effect profile (eg, sedation and dependence liability) because of their nonselective action via multiple GABAA receptor subtypes. GABAA receptors are heteropentamers that can be configured from a repertoire of at least 19 subunits and are often classified into GABAA receptor subtypes based on their α-subunit (α1–α6), which is an essential component of the benzodiazepine binding site (Rudolph and Knoflach, 2011). Classical benzodiazepines such as diazepam bind to and modulate four out of these six subtypes (α1-, α2-, α3-, or α5-containing GABAA receptors, which are from here on referred to as α1-, α2-, α3-, or α5-GABAARs), leading to both therapeutically desired and undesired effects.
The pharmacological functions of GABAA receptor subtypes have so far been deduced from studies using subtype-selective compounds, GABAA receptor subunit knockout mice, and GABAA receptor subunit knock-in mice with mutations preventing binding of benzodiazepines while leaving the sensitivity for the physiological neurotransmitter GABA intact (Rudolph and Knoflach, 2011). Studies with α-subunit knock-in mice with diazepam-insensitive α-subunits, that is, α1(H101R), α2(H101R), α3(H126R), and α5(H105R) mice, demonstrated that the modulation of α2-GABAARs, but not of α1-, α3-, or α5-GABAARs, is required for the anxiolytic-like effects of benzodiazepines (Crestani et al, 2002; Low et al, 2000; McKernan et al, 2000; Morris et al, 2006; Rudolph et al, 1999; Smith et al, 2012; see also Engin et al, 2016). In an apparent contrast, the compound TP003 (4,2′-difluoro-5′-[8-fluoro-7-(1-hydroxy-1-methylethyl)imidazo[1,2-a′]pyridin-3-yl]biphenyl-2-carbonitrile), reported to be selective for α3-GABAARs in in vitro assays on recombinant receptors, was found to have anxiolytic-like effects in several species (Dias et al, 2005), which led to the widely accepted conclusion that α3-GABAARs may also mediate anxiolytic effects of benzodiazepines (eg, Korpi et al, 2015; Sigel and Steinmann, 2012). However, two recent studies were unable to reproduce the α3-selectivity of TP003 in recombinant receptors (Christian et al, 2015; de Lucas et al, 2015), raising the question whether the anxiolytic-like action of TP003 is really dependent on modulation of α3-GABAARs, and, on a broader scale, whether α3-GABAARs are involved at all in the modulation of anxiety-related behaviors. Furthermore, a conditional deletion of the α5-subunit in PKCδ+ neurons in the central amygdala resulted in increased anxiety in the open-field (OF) and elevated plus maze (EPM) tests (Botta et al, 2015), suggesting a role for α5-GABAARs in anxiety regulation. However, it has not been examined whether systemic positive allosteric modulation of α5-GABAARs leads to anxiolysis.
Thus, the role of different GABAA receptor subtypes in anxiety and anxiolytic drug action is still unclear, and the findings from studies using pharmacological agents have been inconclusive, as the currently available ‘subtype-selective' compounds are not truly specific for a given α-subtype. The current study is designed to evaluate whether highly specific positive allosteric modulation of any individual GABAA receptor subtype is sufficient to elicit anxiolytic-like actions using a combined pharmacological and genetic engineering approach, which overcomes the selectivity limitations of earlier pharmacological studies.
Materials and methods
Animals
All experiments and procedures were approved by Kantonales Veterinaramt Zurich or the McLean Hospital Institutional Animal Care and Use Committee following guidelines in the National Research Council Guide for the Care and Use of Laboratory Animals (8th edition). Mice (bred on the 129X1/SvJ background) carry three out of the four point mutations α1(H101R), α2(H101R), α3(H126R), and α5(H105R), which render the respective GABAA receptors insensitive to modulation by diazepam (Crestani et al, 2002; Low et al, 2000; Rudolph et al, 1999). We refer to these mice as DZα1, DZα2, DZα3 and DZα5 based on which of these α-subunits is not mutated and thus diazepam-sensitive (previously described as HRRR, RHRR, RRHR and RRRH, respectively, in Ralvenius et al, 2015). Subjects were male mice aged 8–16 weeks at the time of testing and group-housed with 2–5 mice per cage in Super Mouse 750 cages. Cohort 1 mice were transferred from our breeding colony (12 : 12-h light–dark cycle with lights on at 0700 hours) to a housing room in our behavioral suite where they were kept on a regular 12 : 12-h light–dark cycle (lights on at 0700 hours). Following the stress-induced hyperthermia (SIH) test, the mice were moved to a different housing room where they were kept on a 12 : 12-h reverse light–dark cycle (lights on at 1900 hours) for 3 weeks, after which they were tested in the light–dark box (LDB). In the LDB, animals were balanced for previous drug history. Cohort 2 was moved to the reverse light–dark cycle housing room and were habituated to this cycle for 3 weeks before they were tested in the EPM. Cohort 3 was habituated to the reverse light–dark cycle for at least 3 weeks before undergoing novel OF, forced-swim test (FST), and tail suspension test (TST), with a 1-week hiatus between the tests. The order of tests was counterbalanced for different groups of mice. A fourth cohort was habituated in our regular light–dark cycle behavioral suite for 1 week before undergoing the conditioned place preference (CPP) paradigm.
Drugs
Diazepam (Sigma-Aldrich, St Louis, MO) was dissolved in 10% 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich). Diazepam (3 mg/kg) and vehicle were administered in a volume of 10 ml/kg via PO injection 1 h before testing (SIH) or intraperitoneal injection (2 or 10 mg/kg) 30 min before behavioral testing (LDB, EPM, OF, FST, TST). Cocaine (Sigma-Aldrich) was dissolved in 0.9% saline and administered intraperitoneally immediately before conditioning at 20 mg/kg.
Autoradiography
Mice were deeply anesthetized with isoflurane and killed by decapitation. The dissected brains were immediately frozen in powdered dry ice and stored at −80 °C until used. Parasagittal cryostat-cut sections (16 μm) were thawed, washed two times for 10 min at 4 °C in 50 mM Tris-HCl (pH 7.4), and dried under a stream of cold air. The sections were incubated for 90 min at 4 °C with 5 nM [3H]flumazenil (50 Ci/mmol; ANAWA Trading SA, Wangen, Switzerland) or 8.8 nM [3H]Ro15-4513 (22.7 Ci/mmol; Perkin-Elmer, Schwerzenbach, Switzerland) diluted in 50 mM Tris-HCl (pH 7.4). Nonspecific [3H]flumazenil or [3H]Ro15-4513 binding was determined in the presence of 10 μM clonazepam and 10 μM flumazenil, respectively. The incubation was terminated by washing the sections three times for 20 s in ice-cold 50 mM Tris-HCl (pH 7.4). The sections were thoroughly dried and exposed to a tritium-sensitive phosphor imaging screen along with [3H]-standards for 5–7 days. The screens were scanned with a Packard Cyclone Storage Phosphor imager and images were quantified using OptiQuant (version 4.0). Sections from all genotypes were processed and exposed in parallel and are therefore directly comparable.
Behavioral Experiments
Novel OF
The OF apparatus was a transparent Plexiglas box (42 cm × 42 cm × 31 cm), evenly illuminated at 100 lx. Mice were placed in one corner and allowed to explore freely for 30 min while their distance traveled (cm) was recorded and analyzed using EthoVision XT video tracking system (Noldus Information Technology, Wageningen, The Netherlands). The center zone was 20 cm × 20 cm. The 408 s (22.7% of the time) in the center zone was determined as the chance level based on the sizes of the center zone and the full chamber assuming purely probabilistic movement of the mice.
Elevated plus maze
The EPM apparatus was elevated 1 m above the floor and consisted of two open arms (35 cm long × 6 cm wide), two closed arms (35 cm long × 6 cm wide × 20 cm high), and one center area (5 cm × 5 cm). When all mice were tested under the same lighting conditions (Supplementary Figure S1), the vehicle-treated wild-type (WT) mice appeared to spend more time in the open arms than in any of the vehicle-treated mutant mice. Although this trend did not reach statistical significance in this pilot experiment, we predicted it could become a significant confound in a complete data set. Thus, each genotype was tested under conditions that resulted in a percent time in light that would avoid any floor or ceiling effects (≈20%) after vehicle injection. All mice except WT were habituated to the testing room overnight, and all mice including WT were habituated to the appropriate lighting in the testing room for an hour before testing. Illumination in the open arms was 20 lx for WT mice, 10 lx for DZα1, DZα3, and DZα5 mice, and red light for DZα2 mice. For additional reduction of baseline anxiety in DZα1, DZα2, DZα3, and DZα5 mice, each mouse was placed in a new cage after completion of the behavioral test to separate naïve mice from those who had experienced the EPM test. Each mouse was placed in the maze facing an open arm and allowed to freely explore the maze for 5 min. Behavioral activity was recorded with the EthoVision XT video tracking system. After each trial, the maze was wiped down with 70% ethanol and allowed to dry completely. The percent time in open arms ((time in open arms/5 min) × 100) and the percent open arm entries ((open arm entries)/(open arm entries+closed arm entries) × 100) were calculated as measures of anxiolysis. In addition, total distance traveled in the maze during the test was used to determine the effect of diazepam on locomotion during EPM. The number of total arm entries was recorded as an additional measure of within-test locomotor activity.
Light–dark box test
The LDB apparatus consisted of one clear, brightly lit (250 lx) chamber (28 cm × 28 cm × 31 cm) and one smaller dark (<10 lx) chamber (14 cm × 14 cm × 31 cm) connected by a square opening between the chambers (5 cm × 5 cm). As the lit chamber is two times as large as the dark chamber, mice would spend 67% time in the lit chamber if the distribution of time between the chambers was random (‘chance level'). All mice were habituated to the test room overnight and habituated to the appropriate ambient lighting an hour before testing. At the start of the test, each mouse was placed within the dark chamber and allowed to freely explore the two chambers for 6 min. Each mouse's activity in the visible clear chamber was recorded with the EthoVision XT video tracking system. Between each trial, the chambers were wiped down with 70% ethanol and allowed to dry completely. The percent time in light ((time in clear chamber)/6 min × 100) was calculated as a measure of anxiolysis.
Stress-Induced Hyperthermia
Mice were housed in the room where the SIH test occurred for 1 week before the test, and were single-housed 24 h before the start of the test. Diazepam was administered 1 h before first rectal temperature reading (T1). The second temperature (T2) was taken 10 min after T1. The change in temperature (T2−T1) induced by stress of T1 was calculated and used to measure the autonomic response to stress.
FST and TST
These tests were performed as described previously (Vollenweider et al, 2011). Diazepam or vehicle were administered intraperitoneally 30 min before testing.
Statistical Analysis
For autoradiography quantification, each subregion was analyzed separately using a one-way analysis of variance (ANOVA). Only the [3H]flumazenil binding in the hippocampus data set passed the Shapiro–Wilk normality test, and thus this was the only data set that was analyzed with a parametric ANOVA and post hoc Tukey's test. All other quantification data sets were analyzed with a Kruskal–Wallis ANOVA on ranks followed by post hoc Dunn's method when necessary. Owing to the between-genotype variance in behavioral responses with vehicle injection during LDB and EPM, statistical analyses were only performed for within-genotype drug effects but not between-genotype effects. For OF, EPM, and LDB, each genotype was analyzed separately using a one-way ANOVA followed by the post hoc Tukey's test for pairwise comparison. For data sets that failed the Shapiro–Wilk normality test or the equal variance test (p<0.05), the Kruskal–Wallis one-way ANOVA on ranks was used followed by post hoc pairwise comparison using Tukey's test when the treatment group sizes were equal and Dunn's method when the treatment group sizes were unequal. This nonparametric test was used in LDB data for DZα1, DZα2, and DZα3, and in EPM for DZα2 and DZα3 in % time in open arms, DZα5 distance traveled and DZα1 in % time, % entries, distance traveled, and total entries. For OF, the nonparametric analysis was used for WT center zone duration, DZα1 distance travelled and center duration, DZα2 for distance travelled, and DZα5 for center duration. For CPP (see Supplementary Methods), each genotype was analyzed using a two-way repeated-measures ANOVA. For SIH, the drug effect of each genotype was analyzed using separate t-tests for each genotype. The DZα3 SIH data set did not pass the Shapiro–Willk normality test, and thus it was analyzed with a Mann–Whitney rank-sum test. In all statistical analyses, results were considered significant when p<0.05.
Results
To investigate the pharmacological effects of highly specific positive allosteric modulation of single GABAA receptor subtypes, we used four gene-targeted mouse lines in which three out the four diazepam-sensitive α-subunits were rendered insensitive to modulation by diazepam, which we refer to as DZα1, DZα2, DZα3, or DZα5 mice (the ‘x' in ‘DZαx' indicates the non-mutated α subunit) (Ralvenius et al, 2015).
Receptor Autoradiography
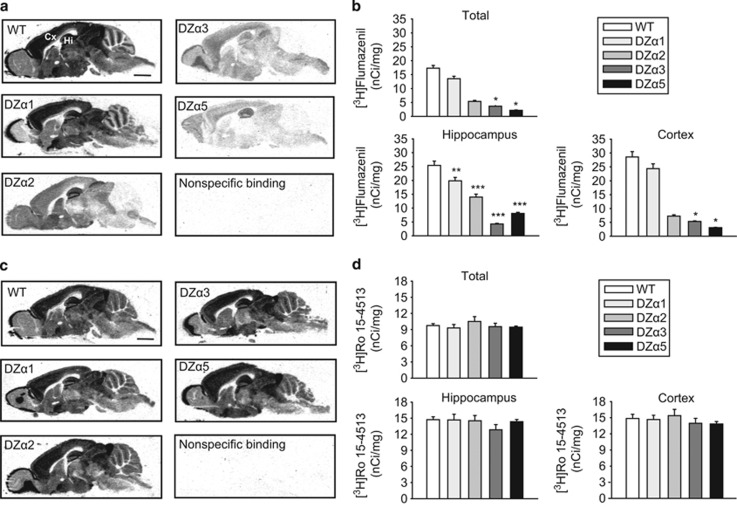
To visualize the benzodiazepine binding sites in mice carrying triple point mutations and WT mice, we performed receptor autoradiography with [3H]flumazenil (Figure 1a). [3H]flumazenil binds with high affinity only to the GABAA receptors containing the WT α1-, α2-, α3-, or α5-subunits. The distribution of [3H]flumazenil binding observed in the DZα1, DZα2, DZα3, and DZα5 triple mutant mice corresponds well to the distribution of the diazepam-sensitive α-subunits seen in immunohistochemistry (Supplementary Figure S2), demonstrating the specificity of [3H]flumazenil binding to the non-mutated α-subtypes in the triple mutant mice. Quantification of [3H]flumazenil binding in total brain (H (4)=26.253, p<0.001) as well as in hippocampal (F (4, 24)=72.281, p<0.001) and cortical subregions (H (4)=25.726, p<0.001) further supports the specificity of [3H]flumazenil binding in the triple mutant mice (Figure 1b). DZα1 and DZα2 mice only showed a statistically significant decrease in [3H]flumazenil binding in the hippocampus (DZα1, p<0.01; DZα2 p<0.001), whereas DZα3 and DZα5 mice showed statistically significant decreases in the hippocampus (p<0.001 for both), cortex (p<0.05 for both), and total brain (p<0.05 for both). The sum of [3H]flumazenil binding for DZα1, DZα2, DZα3, and DZα5 mice is more than [3H]flumazenil binding in WT mice, which is likely due to GABAA receptor complexes that contain two different α-subunits (Benke et al, 2004).
Figure 1.
Receptor autoradiography demonstrating specificity of pharmacogenetic ‘restriction-of-function' approach. (a) [3H]Flumazenil binding to parasagittal brain sections derived from wild-type (WT, 129X1/SvJ) and triple mutant mice (DZα1, DZα2, DZα3, and DZα5). The α-subunit indicated for triple mutant mice is diazepam-sensitive. Nonspecific [3H]flumazenil binding was assessed in the presence of 10 μM clonazepam. Sections from all genotypes were processed in parallel and exposed to the same phosphorimaging screen. Therefore, the gray levels of sections from the different genotypes reflect the abundance of the respective receptor subtype(s) and can be directly compared. Scale bar=2 mm. (b) Quantification of [3H]flumazenil binding in WT and triple mutant mice. [3H]flumazenil binding (nCi/mg) is expressed as mean (±SEM); n=5–6 per genotype. *p<0.05, **p<0.01, and ***p<0.001. (c) [3H]Ro15-4513 binding to parasagittal brain sections derived from WT (129X1/SvJ) and triple mutant mice (DZα1, DZα2, DZα3, and DZα5). Nonspecific [3H]Ro15-4513 binding was assessed in the presence of 10 μM flumazenil. Sections from all genotypes were processed in parallel and exposed to the same phosphorimaging screen. Therefore, the gray levels of sections from the different genotypes reflect the abundance of the respective receptor subtype(s) and can be directly compared. Scale bar=2 mm. (d) Quantification of [3H]Ro15-4513 binding in WT and triple mutant mice. [3H]Ro15-4513 binding (nCi/mg) is expressed as mean (±SEM); n=6 per genotype. Cx, cortex; Hi, hippocampus.
To visualize binding to all GABA receptors, we performed additional receptor autoradiography with [3H]Ro15-4513 (Figure 1c). The distribution of [3H]Ro15-4513 binding in all of the triple mutant mice corresponds well to the [3H]Ro15-4513 binding in the WT mice, suggesting that total GABAA receptor expression levels remain unchanged in the triple mutant mice. Quantification of [3H]Ro15-4513 binding demonstrates no significant difference in total GABAA receptor levels between any of the triple mutants and WT (Figure 1d).
LDB Test
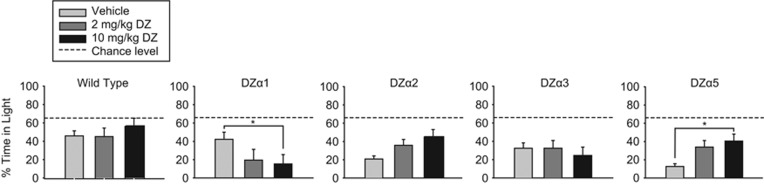
We assessed the effect of diazepam on unconditioned anxiety in the LDB test (Figure 2). One-way ANOVAs indicated a significant effect of diazepam on the percent time spent in light for DZα5 mice (F (2,33)=5.384, p=0.009), and post hoc analysis revealed that 10 mg/kg diazepam significantly increased percent time in light in DZα5 mice compared with vehicle (p<0.05). There was no significant effect of diazepam on percent time in light in either DZα2 mice (H (2)=5.264, p=0.072) or DZα3 mice (H (2)=2.803, p=0.246). In DZα1 mice, diazepam had a significant effect on percent time in light (H (2)=10.47, p=0.007). Post hoc analysis demonstrated that 10 mg/kg decreased the percent time in light (p<0.05), an effect that is likely driven by the strong sedation that diazepam elicits in these mice (Ralvenius et al, 2015). Thus, a statistically significant anxiolytic-like effect was only observed in DZα5 mice, indicating that positive allosteric modulation of α5-GABAARs is sufficient to induce an anxiolytic-like effect in this test.
Figure 2.
Light–dark box (LDB) test. Percent time in the light during LDB test after an injection of vehicle or diazepam (2 and 10 mg/kg) in wild-type (WT) (129X1/SvJ) and triple mutant mice. The dotted line (‘chance level') indicates the percent time in the light chamber that would be expected if the distribution of time between the chambers was random (67%). Percent time in the light side is expressed as mean (±SEM); n=11–12 per treatment group. *p<0.05.
EPM Test
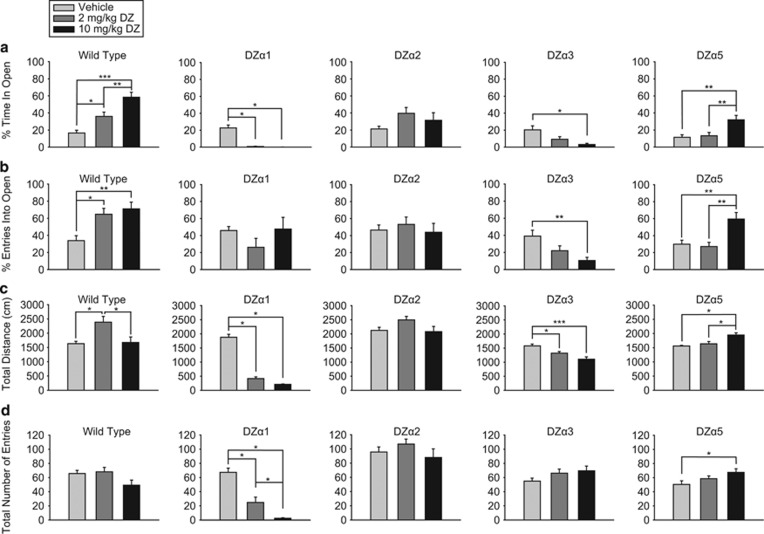
In a second test of unconditioned anxiety, the EPM test (Figure 3), diazepam caused a significant effect on percent time spent in open arms (F (2,33)=19.441, p<0.001) and percent entries into open arms (F (2,33)=8.099, p=0.001) in WT mice. Post hoc analysis revealed that diazepam caused a significant and dose-dependent increase in percent time spent in open arms (2 mg/kg: p<0.05; 10 mg/kg: p<0.001) and in percent entries into the open arms (2 mg/kg: p<0.05; 10 mg/kg: p<0.01), indicative of an anxiolytic-like action in WT mice. In WT mice, diazepam had a significant effect on distance traveled (F (2,33)=6.161, p=0.005) by increasing total distance traveled at 2 mg/kg (p<0.05) but not 10 mg/kg (p=0.983). However, total arm entries were not altered.
Figure 3.
Elevated plus maze test. Effect of diazepam (2 and 10 mg/kg) in the elevated-plus maze in wild-type (WT) (129X1/SvJ) and triple mutant mice on (a) percent time in open arms, (b) percent entries into open arms, (c) distance traveled, and (d) total number of entries into arms. Results are expressed as mean (±SEM); n=11–13 per treatment group. *p<0.05, **p<0.01, and ***p<0.001.
Diazepam had a significant effect in DZα5 mice on percent time in open arms (F (2, 33)=7.679, p=0.002) and percent entries into open arms (F (2,33)=8.944, p<0.001). Post hoc analysis demonstrated that 10 mg/kg diazepam caused a significant increase compared with 2 mg/kg diazepam and vehicle in both percent time in (p<0.01 for both) and percent entries into (p<0.01 for both) the open arms. Diazepam had a significant effect on total distance traveled (H (2)=11.866, p=0.003), and post hoc analysis showed that 10 mg/kg diazepam significantly increased distance traveled compared with both vehicle and 2 mg/kg diazepam (p<0.05 for both). Diazepam also had a significant effect on total number of arm entries in DZα5 mice (F (2,33)=3.341, p=0.048), and post hoc analysis indicated that 10 mg/kg diazepam significantly increased the total number of arm entries compared to vehicle (p<0.05).
In DZα3 mice, diazepam had a significant effect on percent time in open arms (H (2)=10.330, p=0.006) and percent entries into open arms (F (2,34)=6.343, p=0.005). Post hoc analysis revealed that 10 mg/kg diazepam decreased percent time in open arms (p<0.05) and percent entries in open arms (p<0.01) compared with vehicle. Diazepam also had a significant effect on total distance traveled (F (2,34)=12.176, p<0.001), and post hoc analysis revealed that both 2 mg/kg (p<0.05) and 10 mg/kg diazepam (p<0.001) significantly decreased total distanced traveled in DZα3 mice. Interestingly, there was no change in total arm entries (F (2,34)=1.796, p=0.181), suggesting that diazepam induces locomotor changes in DZα3 mice that are different from the nonspecific sedative changes seen in DZα1 mice, in which both distance traveled and total arm entries are reduced. DZα1 mice displayed a significant decrease in percent time in open arms at both 2 and 10 mg/kg diazepam (H (2)=24.023, p<0.001; post hoc Dunn's test p<0.05 for both), but the percent entries into open arms was not altered (H (2)=3.304, p=0.192). In contrast to DZα3 mice, DZα1 mice showed significant effects from diazepam in both total distance traveled (H (2)=28.402, p<0.001) and total arm entries (H (2)=24.688, p<0.001) at both 2 mg/kg (p<0.05) and 10 mg/kg diazepam (p<0.05). The strong reductions in total distance traveled and number of total arm entries indicate that the diazepam effect on percent time in open arms is probably an artifact of sedation. Surprisingly, diazepam had no significant effect on the behavioral measurements of DZα2 mice in the EPM.
Novel OF Test
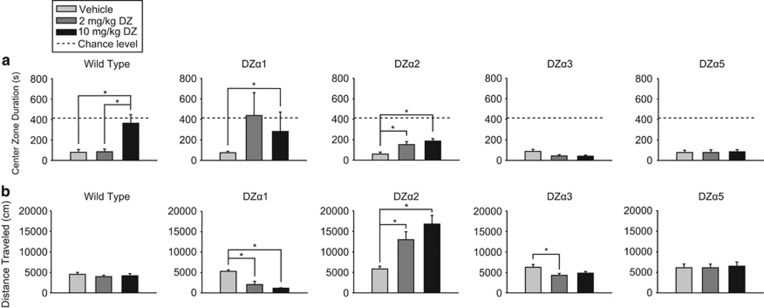
As a third test of unconditioned anxiety, we used a novel OF (Figure 4), where the time spent in the center zone is used as a measure of anxiolysis. In WT mice, 10 mg/kg diazepam significantly increased center zone time (H (2)=10.177, p=0.006) compared with vehicle (p<0.05) and 2 mg/kg diazepam (p<0.05), without a change in total distance traveled, indicative of an anxiolytic-like effect. In DZα2 mice, diazepam significantly increased center zone duration (F (2,31)=7.131, p=0.003) at both 2 mg/kg (p<0.05) and 10 mg/kg (p<0.01) diazepam. These results may indicate that positive allosteric modulation of α2-GABAARs is sufficient to elicit an anxiolytic-like response in a novel OF. In DZα2 mice, diazepam significantly increased total distance traveled (H (2)=15.153, p<0.001) at both 2 mg/kg diazepam (p<0.05) and 10 mg/kg diazepam (p<0.05) compared with vehicle. In contrast to WT mice and DZα2 mice, diazepam had no effect on center zone duration in DZα3 and DZα5 mice. In DZα3 mice, diazepam significantly affected locomotor activity (F (2,27)=3.502, p=0.044) by decreasing total distance traveled at 2 mg/kg diazepam (p<0.05) but not at 10 mg/kg diazepam. Diazepam had no effect on locomotor activity in DZα5 mice in this test. An increase in the time in the center zone was observed at 10 mg/kg diazepam in DZα1 mice (H (2)=8.938, p=0.011, post hoc Dunn's test p<0.05). However, diazepam had a significant dose-dependent effect on distance traveled in DZα1 mice (H (2)=18.030, p<0.001), and post hoc analysis demonstrated that both 2 mg/kg (p<0.05) and 10 mg/kg (p<0.05) diazepam caused significant decreases on total distance traveled. The strong sedation largely precludes meaningful interpretation of the behavior in the DZα1 mice. The current results show that α1-GABAARs are sufficient for mediating the sedative effect of diazepam, that at least at 10 mg/kg diazepam α3-GABAARs and α5-GABAARs individually do not mediate sedation, and that positive modulation of α2-GABAARs results in a substantial and dose-dependent locomotor stimulation. These findings on locomotor activity are in line with those published in an OF to which animals were habituated (Ralvenius et al, 2015).
Figure 4.
Novel open-field test. Effect of diazepam (2 and 10 mg/kg) in novel open field in wild-type (WT) (129X1/SvJ) and triple mutant mice on (a) center zone duration and (b) distance traveled. The dotted line (‘chance level') represents the time in the center zone that would be expected if the distribution of time spent in the center and outside zones was random (408 s in center zone). Results are expressed as mean (±SEM); n=8–13 per treatment group. *p<0.05.
Tests of Predictive Validity of Antidepressant-Like Responses and CPP
The high comorbidity between anxiety and mood disorders leads to a strong therapeutic interest in pharmacological agents that can alleviate both anxiety and symptoms of depression. Some benzodiazepines have also been shown to have antidepressant activity in humans (Amsterdam et al, 1986; Petty et al, 1995), but their sedative action largely precludes testing them in tasks of pharmacological predictability of antidepressant-like responses (also referred to as tests of behavioral despair like the FST and the TST). Thus, we conducted tests to evaluate whether modulation of specific GABAA receptor subtypes, which do not cause sedation (α2, α3, and α5), may lead to antidepressant-like effects in the FST and the TST. Surprisingly, we found that diazepam decreased latency to immobility and increased total immobility in DZα2 mice in the TST, but not the FST, which may thus reflect muscle relaxation (see Supplementary Information and Supplementary Figures S3 and S4 for more details and statistics). Overall, our results in FST and TST do not support a role for modulation of α3- or α5-GABAARs in these paradigms. Moreover, modulation of specific GABAA receptor subtypes did not result in CPP (Supplementary Table S1).
Stress-Induced Hyperthermia
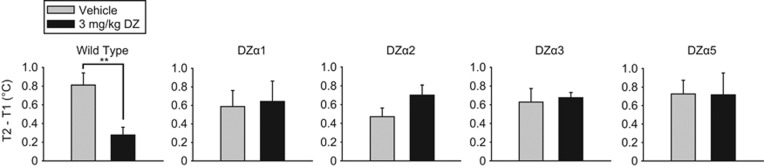
The SIH paradigm is used to measure the autonomic component of anxiolytic-like responses. In this paradigm, we used a dose of diazepam (3 mg/kg) that does not cause hypothermia (see T1 values in Supplementary Figure S5). In WT mice, 3 mg/kg diazepam significantly decreased the SIH (T2−T1) (t (22)=2.989, p=0.007). In the DZα1, DZα2, DZα3, and DZα5 mice, 3 mg/kg diazepam had no significant effect on the stress-induced increase in body temperature (Figure 5). These results indicate that positive allosteric modulation of a single GABAA receptor subtype is not sufficient to attenuate SIH at this dose of diazepam that attenuates SIH in WT mice.
Figure 5.
Stress-induced hyperthermia test. Change in temperature during stress-induced hyperthermia in wild-type (WT) (129X1/SvJ) and triple mutant mice after an injection of vehicle or 3 mg/kg diazepam. Results are expressed as mean (±SEM); n=11–16 per treatment group. **p<0.01.
Discussion
In this study, we used a pharmacogenetic ‘restriction-of-function' approach that allows diazepam-induced modulation of individual GABAA receptor subtypes with very high specificity. Our technique avoids the major limitation of pure pharmacological approaches that no known ‘subtype-selective' compound is really specific for any one receptor subtype. Surprisingly, our findings indicate that positive modulation of α5-GABAARs elicits an anxiolytic-like action, as seen in the EPM and LDB tests. While modulation of α2-GABAARs appears to be sufficient to cause anxiolytic-like effects only in the OF, we did not observe an anxiolytic-like effect of positive modulation of α3-GABAARs in any of the tests. The lack of an effect of specific modulation of α2-GABAARs in the EPM and LDB tests is surprising as it has previously been shown (also in 129X1/SvJ mice, then called 129/SvJ mice) that α2-GABAARs are required for anxiolytic-like responses in these two paradigms (Low et al, 2000). The current results indicate that α2-GABAARs are not fully sufficient for an anxiolytic-like response in the classical EPM and LDB tests. Possibly, for full anxiolytic-like responses in these paradigms, α2-GABAARs need to be modulated in concert with another GABAA receptor subtype (α1, α3, or α5), which may facilitate the α2-mediated response.
It is a well-known limitation of unconditioned, etiological models of anxiety that changes in locomotor activity may confound interpretation of results (Dawson and Tricklebank, 1995). A recent study has shown that while baseline locomotor activity is indistinguishable between mutant and WT mice in a familiar OF in which the animals were habituated to the test chamber, specific modulation of α1-GABAARs in Dzα1 mice by diazepam substantially decreased the locomotor activity and specific modulation of α2-GABAARs in Dzα2 mice by diazepam substantially increased the locomotor activity, whereas specific modulation of α3-GABAARs and of α5-GABAARs had no effect on locomotor activity in mice (Ralvenius et al, 2015). In our experiments, diazepam also had no locomotor effect in the novel OF where conditions are expected to be anxiogenic because of neophobia in DZα5 mice, however, in the EPM diazepam increased total distance traveled and total arm entries. Interestingly, Dzα2 mice do not show increased locomotion in the EPM, suggesting that the hyperlocomotive effects of diazepam may be context-dependent. Although we consider it unlikely, we cannot exclude the possibility that the anxiolytic-like action of diazepam in the DZα2 mice in the novel OF is an artefact of the hyperlocomotion induced by diazepam. Finally, the stronger sedative action of diazepam in Dzα1 mice compared with WT may be due to the fact that in contrast to WT mice in Dzα1 mice diazepam does not modulate α2-GABAARs, which increases locomotor activity. In addition to the effects of these nonspecific or neophobia-induced locomotor changes in the findings, the inconsistency of the anxiolytic-like effects between the three paradigms for the DZα2 and DZα5 mice, respectively, may also, in part, be due to subtype-specific anxiolytic-like activity not being strong enough to robustly manifest itself across all tests.
Our finding—with the potential limitations discussed above—that diazepam does not elicit anxiolytic-like behavior in the DZα3 mice in any of the tests used may thus lend credence to the argument that the anxiety-related effects reported with compounds targeting α3-GABAARs may be due to the limited specificity of the compounds. By far the strongest argument for anxiolysis mediated by α3-GABAARs has been made using compound TP003 (Dias et al, 2005), which was reported to display a very high selective efficacy for α3-GABAARs, with ~80% efficacy compared with chlordiazepoxide, whereas it had essentially no efficacy at α1-, α2-, and α5-GABAARs (Dias et al, 2005). Based on these in vitro data, TP003 was postulated to be an α3-selective modulator and had clear anxiolytic-like effects in rats and squirrel monkeys (Dias et al, 2005). However, two recent studies failed to replicate the findings of the initial report by Dias et al (2005) regarding the subtype selectivity of TP003, and instead demonstrated comparable efficacies at all diazepam-sensitive α-subunits (Christian et al, 2015; de Lucas et al, 2015). Moreover, the presumably α3-selective compound SB-205384 (4-amino-7-hydroxy-2-methyl-5,6,7,8,-tetrahydrobenzo[b]thieno[2,3-b]pyridine-3-carboxylic acid, but-2-ynyl ester), which was shown to have anxiolytic-like actions (Navarro et al, 2006), was later found to be a positive modulator at α5- and α6-GABAARs in addition to α3-GABAARs (Heidelberg et al, 2013). Similarly, while the anxiogenic compound α3IA (6-(4-pyridyl)-5-(4-methoxyphenyl)-3-carbomethoxy-1-methyl-1H-pyridin-2-one) displays some selectivity for α3-GABAARs, it also possesses some efficacy at α2-GABAARs (Atack et al, 2005). Finally, the observation that L-838,417 (3-(2,5-difluorophenyl)-7-(1,1-dimethylethyl)-6-[(1-methyl-1H-1,2,4-triazol-5-yl)methoxy]-1,2,4-triazolo[4,3-b]pyridazine), an α2/α3/α5-selective partial positive allosteric modulator, has an anxiolytic-like action in the conditioned emotional response test in α2(H101R) mice was also interpreted as evidence for a role for α3-GABAARs in anxiolysis (Morris et al, 2006). However, it has not been shown that the α2(H101R) point mutation abolishes modulation of α2-GABAARs by L-838 417. Furthermore, L-838 417 also modulates α5-GABAARs (McKernan et al, 2000), which—as our results may suggest—could mediate the observed anxiolytic-like effects. Thus, our findings combined with these studies suggest that anxiolytic effects cannot be achieved through the positive modulation of α3-GABAA-Rs.
In the SIH paradigm, chlordiazepoxide effectively reduced SIH because of cage-change stress in α2(H101R) mice (Dias et al, 2005), indicating that α2-GABAARs are not required for attenuation of SIH. In our study, we found that while diazepam reduced the SIH caused by restraint and the insertion of the rectal probe during the first temperature measurement in WT mice, the highly subtype-specific modulation of α1-, α2-, α3-, or α5-GABAARs was not sufficient to reduce the SIH, suggesting that the concerted modulation of two or more diazepam-sensitive GABAA receptor subtypes may be required to reduce this autonomic stress response.
The current finding that positive modulation of α5-GABAARs results in anxiolysis is most surprising, as we reported previously that modulation of α5-GABAARs is not required for the anxiolytic-like action of diazepam (Crestani et al, 2002). Presumably, in α5(H105R) mice diazepam exerts anxiolysis via α2-GABAARs, perhaps facilitated by α1-GABAARs or α3-GABAARs. However, the question arises why—if positive modulation of α5-GABAARs is sufficient for anxiolysis—diazepam is not anxiolytic in α2(H101R) mice (Low et al, 2000), in which it modulates α1-, α3-, and α5-GABAARs. One potential interpretation is that simultaneous modulation of α5-GABAARs and of α1- and α3-GABAARs in the α2(H101R) mice results in interactions between different GABAAR subtypes, which mask the anxiolytic-like effect. α1(H101R)/α2(H101R) double mutant mice in which diazepam modulates only α3-GABAARs and α5-GABAARs are also resistant to the anxiolytic-like effect of diazepam (Koester et al, 2013), indicating that simultaneous positive modulation of α3- and α5-GABAARs may not be sufficient to induce anxiolysis. Based on our findings that the modulation of α3-GABAARs reduces open-arm time in the EPM, which is consistent with an anxiogenic-like effect, one possibility is that the diazepam action on α3-GABAARs nullifies the anxiolytic-like action mediated by α5-GABAARs. It is also noteworthy that in DZα5 mice the anxiolytic-like effect in the EPM requires a higher dose compared with WT mice. It is thus conceivable that a higher receptor occupancy of α5-GABAARs is required for anxiolysis if these receptors are the only ones modulated by diazepam, and that this receptor subtype may not necessarily contribute to anxiolysis at low doses of diazepam in WT mice.
α5-GABAARs are strongly expressed in the hippocampus (Figure 1 and Supplementary Figure 2). As the ventral hippocampus has been linked to emotion and stress, whereas the dorsal hippocampus has been linked primarily to cognitive functions (Fanselow and Dong, 2010), α5-GABAARs in the ventral hippocampus may be involved in anxiolysis. Furthermore, a recent study showed that conditional genetic deletion of α5 in the central nucleus of the amygdala (CEA) leads to anxiogenic-like effects and increased fear generalization (Botta et al, 2015). The study concluded that extrasynaptic α5-GABAARs in CEA PKCδ+ neurons control anxiety. Taken together, our results and the study by Botta et al (2015) suggest the possibility that the anxiolytic-like effects of the specific modulation of α5-GABAARs reported here may be due, at least in part, to the positive modulation of the α5-GABAARs in this specific neuronal population.
In conclusion, our findings reveal a role of α5-GABAARs in mediating the affective component of benzodiazepine-induced anxiolysis and no evidence for a role of α3-GABAARs in anxiolysis. Compounds targeting α5- and/or α2-GABAARs should lack both the sedative (Rudolph et al, 1999) and addictive (Tan et al, 2010) properties of benzodiazepines, which have been attributed to the α1-GABAARs. Such compounds may provide additional value in the treatment of conditions such as anxious depression, as positive modulation of α2-GABAARs may enhance reward states (Engin et al, 2014; Reynolds et al, 2012) and positive modulation of α5-GABAARs might reduce cognitive problems commonly observed in psychiatric disorders, such as issues with memory interference and cognitive rigidity (Engin et al, 2015).
Funding and disclosure
This work was supported by Award Numbers R03MH094834, R01MH080006 and R01MH095905 from the National Institute of Mental Health to UR and Award Number R03DA033491 from the National Institute on Drug Abuse to UR. EE was supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award, an Eleanor and Miles Shore Harvard Medical School Fellowship, and an Andrew P Merrill Memorial Research Fellowship. RSB was supported by a Rappaport Mental Health Research Scholar Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse, the National Institute of Mental Health or the National Institutes of Health. The funders had no role in design, execution, and publication of the work. In the past 3 years, UR has received compensation for professional services from Concert Pharmaceuticals. The other authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Author contributions
LMB, RAF, JL, DB, EE, and AJN performed experiments and analyzed data, RSB and EE supervised LMB, RAF, JL, and AJN in these activities. BKY performed initial experiments with triple mutant mice, and HUZ provided the four triple mutant mouse lines used and characterized baseline behavior of these lines. UR conceived, designed, and supervised the work that led to this publication. LMB, EE, and UR drafted the manuscript, and all authors revised the manuscript and approved the final version.
Supplementary Material
References
- Amsterdam JD, Kaplan M, Potter L, Bloom L, Rickels K (1986). Adinazolam, a new triazolobenzodiazepine, and imipramine in the treatment of major depressive disorder. Psychopharmacology 88: 484–488. [DOI] [PubMed] [Google Scholar]
- Atack JR, Hutson PH, Collinson N, Marshall G, Bentley G, Moyes C et al (2005). Anxiogenic properties of an inverse agonist selective for α3 subunit-containing GABAA receptors. Br J Pharmacol 144: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke D, Fakitsas P, Roggenmoser C, Michel C, Rudolph U, Mohler H (2004). Analysis of the presence and abundance of GABAA receptors containing two different types of α subunits in murine brain using point-mutated α subunits. J Biol Chem 279: 43654–43660. [DOI] [PubMed] [Google Scholar]
- Botta P, Demmou L, Kasugai Y, Markovic M, Xu C, Fadok JP et al (2015). Regulating anxiety with extrasynaptic inhibition. Nat Neurosci 18: 1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian EP, Snyder DH, Song W, Gurley DA, Smolka J, Maier DL et al (2015). EEG-β/γ spectral power elevation in the rat: a translatable biomarker elicited by GABAAα2/3-positive allosteric modulators at nonsedating anxiolytic doses. J Neurophysiol 113: 116–131. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L et al (2002). Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci USA 99: 8980–8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GR, Tricklebank MD (1995). Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol Sci 16: 33–36. [DOI] [PubMed] [Google Scholar]
- de Lucas AG, Ahring PK, Larsen JS, Rivera-Arconada I, Lopez-Garcia JA, Mirza NR et al (2015). GABAA α5 subunit-containing receptors do not contribute to reversal of inflammatory-induced spinal sensitization as indicated by the unique selectivity profile of the GABAA receptor allosteric modulator NS16085. Biochem Pharmacol 93: 370–379. [DOI] [PubMed] [Google Scholar]
- Dias R, Sheppard WF, Fradley RL, Garrett EM, Stanley JL, Tye SJ et al (2005). Evidence for a significant role of α 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci 25: 10682–10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Bakhurin KI, Smith KS, Hines RM, Reynolds LM, Tang W et al (2014). Neural basis of benzodiazepine reward: requirement for α2 containing GABAA receptors in the nucleus accumbens. Neuropsychopharmacology 39: 1805–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Smith KS, Gao Y, Nagy D, Foster RA, Tsvetkov E et al (2016). Modulation of anxiety and fear via distinct intrahippocampal circuits. eLife 5: e14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Zarnowska ED, Benke D, Tsvetkov E, Sigal M, Keist R et al (2015). Tonic inhibitory control of dentate gyrus granule cells by α5-containing GABAA receptors reduces memory interference. J Neurosci 35: 13698–13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65: 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberg LS, Warren JW, Fisher JL (2013). SB-205384 is a positive allosteric modulator of recombinant GABAA receptors containing rat α3, α5, or α6 subunit subtypes coexpressed with beta3 and gamma2 subunits. J Pharmacol Expl Ther 347: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester C, Rudolph U, Haenggi T, Papilloud A, Fritschy JM, Crestani F (2013). Dissecting the role of diazepam-sensitive γ-aminobutyric acid type A receptors in defensive behavioral reactivity to mild threat. Pharmacol Biochem Behav 103: 541–549. [DOI] [PubMed] [Google Scholar]
- Korpi ER, den Hollander B, Farooq U, Vashchinkina E, Rajkumar R, Nutt DJ et al (2015). Mechanisms of action and persistent neuroplasticity by drugs of abuse. Pharmacol Rev 67: 872–1004. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA et al (2000). Molecular and neuronal substrate for the selective attenuation of anxiety. Science 90: 131–134. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR et al (2000). Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci 3: 587–592. [DOI] [PubMed] [Google Scholar]
- Morris HV, Dawson GR, Reynolds DS, Atack JR, Stephens DN (2006). Both α2 and α3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm. Eur J Neurosci 23: 2495–2504. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Buron E, Martin-Lopez M (2006). Anxiolytic-like activity of SB-205384 in the elevated plus-maze test in mice. Psicothema 18: 100–104. [PubMed] [Google Scholar]
- Petty F, Trivedi MH, Fulton M, Rush AJ (1995). Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry 38: 578–591. [DOI] [PubMed] [Google Scholar]
- Ralvenius WT, Benke D, Acuna MA, Rudolph U, Zeilhofer HU (2015). Analgesia and unwanted benzodiazepine effects in point-mutated mice expressing only one benzodiazepine-sensitive GABAA receptor subtype. Nat Commun 6: 6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LM, Engin E, Tantillo G, Lau HM, Muschamp JW, Carlezon WA Jr. et al (2012). Differential roles of GABAA receptor subtypes in benzodiazepine-induced enhancement of brain-stimulation reward. Neuropsychopharmacology 37: 2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM et al (1999). Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature 401: 796–800. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F (2011). Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 10: 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shader RI, Greenblatt DJ (1993). Use of benzodiazepines in anxiety disorders. N Engl J Med 328: 1398–1405. [DOI] [PubMed] [Google Scholar]
- Sigel E, Steinmann ME (2012). Structure, function, and modulation of GABAA receptors. J Biol Chem 287: 40224–40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Engin E, Meloni EG, Rudolph U (2012). Benzodiazepine-induced anxiolysis and reduction of conditioned fear are mediated by distinct GABAA receptor subtypes in mice. Neuropharmacology 63: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM et al (2010). Neural bases for addictive properties of benzodiazepines. Nature 463: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider I, Smith KS, Keist R, Rudolph U (2011). Antidepressant-like properties of α2-containing GABAA receptors. Behav Brain Res 217: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.