Abstract
The extent to which cannabis is deleterious to the human brain is not well understood. Here, we test whether cannabis abusers (CA) have impaired frontal function and reactivity to dopaminergic signaling, which are fundamental to relapse in addiction. We measured brain glucose metabolism using PET and [18F]FDG both at baseline (placebo) and after challenge with methylphenidate (MP), a dopamine-enhancing drug, in 24 active CA (50% female) and 24 controls (HC; 50% female). Results show that (i) CA had lower baseline glucose metabolism than HC in frontal cortex including anterior cingulate, which was associated with negative emotionality. (ii) MP increased whole-brain glucose metabolism in HC but not in CA; and group by challenge effects were most profound in putamen, caudate, midbrain, thalamus, and cerebellum. In CA, MP-induced metabolic increases in putamen correlated negatively with addiction severity. (iii) There were significant gender effects, such that both the group differences at baseline in frontal metabolism and the attenuated regional brain metabolic responses to MP were observed in female CA but not in male CA. As for other drug addictions, reduced baseline frontal metabolism is likely to contribute to relapse in CA. The attenuated responses to MP in midbrain and striatum are consistent with decreased brain reactivity to dopamine stimulation and might contribute to addictive behaviors in CA. The gender differences suggest that females are more sensitive than males to the adverse effects of cannabis in brain.
INTRODUCTION
Despite the high prevalence of cannabis consumption worldwide, the effects of cannabis abuse in the human brain are not well understood. The expanding legalization of cannabis for medical or recreational purposes in the USA and in other countries poses a sense of urgency towards addressing the limited evidence regarding potential deleterious effects of cannabis to the human brain. Here, we test the hypothesis that chronic cannabis abusers (CA) would show impaired activity of frontal brain regions, which is a robust finding in addiction, and is associated with impaired self-regulation (Goldstein and Volkow, 2011; Volkow and Morales, 2015a). Reduced glucose metabolism (marker of brain function) in frontal brain regions has been shown in CA even after 3–4 months of abstinence (Volkow et al, 1992b, 1993), alcoholics (Volkow et al, 1992a), and in heavy drinkers (Volkow et al, 2015b). Moreover, in a pilot study, we showed decreased frontal (and cerebellar) metabolism in eight male CA compared with age-matched male controls (Volkow et al, 1996).
We recently showed that CA had attenuated behavioral and cardiovascular responses to a methylphenidate challenge (MP), a stimulant drug that increases dopamine (DA), and an attenuated reduction in MP-induced decreases in the distribution volumes of [11C]raclopride, which is a radiotracer whose binding to DA D2 receptors (D2R) in brain is reduced when DA is increased (Volkow et al, 2014). Here we extend these findings to assess whether MP-induced responses on brain glucose metabolism (Sokoloff et al, 1977) are also attenuated in CA. We hypothesized that blunted responses to MP in regional brain glucose metabolism would be associated with addiction severity and with attenuated behavioral effects of MP (eg, self-reported measures of control, restlessness, and cannabis craving), as previously found in CA (Volkow et al, 1999).
Recent findings suggest higher expression of cannabinoid type-1 (CB1) receptors in the brain of women than men (Normandin et al, 2015). There is also evidence that women compared with men are less likely to be chronic cannabis users (Preston, 2006). This led us to additionally investigate whether there were group by gender interactions in baseline and MP-induced changes in brain metabolism.
For these purposes, we measured regional brain glucose metabolism using PET and [18F]deoxyglucose (FDG) in 24 active CA (50% females) and 24 healthy control participants (HC) (50% females). Regional brain glucose metabolism was measured twice in each participant in a randomized order: after placebo (which was used as baseline) and after challenge with intravenous MP. MP increases DA by blocking DA transporters (Volkow et al, 1998) and was used as challenge to assess the reactivity of the brain to DA stimulation.
We hypothesized that (i) CA compared with HC would show decreased baseline frontal metabolism, (ii) CA compared with HC would show blunted regional brain metabolic responses to MP, and (iii) group differences would be stronger for females than for males.
MATERIALS AND METHODS
Subjects
Twenty-four CA (12 females) and 24 HC (12 females) completed the studies. Participants were recruited from advertisements in local newspapers. At least two clinicians interviewed the patients to ensure that they met DSM-IV diagnostic criteria for cannabis abuse or dependence, with a semi-structured standardized interview. Participants were excluded if they had a history of substance abuse or addiction (other than cannabis abuse/dependence in the cannabis group and nicotine abuse/dependence), a history of psychiatric disease (other than cannabis abuse/dependence), neurological disease, medical conditions that may alter cerebral function (ie, cardiovascular, endocrinological, oncological, or autoimmune diseases), current use of prescribed or over-the-counter medications, and/or head trauma with loss of consciousness of more than 30 min. All subjects had Hamilton's Anxiety and Depression scores<19 (Hamilton, 1960). Exclusion criteria for HC were the same as for CA other than allowance for regular cannabis use (ie, exclusion for controls was use of cannabis more than 1 day a month). All subjects had a physical, psychiatric, and neurologic examination. Drug screens were performed on the days of the PET studies to exclude the use of psychoactive drugs (other than cannabis in CA). Subjects were free from any over-the-counter medication 2 weeks prior to the PET scan. Food and beverages (except for water) were discontinued at least 4 h prior and cigarettes for at least 2 h prior to the study. This study was approved by the Committee on Research in Human Subjects at Stony Brook University (IRB net number 225114) and written informed consent was obtained from all subjects.
Behavioral Measures
Personality measures
Participants completed the Multidimensional Personality Questionnaire (MPQ), which provides rating for three main factors: positive emotional temperament (PEM), negative emotional temperament (NEM), and constraint (Tellegen and Waller, 2008). PEM is a combination of scores for well-being (reward sensitivity), social potency, achievement (motivation), and social closeness; high PEM reflects behavior and temperamental characteristics conducive to joy, and to active and rewarding engagement with social and work environments, whereas low PEM reflects the tendency to experience joylessness, loss of interest, and fatigue, reflecting non-pleasurable and possibly depressive disengagement. NEM is a combination of scores for stress reaction, alienation, and aggression; high NEM reflects proneness to experience anxiety, anger, and related emotional and behavioral negative engagement, whereas low NEM reflects a disposition to calm, relaxation, and other non-pleasurable states of disengagement. Constraint is a combination of scores for self-control, harm avoidance, and traditionalism; high Constraint reflects a tendency to inhibit and restrain impulse expression, unconventional behavior, and risk-taking, whereas a person with low Constraint is inclined to act on impulse, take risks, and ignore conventional restrictions. We also particularly looked at the NEM subscale Alienation; with high scores reflecting people who believe that others wish them harm; being victims of false and nasty rumors; feeling pushed around; feeling used by friends; having been betrayed and deceived; and generally having had a lot of bad luck (Tellegen and Waller, 2008).
Marijuana dependency questionnaire
The Marijuana Dependency Questionnaire (MDQ) scores 7 symptoms of dependence as per DSM IV, each on a range from 0 to 3. The MPQ and MDQ were obtained on the day of screening.
We also assessed subjective ratings and cardiovascular measures 60 min after placebo and MP injection, which are reported in the Supplementary Material.
PET Scanning
PET studies were performed with a Siemens HR+ tomograph. Images were reconstructed using filtered back projection (Hann filter with a 4.9 mm Kernel FWHM). Each subject underwent two PET FDG scans on two separate days. On a given day, each subject was injected with placebo (3 cc saline iv) and then 90 min later with FDG, and on another they were first injected with MP (0.5 mg/kg iv) followed 90 min later with FDG. The order of administration was randomized across group and gender. The study was a single blind design: ie, subjects were blind to the drugs received. Imaging procedure consisted of an emission scan obtained for 20 min and started 35 min after injection of 4–6 mCi of FDG using previously described procedures (Wang et al, 1993). Blood sampling was obtained from a catheter placed in the radial artery, which was used to measure the concentration of radiotracer in plasma. During the uptake period of FDG, subjects remained in a supine position with their eyes open in a darkly lit room and noise was kept to a minimum except for the periodic assessment of drug effects. Metabolic rates were computed using an extension of Sokoloff's model (Phelps et al, 1979). To reduce effects of menstrual cycle, females were scanned during their mid-follicular phase.
The data were normalized to the FDG template in MNI space in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Whole-brain and SPM analyses were performed on the absolute metabolic images. For SPM, the metabolic images were spatially smoothed using an 8-mm Gaussian kernel to account for the variability of the brain anatomy across subjects.
Statistical Analyses
To calculate the main effects of group, gender, and drug challenge on behavioral, cardiovascular, and whole-brain glucose metabolism and its interaction (group × gender × drug-challenge), we used mixed ANOVAs in SPSS 22 (IBM, Armonk, NY). Post hoc t-tests were performed to determine the direction of findings. There was a main effect of gender on body-mass index (F=6.2, p=0.017) and age (F=4.9, p=0.032), with females having lower body-mass indexes and being younger than males. We therefore used body-mass index and age as covariates in gender interactions, for we had previously shown that regional brain glucose metabolism correlated negatively with age (De Santi et al, 1995; Volkow et al, 2000b) and with body-mass index in healthy volunteers (Volkow et al, 2009).
Second-level voxel-wise analyses were performed in SPM8 using a mixed design ANOVA model with group (CA and HC) as between-subject factor and challenge (MP and Placebo) as within subject factor. We also investigated group × gender interactions for baseline and MP-induced differences in metabolism. The statistical significance for the a priori hypotheses: (i) that baseline frontal and cerebellar metabolism would be lower in CA than HC, we set significance at p<0.005 uncorrected, k⩾10; (ii) that metabolic responses to MP would be attenuated in DA regions and their projections targets (caudate, putamen, nucleus accumbens, midbrain) in CA compared with HC was tested with small volume correction (SVC) for caudate, putamen, nucleus accumbens, and midbrain using the WFU Pickatlas (Maldjian et al, 2003) with a significance threshold of p<0.05 family-wise error corrected (FWE); and for the whole brain at p<0.001 uncorrected, k⩾10; (iii) that group differences would be stronger in females than males, we set significance at p<0.001 uncorrected, k⩾10. We computed average metabolic values within clusters that showed significant difference in CA<HC using SPM8 and tested their associations with severity of dependence (MDQ) using Pearson's correlations in SPSS 22. We tested normality for demographics and physiological measures using the Kolmogorov-Smirnov (K-S) test for each group separately. Demographic variables were normally distributed on the K-S test (all p>0.05), except education years for HC (D=0.2, p=0.007) and CA (D=0.3 p<0.001), BDI for HC (D=0.2, p=0.006), and cannabis age initiation (D=0.2 p=0.04), cannabis days/week (D=0.45 p<0.001) joints/day (D=0.3 p<0.001), and years of abuse (D=0.2 p=0.007). Cardiovascular measures and whole-brain measures of glucose metabolism (PL and MP) were distributed normally in both groups (K-S test: all p>0.05). For variables that were not normally distributed, we used the Mann–Whitney U test for group comparisons and Spearman's rank-order rho for correlation analyses.
RESULTS
Group Characteristics
There were no differences in demographics between the groups (Table 1). Groups differed significantly in personality measures on the MPQ; CA had significantly lower scores in Positive Emotionality (PEM) (t=2.4, p=0.02) and higher scores in Negative Emotionality (NEM) (t=3.5, p=0.001) than HC (Table 1). There were no gender or gender by group interactions for the personality measures.
Table 1. Demographics, Clinical Characteristics, and Personality Scores (MPQ) in Male and Female Cannabis Abusers and Healthy Controls.
| Characteristic |
Cannabis abusers |
Healthy controls |
P-value |
||||
|---|---|---|---|---|---|---|---|
| Male N=12 | Female N=12 | Male N=12 | Female N=12 | Group | Gender | Group × Gender | |
| Age, years | 29.0±8.8 | 24.6±4.3 | 30.3±7.0 | 26.2±26.2 | NS | 0.032 | NS |
| Years of education | 12.9±1.3 | 13.5±1.5 | 13.6±1.3 | 14.2±1.8 | NS | NS | NS |
| BMI | 25.7±3.1 | 22.6±3.5 | 24.9±3.1 | 23.7±2.2 | NS | 0.017 | NS |
| Cigarette smokers | 7 active 1 former | 3 active 1 former | 3 active 1 former | 1 active 0 former | NS | NS | NS |
| Cannabis age initiation | 14.8±3.0 | 15.2±2.4 | − | − | NS | ||
| Days/week | 6.7±.7 | 6.8±.5 | − | − | NS | ||
| Joints/day | 4.9±3.8 | 4.8±2.9 | − | − | NS | ||
| Years abuse | 12.9±9.1 | 9.0±4.7 | − | − | NS | ||
| MDQ sum | 6.5±2.7 | 4.3±2.6 | − | − | NS | ||
| BDI | 8.6±4.1 | 9.0±8.2 | 6.1±9.5 | 5.4±5.6 | 0.06 | NS | NS |
| MPQ PEM | 51.3±10.4 | 43.2±9.6 | 50.8±5.6 | 51.7±10.1 | 0.05 | NS | NS |
| MPQ NEM | 22.8±7.7 | 21.9±10.0 | 16.0±10.4 | 11.4±6.0 | 0.001 | NS | NS |
| MPQ Constraint | 45.6±7.4 | 49.8±8.1 | 48.6±10.9 | 54.0±7.9 | NS | NS | NS |
Abbreviations: BDI, Beck's Depression Index; BMI, body mass index; MDQ, Marijuana Dependence Questionnaire; MPQ, Multidimensional Personality Questionnaire; NEM, negative emotionality; NS, non-significant=p⩾0.1; PEM, positive emotionality.
Characteristics are listed with mean±SD. Mann–Whitney U test. Bold values indicate P-values <0.1.
PET Measures
Baseline brain glucose metabolism in CA vs HC
Whole-brain measures of glucose metabolism at baseline did not differ between groups (F=0.05, p=0.82).
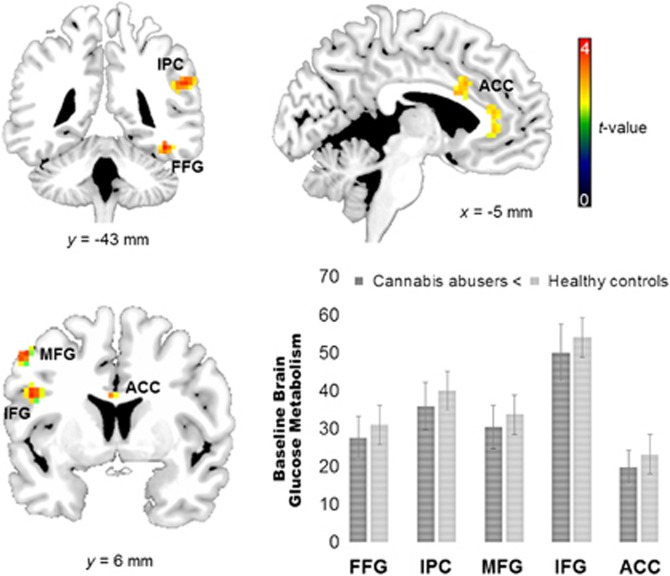
SPM analysis for the a priori analysis (threshold p<0.005 uncorrected, k⩾10) revealed that CA had lower regional metabolic measures in frontal regions, including anterior cingulate cortex (BA 24, BA 32, BA 31), medial and inferior frontal gyrus compared with HC (see Table 2; Figure 1). There were no significant voxels for the reverse contrast of CA>HC. The SPM also revealed differences in fusiform gyrus and inferior parietal cortex, which were regions that we had not identified a priori and thus we are not considering them as significant.
Table 2. Baseline Regional Brain Glucose Metabolism in Cannabis Abusers vs Controls.
| Brain region | BA | Hemi | K | MNI (x y z) | T-value | ||
|---|---|---|---|---|---|---|---|
| CA<HC | |||||||
| Fusiform gyrus | 37 | R | 20 | 42 | −43 | −17 | 3.87 |
| Inferior parietal cortex | 40 | R | 21 | 57 | −43 | 28 | 3.68 |
| Middle frontal gyrus | 8 | L | 22 | −54 | 8 | 46 | 3.60 |
| Inferior frontal gyrus | 44 | L | 11 | −48 | 5 | 25 | 3.43 |
| Anterior cingulate | 24 | R | 12 | 9 | 8 | 22 | 3.34 |
| Anterior cingulate | 24, | L | 41 | −9 | 14 | 22 | 3.28 |
| 32 | |||||||
| HC<CA | |||||||
| No significant voxels | |||||||
Abbreviations: BA, Brodmann area; CA, cannabis abusers; HC, healthy controls; Hemi, hemisphere; K, cluster size; L, left; MNI (x y z), Coordinates in Montreal Neurological Institute space (x y z); R, right.
p<0.005 uncorrected, k⩾10.
Figure 1.
SPM results for the comparison of baseline regional brain glucose metabolism between cannabis abusers and healthy controls. CA had lower metabolism in the fusiform gyrus (FFG), inferior parietal gyrus (IPG), inferior frontal gyrus (IFG), anterior cingulate cortex (ACC), and middle frontal gyrus (MFG) (p<0.005 uncorrected). For visualization purposes, activations were plotted at p<0.01 uncorrected.
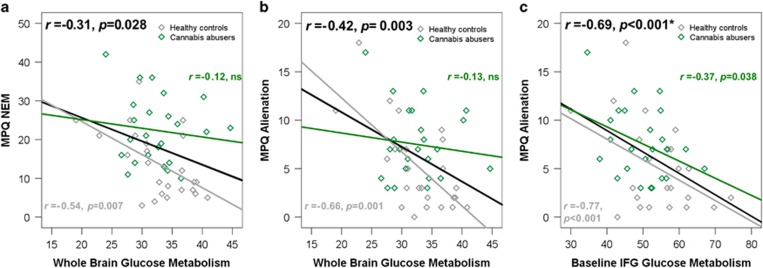
Correlation with MPQ. In both groups pooled together, whole-brain glucose metabolism correlated significantly with NEM (r=−0.314, p=0.028), particularly with the alienation subscale (r=−0.421, p=0.003). This was due to a negative correlation in HC (NEM: r=−0.535, p=0.007, AL: r=−0.656, p=0.001), but not in CA (ns). This pattern was also shown for brain areas that showed lower uptake in CA than HC: alienation correlated with all brain areas in HC (fusiform gyrus r=−0.424; inferior parietal cortex r=−0.439, medial frontal gyrus r=−0.50, and inferior frontal gyrus r=−0.443, anterior cingulate cortex r=−0.375; all p<0.05), but only with inferior frontal gyrus in CA (r=−0.367 p<0.05) (Figure 2).
Figure 2.
Whole-brain glucose metabolism correlated with negative emotionality (NEM) (a) particularly with the alienation subscale (b). Regional glucose metabolism in frontal areas that showed cannabis abusers<healthy controls (CA<HC) group differences correlated with alienation in all brain areas in HC, and only with IFG in CA (c). * Note that this correlation may be artificially high owing to drug group differences in both IFG glucose metabolism and MPQ alienation. Regressions for groups pooled together are depicted in black, for HC in gray and for CA in green. MPQ, Multidimensional Personality Questionnaire.
Effects of MP on brain metabolism in CA and HC
For whole-brain glucose metabolism, there was a main effect of challenge (F=6.94, p=0.012) and an interaction of group × gender (F=4.14, p=0.048). Post hoc paired t-tests showed that MP increased whole-brain metabolism in HC (Placebo: 32.8±5.3 SD vs MP: 36.5±6.5 μmol/g/min; t=3.0, p=0.006), but in CA, the effects were not significant (Placebo: 32.5 ±4.8 vs MP: 33.9 ±5.6 μmol/g/min, t=1.1, p=0.27).
SPM showed that the largest MP-induced increase in regional brain glucose metabolism occurred in hippocampus, bilateral thalamus, bilateral occipital cortex (BA 17), insula, and inferior temporal gyrus (all p<0.001 uncorrected, k⩾10; see Table 3).
Table 3. Effects of MP on Regional Brain Glucose Metabolism: Main Effects and Interaction with Group (Cannabis Abusers vs Controls).
| Brain region | BA | Hemi | K | MNI (x y z) | T-value | ||
|---|---|---|---|---|---|---|---|
| PL<MP | |||||||
| Cerebellum/Midbrain | R/L | 4598 | 6 | −70 | −32 | 7.87a | |
| Hippocampus | 17 | R | 30 | 30 | −28 | −8 | 4.50 |
| Thalamus | L | 33 | −21 | −22 | 7 | 4.14 | |
| Thalamus | R | 35 | 9 | −13 | 7 | 4.06 | |
| Occipital cortex | R | 56 | 12 | −76 | 10 | 4.05 | |
| Mid temp gyrus/insula | 21 | L | 24 | −39 | 2 | −32 | 4.01 |
| Inferior temporal gyrus | 20 | R | 57 | 78 | −25 | −17 | 3.98 |
| Occipital cortex | 17 | L | 13 | −9 | −88 | 7 | 3.51 |
| Interaction PL<MP, CA<HC | |||||||
| Cerebellum | R | 38 | 21 | −49 | −23 | 4.20 | |
| Putamen | R | 10 | 24 | −1 | 13 | 3.80b | |
| Caudate | L | 5 | −21 | 11 | 22 | 3.60b | |
| Midbrain | L | 4 | 15 | −22 | −17 | 3.63b | |
| Thalamus | R | 10 | 9 | −13 | 10 | 3.39 | |
Abbreviations: BA, Brodmann area; CA, cannabis abusers; FWE, family-wise error corrected; HC, healthy controls; Hemi, hemisphere; K, cluster size; L, left; MNI (x y z), Coordinates in Montreal Neurological Institute space (x y z); MP, methylphenidate; PL, placebo; R, right; SVC, small-volume corrected.
p<0.001 uncorrected, k⩾10.
p<0.05 FWE.
p<0.05 FWE, SVC.
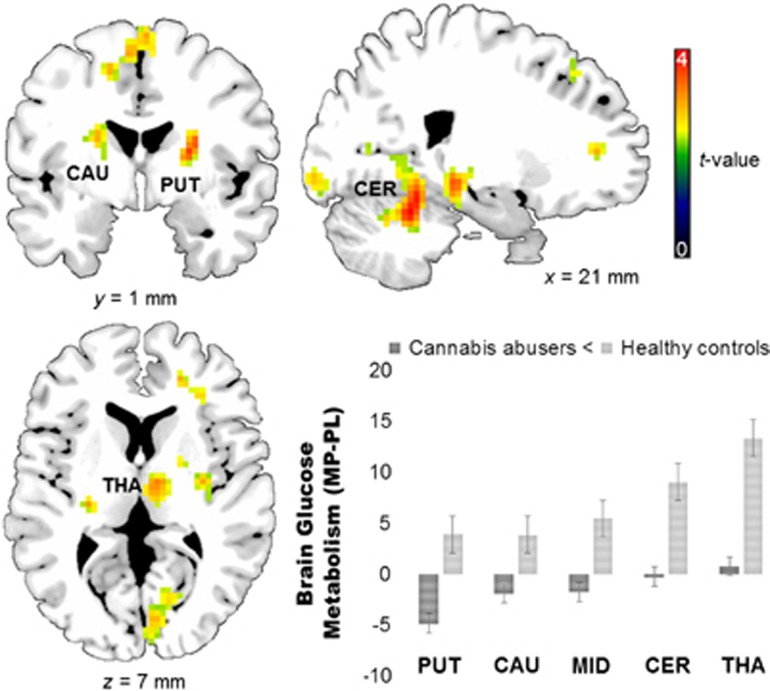
For the a priori hypothesis that CA would have blunted response to MP in DA projection regions, we showed that the right putamen, left caudate, and midbrain (all p<0.05 FWE-corrected SVC) but not nucleus accumbens (p>0.05 FWE-corrected) had lower MP-induced activation in CA vs HC (see Table 3). The exploratory whole-brain analysis showed that increases in metabolism were stronger in HC than CA not just in midbrain, putamen, and caudate (a priori regions), but also in cerebellum and thalamus (all p<0.001 uncorrected) (see Figure 3; Table 3).
Figure 3.
Group comparison cannabis abusers vs healthy controls (CA vs HC) of MP effects (MP-PL) on regional brain glucose metabolism. Cannabis abusers showed lower MP-PL activation in putamen (PUT), caudate (CAU), midbrain (MID), cerebellum (CER), and thalamus (THA) (p<0.001 uncorrected). There were no regions where MP induced stronger metabolic increases in CA vs HC. For visualization purposes, activations were plotted at p<0.01 uncorrected.
Correlations with addiction severity. Metabolic values in the regions that showed a main MP-induced difference of CA<HC (ie, putamen, caudate, midbrain, cerebellum, thalamus) were extracted using SPM8.
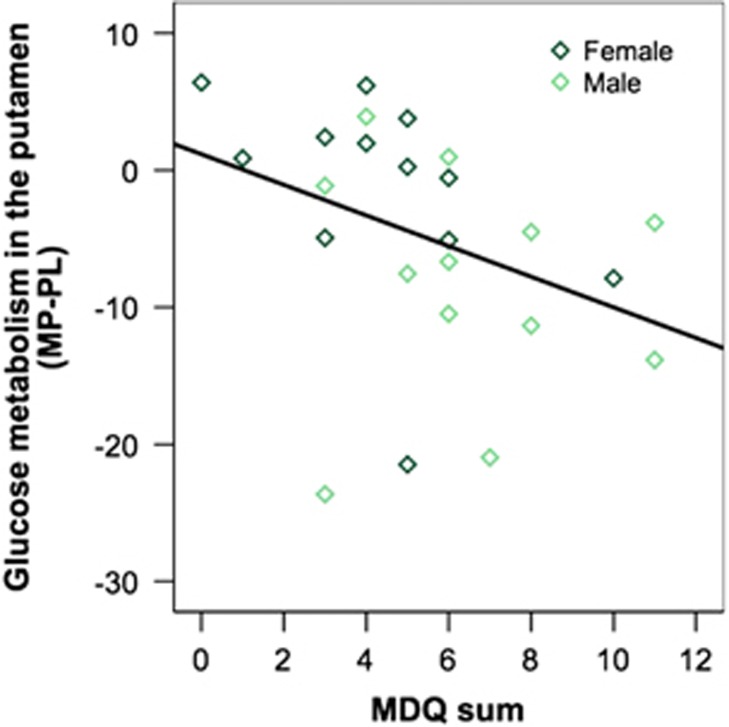
In CA, the measures of addiction severity (MDQ scores) correlated with MP-induced changes in metabolism in putamen (r=−0.37, p=0.04, one-tailed; see Figure 4). Please see Supplementary Material for exploratory correlations between MP-induced changes in behavioral ratings with MP-induced changes in regional brain glucose metabolism.
Figure 4.
Measures of addiction severity (MDQ scores) correlated with MP-induced metabolic changes in putamen (r=−0.37, p<0.05, one-tailed). There were no effects of gender.
Group by gender interactions on baseline and MP-induced changes in regional brain glucose metabolism
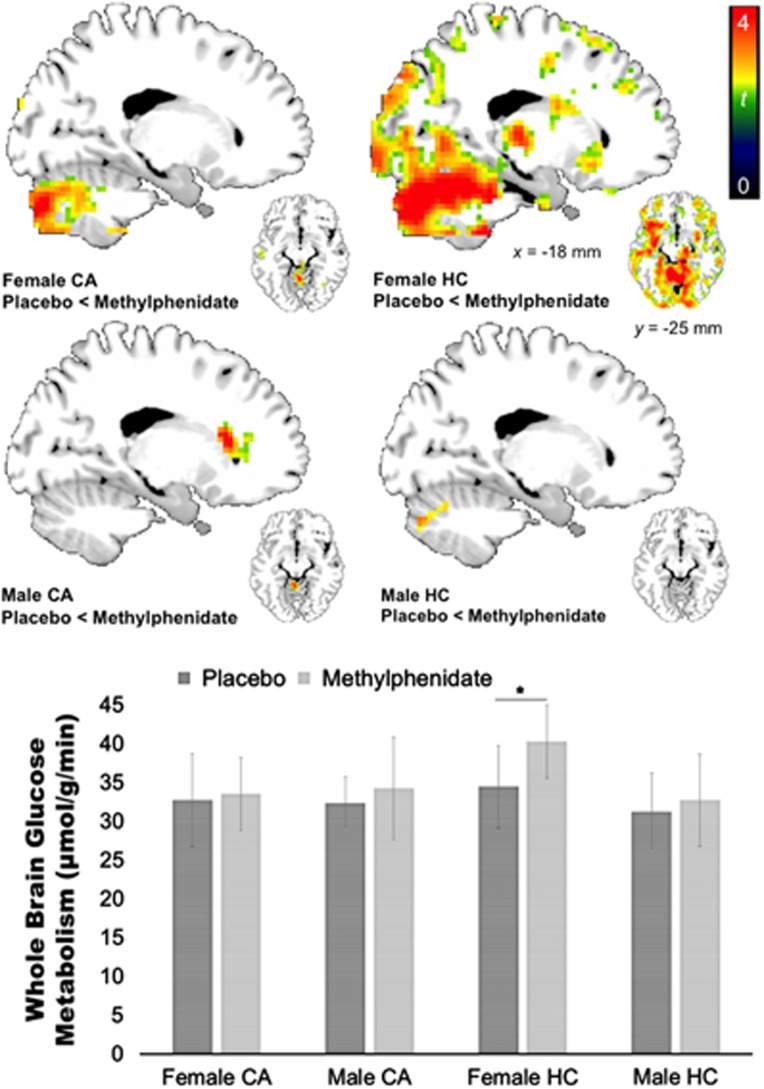
At baseline, there was no group by gender interaction effect for whole-brain glucose metabolism (F=0.51, p=0.48), nor an effect of gender (F=2.43, p=0.13). For the regional brain metabolic measures at baseline, SPM analyses showed a significant group × gender interaction in bilateral medial frontal gyrus, right superior frontal gyrus, and right occipital cortex, all p<0.001 uncorrected, k⩾10 (Table 4). Post hoc contrasts showed no group differences in male CA<HC (all p>0.005 uncorrected), but female CA had significant lower metabolism in left superior frontal gyrus, right occipital cortex, and right anterior cingulate cortex than female HC (Table 4; Figure 5).
Table 4. Gender Effects in Baseline and MP-induced Brain Glucose Metabolism.
| Brain region | BA | Hemi | K | MNI (x y z) | T-value | ||
|---|---|---|---|---|---|---|---|
| Baseline, interaction female<male, CA<HC | |||||||
| Middle frontal gyrus | 8 | R | 24 | 39 | 14 | 49 | 4.33 |
| Superior frontal gyrus | 9 | R | 17 | 0 | 11 | 67 | 3.97 |
| Occipital cortex | 17 | R | 12 | 54 | −85 | −2 | 3.64 |
| Baseline, female CA<HC | |||||||
| Superior frontal gyrus | 9 | L | 13 | −12 | 17 | 55 | 3.79 |
| Occipital cortex | 17 | R | 10 | 45 | −61 | −14 | 3.68 |
| Anterior cingulate | 24 | L | 10 | −9 | 23 | 31 | 3.54 |
| Baseline, male CA<HC | |||||||
| No significant voxels | |||||||
| PL<MP, female CA<HC | |||||||
| Cerebellum | R | 19 | 6 | −61 | −14 | 4.10 | |
| Medial frontal gyrus | R | 50 | 0 | −22 | 55 | 4.08 | |
| Cerebellum | R | 10 | 18 | −46 | −17 | 3.98 | |
| Pons | L | 25 | −18 | −28 | −50 | 3.90 | |
| Hippocampus/thalamus/midbrain | R | 22 | 18 | −25 | −11 | 3.84 | |
| PL<MP, male CA<HC | |||||||
| No significant voxels | |||||||
Abbreviations: BA, Brodmann area; CA, cannabis abusers; HC, healthy controls; Hemi, hemisphere; K, cluster size; L, left; MNI (x y z), Coordinates in Montreal Neurological Institute space (x y z); MP, methylphenidate; PL, placebo; R, right.
p<0.001 uncorrected, k⩾10.
Figure 5.
MP increased whole-brain metabolism in controls (HC) (p<0.01), but the effects in cannabis abusers (CA) were not significant. In HC, the increases in whole-brain metabolism with MP were driven by gender because increases with MP were observed in females (p<0.01) but not in males. Only female HC significantly increased whole-brain metabolism (p<0.01), but not female CA, male CA, or male HC. SPM analyses showed that female CA compared with female HC had lower MP-induced increases in regional metabolism in cerebellum, medial frontal gyrus, pons, hippocampus, and thalamus (p<0.001 uncorrected). For visualization purposes, activations were plotted at p<0.01 uncorrected, cluster size 50 voxels. There were no differences in MP reactivity in male CA vs HC.
There was an interaction of group × challenge × gender at trend level for whole-brain metabolism (F=3.93, p=0.054): Increases in whole-brain metabolism with MP were observed in females in both groups pooled together (9.8±16.4% increase; t=2.9, p=0.007), but not in males (5.4±20.2% increase, t=1.3, p=0.21). However, increases in females were driven by HC who were the only ones showing significant increases with MP (16.9% increase, t=3.9, p=0.002), whereas neither female/male CA nor male HC showed significant effects (all p>0.36; Figure 5).
For regional effects, the exploratory whole-brain SPM analyses also showed a significant gender by group interaction effect. Female CA showed blunted MP-induced responses in cerebellum, medial frontal gyrus, pons, and in a cluster that encompassed hippocampus, thalamus, and midbrain, whereas there were no differences in males (Table 4; Figure 5).
DISCUSSION
Baseline Brain Glucose Metabolism in CA vs HC
Here, we show, first, reduced baseline metabolism in frontal brain regions in CA compared with controls, which is consistent with prior findings of impaired frontal baseline metabolism in other drug addictions (Volkow and Fowler, 2000a; Goldstein and Volkow, 2011), including CA (Volkow et al, 1996; Jacobus et al, 2012). Decreased baseline frontal metabolic activity has been associated with low DA D2 receptors in striatum (Volkow et al, 1993, 2001, 2013) and the associated reduced dopaminergic tonic stimulation (Volkow and Morales, 2015a), and clinically, it is associated with reductions in self-regulation and higher rates of relapse (Goldstein and Volkow, 2011; Volkow and Morales, 2015a). Moreover, enhancing DA signaling with oral MP has been shown to normalize frontal activation and to improve cognitive task performance in cocaine addiction (Goldstein et al, 2010; Moeller et al, 2014).
In the current study, decreased whole-brain and frontal metabolism correlated with negative emotionality (NEM) scores on the MPQ, particularly the subscale of alienation for which CA showed significantly higher scores than HC. These findings are consistent with a study that showed decreased frontal BOLD activation on an emotion-arousal task in CA that mediated the relationship between cannabis abuse and negative emotionality (Heitzeg et al, 2015). They are also in line with previous findings that in CA, reduced brain DA signaling was associated with negative emotionality (Volkow et al, 2014) and negative symptoms and inattention (van de Giessen et al, 2016), and reduced DA synthesis with subjective apathy (Bloomfield et al, 2014b). Further, DA synthesis capacity was negatively associated with higher levels of cannabis use and positively associated with age of onset of cannabis use (Bloomfield et al, 2014a), and stress-induced DA release was shown to be associated with duration of cannabis use in chronic CA (Mizrahi et al, 2013). This suggests that cannabis use leads to impairment in frontal brain function and in DA neurotransmission, which contribute to negative emotional states in CA.
Effects of MP on Brain Metabolism in CA and HC
Second, we show attenuated responses to MP in CA vs HC in the hypothesized striatal DA projections, though not in prefrontal cortex or NAc, which is consistent with reduced reactivity to dopaminergic stimulation previously found in CA (Volkow et al, 2014). MP-induced increases in brain metabolism in the putamen of CA were negatively correlated with Marijuana Dependency Questionnaire scores, suggesting that blunted striatal DA reactivity to MP is associated with addiction severity. The mechanism responsible for reduced striatal reactivity to MP may reflect downregulation of DA transporters, because lower than normal DA transporter has been reported in CA (Leroy et al, 2012). Similarly, we reported an attenuation of MP-induced reduction in the distribution volume of [11C]raclopride, which is an indication of DA release in CA (Volkow et al, 2014), consistent with prior findings in CA with psychosis of reduced DA release when challenged with a stimulant drug or when exposed to a stressor (Thompson et al, 2013), though others have not shown reduced DA release in CA (Urban et al, 2012). In controls, we previously showed that MP-induced increases in brain glucose metabolism in cortical regions were modulated by striatal D2R availability, such that MP increased brain metabolism in subjects with high striatal D2R, whereas it decreased metabolism in subjects with low D2R (Volkow et al, 1997). Thus, downregulation of D2R could also result in an attenuated brain metabolic response to MP. This is, however, unlikely to account for our results because striatal D2R availability was not reduced in CA (Volkow et al, 2014). Finally, it is also possible that the attenuated responses reflect downstream modulation from D2R or other DA receptors (D1, D3, D4, or D5). In this respect, our findings are opposite to what we observed in alcoholics in whom changes in regional brain metabolism induced by MP were enhanced compared with controls even though DA release to MP was attenuated (Volkow et al, 2013).
The exploratory analyses also revealed group differences in MP-induced metabolic changes in the cerebellum and thalamus, which are regions that receive dense noradrenergic innervation (Olson and Fuxe, 1971; Sara, 2009). Moreover, the cerebellum has minimal DA innervation, which suggests that reduced metabolic increases to MP in CA could also reflect attenuated reactivity to MP-induced noradrenergic stimulation (Tilley and Gu, 2008). The cannabinoid system is an important regulator of noradrenergic systems in part via CB1 receptors (Reyes et al, 2009; Carvalho and Van Bockstaele, 2012; Cathel et al, 2014) and downregulation of CB1 receptors in CA (Hirvonen et al, 2012) could underlie the attenuated responses to MP-induced changes in metabolism observed in these brain regions. A prior study in CA showed that co-administration of Δ-9-tetrahydrocannabinol (THC) with ecstasy, which like MP is a monoamine transporter blocker, enhanced its cardiovascular effects and the increases in epinephrine and norepinephrine in plasma (Dumont et al, 2009). This is indicative of enhanced effects to ecstasy when CB1 receptors are co-stimulated by THC. Thus, downregulation of CB1 receptors in CA could contribute to their attenuated responses to MP.
Group by Gender Interactions on Baseline and MP-Induced Changes in Regional Brain Glucose Metabolism
A third important finding was the interaction between gender and drug effects as well as an interaction between gender and group on both baseline and MP-induced changes in regional brain metabolism. Female CA showed lower baseline metabolism in frontal and occipital cortices than HC, whereas these baseline differences were not significant for males. Females also had significantly higher MP-induced increases in brain glucose metabolism than males, which was an effect driven by the HC. Specifically, MP produced greater metabolic increases in female HC than in female CA or in male HC or CA. In turn, this drove the significant gender by group drug interaction because only in females was the difference between the groups significant. Female CA showed decreased responses to MP in the cerebellum, medial frontal gyrus, pons, hippocampus, and thalamus, compared with female HC. In contrast, there were no group differences in male participants on MP-induced metabolism. Preclinical studies have shown higher sensitivity of females than males to the rewarding effects of cannabinoids and a greater vulnerability to reinstatement during abstinence (Fattore et al, 2008). However, adolescent male rats were recently shown to be more sensitive to the anxiolytic and antidepressant effects of THC, as measured by the elevated plus maze and forced swim test (Silva et al, 2016). Clinical studies have shown that male CA are more likely than female CA to be chronic cannabis users (eg, Preston, 2006), experience more withdrawal symptoms (Crowley et al, 1998), and suffer a higher prevalence of panic attacks and personality disorders (Hasin et al, 2008). Gender differences have also been reported in subjective responses to oral THC administration: while female CA reported greater responses to a 5 mg THC dose than male CA, male CA were more sensitive to a higher (ie, 15 mg) THC dose than female CA (Fogel et al, 2016). Epidemiologic studies have shown important gender differences in clinical characteristics and psychiatric comorbidities in CA. That is, smoking cannabis was associated with decreased quality of life, particularly in female CA (Lev-Ran et al, 2012). Further, male CA were more likely diagnosed with any psychiatric disorder, any substance use disorder and antisocial personality disorder compared with female CA, whereas female CA had more mood, anxiety, and externalizing disorders (Khan et al, 2013). Male CA also had longer episodes of abuse, smoked more joints, and were older at remission than female CA (Khan et al, 2013). Nevertheless, it has to be noted that some of these gender differences might reflect social and cultural factors influencing exposure and responses to cannabis rather than biological differences in the sensitivity to cannabis.
A recent brain imaging study documented higher CB1 receptor levels in the brains of women compared with men (Neumeister et al, 2013; Normandin et al, 2015), and a preclinical study showed that THC altered CB1 receptor expression and function in females but not in males (Silva et al, 2016). This could provide a neurobiological basis for differences in sensitivity to the effects of cannabis between the genders. Sex differences in cannabinoid action have been ascribed in part to menstrual phase and fluctuating hormonal levels (Fattore and Fratta, 2010; Riebe et al, 2010). In our study, we scanned women during the mid-follicular phase when levels of estrogen, FSH, LH, and progesterone are low, to minimize the effects of menstrual cycle. The study reporting higher brain CB1 receptors in females than males was also performed during the follicular phase. Preclinical studies also support sex effects on cannabinoid-induced changes in regional brain glucose in responses to a stimulant drug. Interestingly, however, the findings were opposite to our results; in male rats but not in females, cannabinoid exposure decreased the metabolic responses to cocaine (Higuera-Matas et al, 2011).
Gender differences in regional brain metabolic response to drug cues have also been reported: female but not male cocaine abusers show significant metabolic decreases in cognitive control networks (ie, fronto-parietal and cingulo-opercular network) (Volkow et al, 2011). In the current study, we also found preliminary evidence for a gender by group effect in subjective ratings of control: MP significantly decreased control in female HC and in male CA, but not in female CA and male HC (see Supplementary Material). In this respect, female CA reacted similarly to male HC for MP-induced changes in self-reports of control as well as restlessness, desire for MP and cannabis craving. Although the particulars of the sex and gender differences in responses to acute and chronic cannabis are far from elucidated, it is clear that there are important differences between males and females that might reflect in part the interaction of gonadal hormones with the endocannabinoid system (Gorzalka and Dang, 2012). Future studies investigating the influence of gender on the effects of cannabis in brain are needed to elucidate the nature of the differences and the mechanisms underlying them.
Study Limitations
Study limitations include that we did not quantify hormonal measures from blood of participants. This would have given us the opportunity to assess whether there was a relationship between estrogen and progesterone levels and MP-induced changes in brain glucose metabolism and to assess whether these differed between controls and CA. Further, despite a lack of group differences in tobacco smoking between CA and HC (χ2=4.48, p=0.11, ns; see Table 1) and most CA expressed smoking joints with cannabis only, we did not systematically assess whether CA smoked cannabis together with tobacco on a regular basis. As such, brain metabolism differences between CA and HC reported in this study may, partly, be due to tobacco use. In our study, the administration of saline and MP was single rather than double-blind, which may have influenced our measures. However, we chose a single-blind design to ensure that medical and nursing support was present for potential adverse reactions to MP, which can result in tachychardia and in blood pressure increases. Finally, though we interpret our findings of reduced brain metabolic changes in response to MP challenge to reflect blunting of dopaminergic and/or noradrenergic signaling because these neurotransmitters are increased by MP (Berridge and Arnsten, 2013), we cannot rule out the possibility that the blunting reflects a downstream effect because CB1 receptors, which are the targets for cannabis, modulate multiple signaling pathways (Puighermanal et al, 2012).
MP was given 90 min prior to FDG. Because the initial brain uptake of iv MP is associated with its rewarding effects, which return to baseline 20–30 min after its injection even when MP brain levels are still high (Volkow et al, 1995), the metabolic changes have to be interpreted as reflecting changes in activity that follow the fast DA increases induced by MP. We chose this design because we wanted to assess MP's effects when the levels in brain had stabilized (Volkow et al, 1995). The pharmacological effects of MP persist for at least 2–3 h (Patrick and Markowitz, 1997), which is within the time window of the FDG measures. Interestingly, when we measured the effects of MP 1 min after its intravenous administration, we observed a similar pattern of changes in regional brain glucose metabolism to those reported in this study (Volkow et al, 2003).
CONCLUSION
In summary, we show evidence of decreased baseline activity in frontal brain regions in CA, which has been implicated in other types of addiction. We also report attenuated regional brain metabolic responses to MP challenge, which are consistent not only with reduced neuronal reactivity to DA but also because of noradrenergic stimulation in CA. Finally, consistent with greater sensitivity of the female brain to the effects of repeated cannabis exposure, we showed that the reduced baseline frontal activity and the blunted MP-induced changes in brain metabolism in CA were predominantly driven by differences in females but not male participants. This suggests that women are more sensitive to the adverse effects of cannabis on the brain than men.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We thank Karen Torres, Barbara Hubbard, Millard Jayne, Yana Studentsova, Payton King, Pauline Carter, Donald Warner, Joanna Fowler, and Ruben Baler for their contributions. The work was supported by NIH/NIAAA intramural grant Y1AA-3009 to NDV. CEW received a scholarship from the German Research Foundation.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Berridge CW, Arnsten AF (2013). Psychostimulants and motivated behavior: arousal and cognition. Neurosci Biobehav Rev 37(9 Pt A): 1976–1984. [DOI] [PubMed] [Google Scholar]
- Bloomfield MA, Morgan CJ, Egerton A, Kapur S, Curran HV, Howes OD (2014. a). Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol Psychiatry 75: 470–478. [DOI] [PubMed] [Google Scholar]
- Bloomfield MA, Morgan CJ, Kapur S, Curran HV, Howes OD (2014. b). The link between dopamine function and apathy in cannabis users: an [18F]-DOPA PET imaging study. Psychopharmacology 231: 2251–2259. [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Van Bockstaele EJ (2012). Cannabinoid modulation of noradrenergic circuits: implications for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 38: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathel AM, Reyes BA, Wang Q, Palma J, Mackie K, Van Bockstaele EJ et al (2014). Cannabinoid modulation of alpha2 adrenergic receptor function in rodent medial prefrontal cortex. Eur J Neurosci 40: 3202–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ, Macdonald MJ, Whitmore EA, Mikulich SK (1998). Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. Drug Alcohol Depend 50: 27–37. [DOI] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Convit A, Tarshish C, Rusinek H, Tsui WH et al (1995). Age-related changes in brain: II. Positron emission tomography of frontal and temporal lobe glucose metabolism in normal subjects. Psychiatr Q 66: 357–370. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, Kramers C, Sweep FC, Touw DJ, van Hasselt JG, de Kam M et al (2009). Cannabis coadministration potentiates the effects of "ecstasy" on heart rate and temperature in humans. Clin Pharmacol Ther 86: 160–166. [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W (2008). Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond Engl) 4: 51–65. [DOI] [PubMed] [Google Scholar]
- Fattore L, Fratta W (2010). How important are sex differences in cannabinoid action? Br J Pharmacol 160: 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel JS, Kelly TH, Westgate PM, Lile JA (e-pub ahead of print 15 January 2016). Sex differences in the subjective effects of oral Delta-THC in cannabis users. Pharmacol Biochem Behav. [DOI] [PMC free article] [PubMed]
- Goldstein RZ, Volkow ND (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12: 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Maloney T, Tomasi D, Alia-Klein N, Shan J et al (2010). Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Natl Acad Sci USA 107: 16667–16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Dang SS (2012). Minireview: Endocannabinoids and gonadal hormones: bidirectional interactions in physiology and behavior. Endocrinology 153: 1016–1024. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF (2008). Cannabis withdrawal in the United States: results from NESARC. J Clin Psychiatry 69: 1354–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Cope LM, Martz ME, Hardee JE, Zucker RA (2015). Brain activation to negative stimuli mediates a relationship between adolescent marijuana use and later emotional functioning. Dev Cogn Neurosci 16: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuera-Matas A, Soto-Montenegro ML, Montoya GL, Garcia-Vazquez V, Pascau J, Miguens M et al (2011). Chronic cannabinoid administration to periadolescent rats modulates the metabolic response to acute cocaine in the adult brain. Mol Imaging Biol 13: 411–415. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C et al (2012). Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 17: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, Tapert SF (2012). Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology 222: 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, Secades-Villa R, Okuda M, Wang S, Perez-Fuentes G, Kerridge BT et al (2013). Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug Alcohol Depend 130: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy C, Karila L, Martinot JL, Lukasiewicz M, Duchesnay E, Comtat C et al (2012). Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: a high-resolution PET study. Addict Biol 17: 981–990. [DOI] [PubMed] [Google Scholar]
- Lev-Ran S, Imtiaz S, Taylor BJ, Shield KD, Rehm J, Le Foll B (2012). Gender differences in health-related quality of life among cannabis users: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 123: 190–200. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Suridjan I, Kenk M, George TP, Wilson A, Houle S et al (2013). Dopamine response to psychosocial stress in chronic cannabis users: a PET study with [11C]-+-PHNO. Neuropsychopharmacology 38: 673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Honorio J, Tomasi D, Parvaz MA, Woicik PA, Volkow ND et al (2014). Methylphenidate enhances executive function and optimizes prefrontal function in both health and cocaine addiction. Cerebral Cortex 24: 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A et al (2013). Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry 18: 1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normandin MD, Zheng MQ, Lin KS, Mason NS, Lin SF, Ropchan J et al (2015). Imaging the cannabinoid CB1 receptor in humans with [11C]OMAR: assessment of kinetic analysis methods, test-retest reproducibility, and gender differences. J Cereb Blood Flow Metab 35: 1313–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L, Fuxe K (1971). On the projections from the locus coeruleus noradrealine neurons: the cerebellar innervation. Brain Res 28: 165–171. [DOI] [PubMed] [Google Scholar]
- Patrick KS, Markowitz JS (1997). Pharmacology of methylphenidate, amphetamine enantiomers and pemoline in attention-deficit hyperactivity disorder. Hum Psychopharm Clin 12: 527–546. [Google Scholar]
- Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE (1979). Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol 6: 371–388. [DOI] [PubMed] [Google Scholar]
- Preston P (2006). Marijuana use as a coping response to psychological strain: racial, ethnic, and gender differences among young adults. Deviant Behav 27: 397–421. [Google Scholar]
- Puighermanal E, Busquets-Garcia A, Maldonado R, Ozaita A (2012). Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Philos Trans R Soc Lond B Biol Sci 367: 3254–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Rosario JC, Piana PM, Van Bockstaele EJ (2009). Cannabinoid modulation of cortical adrenergic receptors and transporters. J Neurosci Res 87: 3671–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe CJ, Hill MN, Lee TT, Hillard CJ, Gorzalka BB (2010). Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology 35: 1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ (2009). The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10: 211–223. [DOI] [PubMed] [Google Scholar]
- Silva L, Black R, Michaelides M, Hurd YL, Dow-Edwards D (e-pub ahead of print 16 February 2016). Sex and age specific effects of delta-9-tetrahydrocannabinol during the periadolescent period in the rat: The unique susceptibility of the prepubescent animal. Neurotoxicol Teratol. [DOI] [PubMed]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD et al (1977). The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28: 897–916. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Waller NG (2008). Exploring personality through test construction: Development of the Multidimensional Personality Questionnaire. In: Boyle GJ, Matthews G, Saklofske DH (eds). The SAGE Handbook of Personality Theory and Assessment 2. Sage Publications, Inc: Thousand Oaks, CA, USA, pp 261–292.
- Thompson JL, Urban N, Slifstein M, Xu X, Kegeles LS, Girgis RR et al (2013). Striatal dopamine release in schizophrenia comorbid with substance dependence. Mol Psychiatry 18: 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley MR, Gu HH (2008). The effects of methylphenidate on knockin mice with a methylphenidate-resistant dopamine transporter. J Pharmacol Exp Ther 327: 554–560. [DOI] [PubMed] [Google Scholar]
- Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S et al (2012). Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biol Psychiatry 71: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Giessen E, Weinstein JJ, Cassidy CM, Haney M, Dong Z, Ghazzaoui R et al (e-pub ahead of print 22 March 2016). Deficits in striatal dopamine release in cannabis dependence. Mol Psychiatry. [DOI] [PMC free article] [PubMed]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M et al (2001). Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158: 2015–2021. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS et al (1995). Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry 52: 456–463. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS (2000. a). Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral Cortex 10: 318–325. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ et al (1993). Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14: 169–177. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A et al (1996). Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res 67: 29–38. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Burr G, Pascani K et al (1992. a). Decreased brain metabolism in neurologically intact healthy alcoholics. Am J Psychiatry 149: 1016–1022. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL et al (1992. b). Long-term frontal brain metabolic changes in cocaine abusers. Synapse 11: 184–190. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C et al (2000. b). Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry 157: 75–80. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M (2015. a). The brain on drugs: from reward to addiction. Cell 162: 712–725. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Goldstein RZ et al (2011). Reduced metabolism in brain "control networks" following cocaine-cues exposure in female cocaine abusers. PloS One 6: e16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Logan J et al (2013). Predominance of D2 receptors in mediating dopamine's effects in brain metabolism: effects of alcoholism. J Neurosci 33: 4527–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS et al (1998). Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry 155: 1325–1331. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ et al (1999). Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry 156: 19–26. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Angrist B, Hitzemann R et al (1997). Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry 154: 50–55. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L et al (2003). Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci 23: 11461–11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Shokri Kojori E, Fowler JS, Benveniste H, Tomasi D (2015. b). Alcohol decreases baseline brain glucose metabolism more in heavy drinkers than controls but has no effect on stimulation-induced metabolic increases. J Neurosci 35: 3248–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Alexoff D, Logan J et al (2014). Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc Natl Acad Sci USA 111: E3149–E3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N et al (2009). Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity 17: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Roque CT, Cestaro VL, Hitzemann RJ, Cantos EL et al (1993). Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MR imaging, and neuropsychologic testing. Radiology 186: 59–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.