Abstract
Neuroimaging studies investigating patients with schizophrenia often report appreciable volumetric reductions and cortical thinning, yet the cause of these deficits is unknown. The association between subcortical and cortical structural alterations, and glutamatergic neurometabolites is of particular interest due to glutamate's capacity for neurotoxicity; elevated levels may be related to neuroanatomical compromise through an excitotoxic process. To this end, we explored the relationships between glutamatergic neurometabolites and structural measures in antipsychotic-naive patients experiencing their first non-affective episode of psychosis (FEP). Sixty antipsychotic-naive patients with FEP and 60 age- and sex-matched healthy controls underwent a magnetic resonance imaging session, which included a T1-weighted volumetric image and proton magnetic resonance spectroscopy in the precommissural dorsal caudate. Group differences in precommissural caudate volume (PCV) and cortical thickness (CT), and the relationships between glutamatergic neurometabolites (ie, glutamate+glutamine (Glx) and glutamate) and these structural measures, were examined. PCV was decreased in the FEP group (p<0.001), yet did not differ when controlling for total brain volume. Cortical thinning existed in the FEP group within frontal, parietal, temporal, occipital, and limbic regions at a 5% false discovery rate. Glx levels were negatively associated with PCV only in the FEP group (p=0.018). The observed relationship between Glx and PCV in the FEP group is supportive of a focal excitotoxic mechanism whereby increased levels of glutamatergic markers are related to local structural losses. This process may be related to the prominent structural deficits that exist in patients with schizophrenia.
Introduction
Previous studies have identified appreciable volumetric deficits and cortical thinning in patients with schizophrenia (Rimol et al, 2010; van Erp et al, 2016). However, chronicity and medication intake may confound the assessment of these measures and thus true illness pathophysiology. The investigation of antipsychotic-naive patients experiencing their first episode of psychosis (FEP) is beneficial in that confounding effects are substantially reduced. Previous meta-analyses have specifically identified volumetric deficits in patients with FEP (Ellison-Wright et al, 2008; Olabi et al, 2012) and in antipsychotic-naive patients with schizophrenia (Haijma et al, 2013). Similarly, cortical thickness (CT) alterations have been observed in antipsychotic-naive patients with FEP (Song et al, 2015; Xiao et al, 2015). However, the mechanisms through which these structural changes occur are presently unknown.
Glutamate is an abundant excitatory neurotransmitter that exists in large intracellular concentrations in the brain (Mehta et al, 2013). When present in abnormally high extracellular concentrations, glutamate may have a neurotoxic effect through a process referred to as excitotoxicity, whereby overstimulation by glutamate increases intracellular calcium and triggers a cascade of events that lead to cell death (Lahti and Reid, 2011; Mehta et al, 2013). Several studies have quantified glutamate in patients with schizophrenia using proton magnetic resonance spectroscopy (1H-MRS). Studies investigating patients in the early stages of the disorder, in which subjects are either antipsychotic-naive or have been minimally exposed to antipsychotic medication, have observed increased levels of glutamatergic neurometabolites (de la Fuente-Sandoval et al, 2013, 2011; Kegeles et al, 2012; Kraguljac et al, 2013; Plitman et al, 2016); this increase may have an excitotoxic effect in several brain regions. However, as previously reviewed by our group, only a few human studies currently exist that have investigated the relationship between glutamatergic neurometabolites and measures of brain structure, collectively providing inconclusive support for the role of glutamate-mediated excitotoxicity in the structural deficits present in schizophrenia (Plitman et al, 2014). Our group has also previously reported increases in glutamate and glutamate+glutamine (Glx) levels in patients with FEP within the precommissural dorsal caudate (PDC) (de la Fuente-Sandoval et al, 2013, 2011; Plitman et al, 2016), an area highly implicated in the pathophysiology of schizophrenia that has been reported to specifically contain communications with cortical brain regions (Kegeles et al, 2010).
Given the overwhelming evidence for structural compromise in patients with schizophrenia, there is a pressing need to better understand the role of glutamate-mediated excitotoxicity in neuroanatomical alteration. To answer this question, this study examined the relationships between levels of glutamatergic neurometabolites (ie, glutamate and Glx) in the PDC and structural measures (ie, precommissural caudate volume (PCV) and CT) in the largest sample to date of antipsychotic-naive patients with FEP in which 1H-MRS was performed. The study of PCV provides a specific and local examination of this phenomenon, as the 1H-MRS voxel was preferentially located within the PDC, while CT assesses a more global excitotoxic mechanism. To the best of our knowledge, no previous study has investigated these relationships. On the basis of previous literature (Ellison-Wright et al, 2008; Haijma et al, 2013; Olabi et al, 2012; Song et al, 2015; Xiao et al, 2015), we hypothesized that the FEP group would demonstrate PCV deficits in addition to widespread cortical thinning. Also, in accordance with a local excitotoxic process (Kraguljac et al, 2013; Plitman et al, 2014), we hypothesized that increased levels of glutamatergic neurometabolites would be associated with PCV loss in the patient group, while no such relationship would exist with CT. Finally, as an exploratory investigation, we examined the relationships between structural measures and clinical symptoms, hypothesizing that lower PCV and cortical thinning would be related to higher symptom scores.
Materials and methods
Participants, Clinical Assessment, Magnetic Resonance Studies, and 1H-MRS Data Analysis
Information concerning participants, ethical approval, clinical assessment, imaging parameters, and 1H-MRS data analysis has been presented within a previous manuscript (Plitman et al, 2016); however, detailed methodology is included within the Supplementary Information. The present study included 60 antipsychotic-naive patients with FEP and 60 age- and sex-matched healthy controls. 1H-MRS spectra were acquired in the right PDC using an 8-ml isotropic voxel, point-resolved spectroscopy, and a TE of 35 ms. Water suppressed spectra were analyzed with LCModel version 6.3-0E (Provencher, 2001). Spectra were normalized to the unsuppressed water signal. Spectroscopic values were corrected for cerebrospinal fluid (CSF) fraction within the 1H-MRS voxel (de la Fuente-Sandoval et al, 2011). A %SD of 20% or greater, or a full-width at half maximum (FWHM) value exceeding 12 Hz, resulted in exclusion. Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS) (Kay et al, 1987).
Image Preprocessing
All structural imaging analysis was done in the minc format. T1-weighted structural images were first preprocessed using an intensity correction tool. Intensity correction was performed using the N4ITK algorithm (Tustison et al, 2010), an improved version of the popular N3 correction algorithm (Sled et al, 1998). This updated version provides improved bias field correction via a robust B-spline approximation routine with the capability to handle a range of resolutions. It also provides an optimization scheme that leads to better convergence and a more accurate estimation of the overall bias field (Tustison et al, 2010).
PCV Analysis
For volume analyses, T1-weighted structural images were additionally preprocessed using an autocrop tool part of the minc toolkit (https://github.com/BIC-MNI/mni_autoreg), which resampled each image to a 1-mm isotropic slicing. This resampling was done in order to satisfy computational resource limits, as well as to achieve consistency throughout the data set. Note that this is a stage that is carried out in various other image processing tool chains (Lerch and Evans, 2005). Fully-automated segmentation of striatal subdivisions was carried out using the Multiple Automatically Generated Templates (MAGeT-Brain) algorithm (Chakravarty et al, 2013). This technique is a modified multi-atlas segmentation technique designed to use a limited number of high-quality manually segmented atlases as an input. In this case, the striatal subdivisions atlas—based on a histology-based atlas warped to fit the Colin-27 Subcortical Atlas (Chakravarty et al, 2006)—was used as the single atlas input. A subset of the population under study is used as a template library through which the final segmentation is bootstrapped. Each subject in the template library is segmented through non-linear atlas-to-template registration followed by label propagation, yielding a unique definition of the subdivisions for each of the templates. For this work, 21 templates were used from the overall subject pool. A matched set of 10 patients with FEP and 11 healthy controls were chosen so as to ensure a representative template set. The bootstrapping of the final segmentations through the template library results in 21 candidate labels produced for each subject and labels are then fused using a majority vote to complete the segmentation process. Non-linear registration was performed using a version of the Automatic Normalization Tools (ANTS) registration technique (Avants et al, 2008) that is compatible with the minc toolkit (https://github.com/vfonov/mincANTS). To focus on most local phenomena, only right PCV was examined in primary analyses, given that the 1H-MRS voxel was preferentially placed in the right PDC (Supplementary Figure S1).
Total Brain Volume Analysis
Total brain volume (TBV) was obtained using the Brain Extraction based on non-local Segmentation Technique (BEaST) method (Eskildsen et al, 2012), which is based on non-local segmentation in a multi-resolution framework. In BEaST, each voxel is labeled based on the similarity of its neighborhood of voxels to all the neighborhoods in a library of pre-defined priors and a non-local means estimator is used to estimate the label at the voxel. Inputs are downsampled to a lower resolution, segmentation is performed, and results are propagated up to higher resolutions (Eskildsen et al, 2012). BEaST is designed to include CSF (in the ventricles, cerebellar cistern, deep sulci, along surface of brain, and brainstem), the brainstem, and cerebellar white matter (WM) and gray matter (GM) in the brain mask, while excluding the skull, skin, fat, muscles, dura, eyes, bone, exterior blood vessels, and exterior nerves. Images preprocessed for MAGeT were similarly used for BEaST.
CT Analysis
CT was estimated using the CIVET processing pipeline (version 1.1.12; Montreal Neurological Institute). T1-weighted images were aligned linearly to the ICBM 152 average template through a nine-parameter transformation (three translations, rotations, and scales) (Collins et al, 1994) and preprocessed to reduce intensity non-uniformity effects (Sled et al, 1998). Next, images were classified into GM, WM, and CSF (Zijdenbos et al, 2002). Hemispheres were then modeled as GM and WM surfaces using a deformable model strategy, which generates 4 separate surfaces, each defined by 40 962 vertices (Kim et al, 2005). CT was determined in native space through non-linear surface-based normalization that uses a midsurface between pial and WM surfaces. Images were then smoothed with a 20-mm surface-based diffusion kernel and non-linearly registered to a minimally biased surface-based template (Boucher et al, 2009). Native-space thicknesses were used in all analyses, considering that normalizing for head or brain volume has little relationship to CT and risks introducing noise.
Statistical Analysis
Analyses were performed using SPSS Statistics version 21 (IBM Corporation). Independent-sample t tests were used to compare demographic characteristics, Cramer-Rao lower bounds (CRLBs), FWHM values, signal-to-noise ratios, and GM, WM, and CSF percentages between groups. χ2 or Fisher's exact tests were used to compare frequency data. Glutamatergic neurometabolite levels were compared between groups using analyses of variance.
Group differences in TBV were assessed using an analysis of covariance (ANCOVA), controlling for original T1-weighted image voxel dimensions (henceforth, dimensions), age, and sex. Group differences in PCV were assessed with an ANCOVA, controlling for dimensions, TBV, age, and sex; here, analyses were also performed without TBV as a covariate. Additionally, multiple regressions were performed separately for each group to investigate the relationships between glutamatergic neurometabolite levels and PCV, controlling for dimensions, TBV, age, and sex. Finally, multiple regressions were utilized to measure the relationships between PCV and PANSS subscale total scores, controlling for dimensions, TBV, age, and sex. Owing to our a priori hypotheses that PCV would be smaller in the FEP group and that glutamatergic neurometabolites would have a negative relationship with PCV in the FEP group, significance thresholds set at p<0.05 and p<0.025 (p=0.05÷2 to correct for the examination of both glutamate and Glx), respectively, were used for these investigations. The assessment of the relationships between PCV and clinical symptoms was considered exploratory; thus, a Bonferroni correction for multiple comparisons was employed.
CT vertexwise analyses were performed with the RMINC package (https://github.com/mcvaneede/RMINC). Using a general linear model, separate CT regression analyses were carried out for diagnosis, glutamate, Glx, and PANSS subscale total scores, each controlling for dimensions, age, and sex. Glutamate and Glx analyses were performed independently for FEP and control groups. Maps of t statistics at each vertex were projected onto an average brain template. Analyses were corrected for multiple comparisons using a false discovery rate (FDR<0.05).
Outliers were defined as greater than three times the interquartile range; where applicable, outliers were removed in a neurometabolite-specific manner.
Results
Demographic, Clinical, and 1H-MRS Group Differences
Demographic, clinical, and 1H-MRS results have been reported upon in another publication (Plitman et al, 2016), although data pertinent to the present study are included in Supplementary Tables S1–S3. DSM-IV diagnoses of patients with FEP were brief psychotic disorder (n=14), schizophreniform disorder (n=21), and schizophrenia (n=25). No group differences existed in age, sex, handedness, and cannabis use. Education years were higher in the control group (t(118)=6.40, p<0.001), whereas tobacco use was greater in the FEP group (χ2=5.21, p=0.039). The FEP group had a mean duration of untreated psychosis of 33.03±52.70 weeks, and mean PANSS positive, negative, and general psychopathology subscale total scores of 24.13±4.97, 24.33±5.66, and 48.75±8.38, respectively. One glutamate outlier was removed before analysis; however, removal of this outlier did not affect results. Glutamate levels were higher in the FEP group (F(1,117)=11.63, p<0.001), whereas Glx levels were not different between groups (F(1,118)=1.84, p=0.18). Neurometabolite CRLBs, FWHM values, signal-to-noise ratios, and GM, WM, and CSF voxel percentages did not differ between groups.
Group Differences in PCV and TBV
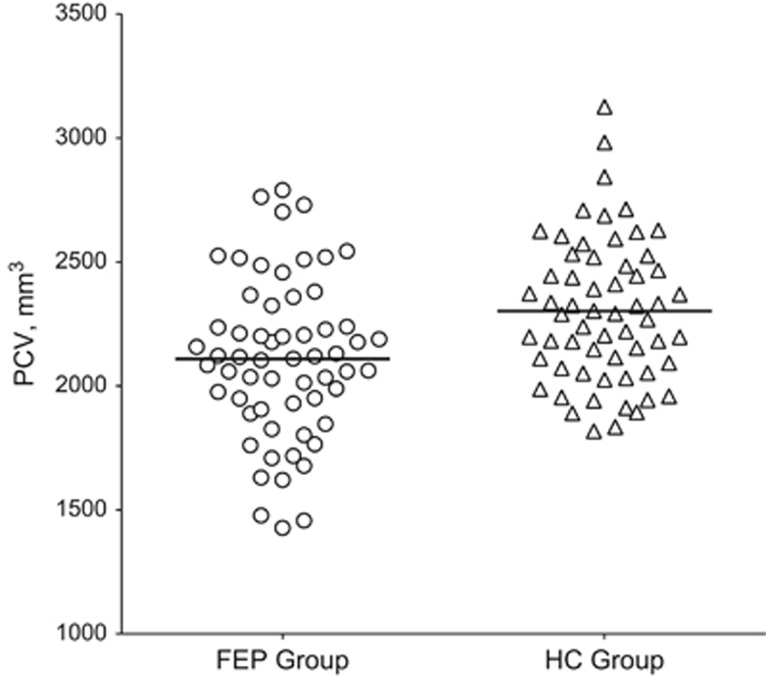
No outliers were identified for PCV or TBV. PCV and TBV values are presented in Table 1; mean PCV values are also displayed in Figure 1. Without TBV as a covariate, PCV was smaller in the FEP group (F(1,114)=11.88, p<0.001). However, controlling for TBV rendered the group difference insignificant (F(1,113)=1.18, p=0.28). TBV was smaller in the FEP group (F(1,114)=16.80, p<0.001).
Table 1. Precommissural Caudate Volume and Total Brain Volume in Patients with First-Episode Psychosis and Healthy Controls.
| Mean (SD) (mm3) | ||
|---|---|---|
| PCV | TBV | |
| FEP group | 2109.80 (320.91)a | 1 354 333.20 (142 317.05)a |
| HC group | 2302.63 (287.98) | 1 442 801.00 (137 349.56) |
Abbreviations: FEP, first-episode psychosis; HC, healthy control; PCV, precommissural caudate volume; TBV, total brain volume.
Denotes p<0.001.
Figure 1.
Precommissural caudate volume (PCV) in patients with first-episode psychosis and healthy controls.
Group Differences in CT
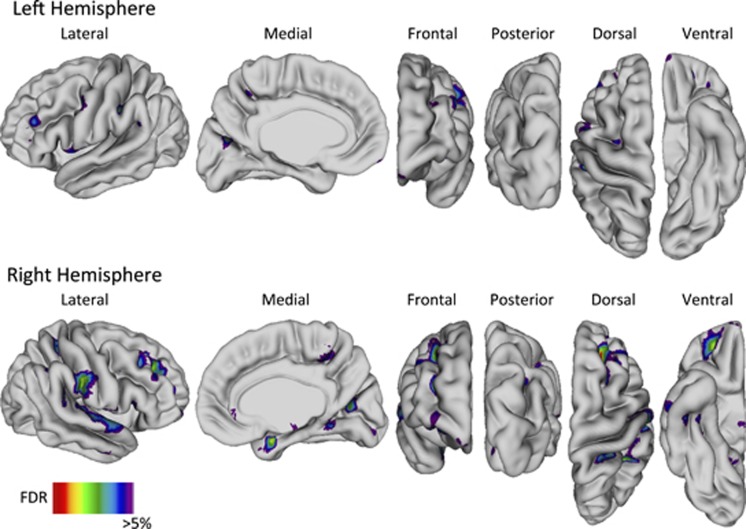
Widespread thinning was identified in the FEP group within the frontal gyri (right superior, bilateral middle, bilateral inferior, bilateral precentral), parietal gyri (bilateral postcentral, bilateral supramarginal, bilateral angular), temporal gyri (bilateral superior, right middle, right inferior), bilateral gyrus rectus, bilateral orbitofrontal cortex, bilateral precuneus, bilateral cuneus, right superior parietal lobule, right paracentral lobule, right parahippocampal gyrus, right lingual gyrus, and right fusiform gyrus (Figure 2). CT was not increased at any vertex in the FEP group.
Figure 2.
Cortical thinning in patients with first-episode psychosis compared with healthy controls. Abbreviation: FDR, false discovery rate.
Relationships Between Neurometabolite Levels and Structural Measures
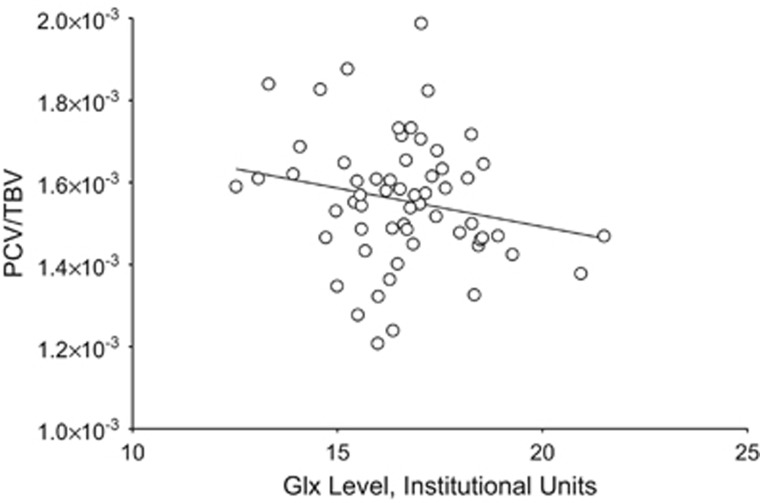
The relationships between glutamatergic neurometabolite levels and PCV are presented in Table 2. Glutamate levels were not related to PCV in either group. Glx levels were negatively associated with PCV only in the FEP group (β=−0.25, t=−2.44, p=0.018). The relationship between PCV/TBV and Glx levels in the FEP group is displayed in Figure 3. No relationships were identified between glutamatergic markers and CT in either group.
Table 2. Relationships Between Glutamatergic Neurometabolite Levels and Precommissural Caudate Volumea.
| Variable | Glu | Glx |
|---|---|---|
| FEP group | ||
| PCV | β=−0.08, t=−0.81, p=0.42 | β=−0.25, t=−2.44, p=0.018b,c |
| HC group | ||
| PCV | β=0.11, t=0.88, p=0.38 | β=0.01, t=0.09, p=0.93 |
Abbreviations: FEP, first-episode psychosis; Glu, glutamate; Glx, glutamate+glutamine; HC, healthy control; PCV, precommissural caudate volume.
For ease of presentation, only model statistics for significant relationships are included; others are in Supplementary Table S4.
Model statistics: adjusted R2=0.597, F5,54=18.51, p<0.001.
Denotes p<0.05.
Figure 3.
Relationship between glutamate+glutamine (Glx) levels and precommissural caudate volume (PCV) relative to total brain volume (TBV) in patients with first-episode psychosis.
Relationships Between Clinical Symptoms and Structural Measures
No relationships between PANSS subscale total scores and PCV were identified (Supplementary Table S5). Similarly, no relationships between PANSS subscale total scores and CT were identified.
Discussion
The present study aimed to investigate whether glutamatergic markers in the PDC were related to PCV and CT in a sample of antipsychotic-naive patients with FEP. Expectedly, we found decreased PCV and widespread cortical thinning within the FEP group; however, when TBV was included as a covariate, PCV did not differ between groups. Glx levels were negatively associated with PCV in the FEP group, while no relationships involving glutamate or CT were identified.
To the best of our knowledge, this is the first study to examine the relationships between glutamatergic neurometabolite levels and volume in this population within the precommissural caudate. Also, though MAGeT-Brain has been recently used to investigate patients with childhood-onset schizophrenia and their non-psychotic siblings (Chakravarty et al, 2015), the present study is the first to use this algorithm to assess a measure of striatal volume in an antipsychotic-naive sample of patients with FEP. Likewise, we believe this is the first study to investigate the relationships between glutamatergic neurometabolite levels and CT in patients with FEP or schizophrenia.
Our finding of decreased PCV in the FEP group is consistent with the current literature. Two meta-analyses found bilateral caudate head GM volume decreases in patients with FEP (Ellison-Wright et al, 2008; Olabi et al, 2012), one of which identified that deficits were more pronounced in FEP than in chronic schizophrenia (Ellison-Wright et al, 2008). Another meta-analysis reported that caudate nucleus reductions were greater in antipsychotic-naive patients than in medicated patients with schizophrenia (Haijma et al, 2013). Caudate volume reductions have been similarly observed in studies including antipsychotic-naive patients with FEP that have adjusted for TBV-analogous measures (Ebdrup et al, 2010; Keshavan et al, 1998). However, other studies considering the effect of TBV have shown no caudate volumetric deficits in antipsychotic-naive or minimally treated patients with FEP (Glenthoj et al, 2007; Gunduz et al, 2002). In our study, group differences in PCV failed to reach significance once TBV was considered as a covariate, suggesting that group volumetric differences may not be specific to PCV and might be occurring simultaneously in other brain regions, such that controlling for TBV masks the PCV deficit in the patient population.
Only one prior study has investigated striatal subdivision volumes in patients with schizophrenia; this study examined medicated male patients with chronic schizophrenia and found no reduction in PDC volume relative to total intracranial contents (Levitt et al, 2013). This is in line with a recent meta-analysis that found no caudate volumetric abnormalities in a large sample of mostly medicated patients with schizophrenia regardless whether intracranial volume was included as a covariate (van Erp et al, 2016). Of note, in this meta-analysis, the sample proportions of medication-naive patients were not associated with caudate volume.
Furthermore, the PDC has been reported to have elevated dopaminergic function and is highly implicated in the pathophysiology of schizophrenia (Kegeles et al, 2010). Excitotoxicity in this region may impact dopaminergic activity and thereby contribute to symptomatology. In the present study, we observed a negative relationship between Glx levels in the PDC and PCV in the FEP group, providing support for a local excitotoxic process. Glutamate-mediated excitotoxicity may contribute to the neuroanatomical deviations present in schizophrenia. It is currently posited that hypofunctioning N-methyl-D-aspartate receptors (NMDARs) on gamma-aminobutyric acid-ergic inhibitory interneurons lead to the disinhibition of downstream pyramidal neurons, a subsequent increase in glutamate release, and consequent excitotoxicity (Stone et al, 2007). Evidence for this phenomenon arises from pharmacological studies using NMDAR antagonists, which lead to the emergence of schizophrenia-like symptomatology in healthy volunteers (Krystal et al, 1994; Lahti et al, 2001) and elicit symptom exacerbation in patients with schizophrenia (Lahti et al, 1995, 2001). Studies in which rodents are exposed to an NMDAR antagonist have shown elevations in extracellular glutamatergic markers (Adams and Moghaddam, 1998; Bustos et al, 1992). Comparably, 1H-MRS studies in healthy humans have shown increased glutamate and glutamine following acute exposure to ketamine (Rowland et al, 2005; Stone et al, 2012). Moreover, studies administering an NMDAR antagonist to rodents have demonstrated resultant neurotoxic injury, which resembles features of schizophrenia and is attenuated by agents inhibiting glutamate release (Plitman et al, 2014).
While preclinical literature strongly supports the role of glutamate-mediated excitotoxicity in schizophrenia, human studies have provided insufficient evidence for the phenomenon (Plitman et al, 2014). Kraguljac et al (2013) reported a negative relationship between left hippocampal Glx and two clusters of left hippocampal GM volume adjusted for total intracranial volume, only in the unmedicated patient group and not in controls. However, Klar et al (2010) did not find a relationship between hippocampal glutamate and hippocampal volume in a medicated sample. Two longitudinal studies investigating a sample of initially unmedicated patients who were then medicated at follow-up visits noted positive correlations between decreasing thalamic glutamate and GM volume within frontal, temporal, parietal, and limbic areas (Aoyama et al, 2011; Theberge et al, 2007). The observed negative relationship between Glx levels and local PCV in the present study is most comparable to the findings of Kraguljac et al (2013) and supports an excitotoxic process in schizophrenia. Our analysis similarly included TBV as a covariate, suggesting that whole brain effects do not drive the relationship between Glx and PCV, and indicating a localized effect. However, the lack of a group difference in PCV when TBV was included as a covariate is not wholly supportive of a specific excitotoxic process in this brain region.
Notably, Glx levels were not significantly elevated in the FEP group, though mean levels were higher than the control group; this group difference may have been underpowered to achieve statistical significance. Thus, the proposed excitotoxicity in the patient group might be a result of elevated Glx levels or a pathological process specific to individuals with FEP that increases susceptibility to the excitotoxicity phenomenon. It is unclear why a similar relationship did not exist for glutamate levels, which were significantly higher in the FEP group. We speculate that glutamine's influence on the Glx peak had a contributing effect; abnormalities in glutamatergic metabolism might account for the observed results in addition to or instead of excitotoxicity, especially considering that 1H-MRS cannot distinguish synaptic from extra-synaptic pools of neurometabolites. Further, neurotransmitter glutamate likely has a greater role in excitotoxicity than intracellular glutamate, and it has previously been suggested that glutamine (contained in Glx) may more robustly reflect the neurotransmitter pool of glutamate than 1H-MRS glutamate itself, which may be mainly intracellular (Wijtenburg et al, 2015).
The cortical thinning identified in the FEP group was diffuse, involving frontal, parietal, temporal, occipital, and limbic brain regions. Our findings are akin to those of previous studies investigating CT in antipsychotic-naive patients with FEP, in which widespread thinning has been reported (Song et al, 2015; Xiao et al, 2015). Within our investigation, the lack of any observed relationships between glutamatergic markers and CT suggests that PDC glutamatergic activity does not account for widespread cortical thinning. Thus, cortical thinning might occur through an alternate mechanism. However, glutamatergic neurometabolites were not measured in cortical areas; consequently, a local inverse relationship between cortical glutamatergic neurometabolites and cortical structural measures cannot be excluded.
Finally, neither PCV nor CT was related to any PANSS subscale total score. Previous studies have similarly failed to identify an association between caudate volumes and symptom scores in untreated patients with schizophrenia (Ballmaier et al, 2008; Venkatasubramanian et al, 2003). With respect to CT, while this null finding is comparable to those from previous CT investigations of patients with FEP or chronic schizophrenia (Rimol et al, 2010; Song et al, 2015), it contrasts a recent CIVET examination of antipsychotic-naive FEP patients in which cortical thinning within frontal regions was related to higher PANSS-positive symptom scores (Xiao et al, 2015).
This study must be considered in light of its limitations. First, the adopted study design does not provide insight toward illness progression or causation. Second, tobacco use was greater in the FEP group. Importantly, when tobacco use was examined as a covariate in all analyses, the only deviation from the presented results was additional cortical thinning observed in the patient group within the left parahippocampal gyrus. Third, to reduce imaging time in patients with active psychosis, 1H-MRS was only performed in the right PDC, although glutamatergic neurometabolite laterality differences were not previously observed in this region (de la Fuente-Sandoval et al, 2009). Fourth, the inclusion of patients with brief psychotic disorder presents an important limitation given their short duration of illness. Fifth, since the 1H-MRS voxel was located in the right PDC, the present study focused on the examination of right PCV. However, an exploratory analysis involving left PCV demonstrated no laterality effects: left PCV was reduced in the FEP group without TBV as a covariate (F(1,114)=8.27, p=0.005); controlling for TBV rendered this difference insignificant; Glx levels were negatively associated with left PCV only in the FEP group (β=−0.23, t=−2.17, p=0.035; Model Statistics: adjusted R2=0.541, F5,54=14.91, p<0.001); and no relationships existed between left PCV and glutamate or PANSS subscale total scores. Sixth, despite the preferential placement of the 1H-MRS voxel in the PDC, it encompassed other associative striatum subregions and included components of other structures (eg, putamen and internal capsule). The 1H-MRS voxel location was referred to as the PDC to maintain consistency in nomenclature. Seventh, to focus on most local phenomena and examine a highly implicated subregion in schizophrenia, the current investigation employed an a priori hypothesis concerning the precommissural caudate. Although the present study is limited in its investigation of regionally specific excitotoxicity in that 1H-MRS was not collected elsewhere, other subcortical structure volumes were examined in exploratory analyses to provide support for this phenomenon. As shown in Supplementary Tables S6 and S7, no associations were identified between PDC glutamatergic neurometabolites and other subcortical structure volumes after correcting for multiple comparisons. Eighth, with PRESS at 3T, chemical shift artifact might result in varying volumes of interest between neurometabolites and the water signal; however, we anticipate that such would be the case for both patients and controls. Finally, though our sample size was not small, our study may have been underpowered.
Taken together, our findings offer evidence in support of a local excitotoxic process in schizophrenia. This mechanism may contribute to structural compromise in the illness. Going forward, a longitudinal study following FEP patients as they transition to schizophrenia, investigating several brain regions using 1H-MRS, is warranted. An improved understanding of dysregulated neurometabolites and their consequent impact on structural losses in schizophrenia may aid in furthering our knowledge of illness pathophysiology and might identify future diagnostic and therapeutic strategies.
Funding and disclosure
Computations were performed on the gpc supercomputer at the SciNet HPC Consortium. SciNet is funded by the Canada Foundation for Innovation under the auspices of Compute Canada, the Government of Ontario, Ontario Research Fund—Research Excellence, and the University of Toronto.
Acknowledgments
We would like to acknowledge the technical assistance provided by Gabriel Devenyi and Robert Amaral, in addition to the invaluable support provided by the entire CoBrA Lab at the Douglas Mental Health University Institute, Montreal, QC, Canada. We would also like to acknowledge the important and frequent assistance provided by Shinichiro Nakajima, Yusuke Iwata, Fernando Caravaggio, and Philip Gerretsen. This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT) (182279 to CdlF-S and AG-G), CONACyT project 261895 (CdlF-S), the CONACyT Scholarship (FR-M and PL-O), the Sistema Nacional de Investigadores (CdlF-S and AG-G), the Canadian Institute of Health Research (CIHR) (MOP-114989 to AG-G), the Vanier Canada Graduate Scholarship (EP), the Ontario Graduate Scholarship (EP), and the Canada Graduate Scholarship – Master's (EP). These sources were not involved in the study design, data collection, data analysis, or the decision to publish the manuscript. EP has received funding from the Vanier Canada Graduate Scholarship, the Ontario Graduate Scholarship, and the Canada Graduate Scholarship – Master's. FR-M has served as a speaker for AstraZeneca. CdlF-S has received support from the United States National Institute of Health (US-NIH), CONACyT, the Instituto de Ciencia y Tecnología del DF (ICyTDF), Janssen, AstraZeneca, and Eli Lilly. AG-G has received support from US-NIH, CIHR, the Ontario Mental Health Foundation, CONACyT, ICyTDF, the Brain & Behavior Research Foundation (Formerly NARSAD), the Ontario Ministry of Health and Long-Term Care, the Ontario Ministry of Research and Innovation Early Research Award, and Janssen. EP, MMC, CdlF-S, and AG-G contributed to the study concept and design. FR-M, GG-C, PL-O, CdlF-S, and AG-G participated in data acquisition. EP, RP, JKC, JP, SC, MMC, CdlF-S, and AG-G analyzed and interpreted the data. EP, RP, MMC, CdlF-S, and AG-G drafted the manuscript. All authors critically revised the manuscript. SC provided technical support. CdlF-S and AG-G supervised the study. All authors have approved the final version of this manuscript and have agreed to its submission.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adams B, Moghaddam B (1998). Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci 18: 5545–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama N, Theberge J, Drost DJ, Manchanda R, Northcott S, Neufeld RW et al (2011). Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psychiatry 198: 448–456. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC (2008). Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12: 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier M, Schlagenhauf F, Toga AW, Gallinat J, Koslowski M, Zoli M et al (2008). Regional patterns and clinical correlates of basal ganglia morphology in non-medicated schizophrenia. Schizophr Res 106: 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher M, Whitesides S, Evans A (2009). Depth potential function for folding pattern representation, registration and analysis. Med Image Anal 13: 203–214. [DOI] [PubMed] [Google Scholar]
- Bustos G, Abarca J, Forray MI, Gysling K, Bradberry CW, Roth RH (1992). Regulation of excitatory amino acid release by N-methyl-D-aspartate receptors in rat striatum: in vivo microdialysis studies. Brain Res 585: 105–115. [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Bertrand G, Hodge CP, Sadikot AF, Collins DL (2006). The creation of a brain atlas for image guided neurosurgery using serial histological data. NeuroImage 30: 359–376. [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Rapoport JL, Giedd JN, Raznahan A, Shaw P, Collins DL et al (2015). Striatal shape abnormalities as novel neurodevelopmental endophenotypes in schizophrenia: a longitudinal study. Hum Brain Mapp 36: 1458–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty MM, Steadman P, van Eede MC, Calcott RD, Gu V, Shaw P et al (2013). Performing label-fusion-based segmentation using multiple automatically generated templates. Hum Brain Mapp 34: 2635–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994). Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Favila R, Alvarado P, Leon-Ortiz P, Diaz-Galvis L, Amezcua C et al (2009). [Glutamate increase in the associative striatum in schizophrenia: a longitudinal magnetic resonance spectroscopy preliminary study]. Gac Med Mex 145: 109–113. [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L et al (2013). Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry 70: 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, Stephano S, Mamo D, Ramirez-Bermudez J et al (2011). Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology 36: 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebdrup BH, Glenthoj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson OB et al (2010). Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci 35: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E (2008). The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry 165: 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskildsen SF, Coupe P, Fonov V, Manjon JV, Leung KK, Guizard N et al (2012). BEaST: brain extraction based on nonlocal segmentation technique. NeuroImage 59: 2362–2373. [DOI] [PubMed] [Google Scholar]
- Glenthoj A, Glenthoj BY, Mackeprang T, Pagsberg AK, Hemmingsen RP, Jernigan TL et al (2007). Basal ganglia volumes in drug-naive first-episode schizophrenia patients before and after short-term treatment with either a typical or an atypical antipsychotic drug. Psychiatry Res 154: 199–208. [DOI] [PubMed] [Google Scholar]
- Gunduz H, Wu H, Ashtari M, Bogerts B, Crandall D, Robinson DG et al (2002). Basal ganglia volumes in first-episode schizophrenia and healthy comparison subjects. Biol Psychiatry 51: 801–808. [DOI] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS (2013). Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull 39: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M et al (2010). Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 67: 231–239. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X et al (2012). Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 69: 449–459. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Rosenberg D, Sweeney JA, Pettegrew JW (1998). Decreased caudate volume in neuroleptic-naive psychotic patients. Am J Psychiatry 155: 774–778. [DOI] [PubMed] [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab'bagh Y, MacDonald D et al (2005). Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. NeuroImage 27: 210–221. [DOI] [PubMed] [Google Scholar]
- Klar AA, Ballmaier M, Leopold K, Hake I, Schaefer M, Bruhl R et al (2010). Interaction of hippocampal volume and N-acetylaspartate concentration deficits in schizophrenia: a combined MRI and 1H-MRS study. NeuroImage 53: 51–57. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Reid MA, Lahti AC (2013). Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry 70: 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD et al (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199–214. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA (1995). Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13: 9–19. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Reid MA (2011). Is there evidence for neurotoxicity in the prodromal and early stages of schizophrenia? Neuropsychopharmacology 36: 1779–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA (2001). Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 25: 455–467. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Evans AC (2005). Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage 24: 163–173. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, Rosow LK, Nestor PG, Pelavin PE, Swisher TM, McCarley RW et al (2013). A volumetric MRI study of limbic, associative and sensorimotor striatal subregions in schizophrenia. Schizophr Res 145: 11–19. [DOI] [PubMed] [Google Scholar]
- Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL (2013). Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol 698: 6–18. [DOI] [PubMed] [Google Scholar]
- Olabi B, Ellison-Wright I, Bullmore E, Lawrie SM (2012). Structural brain changes in first episode schizophrenia compared with fronto-temporal lobar degeneration: a meta-analysis. BMC Psychiatry 12: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitman E, de la Fuente-Sandoval C, Reyes-Madrigal F, Chavez S, Gomez-Cruz G, Leon-Ortiz P et al (2016). Elevated myo-inositol, choline, and glutamate levels in the associative striatum of antipsychotic-naive patients with first-episode psychosis: a proton magnetic resonance spectroscopy study with implications for glial dysfunction. Schizophr Bull 42: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitman E, Nakajima S, de la Fuente-Sandoval C, Gerretsen P, Chakravarty MM, Kobylianskii J et al (2014). Glutamate-mediated excitotoxicity in schizophrenia: a review. Eur Neuropsychopharmacol 24: 1591–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW (2001). Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14: 260–264. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvag R, Fennema-Notestine C, Hagler DJ Jr., Pung CJ et al (2010). Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry 68: 41–50. [DOI] [PubMed] [Google Scholar]
- Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E et al (2005). Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry 162: 394–396. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17: 87–97. [DOI] [PubMed] [Google Scholar]
- Song X, Quan M, Lv L, Li X, Pang L, Kennedy D et al (2015). Decreased cortical thickness in drug naive first episode schizophrenia: in relation to serum levels of BDNF. J Psychiatr Res 60: 22–28. [DOI] [PubMed] [Google Scholar]
- Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ et al (2012). Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry 17: 664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS (2007). Glutamate and dopamine dysregulation in schizophrenia—a synthesis and selective review. J Psychopharmacol (Oxford, England) 21: 440–452. [DOI] [PubMed] [Google Scholar]
- Theberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK et al (2007). Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry 191: 325–334. [DOI] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA et al (2010). N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29: 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA et al (2016). Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 21: 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian G, Gangadhar BN, Jayakumar PN, Janakiramaiah N, Keshavan MS (2003). Reduced caudate volume in never-treated schizophrenia: evidence for neuro developmental etiopathogenesis. Indian J Psychiatry 45: 20–26. [PMC free article] [PubMed] [Google Scholar]
- Wijtenburg SA, Yang S, Fischer BA, Rowland LM (2015). In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neurosci Biobehav Rev 51: 276–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Lui S, Deng W, Yao L, Zhang W, Li S et al (2015). Altered cortical thickness related to clinical severity but not the untreated disease duration in schizophrenia. Schizophr Bull 41: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC (2002). Automatic "pipeline" analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imag 21: 1280–1291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.